Abstract
Nef, an important pathogenicity factor of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV), elevates virus replication in vivo. Among other activities, Nef affects T-cell receptor (TCR) signaling via several mechanisms. For HIV-1 Nef these include alteration of the organization and function of the immunological synapse (IS) such as relocalization of the Lck kinase, as well as early inhibition of TCR/CD3 complex (TCR-CD3)-mediated actin rearrangements and tyrosine phosphorylation. Although most SIV and HIV-2 Nef alleles (group 2) potently downregulate cell surface TCR-CD3, this activity was lost in the viral lineage that gave rise to HIV-1 and its SIV counterparts (group 1). To address the contribution of TCR-CD3 downregulation to Nef effects on TCR signal initiation, we compared the activities of 18 group 1 and group 2 Nef proteins, as well as SIV Nef mutants with defects in TCR-CD3 downmodulation. We found that alteration of Lck's subcellular localization is largely conserved and occurs independently of actin remodeling inhibition or TCR-CD3 downregulation. Surprisingly, Nef proteins of both groups also strongly reduced TCR-induced actin remodeling and tyrosine phosphorylation on TCR-stimulatory surfaces and TCR-CD3 downmodulation competence by group 2 Nef proteins only slightly elevated these effects. Furthermore, Nef proteins from HIV-1 and SIV reduced conjugation between infected primary human T lymphocytes and Raji B cells and potently prevented F-actin polarization at the IS independently of their ability to downmodulate TCR-CD3. These results establish alterations of early TCR signaling events at the IS, including F-actin remodeling and relocalization of Lck, as evolutionary conserved activities of highly divergent lentiviral Nef proteins.
The Nef protein is a 25- to 35-kDa myristoylated accessory gene product that is exclusively expressed by the lentiviruses human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIV). Largely dispensable for virus replication in ex vivo cell cultures, Nef expression potently elevates lentiviral replication in the infected host and represents a crucial parameter for disease progression (8, 27, 29). Consistent with a central role as lentiviral pathogenesis factor, expression of Nef as sole gene product of HIV-1 or SIV in transgenic mice causes AIDS-like depletion of CD4+ T lymphocytes (21). A large array of effector functions of Nef, all mediated by interactions of this viral adaptor protein with host cell factors, have been described that, in cumulative fashion, could account for this vital role in AIDS pathogenesis (28, 30). Overall, Nef exerts independent effects on host cell vesicular transport and signal transduction processes that directly facilitate virus spread and immune evasion of productively infected cells. In T lymphocytes, one of the main target cell populations of HIV, Nef predominantly affects signal transduction via the T cell receptor (TCR) (10). Both positive and negative effects of Nef on TCR signal transduction have been reported and the reasons for these discrepant observations are not entirely clear. One view that reconciles many of these findings is that Nef may generally elevate basal states of T-cell activation, while its expression limits the response to exogenous TCR engagement (12, 13, 20, 25, 36). By the combination of these effects, Nef may reduce premature activation-induced death of infected cells while simultaneously increasing their permissivity for HIV-1 replication (9). However, possibly depending on the experimental system and the Nef allele used (see below), potent sensitization of T lymphocytes to TCR stimulation has also been observed (38).
In a physiological context, exogenous T-cell activation occurs in the context of a close contact between an antigen-presenting cell (APC) and a T cell, referred to as the immunological synapse (IS). Engagement of the TCR by major histocompatibility complex (MHC)-bound antigenic peptides triggers profound reorganization of the protein and lipid composition at the IS, leading to the formation of signaling competent protein microclusters (22, 35). Lateral sorting processes at the IS, as well as the stabilization of cell-cell contacts are mediated by massive actin rearrangements, and TCR-induced actin dynamics are prerequisites for subsequent signaling, including the induction of a characteristic cascade of tyrosine phosphorylation events, Ca2+ release and target gene expression (2, 5, 24). A series of recent studies linked the ability of HIV-1 Nef to reduce exogenous TCR stimulation of infected T lymphocytes to the inhibition of IS formation and organization. Using APC-T-cell conjugates, Thoulouze et al. demonstrated that Nef expression reduces the frequency of conjugate formation but also causes the pronounced accumulation of the TCR proximal Src kinase Lck in an intracellular endosomal compartment. These alterations were paralleled by a significant reduction of TCR-induced tyrosine phosphorylation and interleukin-2 (IL-2) production (41). By studying Nef effects on TCR stimulatory surfaces, an experimental approach that closely mimics events at T-cell-APC contacts (4), we were able to confirm these effects of Nef on TCR-induced tyrosine phosphorylation and subcellular distribution of Lck and observed that, in particular, tyrosine phosphorylation of the TCR proximal kinase Zap70 and recruitment of N-Wasp to the sites of TCR engagement was reduced in the presence of Nef (20). Moreover, we found that HIV-1 Nef potently disrupts F-actin remodeling and cell spreading in response to TCR engagement. This effect, however, occurred independently of Nef's effect on intracellular Lck accumulation (19). Together, these studies revealed profound and independent effects of HIV-1 Nef on intracellular sorting and actin remodeling at the IS; however, these studies did not address how these effects contribute to Nef-mediated alterations in IS function and downstream signaling (18).
Although most activities are conserved among Nef proteins of HIV-1, HIV-2, and SIV, functional differences have also been identified. Importantly, HIV-2 and SIV Nef proteins interact with the TCR zeta chain and exert potent downmodulation of cell surface TCR/CD3 complexes (TCR-CD3), an activity lacking in all HIV-1 Nef proteins analyzed to date (1, 23, 31). This ability to reduce cell surface densities of TCR-CD3 correlates to some extent with the induction of AIDS-like disease by these viruses in their natural host (36, 37), suggesting that the loss of this activity during viral transmission from monkeys to humans contributes to the development of AIDS in human HIV-1 infection (28). Based on these criteria, Nef proteins can be stratified into a TCR-CD3 downregulation incompetent class (group 1, HIV-1 and SIVcpz) and a class that potently downregulates TCR-CD3 (group 2, HIV-2 and SIV). Although group 1 viruses are generally considered pathogenic, group 2 viruses typically display at least reduced pathogenic potential in their natural host. Consistent with this model, T lymphocytes expressing group 2 Nef proteins are markedly less sensitive to exogenous TCR-CD3 stimulation, as measured by induction of early and late T-cell activation markers CD69 and IL-2R and activation of the NFAT transcription factor, than cells expressing Nef proteins of group 1 (31, 36). An important implication of this reduced sensitivity to activation could be that group 2 Nef proteins protect cells from hyperactivation, leading to activation-induced cell death, thereby prolonging the life span of infected cells and thus increasing the release of viral progeny. As discussed above, strength and specificity of TCR signal transduction are determined in the context of the IS. Given their different potency to affect TCR signaling, one straightforward model would therefore predict that disruption of the IS is more potent with Nef proteins from group 2 than with those from group 1. In the present study, we tested this hypothesis by a comprehensive characterization of a panel of group 1 and group 2 Nef proteins for their ability to affect early events following TCR-CD3 engagement that are known to be affected by HIV-1 Nef.
MATERIALS AND METHODS
Cells, reagents, and plasmids.
