Abstract
Hypoxia induces apoptosis but also triggers adaptive mechanisms to ensure cell survival. Here we show that the prosurvival effects of hypoxia-inducible factor 1 (HIF-1) in endothelial cells are mediated by neuron-derived orphan receptor 1 (NOR-1). The overexpression of NOR-1 decreased the rate of endothelial cells undergoing apoptosis in cultures exposed to hypoxia, while the inhibition of NOR-1 increased cell apoptosis. Hypoxia upregulated NOR-1 mRNA levels in a time- and dose-dependent manner. Blocking antibodies against VEGF or SU5614 (a VEGF receptor 2 inhibitor) did not prevent hypoxia-induced NOR-1 expression, suggesting that NOR-1 is not induced by the autocrine secretion of VEGF in response to hypoxia. The reduction of HIF-1α protein levels by small interfering RNAs, or by inhibitors of the phosphatidylinositol-3 kinase (PI3K)/Akt pathway or mTOR, significantly counteracted hypoxia-induced NOR-1 upregulation. Intracellular Ca2+ was involved in hypoxia-induced PI3K/Akt activation and in the downstream NOR-1 upregulation. A hypoxia response element mediated the transcriptional activation of NOR-1 induced by hypoxia as we show by transient transfection and chromatin immunoprecipitation assays. Finally, the attenuation of NOR-1 expression reduced both basal and hypoxia-induced cIAP2 (cellular inhibitor of apoptosis protein 2) mRNA levels, while NOR-1 overexpression upregulated cIAP2. Therefore, NOR-1 is a downstream effector of HIF-1 signaling involved in the survival response of endothelial cells to hypoxia.
Mammalian cells require a constant supply of oxygen to maintain their energy balance. Low oxygen (hypoxia) leads to reduced oxidative phosphorylation and the depletion of cellular ATP that can result in cell death. To ensure cell survival during hypoxia, cells have evolved complex adaptive mechanisms (54). Indeed, hypoxia coordinately regulates a large number of genes whose products have widespread roles, including the regulation of vascular function, cell metabolism, cell survival, and cell growth and motility. In response to hypoxia, cells secrete vascular endothelial growth factor (VEGF), a cytokine that modulates gene expression and elicits an array of biologic activities such as cell survival and angiogenesis (15). These cellular effects of VEGF are mediated by a set of transcription factors, among which are cyclic AMP response element binding protein (CREB) (35) and the subfamily 4 group A of nuclear receptors (NR4A) (26, 50, 61). However, the transcriptional response to hypoxia is mediated primarily by the hypoxia-inducible factor (HIF) family of transcription factors. HIF-1, the prototype of this family, is a heterodimeric basic helix-loop-helix transcription factor composed of HIF-1β (constitutive subunit) and HIF-1α (oxygen-sensitive subunit) (48). In normoxic conditions, HIF-1α is degraded by a mechanism involving the hydroxylation of two prolyl residues by specific prolyl hydroxylases, ubiquitylation, and proteasomal degradation through a von Hippel-Lindau-dependent pathway (51, 53). In hypoxic conditions, HIF-1α is stabilized and translocates to the nucleus where it dimerizes with HIF-1β, transactivating the hypoxia response element (HRE) present in the promoter of many hypoxia-responsive genes (48). In the last years, a growing number of genes regulated by hypoxia/HIF have been identified (29); however, the regulatory network of transcription factors that cooperates in the response of vascular endothelial cells to hypoxia is not completely understood.
The zinc finger transcription factor neuron-derived orphan receptor 1 (NOR-1; also known as NR4A3, Minor, TEC, and CHN) is a nuclear receptor originally identified as an early-response gene in forebrain neurons undergoing apoptosis (42). NOR-1, together with Nur77 and Nurr1, form the NR4A subfamily of nuclear orphan receptors within the steroid/thyroid receptor superfamily (30). Unlike most nuclear receptors, whose transcriptional activity is regulated by direct modulatory ligands, NR4A genes do not appear to require ligand binding for activation (56), and they are immediate-early genes highly responsive to extracellular stimuli (31). Various lines of evidence have suggested a role for NOR-1 in cellular proliferation and apoptosis. In the vascular system, NOR-1 is upregulated by percutaneous transluminal coronary angioplasty (32), is overexpressed in atherosclerotic lesions from patients with coronary artery disease (5, 32, 39), and is induced by growth factors, cytokines, and low-density lipoproteins (5, 12, 26, 32, 33, 39, 49, 50, 61). NOR-1 seems to be a key transcription factor involved in vascular smooth muscle cell (12, 32, 39, 49) and endothelial cell (33, 50) proliferation. Furthermore, NOR-1-dependent oncogenic transformation has been described, as a result of its fusion with various N-terminal partners (28). NOR-1 has been implicated in the apoptosis of neural cells (42), T cells (10), and MCF-7 breast cancer cells (41). Finally, NOR-1 has also been involved in neuron survival in the developing murine hippocampus (46), and recently, it has been shown that it is transiently and selectively induced in brain regions resistant to transient global ischemia (22). The aim of this study was to analyze whether NOR-1 plays a role in the adaptive response of endothelial cells to hypoxia. We show that NOR-1 is a downstream target of HIF-1α that could play an important role in the regulation of endothelial cell survival under hypoxic stress.