Jurkat TAg cells and Raji B cells were cultivated in RPMI 1640 plus GlutaMAX-I supplemented with 10% fetal calf serum (FCS) and 1% penicillin-streptomycin (all from Invitrogen). For T-cell spreading, the monoclonal antibody (MAb) anti-CD3 (clone HIT3a) was used (BD Pharmingen). Further analyses were performed with the following antibodies: rabbit anti-phospho-tyrosine (BD Pharmingen) and MAb anti-Lck (clone 3A5) (Santa Cruz). Secondary goat anti-mouse and anti-rabbit Alexa Fluor 568-conjugated antibodies were purchased from Invitrogen. For F-actin stain, TRITC (tetramethyl rhodamine isothiocyanate)-phalloidin (Sigma) was used. The expression constructs for HIV-1 SF2 Nef.GFP wild-type (wt) and mutant proteins were described elsewhere (20, 34). Proviral plasmids based on pBR-NL4.3 Nef.IRES.eGFP encoding for all HIV-1, HIV-2, and SIV Nef alleles used were as described previously (36). Nef.YFP expression plasmids of these alleles were generated by subcloning the nef open reading frame into pEYFP-C1 using NheI/AgeI restriction sites. For Nef proteins of SIVmac239 and SIVblue, site-directed mutagenesis was used to introduce various point mutations.
Immunofluorescence analyses.
A total of 5 × 106 Jurkat TAg cells were transfected with 15 to 20 μg of total plasmid DNA via electroporation (960 μF, 250 V; Bio-Rad GenePulser). Microscope cover glasses (Marienfeld) were prepared by cleaning with 1 M HCl-70% ethanol, drying at 60°C, incubation in 0.01% poly-l-lysine (Sigma) solution for 10 min at room temperature, and subsequent drying at 60°C. At 36 h posttransfection, 3 × 105 Jurkat cells in 50 μl of medium without additives were plated on the cover glasses, incubated for 5 min, and subsequently fixed for 10 min by directly adding Tris-buffered saline (TBS)-3% paraformaldehyde. After permeabilization with TBS-0.1% Triton X-100 for 1 min, the cells were blocked with TBS-1% bovine serum albumin for 30 min. Indirect immunofluorescence was performed by incubating cells with 1:50 (anti-Lck)- and 1:100 (anti-phospho-tyrosine)-diluted primary antibodies for 4 h and overnight, respectively. After being washed with TBS, fluorochrome-labeled secondary antibodies (1:2,000) were added for 1 h. Cover glasses were mounted in LinMount (Linaris) and analyzed with an IX81 fluorescence microscope (Olympus) or a LSM 510 confocal laser scanning microscope (Zeiss). Images were taken by using a ×60 or ×100 oil immersion objective lens and processed by using Adobe Photoshop.
Analysis of TCR-mediated actin ring formation.
T-cell spreading on stimulatory surfaces was performed essentially as described previously (19, 20). Briefly, microscope cover glasses were prepared as described above and subsequently coated with anti-CD3 antibody diluted in TBS (10 μg/ml) for 3 h at 37°C. After being washed with TBS, the cover glasses were stored in TBS at 4°C. Cells were added in a volume of 50 μl onto the glasses, incubated for 5 min at 37°C, and fixed by the direct addition of paraformaldehyde. Further steps were performed as described above.
Analysis of TCR-mediated pTyr induction.
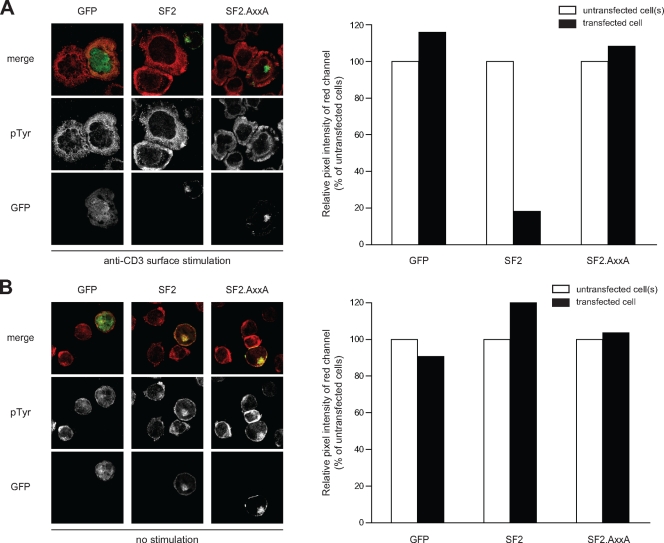
For quantification of pTyr levels, cells on TCR stimulatory cover glasses were stained for pTyr as described above. pTyr levels were judged by microscopic analysis, estimating whether Nef expressing cells displayed pTyr levels similar (high pTyr) or significantly reduced (low pTyr) relative to adjacent neighboring cells As shown in Fig. 3, quantification of pTyr pixel intensities using ImageJ software in Nef-expressing versus control cells revealed that wt Nef.GFP- but not NefAxxA.GFP- or green fluorescent protein (GFP)-expressing cells typically displayed <20% of the pTyr signal detected in Nef-negative neighboring cells.
FIG. 3.
Validation of pTyr quantification. (A) Jurkat T cells transfected with the indicated expression plasmids were incubated on TCR-stimulatory surfaces, fixed, and analyzed for the subcellular distribution of pTyr-positive signaling complexes by confocal microscopy. The left panel depicts merged confocal micrographs of the GFP/YFP (green) and pTyr (red) channels 5 min after TCR stimulation of representative cells. The panel on the right shows the quantification of pTyr pixels of the cells on the left. GFP- and AxxA-expressing cells displayed pTyr levels similar to their untransfected neighboring cells and were thus scored as containing “high” levels of pTyr in Fig. 4B and 5D. pTyr levels were significantly lower in cells expressing Nef and were thus considered as “low” levels of pTyr. (B) Analysis analogous to panel A but on nonstimulatory surfaces. pTyr levels are generally lower than after TCR stimulation, and no significant effect was detected on these basal pTyr levels by the expression of Nef.
Virus production and infection.
Virus stocks were generated by transfection of 293T cells with proviral plasmids as described previously (11). Isolation, activation, and HIV-1 infection of PBL was carried out as described previously (20). Virus was removed 24 h postinfection, and new medium containing 10 ng of IL-2/ml was added 1 and 2 days postinfection. On day 3 postinfection cells were used for the conjugate and immunological synapse assay (see below).
Conjugate assay.
Conjugate and immunological synapse formation between Staphylococcus aureus enterotoxin E (SEE; Toxin Technology)-loaded Raji B cells and Jurkat TAg cells or PBL was carried out as described previously (7, 41). Briefly, Raji B cells were stained with CellTracker Blue (Invitrogen) and subsequently incubated with SEE (10 ng/ml) in 0.5% FCS-RPMI GlutaMAX-I for 1 h. For conjugate and immunological synapse formation, SEE-loaded Raji B cells were incubated with Jurkat TAg cells or PBL in a 1:1 ratio in 0.5% FCS-RPMI GlutaMAX-I for 45 min at 37°C. Seeding of cells on cover glasses and further steps were done as described above.
NFAT activity assay.
NFAT assays were essentially performed as described previously (36, 37). Briefly, 5 × 104 Jurkat cells stably transfected with an NFAT-dependent luciferase reporter gene (13) were infected with the indicated HIV-1 NL4.3.Nef.IRES.eGFP viruses. At 2 days postinfection the cells were either left untreated or stimulated with 1 μg of phytohemagglutinin (PHA)/ml. At 16 h poststimulation the cells were lysed and assessed for luciferase activity.
Statistical analysis.
Data evaluation and statistical analysis using the Mann-Whitney U test or Pearson correlation analysis was done by using the GraphPad Prism software.
RESULTS
Alteration of Lck subcellular localization is a conserved and TCR-CD3 downregulation-independent Nef activity.