MATERIALS AND METHODS
Cell culture.
Human umbilical vein endothelial cells (HUVEC; Advancell) and human dermal microvascular endothelial cells (HDMEC; PromoCell) were cultured in medium M199 (Kibbutz Industries) supplemented with 20 mM HEPES (pH 7.4; Gibco), 30 μg/ml endothelial growth factor supplement (Sigma), 2 mM glutamine (Gibco), 1 mM pyruvate (Gibco), 100 μg/ml heparin (Sigma), 20% fetal calf serum (Biological Industries), and antibiotics (0.1 mg/ml streptomycin, 100 U/ml penicillin G; Gibco) as described previously (33, 50). The cells were used between passages 2 and 5. The cells were seeded in multiwell plates and were maintained under standard culture conditions (21% O2, 5% CO2, 95% humidity) until subconfluence. The cells were arrested overnight in M199 supplemented with glutamine, pyruvate, antibiotics, and fetal calf serum (10% for HUVEC and 5% for HDMEC). Finally, the cells were stimulated with CoCl2 (0.1 to 1 mM; Sigma), deferoxamine mesylate (DFO; 100 μM; Calbiochem), or VEGF-A (50 ng/ml; R&D Systems) or were exposed to hypoxia (1 to 5% O2, 5% CO2, balanced with N2) in a Forma Series II hypoxic incubator (model 3141; Thermo Electron Corp.). When needed, the cells were pretreated with inhibitors for 30 min. The inhibitors used were SU5614 (a VEGF receptor-2 inhibitor; 10 μM; Calbiochem), 1,2-bis(2-aminophenoxy)ethano-N,N,N′,N′-tetraacetic acid tetrakis acetoxymethyl ester (BAPTA-AM, a Ca2+ chelator; 15 μM; Sigma), bisindolylmaleimide I (GF10933X, a protein kinase C [PKC] inhibitor; 5 μM; Sigma), SB203580 (a p38 mitogen-activated protein kinase [MAPK] inhibitor; 5 μM; Oxford Biomedical Research, Inc.), U0128 (a mitogen-activated protein kinase kinase [MEK1/2] inhibitor that prevents extracellular signal-regulated kinase1/2 [ERK1/2] activation; 10 μM; Calbiochem), 12-(2-cyanoethil)- 6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole (Gö6976, an inhibitor of calcium-dependent PKCs; 3 μM; Biomol); rapamycin (a mammalian target of rapamycin [mTOR] inhibitor; 100 nM; Sigma), and wortmannin. (100 μM; Sigma) or LY294002 (25 μM; Calbiochem), two phosphatidylinositol-3 kinase (PI3K)/Akt inhibitors. Neutralizing antibodies against VEGF (AF-293-NA; 200 ng/ml; R&D Systems) were used to block VEGF secreted by endothelial cells as described previously (8).
Real-time PCR.
Total RNA was isolated with an RNAeasy kit (Qiagen) according to the manufacturer's recommendations and was reverse transcribed with a High-Capacity cDNA archive kit (Applied Biosystems) with random hexamers (33). Assays-on-Demand (Applied Biosystems) of TaqMan fluorescent real-time PCR primers and probes were used for NOR-1 (Hs00175077_m1), Nur77 (Hs00172437_m1), Nurr-1 (Hs00128691_m1), VEGF-A (Hs00173626_m1), HIF-1α (Hs00153153_m1), and cyclin D1 (Hs00277039_m1). Primers for the real-time PCR analysis of the cellular inhibitor of apoptosis protein-1 (cIAP1, BIRC2) and -2 (cIAP2, BIRC3) using SYBR green have been described previously (13). The results were normalized by 18S rRNA (4319413E); alternatively, TATA binding protein (Hs99999910_m1) was also used.