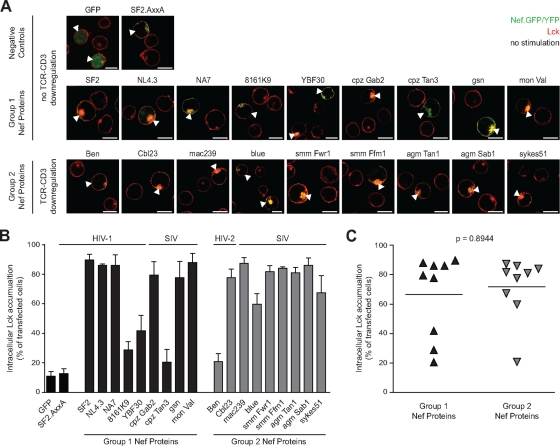
To address which aspects of IS modulation are conserved activities of lentiviral Nef proteins and to assess the specific contribution of TCR-CD3 cell surface downmodulation to these processes, we analyzed a panel of previously extensively characterized HIV-1, HIV-2, and SIV Nef proteins (36). In all, nine Nef proteins of group 1 (HIV-1 and SIVcpz, no downregulation of cell surface TCR-CD3 [36]) and nine Nef proteins of group 2 (HIV-2 and SIV, downregulation of cell surface TCR-CD3 [36]) were analyzed. To allow for concomitant functional and microscopic analyses, fusion proteins of Nef with GFP or yellow fluorescent protein (YFP), respectively, were used. As a first parameter of modulation of TCR-proximal machinery by Nef we analyzed the relocalization of the Src kinase Lck (19, 41). Since HIV-1 Nef induces a pronounced accumulation of Lck in endosomal compartments already prior to CD3 engagement, this analysis was performed in Jurkat T lymphocytes that transiently expressed Nef.GFP/YFP in the absence of anti-CD3 stimulation. Expectedly, Lck was distributed mostly at the plasma membrane with some additional endosomal localization in control cells expressing GFP or the Nef mutant AxxA (prolines 76 and 79 mutated to alanine). In contrast, wt Nef from HIV-1 SF2 caused a marked intracellular enrichment of Lck that was observed in 89.7% ± 3.8% of all cells analyzed and apparently colocalized with the kinase (Fig. 1). Independent of their capacity to downregulate TCR-CD3, most group 1 and group 2 Nef proteins potently induced intracellular Lck accumulation that was indistinguishable in magnitude and localization from that caused by HIV-1 SF2 Nef. However, three Nef proteins (from HIV-1 8161K9 and SIVcpzTan3 [group 1] and HIV-2Ben [group 2]) were defective in inducing Lck accumulation (<30% of cells with intracellular Lck accumulation), and the Nef proteins from HIV-1 YBF30 (group 1) and SIVblue (group 2) displayed intermediate Lck accumulation activity (between 30 and 60% of cells with intracellular Lck accumulation). A comparison of Lck accumulation between Nef proteins of groups 1 and 2 revealed no statistically significant difference (P = 0.8944 [Mann-Whitney U test]) (Fig. 1C). Together, these results demonstrate that, although individual Nef proteins in both groups lost this activity, alteration of Lck subcellular localization is generally a conserved activity of Nef proteins from HIV-1, HIV-2, and SIV that does not require downregulation of cell surface TCR-CD3.
FIG. 1.
Nef proteins of the HIV and SIV lineages induce intracellular accumulation of Lck in T lymphocytes. (A) Confocal microscopy analysis of Jurkat T lymphocytes transfected with expression plasmids for GFP or the indicated Nef.GFP/YFP fusion proteins after staining for endogenous Lck. Depicted are merged pictures of the GFP/YFP (green) and Lck (red) channels of representative confocal sections through the middle of the cell. Arrowheads indicate transfected cells. Scale bar, 10 μm. (B) Quantification of cells displaying pronounced intracellular Lck accumulation. Values are the arithmetic means of at least three independent experiments plus the standard deviation (SD) in which more than 100 cells were counted per condition are given. (C) Comparison of Lck accumulation by Nef proteins of groups 1 and 2. Bars indicate the mean values of the representative group, with the statistical significance indicated by the P value (Mann-Whitney U test).
Interference with TCR-induced actin remodeling and pTyr induction by group 1 and group 2 Nef proteins.
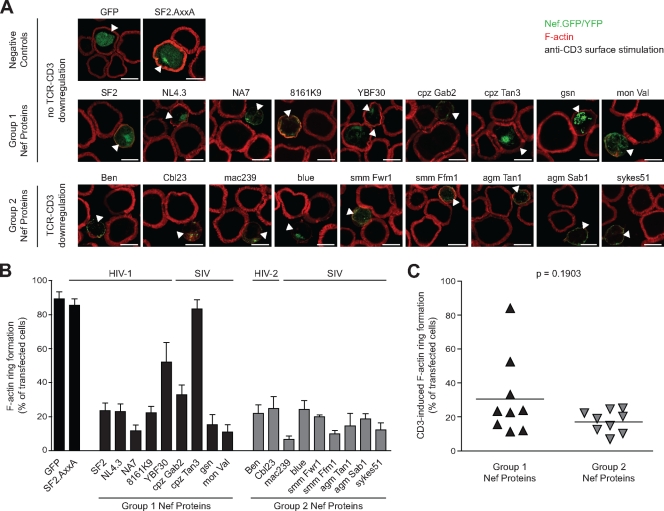
We next assessed the ability of group 1 and group 2 Nef proteins to affect the functional interaction of T lymphocytes with anti-CD3 antibody-coated stimulatory surfaces, an established model system for the visualization of TCR-induced actin remodeling that reflects key aspects of APC-T-lymphocyte interactions (3, 4, 32). In this experimental system, T lymphocytes rapidly spread on the stimulatory surface in response to TCR-CD3 engagement to form characteristic F-actin rich circumferential rings and these processes are inhibited by HIV-1 Nef (Fig. 2A) (20). While F-actin ring formation was efficiently observed in control cells expressing GFP or Nef.AxxA (89.2% ± 4.3% and 85.4% ± 3.9%, respectively), wt Nef of HIV-1 SF2 potently interfered with cell spreading and actin remodeling (23.5% ± 4.5%), as reported earlier (20). Analysis of our panel of group 1 and group 2 Nef proteins revealed a good conservation of the ability to disrupt TCR-induced actin remodeling, with the exception of the group 1 Nef proteins of HIV-1 YBF30 and cpzTan3 that displayed intermediate or absent activity (52.0% ± 11.6% and 83.4% ± 5.3%, respectively). Importantly, no statistically significant difference was observed between group 1 and 2 Nef proteins in their ability to disrupt TCR-induced F-actin rearrangements on stimulatory surfaces (P = 0.1903 [Mann-Whitney U test]) (Fig. 2C). Titration experiments revealed that this was independent of the amount of anti-CD3 antibody used for stimulation (data not shown). However, the block in formation of circumferential F-actin rings appeared generally more complete for group 2 Nef proteins than for their group 1 counterparts, resulting in only trace amounts of subcortical F-actin following TCR-CD3 stimulation.
FIG. 2.
Inhibition of TCR-induced actin remodeling by group 1 and 2 Nef proteins. (A) Confocal microscopy analysis of Jurkat T lymphocytes transfected with the indicated expression plasmids after 5 min of incubation on anti-CD3-coated cover glasses and subsequent staining for F-actin. Depicted are merged pictures of the GFP/YFP (green) and F-actin (red) channels of representative confocal sections near the cover glass. Arrowheads indicate transfected cells. Scale bar, 10 μm. (B) Quantification of the experiment shown in panel A. Shown is the percentage of cells exhibiting pronounced F-actin ring formation. Values are the arithmetic means of at least three independent experiments plus the SD in which more than 100 cells were counted per condition. (C) Comparison of F-actin ring formation in the presence of group 1 and group 2 Nef proteins. Bars indicate the mean values of the representative group, with the statistical significance indicated by the P value (Mann-Whitney U test).
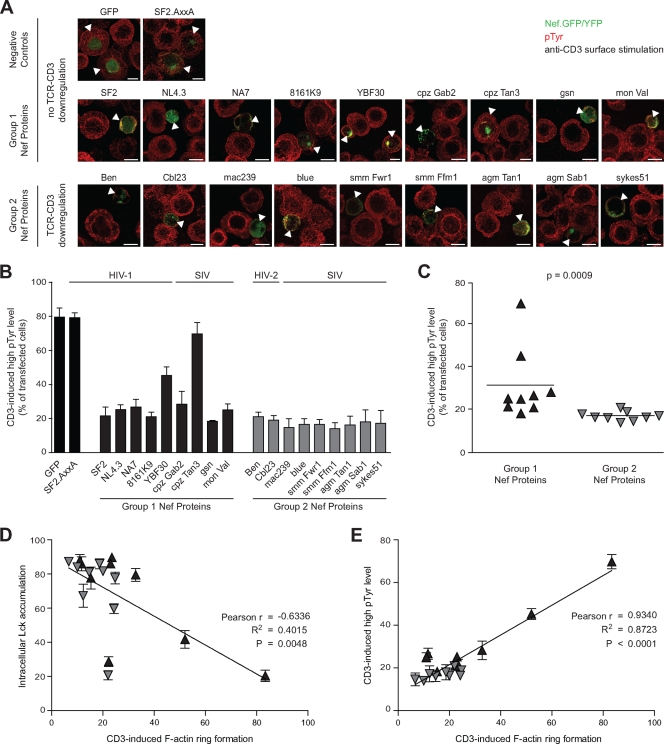
Since we had previously identified that the disruption of F-actin ring formation correlates with a reduced induction of tyrosine phosphorylation following TCR-CD3 stimulation (19, 20), we also performed a semiquantitative analysis of total phosphotyrosine (pTyr) levels in cells expressing group 1 and group 2 Nef proteins. After immunostaining for pTyr, the pTyr levels were judged by microscopic analysis, estimating whether Nef expressing cells displayed pTyr levels similar (high pTyr) or significantly reduced (low pTyr) relative to untransfected adjacent neighboring cells. Figure 3 presents the validation of this strategy with a quantification of pTyr pixel intensities using ImageJ software in Nef-expressing versus control cells that reveals that wt Nef.GFP- but not NefAxxA.GFP- or GFP-expressing cells typically displayed <20% of the pTyr signal detected in Nef-negative neighboring cells (Fig. 3A). In the absence of TCR stimulation, pTyr levels were generally lower and not affected by the expression of Nef (Fig. 3B). Using this stratification to define cells with high (normal) pTyr levels following surface-mediated TCR-CD3 stimulation, expression of the negative controls GFP or AxxA.GFP did not affect the robust induction of pTyr since the levels were indistinguishable from those in untransfected neighboring cells in 79.5% ± 5.5% and 79.3% ± 2.8% of the cells, respectively (Fig. 4A and B). In contrast, Nef proteins from both groups in general markedly reduced the amounts of pTyr induced by anti-CD3 stimulation, resulting in >70% of transfected cells displaying significantly lower pTyr levels than untransfected neighboring cells (Fig. 4A and B). Of note, the results obtained for the inhibition of F-actin ring formation and pTyr induction gave remarkably similar results, including the partial or complete loss of activity for Nef from HIV-1 YBF30 and cpzTan3 (45.3% ± 5.0% and 69.8% ± 6.6% with high pTyr levels, respectively) (Fig. 4B). However, in the case of disruption of pTyr induction, group 2 Nef proteins caused significantly more pronounced effects than their group 1 counterparts (P = 0.0009) (Fig. 4C). Cross-correlating the three effects of Nef on early TCR signaling among all Nef proteins analyzed revealed a weak correlation between inhibition of actin remodeling and inducing Lck accumulation (Pearson's r = −0.6336, R2 = 0.4015, P = 0.0048) (Fig. 4D). In contrast, a strong correlation was observed between the inhibition of actin remodeling and the induction of pTyr (Pearson's r = −0.9340, R2 = 0.8723, P < 0.0001) (Fig. 4E). Together, these results demonstrate that Nef does not require TCR-CD3 downmodulation activity to disrupt TCR-induced actin remodeling and pTyr induction.
FIG. 4.
Correlation between Nef-mediated inhibition of TCR-induced actin dynamics and tyrosine phosphorylation. (A) Jurkat T cells transfected with the indicated expression plasmids were incubated on TCR-stimulatory surfaces, fixed, and analyzed for the subcellular distribution of pTyr-positive signaling complexes by confocal microscopy. Depicted are merged pictures of the GFP/YFP (green) and pTyr (red) channels 5 min after TCR stimulation. Arrowheads indicate transfected cells. Scale bar, 10 μm. (B) Quantification of the experiment shown in panel A. Values are the arithmetic means of at least three independent experiments plus the SD in which more than 100 cells were counted per condition. The pTyr levels of transfected cells were scored as high and low, respectively, by comparing the intensity of their pTyr staining to that of untransfected neighboring cells. (C) Comparison of pTyr levels in the presence of Nef proteins of groups 1 and 2. Bars indicate the mean values of the representative group, with the statistical significance indicated by the P value (Mann-Whitney U test). (D) Correlation analysis of the efficacy of Nef-mediated inhibition of F-actin rings with intracellular Lck accumulation (Pearson's r, R2). (E) Correlation analysis of the efficacy of Nef-mediated inhibition of F-actin rings with induction of high pTyr levels (Pearson's r, R2).
Downmodulation of cell surface TCR-CD3 is a minor determinant for disruption of TCR-induced actin remodeling by SIV Nef.