Western blot analysis.
Endothelial cells were stimulated with CoCl2 or were exposed to hypoxia in the presence or in the absence of signaling inhibitors, and protein extracts were analyzed by Western blotting as described previously (32, 33). Briefly, cell cultures were washed twice with wash buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 100 mM NaF, 10 mM NaPPi, 10 mM EDTA, 2 mM Na3VO4) and lysed with lysis buffer (wash buffer containing 1 mM phenylmethylsulfonyl fluoride, 5 μM leupeptin, 1% Triton X-100). The protein concentration was determined by the bicinchoninic acid protein assay (Pierce). Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred onto nitrocellulose membranes (Bio-Rad Laboratories). The membranes were incubated with antibodies against CREB phosphorylated in Ser133 (Sigma), total CREB (Santa Cruz Biotechnology), Akt phosphorylated in Ser473 (Cell Signaling), total Akt (Millipore), HIF-1α (Abcam), or NOR-1 (PP-H7833; R&D Systems) (40). Bound antibody was detected using the appropriate horseradish peroxidase-conjugated antibody (Dako) and a chemiluminescent detection system (Supersignal West Dura; Pierce). The equal loading of protein in each lane was verified by reblotting the filters with an anti-β-actin antibody (Sigma).
siRNA transfection.
Silencer predesigned small interfering RNA (siRNA; Ambion) targeting HIF-1α (ID nos. 42840 [siHIF-11] and S6539 [siHIF-12]), CREB (ID no. 6690), NOR-1 (ID nos. 41668 [siNOR-11] and S15541 [siNOR-12]), or silencer negative control 1 siRNA (siControl; ID no. 4390843) were used in the knockdown experiments. HUVEC and HDMEC were transfected with siRNAs using Nucleofector (Amaxa) and the corresponding kits according to the manufacturer's recommendations (32). In brief, 1 × 106 cells were electroporated in 100 μl buffer containing 1 μg siRNA. Afterwards, the cells were seeded in complete medium for 24 h, were arrested, and were exposed to hypoxia or CoCl2 overnight.
Constructs of NOR-1 promoter.
The human NOR-1 promoter sequence contained in the plasmid pNORα/-1703, kindly provided by N. Ohkura (National Cancer Center Research Institute, Tokyo, Japan), was cloned into pGL3-Luciferase (Promega) (pGL3-NOR/-1703), and deletions were generated by PCR. The putative HRE site present in the NOR-1 promoter was mutated using the QuikChange II site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The primers used were GTTAAGAAACCCACGCCGTACcTcTAAAGAAATCAAACCTTATC (forward) and GATAAGGTTTGATTTCTTTAgAgGTACGGCGTGGGTTTCTTAAC (reverse) (the putative HRE is underlined, and changes are indicated by lowercase letters). Mutation was confirmed by DNA sequencing. CRE motifs were mutated as previously described (32, 49).
Transient transfection and luciferase assays.
Cells were transfected with the luciferase reporter plasmids using Lipofectin reagent (Invitrogen) as described previously (33). Briefly, transient transfections were performed in subconfluent cells seeded in six-well plates using 1 μg/well of the luciferase reporter plasmid, 0.3 μg/well of pSVβ-gal (Promega) as an internal control, and 3 μl of Lipofectin (Invitrogen). The DNA-liposome complexes were added to the cells for 6 h. The cells were washed once with arrest medium, were arrested overnight, and then were exposed to hypoxia or CoCl2. Luciferase activity was measured in the cell lysates using a luciferase assay kit (Promega) and a luminometer (Orion I; Berthold Detection Systems) according to the manufacturers’ instructions. The results were normalized by β-galactosidase activity using an enzyme assay system (Promega). In the cotransfection assays, an expression plasmid of HIF-1α (pCDNA3-HIF-1α) kindly provided by E. Huang (Department of Health & Human Services, NIH, Bethesda, MD) was used.
Overexpression of NOR-1.
A NOR-1 full-length cDNA (32) was subcloned in the bicistronic vector pIRES2-EGFP (Clontech) (where EGFP is enhanced green fluorescent protein). Cells were transfected with the recombinant plasmid expressing GFP and NOR-1 (pEGFP-NOR) using Nucleofector (Amaxa) and the corresponding kits according to the manufacturer's recommendations. NOR-1 overexpression was checked by Western blotting using an antibody specific for NOR-1 (PP-H7833; R&D Systems).
Annexin V binding: FACS and confocal analysis.
Annexin V binding was used as an index of cell apoptosis. HUVEC were transfected with pIRES2-EGFP or pEGFP-NOR, grown in complete medium for 36 h, arrested, and exposed to hypoxia or CoCl2 overnight. Finally, the cells were trypsinized, pooled with cells present in the cell supernatants, and resuspended in 1× binding buffer (10 mM HEPES [pH 7.4], 2.5 mM NaCl, 2.5 mM CaCl2). The cells were incubated with annexin V conjugated with R-phycoerythrin-cyanine 5 (annexin V-PC5; BioVision, Inc.) and propidium iodide (PI; Molecular Probes) according to the manufacturer's recommendations. Annexin V-PC5 and PI binding was analyzed in GFP-positive cells by fluorescence-activated cell sorting (FACS) (Epics XL flow cytometer; Beckman Coulter), and the results were expressed as a percentage of GFP-positive cells. Annexin V and PI binding were also analyzed in NOR-1 knockdown experiments. Cells were transfected with siRNA (siNOR-1 or siControl), arrested, and exposed to hypoxia or CoCl2 overnight. Finally, cells were trypsinized and incubated with annexin V-PC5 and PI as indicated above. Data were expressed as percentages of the total cell population. Annexin V-PC5 fluorescence (abscissa) was plotted versus PI uptake (ordinate). Data were gated for damaged cells (annexin V− and PI+), necrotic cells (annexin V+ and PI+), viable cells (annexin V− and PI−), and apoptotic cells (annexin V+ and PI−).
Annexin V binding was also analyzed by confocal microscopy. HUVEC were transfected with the recombinant plasmid expressing NOR-1 or with siRNAs against NOR-1 and were cultured in glass-bottom dishes (Willco Wells B.V.). The cells were arrested and were exposed to hypoxia overnight. Afterwards, the cells were washed with 1× annexin binding buffer and were processed in situ for annexin V-R-phycoerythrin (annexin V-R-PE; Molecular Probes) binding according to the manufacturer's recommendations for adherent cell cultures. The cells were fixed with a 4% paraformaldehyde solution containing a Hoechst dye (no. 33342; Molecular Probes) for nuclear staining and finally were washed with phosphate-buffered saline and mounted with ProLong mounting medium (Molecular Probes).
Cell cycle analysis.
HUVEC transfected with the recombinant plasmid expressing NOR-1 (or a control plasmid, pIRES2-EGFP) or with siRNA against NOR-1 (ID no. 41668; Ambion) (or silencer negative control, ID no. 4390843; Ambion) were exposed to hypoxia overnight. Then the cells were trypsinized, and DNA was stained with PI (Molecular Probes). The cell cycle phases were assessed by flow cytometry (Epics XL flow cytometer; Beckman Coulter) as described previously (32). A cell cycle distribution analysis was performed with Cylchred software from the Department of Hematology of the University of Cardiff (United Kingdom).
ChIP assay.
Chromatin immunoprecipitation (ChIP) assays were performed using the ExactaChIP kit (R&D Systems) according to the manufacturer's protocol. Briefly, arrested HUVEC were cultured under normoxia or exposed to hypoxia or CoCl2 for 4 h in the presence or in the absence of the PI3K inhibitor LY294002. Cells were cross-linked with 1% formaldehyde for 15 min. Cross-link reactions were stopped by adding glycine to a final concentration of 0.125 mM. Then, the cells were washed, harvested by scraping, lysed, and sonicated to shear chromatin to DNA fragments of 0.5 to 1 kb. Lysates were centrifuged, and an aliquot of supernatant was saved as the input DNA. Supernatants were then immunoprecipitated with an anti-HIF-1α antibody (5 μg) or with goat immunoglobulin G (IgG; control for nonspecific binding). Immunoprecipitates were recovered by the addition of streptavidin agarose beads (Sigma). After extensive washing was completed, 100 μl of chelating resin solution (R&D Systems) was added to the beads and boiled for 10 min. Finally, DNA was purified and concentrated using the QIAquick DNA purification system (Qiagen). The purified DNA was analyzed by conventional PCR and real-time PCR for the presence of the human NOR-1 promoter fragment (−801/−612 bp) using primers 5′-TGTTCAACACGTGTGTGTTTGT-3′ (forward) and 5′-CATTTACTGGGTGCGTGTTTC-3′ (reverse). The PCR amplification of 4% of the soluble chromatin prior to immunoprecipitation was used as the input control. The ChIP-precipitated DNA and input DNA were subjected to real-time PCR analyses using the Quantifast SYBR green PCR kit (Qiagen), and samples from two individual ChIP assays were analyzed in triplicate. The results were normalized by input and were expressed as the n-fold increase over those of the controls (normoxia).