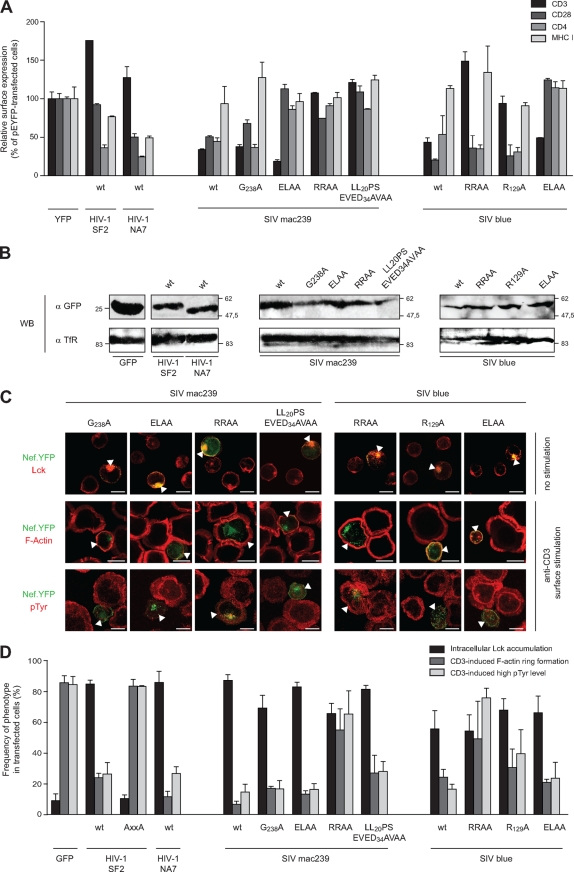
To address more directly the specific role of TCR-CD3 downmodulation in the modulation of IS organization and function by group 2 Nef proteins, we made use of mutant variants of Nef from SIVmac239 and SIVblue that display specific defects in receptor downmodulation. We first characterized the ability of these Nef.YFP proteins to downmodulate cell surface CD3, CD4, CD28, and MHC-I (Fig. 5A) when expressed to comparable levels in Jurkat T lymphocytes (Fig. 5B). Expectedly (36), Nef proteins from HIV-1 NA7 and SF2 downmodulated all of theses receptors except CD3. In contrast, both Nef proteins from SIVmac239 and SIVblue potently reduced cell surface CD3 levels but were rather inactive toward MHC-I. This loss of MHC-I downregulation presumably represents a consequence of the fusion to YFP that may mask molecular surfaces in the C terminus of SIV Nef required for this activity. Although the G238A mutant of Nef from SIVmac239 behaved similarly to the wt, mutation of the ExxxL motif (E190A/L194A) resulted in the ELAA Nef variant that was largely deficient in receptor downmodulation but maintained the ability to downregulate cell surface CD3. In contrast, the R137/138A (RRAA) and LL20PS/EVED34AVAA SIVmac239 Nef variants had lost all receptor downmodulation activity, including CD3. Similarly for Nef from SIVblue, mutating the ExxxL motif (E181A/L185A; ELAA) resulted in a Nef variant with selective CD3 downmodulation activity, while the R129/130A (RRAA) and R129A mutants were specifically deficient in CD3 downmodulation. Functional analysis of this panel of Nef proteins revealed that all of them potently caused endosomal accumulation of Lck in >60% of all transfected cells (Fig. 5C and D). In contrast, a more specific activity pattern emerged upon analysis of disruption of TCR-induced actin remodeling and pTyr induction. First, for both SIVNef proteins tested, the RRAA mutation caused a general loss of activity, presumably reflecting pleiotropic defects caused by this particular mutation (33). Mutating the ExxxL motif in SIVNefmac239 and SIVNefblue had no effect, excluding a role of downregulation of CD4 and CD28 in these processes. In contrast, the mutations that disrupted downmodulation of cell surface CD3 (LL20PS/EVED34AVAA for mac239 and R129A for blue) resulted in a slightly reduced activity in the disruption of TCR-induced actin remodeling (SIVmac239 [6.7% ± 2.1%] versus LL20PS/EVED34AVAA [27.0% ± 11.5%]; SIVblue [24.3% ± 5.1%] versus R129A [30.7% ± 12.1%]). Similarly, these mutations also moderately reduced the ability of these Nef proteins to affect TCR-mediated pTyr induction (wt SIVmac239 [14.7% ± 5.1%] versus LL20PS/EVED34AVAA [28.0% ± 6.6%]; wt SIVblue [16.5% ± 3.3%] versus R129A [39.7% ± 15.6%]). Of note, both TCR-CD3 downregulation-deficient SIV Nef mutants retained strong inhibitory activity toward TCR-induced actin remodeling and elevation of pTyr levels. Similar results were obtained with a wide range of anti-CD3 antibody concentrations used for surface stimulation (data not shown). Thus, TCR-CD3 downmodulation moderately contributes to the defects SIV Nef proteins impose on early events after surface mediated CD3-stimulation in T lymphocytes but does not represent the driving force for these modifications.
FIG. 5.
Downmodulation of surface TCR-CD3 is a minor determinant for Lck accumulation, as well as for inhibition of TCR-induced actin remodeling and pTyr induction by SIV Nef proteins. (A) Characterization of cell surface receptor downmodulation by the indicated Nef.GFP/YFP fusion proteins used. The results show the cell surface levels of CD3, CD28, CD4, and MHC-I in transfected Jurkat T lymphocytes relative to the YFP control (means and SDs of two independent experiments). (B) Western blot analysis of the indicated Nef.GFP/YFP fusion proteins. Lysates of transiently transfected Jurkat T lymphocytes were probed with antibodies against GFP (upper panel) or transferrin receptor (TfR) as a loading control (bottom panel). (C) Effects of SIVmac239 and SIVblue Nef mutants on Lck localization (top panel), TCR-induced F-actin ring formation (middle panel), and pTyr induction (bottom panel). Analysis and presentation in analogy to Fig. 1 to 3. Arrowheads indicate transfected cells. Scale bar, 10 μm. (D) Quantification of the experiments shown in panel C. Values are the arithmetic means of at least three independent experiments plus the SD in which more than 100 cells were counted per condition.
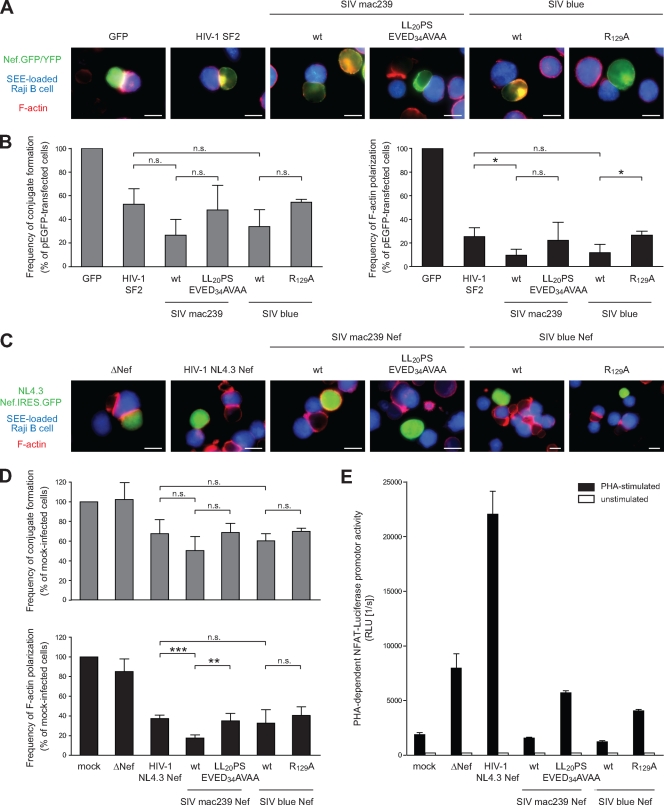
Reduction of IS formation and disruption of F-actin polarization by Nef is largely independent of TCR-CD3 downmodulation.