Statistical analysis.
The results are expressed as means ± standard errors of the means (SEM) (unless otherwise stated). A STAT VIEW II (Abacus Concepts) statistical package for the Macintosh computer system was used for all analysis. Multiple groups were compared by one-factor analysis of variance, followed by the Fisher protected least significant difference to assess specific group differences.
RESULTS
NOR-1 overexpression reduced hypoxia-induced apoptosis in HUVEC.
We had previously reported that VEGF-A, a key cytokine upregulated by hypoxia involved in the proliferation and survival of endothelial cells, is a strong inducer of NOR-1 (50). Here we analyzed whether NOR-1 plays a role in the response of endothelial cells to hypoxia. The overexpression of NOR-1 in endothelial cells transfected with a bicistronic vector encoding GFP and NOR-1 reduced the percentage of apoptotic cells in cultures exposed to either hypoxia (from 40.3% ± 3.7% to 20.4% ± 4.3%) or CoCl2 (from 71.9% ± 3.8% to 46.4% ± 5.8%), as determined by FACS analysis (Fig. 1A and B). In cells maintained under normoxia, the effect was lower but also significant (from 19.8% ± 3.5% to 12.2% ± 3.0%). Figure 1C shows the levels of NOR-1 protein reached in cells transfected with the bicistronic vector comparatively with those from cells maintained under normoxia or exposed to hypoxia or CoCl2. Alternatively, the reduction of annexin binding in NOR-1 overexpressing cells exposed to hypoxia was also qualitatively appreciated using confocal microscopy (Fig. 1D). Conversely, two different siRNA against NOR-1 (siNOR-11 and siNOR-12), that efficiently inhibit NOR-1 expression without affecting Nur77 or Nurr1 expression (Fig. 2C), increased the percentage of apoptotic cells in cultures exposed to hypoxia or CoCl2 as determined by FACS analysis (Fig. 2A and B). In cells maintained under normoxia, the effect was also significant. The increase of annexin binding by NOR-1 inhibition in cells exposed to hypoxia was also qualitatively observed using confocal microscopy (Fig. 2D). Therefore, NOR-1 seems to be involved in the cell survival response to hypoxia.
FIG. 1.
NOR-1 overexpression reduced hypoxia-induced apoptosis. (A) HUVEC were transfected with pIRES2-EGFP (GFP) or with pEGFP-NOR-1 (GFP-NOR-1), a recombinant plasmid expressing GFP and NOR-1, and exposed to normoxia, hypoxia (1% O2), or 1 mM CoCl2 for 16 h. Apoptosis and cell death were evaluated quantitatively by FACS after PI/annexin V-PC5 staining. Representative FACS profiles corresponding to one experiment are shown. Data were gated for damaged cells (annexin V− and PI+; top left quadrant, purple), necrotic cells (annexin V+ and PI+; top right quadrant, red), viable cells (annexin V− and PI−; bottom left quadrant, blue) and apoptotic cells (annexin V+ and PI−; bottom right, green). (B) Bar graph showing the percentage of apoptotic cells determined by FACS in GFP-positive cells expressing GFP or GFP-NOR-1 and treated as described for panel A. Data represent means ± SEM (n = 6). Untrans, untransfected cells; *, P < 0.05 versus cells transfected with the same construct and exposed to normoxia; #, P < 0.05 versus cells exposed to the same treatment but transfected with pIRES2-EGFP (GFP). (C) Western blot showing NOR-1 protein levels in HUVEC exposed to normoxia, hypoxia, and CoCl2 and in cells transfected with pIRES2-EGFP (GFP) or pEGFP-NOR-1 (GFP-NOR-1). Levels of β-actin are shown as a loading control. (D) HUVEC were cultured in glass-bottom dishes, were transfected, and were exposed to hypoxia. Cells were processed for annexin V binding and nuclear staining. Representative confocal images showing annexin V-R-PE binding (red) from cells transfected with pIRES2-EGFP (GFP) or pEGFP-NOR-1 (GFP-NOR-1) and exposed to hypoxia. Bar = 50 μm.