Since the results described above were obtained in a T-cell line following surface stimulation, we sought to corroborate these results in T lymphocytes that undergo IS formation with antigen-presenting B lymphocytes (Fig. 6). First, Jurkat T lymphocytes expressing GFP/YFP fusion proteins were used in conjugation experiments with SEE-loaded Raji B lymphocytes (Fig. 6A). Using GFP as a control, microscopic analysis of such conjugation reactions revealed the efficient formation of close B/T-cell conjugates with characteristic polymerization of F-actin at the cell-cell contact. We next compared the effects of Nef proteins from HIV-1 SF2, SIVmac239, and SIVblue, as well as the TCR-CD3 downregulation-deficient mutants of SIVmac239 and SIVblue, in this assay. In all cases, B/T-cell conjugates appeared less frequently and formed conjugates rarely displayed polarization of F-actin at the IS. Quantification of conjugate formation and F-actin polarization in this experimental system (Fig. 6B) closely matched those obtained upon TCR surface stimulation and revealed that Nef from SIVmac239 and SIVblue tended to be slightly more potent in inhibiting formation of B/T-cell conjugates than Nef from HIV-1SF2, although these differences did not reach statistical significance (26.6% ± 13.3% and 33.8% ± 14.3% versus 52.8% ± 13.2% Nef-expressing cells in conjugates). Scoring for F-actin polarization at the IS in the cell population found in conjugates with B cells as a measure for formation of a functional IS revealed a more potent inhibitory effect of Nef, resulting in an at least fourfold reduction of F-actin polarization relative to the GFP control. Again, Nef proteins of SIVmac239 and SIVblue were slightly more potent than Nef from HIV-1 SF2 in this assay (9.6% ± 5.1% and 11.8% ± 6.9% versus 25.5% ± 7.3% Nef-expressing cells in conjugates with polarized F-actin), with a low statistically significant difference observed only for Nef from SIVmac239. Although the AxxA mutant of Nef from HIV-1 SF2 did not have any effect in both types of analysis (data not shown), the two TCR-CD3 downregulation-deficient SIV Nef variants displayed inhibitory activities that were virtually indistinguishable from that of HIV-1 SF2 Nef (Fig. 6B). Loss of TCR-CD3 downmodulation caused a slight reduction in the ability to interfere with F-actin polarization relative to the corresponding wt SIV Nef proteins; however, these differences were barely (SIVblue) or not at all (SIVmac239) statistically significant. Remarkably similar results were obtained when infected primary human T lymphocytes from five independent donors were used in conjugation reactions with SEE-loaded Raji B cells (Fig. 6C and D). T lymphocytes infected with HIV-1 from which nef had been deleted (ΔΝef) essentially conjugated with B cells and polarized F-actin to the IS as efficiently as uninfected control cells. Infection with HIV-1 encoding for Nef from HIV-1 NL4.3, in contrast, displayed mild and pronounced defects in IS formation and F-actin polarization, respectively. Reduction of conjugation efficiency was again, if at all, slightly more pronounced when these viruses expressed Nef from SIVmac239 or SIVblue, and no statistically significant difference was observed whether or not the respective Nef proteins were able to downmodulate cell surface TCR-CD3. Prevention of F-actin polarization by Nef was markedly more pronounced, with Nef from SIVmac239 but not from SIVblue displaying significantly increased disruption relative to Nef from HIV-1 NL4.3 (NL4.3, 37.2% ± 3.6%; SIVmac239, 17.4% ± 3.2%; and SIVblue, 32.5% ± 13.7%). Loss of TCR-CD3 downmodulation slightly elevated levels of F-actin polarization to those observed in the presence of Nef from HIV-1 NL4.3 for Nef from SIVmac239, while this mutation had no effect in the context of Nef from SIVblue (SIVmac239 LL20PS/EVED34AVAA, 35.0% ± 7.6%; SIVblueR129A, 40.4% ± 8.7%). The effects of Nef on F-actin polarization were thus remarkably independent of its ability to downregulate TCR-CD3 cell surface levels. Finally, since group 2 Nef proteins were previously shown to disrupt TCR downstream signaling such as activation of the NFAT transcription factor more potently than group 1 Nef proteins (36, 37), we tested whether this ability correlated with downmodulation of cell surface TCR-CD3. Indeed, while Nef from HIV-1 NL4-3 increased PHA-stimulated induction of NFAT upon infection of NFAT reporter Jurkat T lymphocytes, Nef proteins from SIVmac239 and SIV blue markedly reduced NFAT induction (Fig. 6E). In contrast, the TCR-CD3 downregulation-deficient SIV Nef variants did not display such effects, resulting in NFAT activity levels similar to those observed upon infection with HIV-1ΔNef. In sum, these results demonstrate that in the context of IS formation between T lymphocytes and SEE-pulsed Raji B cells, Nef proteins from HIV-1 and SIV potently disrupt formation and in particular organization of the IS. Cell surface downregulation of TCR-CD3 plays only a minor role in these processes; however, it correlates with significant alterations in downstream TCR signal transduction.
FIG. 6.
Modulation of the IS between T lymphocytes and Raji B cells by Nef proteins of the HIV-1 and SIV lineages. (A) Shown are representative micrographs of Jurkat T lymphocytes expressing GFP or the indicated Nef.GFP/YFP fusion proteins in conjugates with SEE-loaded Raji B cells (in blue). F-actin is depicted in red. Note the marked F-actin polarization at the cell-cell contact observed with GFP is lacking with Nef expressing cells. Scale bar, 10 μm. (B) Quantification of frequency of overall B/T-cell conjugate formation (left panel) and F-actin polarization in successfully formed conjugates (right panel) of the experiment shown in panel A. Values are the arithmetic means of at least three independent experiments plus the SD in which more than 800 cells were counted per condition expressed relative to GFP-expressing control cells. (C) Conjugates between SEE-pulsed Raji B cells (blue) with peripheral blood mononuclear cells infected with the indicated variants of HIV-1 NL4.3 Nef.IRES.GFP (green). F-actin is depicted in red. Note that HIV-1 infection in the absence of Nef (ΔΝef) allows F-actin polarization at the IS, while Nef-expressing HIV-1 interferes with this process. Scale bar, 10 μm. (D) Quantification of frequency of overall B/T-cell conjugate formation (upper panels) and F-actin polarization in successfully formed conjugates (lower panels) as shown in panel C. The results depict the arithmetic means of experiments with cells from five independent donors plus the SD expressed relative to mock-infected control cells. A total of 100 to 200 (mock, ΔNef) or 100 to 500 (other conditions) cells were analyzed per condition and donor. (E) Analysis of NFAT activation after PHA stimulation. Jurkat T lymphocytes stably expressing a NFAT luciferase reporter were infected with HIV-1 NL4.3 IRES.GFP viruses expressing the indicated Nef proteins and subsequently stimulated with PHA or left unstimulated, respectively. The levels of NFAT-dependent luciferase reporter activity are the means plus the SDs of triplicate infections. ***, P < 0.0005; **, P < 0.005; *, P < 0.05; n.s. (not significant), P > 0.05.
DISCUSSION
Prompted by the functional difference between Nef proteins from HIV-1 and SIV in downmodulation of cell surface TCR-CD3, we conducted a systematic comparison of Nef's ability to affect formation and organization of the IS. In total, 18 Nef alleles, as well as specific SIVNef mutants lacking TCR-CD3 downmodulation activity, were compared for their ability to affect a set of parameters relevant to IS function, including the subcellular localization of Lck, TCR-induced actin remodeling and TCR-induced induction of tyrosine phosphorylation. The use of surface stimulation of the Jurkat T-cell line overexpressing Nef.GFP/YFP fusion proteins, as well as conjugation of SEE-pulsed Raji B cells with primary human T lymphocytes infected with HIV-1 encoding for native Nef proteins, resulted in remarkably similar findings. First, the accumulation of Lck in intracellular endosomal compartments is a conserved activity of lentiviral Nef proteins that is not governed by TCR-CD3 downmodulation or inhibition of actin remodeling. Second, inhibition of actin remodeling by Nef is also strongly conserved, and competence for TCR-CD3 downregulation only slightly enhances this activity. Third, inhibition of actin remodeling by Nef correlates with inhibition of tyrosine phosphorylation. We conclude that TCR-CD3 downmodulation contributes surprisingly little to these early aspects of IS function and that inhibition of actin remodeling upon TCR engagement is a highly conserved activity of lentiviral Nef proteins.