FIG. 2.
NOR-1 inhibition increased hypoxia-induced apoptosis. (A) HUVEC were transfected with either siRNA control silencer (siControl) or two different siRNA against NOR-1 (siNOR-11 and siNOR-12 [see Materials and Methods]) and exposed to normoxia, hypoxia (1% O2), or 1 mM CoCl2 for 16 h. Apoptosis and cell death were evaluated quantitatively by FACS after PI/annexin V-PC5 staining. Representative FACS profiles corresponding to one experiment using siControl and siNOR-11 are shown. Data were gated as indicated in the Fig. 1 legend. (B) Bar graphs showing the percentages of apoptotic cells in HUVEC transfected with either siRNA control silencer (siControl) or siRNAs against NOR-1 and treated as described for panel A. Data represent means ± SEM (n = 6). Untrans, untransfected cells; *, P < 0.05 versus cells transfected with the same siRNA and exposed to normoxia; #, P < 0.05 versus cells exposed to the same treatment but transfected with siControl. (C) mRNA levels of NOR-1, Nur77, and Nurr1 from HUVEC transfected with siControl (white bars), siNOR-11 (black bars), or siNOR-12 (shaded bars). *, P < 0.05 versus cells transfected with control silencer. (D) HUVEC were cultured in glass-bottom dishes, were transfected with siRNA, and were exposed to hypoxia. Cells were processed for annexin V binding and nuclear staining. Representative confocal images showing annexin V-R-PE binding (red) from cells transfected with siControl or siNOR-11 and exposed to hypoxia. Bar = 50 μm.
Hypoxia induces NOR-1 expression in HUVEC.
The exposure of HUVEC to hypoxia upregulated NOR-1 expression (Fig. 3A) to an extent similar to VEGF-A expression (Fig. 3B). Two compounds that mimic hypoxia either through competition for (CoCl2) or chelation of (DFO) free iron, inhibiting Fe(II)-2-oxoglutarate-dependent dioxygenases and stabilizing HIF-1α, also upregulated NOR-1 and VEGF (Fig. 3A and B). Hypoxia and CoCl2 induced NOR-1 in a dose- and time-dependent manner (Fig. 3C and D). Hypoxia and CoCl2 also induced VEGF-A (used as a positive control) in a dose- and time-dependent manner (Fig. 3E and F). Interestingly, NOR-1 expression reached a peak at 4 h (∼3.2- and 6.7-fold over normoxia levels in cells treated with hypoxia [1% O2] and 1 mM CoCl2, respectively) and decreased 8 h after stimulus, while VEGF-A expression progressively increased from 1 to 8 h.
FIG. 3.
Hypoxia induces NOR-1 expression in HUVEC. NOR-1 (A) and VEGF (B) mRNA levels from HUVEC cultured under normoxia (Norm) or exposed to hypoxia (1% O2), CoCl2 (1 mM), or deferoxamine mesylate (DFO; 100 μM) for 4 h. (C and D) Hypoxia induces NOR-1 expression in a dose- and time-dependent manner. (C) HUVEC were cultured under normoxia (Norm) or exposed to hypoxia or increasing concentrations of CoCl2 for 4 h. (D) Time-dependence experiments performed in cells subjected to hypoxia (1% O2) or CoCl2 (1 mM) for increasing times. (E and F) VEGF mRNA levels from cells cultured as indicated for panels C and D. Results were normalized by 18S rRNA. Data are from three independent experiments performed in triplicate. *, P < 0.05 versus controls.
Signaling pathways involved in NOR-1 induction by hypoxia in HUVEC.