In the present study we investigated several aspects of TCR-proximal events relevant to IS formation and function that were previously reported to be affected by the expression of HIV-1 Nef. As a first parameter, we addressed the subcellular localization of Lck, a Src tyrosine kinase with essential roles at early steps of TCR signaling that is retargeted from the plasma membrane to intracellular endosomal compartments in the presence of Nef, preventing its efficient recruitment to the IS upon TCR engagement (41). The results presented clearly demonstrate that such intracellular accumulation of Lck is efficiently induced by almost all group 1 and group 2 Nef proteins tested, demonstrating that this activity occurs independently of Nef's ability to affect TCR-CD3 cell surface levels. This result is corroborated by the fact that Lck accumulation is readily induced by Nef in T lymphocytes prior to engagement of TCR-CD3. Furthermore, no strong correlation was observed between Nef's ability to alter Lck localization and actin remodeling, supporting our previous conclusion that these two activities are mechanistically uncoupled (19). Given this high degree of conservation it will be of interest to address in future studies the mechanism of Lck retargeting and to define the specific functional consequences of this Nef activity. The identification of two Nef proteins that are specifically defective in inducing intracellular accumulation of Lck (HIV-1 8161K9 and HIV-2 Ben) will provide valuable tools for such analyses.
Given that TCR-CD3 is the main receptor engaged during IS formation with antigen-presenting B cells (6) and is specifically triggered on our TCR-stimulatory surfaces, the finding that TCR-CD3 downregulation by Nef has only minor effects on early events of IS formation and organization is surprising at first. One reason for this could be the efficacy of TCR-CD3 downmodulation by group 2 Nef proteins. Although this is a relatively efficient process, it is by no means complete, retaining 10 to 30% of the TCR-CD3 cell surface signal observed in the absence of Nef (25, 36) (Fig. 5A). Provided their efficient recruitment to sites of cell-cell or cell-surface contacts takes place, the local concentration of cell surface TCR-CD3 molecules might thus not be limiting for robust signal initiation even in the presence of SIV Nef. However, based on the high degree of conservation of inhibition of TCR-induced actin remodeling by lentiviral Nef proteins, we favor another scenario. F-actin relaxation and polymerization are essential during signal ignition since they facilitate lateral movement of enhancing and inhibitory components of TCR signaling machineries (14, 39). Interfering with actin dynamics, as by expression of Nef, is therefore expected to generally impact the ability to form a signaling-competent IS and thus to initiate and sustain signaling. In this scenario, inhibition of actin remodeling by Nef acts even before the local concentration of TCR-CD3 becomes relevant for the specific signal transduction output, resulting in altered signal transduction independently of the cell surface density of TCR-CD3. Consistent with this scenario, we observed potent reduction of early tyrosine phosphorylation by Nef proteins of both groups. Although this model likely explains why TCR-CD3 downregulation by Nef does not dictate the earliest parameters of IS formation and function addressed in our study, it does not include downstream signal transduction outcomes triggered by TCR engagement. At the level of gene expression and apoptosis, group 2 Nef proteins reduce TCR-CD3-mediated cell activation with significantly higher potency than group 1 Nef proteins (36), and the correlation of these effects with TCR-CD3 downregulation was confirmed here by the use of downregulation deficient Nef variants. Conceivably, these functional differences reflect that, independent of the initial block imposed by Nef to IS formation via inhibition of actin remodeling, the cell surface concentration of TCR-CD3 determines the signal output in cells that undergo efficient conjugation. In line with this model, group 2 Nef proteins were significantly more effective in reducing pTyr levels than their group 1 counterparts, an early functional difference that could well translate into the opposing outcomes of TCR signaling such as NFAT activation observed in the presence of both types of Nef proteins. More detailed analyses of the magnitude and specificity of the individual pTyr species involved should reveal the molecular basis of the different TCR downstream signals generated in the presence of Nef proteins from divergent lentiviral lineages. On the other hand, it appears likely that yet-unknown TCR downstream activities that strictly depend on actin remodeling are potently affected by expression of all Nef proteins. Although it is clear that full disruption of actin remodeling abrogates all TCR downstream signaling (42), variable functional consequences have been observed after interfering with individual components involved in actin dynamics (15-17, 26, 32). The overall relationship between actin remodeling and signal output is therefore far from being completely understood. Inhibition of actin remodeling by Nef has recently been shown to restrict host cell motility (40). Deciphering the precise role of this highly conserved Nef activity in the context of TCR signaling thus warrants a more comprehensive analysis of individual TCR effector functions, addressing the functional consequences of Nef expression in a time resolved analysis following engagement of T lymphocytes by APCs. These analyses will also have to consider potential functional alterations at the side of the APC that may result from effects of Nef exerted during IS formation. Finally, since the results presented were generated exclusively using SEE-pulsed Raji B cells as APCs, it will be important to address whether the functional consequences of Nef expression at the IS depend on the nature of the APC that engages infected T lymphocytes. Besides adding to our understanding of how HIV and SIV manipulate T-lymphocyte activation states, such studies on Nef will also be useful to further define the specific contribution of actin remodeling to TCR signaling.
In sum, our results establish a hierarchy of events that Nef affects at the T-B cell IS to reduce early TCR signaling. Conserved among highly divergent lentiviral Nef proteins, altered subcellular localization of Lck and inhibition of actin remodeling affect early events following TCR engagement, the functional consequences of which have yet to be determined. These early modifications are further emphasized by Nef proteins of the SIV/HIV-2 lineage that, in addition, reduce cell surface exposure of TCR-CD3 to cause select inhibition of subsequent downstream signaling events, which in turn can be activated by HIV-1 Nef proteins. Future efforts will attempt to decipher how orchestration of these individual measures results in the specific functional outcomes induced by different Nef proteins.
Acknowledgments
We are grateful to Frank Kirchhoff for generously providing the group 1 and group 2 Nef expression constructs and proviruses, Bettina Stolp for helpful instructions, Oliver Keppler for critical comments on the manuscript, SFB638 for access to the LSM confocal microscope, and Nadine Tibroni for expert technical help.
This project was supported by the Deutsche Forschungsgemeinschaft (SFB638 project A11). O.T.F is a member of the Cell Networks Cluster of Excellence (EXC81). M.S. is funded by grants from the Deutsche Forschungsgemeinschaft and the Stiftung zur Bekämpfung Neuroviraler Erkrankungen.
Footnotes
Published ahead of print on 2 September 2009.