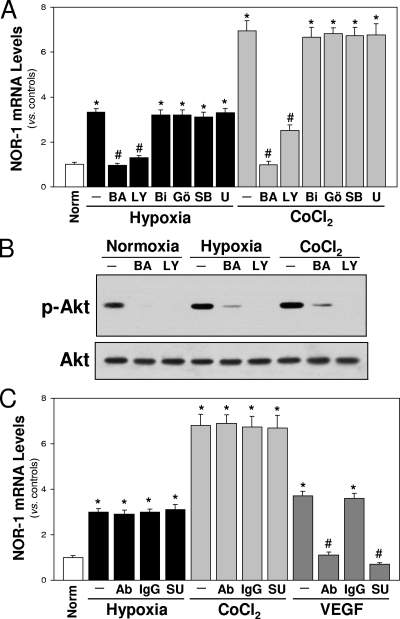
Hypoxia is able to induce different signaling pathways in endothelial cells, including activation of the PKC and MAPK pathways (ERK1/2 and p38 MAPK) and increases free intracellular Ca2+ levels (47). To address which of these pathways are involved in NOR-1 induction, we used specific inhibitors. The results shown in Fig. 4A indicate that NOR-1 upregulation by hypoxia (or CoCl2) is dependent on calcium mobilization (inhibited by a calcium chelator, BAPTA-AM) and PI3K (inhibited by LY294002) but independent of MAPK pathways or PKC. To determine if there is a link between calcium and the PI3K/Akt pathway, we analyzed the effect of BAPTA-AM on the activation of Akt (phosphorylation in Ser-473). Figure 4B shows that BAPTA-AM significantly prevent the activation of Akt induced by hypoxia or CoCl2, suggesting that calcium mobilization is required for PI3K/Akt activation.
FIG. 4.
Pathways involved in hypoxia-induced NOR-1 expression in HUVEC. (A) NOR-1 mRNA levels from HUVEC cultured under normoxia (Norm) or exposed to hypoxia (1% O2) or CoCl2 (1 mM) in the presence or absence (−) of different inhibitors: BAPTA-AM (BA; Ca2+ chelator), LY294002 (LY; PIK3/Akt inhibitor), bisindolylmaleimide I (BI; PKC inhibitor), Gö6976 (Gö; inhibitor of calcium-dependent PKCs), SB203580 (SB; p38 MAPK inhibitor), and U0128 (U; ERK1/2 inhibitor). Data are from three independent experiments performed in triplicate. *, P < 0.05 versus normoxia; #, P < 0.05 versus cells exposed to hypoxia or CoCl2 in the absence of inhibitors. (B) Western blot showing the activation of Akt (phosphorylated Akt [p-Akt]) by hypoxia (1% O2) and CoCl2 (1 mM). Akt activation was prevented by preincubation with BAPTA-AM (BA) and LY294002 (LY). Unchanged levels of total Akt (Akt) were used as loading control. A representative autoradiogram of the results from two experiments performed in duplicate is shown. (C) Inhibition of VEGF or VEGF-R does not prevent hypoxia-induced NOR-1 upregulation. NOR-1 mRNA levels from cells cultured under normoxia (Norm) or exposed to hypoxia (1% O2) or CoCl2 (1 mM) for 4 h in the presence or absence (−) of neutralizing antibodies against VEGF (Ab) or SU5614 (SU, VEGF receptor inhibitor). As a control, the inhibitory effect of anti-VEGF antibodies and SU5614 on NOR-1 expression induced by VEGF is shown. The absence of effect of control IgG is shown. Data are from three independent experiments performed in triplicate. *, P < 0.05 versus normoxia; #, P < 0.05 versus cells treated with VEGF alone.
Autocrine VEGF secretion is not involved in hypoxia-induced NOR-1 upregulation in HUVEC.
In order to assess whether VEGF secreted by endothelial cells in response to hypoxia plays a role in hypoxia-induced NOR-1 expression, we blocked either VEGF (using an anti-VEGF antibody) or VEGFR-2 (using SU5614). Neither the anti-human VEGF antibody nor SU5614 prevented the upregulation of NOR-1 induced by hypoxia or CoCl2 (Fig. 4C). By contrast, as expected, NOR-1 expression induced by stimulation with VEGF was abolished when VEGF or VEGFR-2 was blocked.
NOR-1 upregulation by hypoxia prevents apoptosis in HDMEC.
To determine whether NOR-1 is involved in the survival response of endothelial cells other than HUVEC to hypoxia, we analyzed the regulation of NOR-1 by hypoxia in HDMEC and determined the effect of NOR-1 inhibition on hypoxia-induced apoptosis. Hypoxia and CoCl2 induced NOR-1 in a dose- and time-dependent manner in HDMEC (Fig. 5A and B). Furthermore, the results shown in Fig. 5C indicate that NOR-1 upregulation by hypoxia (or CoCl2) is dependent on calcium mobilization (inhibited by calcium chelator BAPTA-AM) and PI3K (inhibited by LY294002) but is independent of MAPK pathways or PKC. In agreement with the results for HUVEC, SU5614 (inhibitor of VEGFR-2) did not affect NOR-1 upregulation induced by hypoxia (or CoCl2), suggesting that autocrine VEGF secretion is not involved in NOR-1 upregulation by hypoxia. Finally, the inhibition of NOR-1 upregulation using two specific siRNA against NOR-1 increased the percentage of apoptotic cells in cultures exposed to hypoxia (or CoCl2), as determined by FACS analysis (Fig. 5D).