REFERENCES
- 1.Bell, I., C. Ashman, J. Maughan, E. Hooker, F. Cook, and T. A. Reinhart. 1998. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR downmodulation. J. Gen. Virol. 79(Pt. 11):2717-2727. [DOI] [PubMed] [Google Scholar]
- 2.Billadeau, D. D., J. C. Nolz, and T. S. Gomez. 2007. Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 7:131-143. [DOI] [PubMed] [Google Scholar]
- 3.Bunnell, S. C., V. Kapoor, R. P. Trible, W. Zhang, and L. E. Samelson. 2001. Dynamic actin polymerization drives T-cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity 14:315-329. [DOI] [PubMed] [Google Scholar]
- 4.Campi, G., R. Varma, and M. L. Dustin. 2005. Actin and agonist MHC-peptide complex-dependent T-cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 202:1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantrell, D. 1996. T-cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 14:259-274. [DOI] [PubMed] [Google Scholar]
- 6.Choi, Y. W., A. Herman, D. DiGiusto, T. Wade, P. Marrack, and J. Kappler. 1990. Residues of the variable region of the T-cell-receptor beta-chain that interact with Staphylococcus aureus toxin superantigens. Nature 346:471-473. [DOI] [PubMed] [Google Scholar]
- 7.Das, V., B. Nal, A. Dujeancourt, M. I. Thoulouze, T. Galli, P. Roux, A. Dautry-Varsat, and A. Alcover. 2004. Activation-induced polarized recycling targets T-cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity 20:577-588. [DOI] [PubMed] [Google Scholar]
- 8.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 9.Fackler, O. T., A. Alcover, and O. Schwartz. 2007. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat. Rev. Immunol. 7:310-317. [DOI] [PubMed] [Google Scholar]
- 10.Fackler, O. T., and A. S. Baur. 2002. Live and let die: Nef functions beyond HIV replication. Immunity 16:493-497. [DOI] [PubMed] [Google Scholar]
- 11.Fackler, O. T., A. Moris, N. Tibroni, S. I. Giese, B. Glass, O. Schwartz, and H. G. Krausslich. 2006. Functional characterization of HIV-1 Nef mutants in the context of viral infection. Virology 351:322-339. [DOI] [PubMed] [Google Scholar]
- 12.Fenard, D., W. Yonemoto, C. de Noronha, M. Cavrois, S. A. Williams, and W. C. Greene. 2005. Nef is physically recruited into the immunological synapse and potentiates T-cell activation early after TCR engagement. J. Immunol. 175:6050-6057. [DOI] [PubMed] [Google Scholar]
- 13.Fortin, J. F., C. Barat, Y. Beausejour, B. Barbeau, and M. J. Tremblay. 2004. Hyper-responsiveness to stimulation of human immunodeficiency virus-infected CD4+ T cells requires Nef and Tat virus gene products and results from higher NFAT, NF-κB, and AP-1 induction. J. Biol. Chem. 279:39520-39531. [DOI] [PubMed] [Google Scholar]
- 14.Gomez, T. S., and D. D. Billadeau. 2008. T-cell activation and the cytoskeleton: you can't have one without the other. Adv. Immunol. 97:1-64. [DOI] [PubMed] [Google Scholar]
- 15.Gomez, T. S., K. Kumar, R. B. Medeiros, Y. Shimizu, P. J. Leibson, and D. D. Billadeau. 2007. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity 26:177-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez, T. S., S. D. McCarney, E. Carrizosa, C. M. Labno, E. O. Comiskey, J. C. Nolz, P. Zhu, B. D. Freedman, M. R. Clark, D. J. Rawlings, D. D. Billadeau, and J. K. Burkhardt. 2006. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity 24:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grakoui, A., S. K. Bromley, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 1999. The immunological synapse: a molecular machine controlling T-cell activation. Science 285:221-227. [DOI] [PubMed] [Google Scholar]
- 18.Haller, C., and O. T. Fackler. 2008. HIV-1 at the immunological and T-lymphocytic virological synapse. Biol. Chem. 389:1253-1260. [DOI] [PubMed] [Google Scholar]
- 19.Haller, C., S. Rauch, and O. T. Fackler. 2007. HIV-1 Nef employs two distinct mechanisms to modulate Lck subcellular localization and TCR induced actin remodeling. PLoS ONE 2:e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haller, C., S. Rauch, N. Michel, S. Hannemann, M. J. Lehmann, O. T. Keppler, and O. T. Fackler. 2006. The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. J. Biol. Chem. 281:19618-19630. [DOI] [PubMed] [Google Scholar]
- 21.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 22.Harder, T., C. Rentero, T. Zech, and K. Gaus. 2007. Plasma membrane segregation during T-cell activation: probing the order of domains. Curr. Opin. Immunol. 19:470-475. [DOI] [PubMed] [Google Scholar]
- 23.Howe, A. Y., J. U. Jung, and R. C. Desrosiers. 1998. Zeta chain of the T-cell receptor interacts with nef of simian immunodeficiency virus and human immunodeficiency virus type 2. J. Virol. 72:9827-9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, Y., and J. K. Burkhardt. 2007. T-cell-receptor-dependent actin regulatory mechanisms. J. Cell Sci. 120:723-730. [DOI] [PubMed] [Google Scholar]
- 25.Iafrate, A. J., S. Bronson, and J. Skowronski. 1997. Separable functions of Nef disrupt two aspects of T-cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 16:673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilani, T., G. Vasiliver-Shamis, S. Vardhana, A. Bretscher, and M. L. Dustin. 2009. T-cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat. Immunol. 10:531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 28.Kirchhoff, F. 2009. Is the high virulence of HIV-1 an unfortunate coincidence of primate lentiviral evolution? Nat. Rev. Microbiol. 7:467-476. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 30.Malim, M. H., and M. Emerman. 2008. HIV-1 accessory proteins-ensuring viral survival in a hostile environment. Cell Host Microbe 3:388-398. [DOI] [PubMed] [Google Scholar]
- 31.Munch, J., M. Schindler, S. Wildum, E. Rucker, N. Bailer, V. Knoop, F. J. Novembre, and F. Kirchhoff. 2005. Primary sooty mangabey simian immunodeficiency virus and human immunodeficiency virus type 2 nef alleles modulate cell surface expression of various human receptors and enhance viral infectivity and replication. J. Virol. 79:10547-10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolz, J. C., T. S. Gomez, P. Zhu, S. Li, R. B. Medeiros, Y. Shimizu, J. K. Burkhardt, B. D. Freedman, and D. D. Billadeau. 2006. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T-cell activation. Curr. Biol. 16:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Neill, E., L. S. Kuo, J. F. Krisko, D. R. Tomchick, J. V. Garcia, and J. L. Foster. 2006. Dynamic evolution of the human immunodeficiency virus type 1 pathogenic factor, Nef. J. Virol. 80:1311-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rauch, S., K. Pulkkinen, K. Saksela, and O. T. Fackler. 2008. Human immunodeficiency virus type 1 Nef recruits the guanine exchange factor Vav1 via an unexpected interface into plasma membrane microdomains for association with p21-activated kinase 2 activity. J. Virol. 82:2918-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito, T., and T. Yokosuka. 2006. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr. Opin. Immunol. 18:305-313. [DOI] [PubMed] [Google Scholar]
- 36.Schindler, M., J. Munch, O. Kutsch, H. Li, M. L. Santiago, F. Bibollet-Ruche, M. C. Muller-Trutwin, F. J. Novembre, M. Peeters, V. Courgnaud, E. Bailes, P. Roques, D. L. Sodora, G. Silvestri, P. M. Sharp, B. H. Hahn, and F. Kirchhoff. 2006. Nef-mediated suppression of T-cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell 125:1055-1067. [DOI] [PubMed] [Google Scholar]
- 37.Schindler, M., J. Schmokel, A. Specht, H. Li, J. Munch, M. Khalid, D. L. Sodora, B. H. Hahn, G. Silvestri, and F. Kirchhoff. 2008. Inefficient Nef-mediated downmodulation of CD3 and MHC-I correlates with loss of CD4+ T cells in natural SIV infection. PLoS Pathog. 4:e1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 Nef increases T-cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 96:8167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sechi, A. S., and J. Wehland. 2004. Interplay between TCR signalling and actin cytoskeleton dynamics. Trends Immunol. 25:257-265. [DOI] [PubMed] [Google Scholar]
- 40.Stolp, B., M. Reichman-Fried, L. Abraham, X. Pan, S. I. Giese, S. Hannemann, P. Goulimari, E. Raz, R. Grosse, and O. T. Fackler. 2009. HIV-1 Nef interferes with host cell motility by deregulation of cofilin. Cell Host Microbe 6:174-186. [DOI] [PubMed] [Google Scholar]
- 41.Thoulouze, M. I., N. Sol-Foulon, F. Blanchet, A. Dautry-Varsat, O. Schwartz, and A. Alcover. 2006. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity 24:547-561. [DOI] [PubMed] [Google Scholar]
- 42.Valitutti, S., M. Dessing, K. Aktories, H. Gallati, and A. Lanzavecchia. 1995. Sustained signaling leading to T-cell activation results from prolonged T-cell receptor occupancy: role of T-cell actin cytoskeleton. J. Exp. Med. 181:577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]