FIG. 5.
NOR-1 is upregulated by hypoxia in HDMEC and prevents hypoxia-induced apoptosis. (A) NOR-1 mRNA levels from HDMEC cultured under normoxia (Norm) or exposed to hypoxia or increasing concentrations of CoCl2 for 4 h. (B) Time-dependence induction of NOR-1 mRNA levels by hypoxia (1% O2) or CoCl2 (1 mM). (C) Pathways involved in hypoxia-induced NOR-1 expression in HDMEC. NOR-1 mRNA levels from HDMEC cultured under normoxia (Norm) or exposed to hypoxia (1% O2) or CoCl2 (1 mM) in the presence or absence of different inhibitors: BAPTA-AM (Ca2+ chelator), LY294002 (PIK3/Akt inhibitor), bisindolylmaleimide I (Bisindo; PKC inhibitor), Gö6976 (inhibitor of calcium-dependent PKCs), SB203580 (p38 MAPK inhibitor), U0128 (ERK1/2 inhibitor), and SU5614 (VEGF receptor inhibitor). Data are from three independent experiments performed in triplicate. *, P < 0.05 versus normoxia; #, P < 0.05 versus cells exposed to hypoxia or CoCl2 in the absence of inhibitors. (D) Bar graph showing the percentages of apoptotic cells determined by FACS in HDMEC transfected with either siRNA control silencer (siControl) or two different siRNA against NOR-1 (siNOR-11 and siNOR-12) and exposed to normoxia, hypoxia (1% O2), or 1 mM CoCl2 for 16 h. Data represent means ± SEM (n = 6). Untrans, untransfected cells. *, P < 0.05 versus cells transfected with the same siRNA and exposed to normoxia; #, P < 0.05 versus cells exposed to the same treatment but transfected with siControl.
Hypoxia-induced NOR-1 expression is not dependent on CREB activation.
Neither CoCl2 nor hypoxia significantly induced CREB activation in a time frame of 10 min to 2 h (Fig. 6A). In addition, the downregulation of CREB by siRNA (Fig. 6B) did not modify the hypoxia-induced expression of NOR-1 (Fig. 6C). Hypoxia-induced VEGF-A mRNA levels were not modified either. Taken together, these results suggest that under our experimental conditions, CREB is not involved in hypoxia-induced NOR-1 expression.
FIG. 6.
CREB is not involved in hypoxia-induced NOR-1 upregulation. (A) Representative Western blot showing the effect of hypoxia (1% O2) or CoCl2 (1 mM) on CREB activation for increasing times. The activation of CREB by VEGF (50 ng/ml) is shown as a positive control. Norm, normoxia. (B) Representative Western blot showing the reduction of CREB protein levels in cells transfected with an siRNA against CREB (siCREB) and cultured under normoxia (Norm) or exposed to hypoxia (1% O2) or CoCl2 (1 mM) for 4 h. An siRNA against a control silencer sequence (siControl) was used. Unchanged levels of β-actin are shown as a loading control. (C and D) mRNA levels of NOR-1 (C) and VEGF (D) from cells transfected with siCREB (+) and exposed to hypoxia for 4 h. Data are from three independent experiments performed in triplicate. siCREB (−) indicates cells transfected with siControl. *, P < 0.05 versus cells transfected with siControl and exposed to normoxia.
Inhibition of HIF reduces hypoxia-induced NOR-1 expression.
Hypoxia and CoCl2 upregulated HIF-1α protein levels in a dose- and time-dependent manner (Fig. 7A and B). This early upregulation of HIF-1α correlates with the early induction of NOR-1 mRNA levels by hypoxia and CoCl2 (Fig. 3D). Two different siRNA against HIF-1α (siHIF-1α1 and siHIF-1α2) prevented hypoxia- and CoCl2-induced upregulation of HIF-1α protein levels (Fig. 7C) and concomitantly inhibited hypoxia- and CoCl2-induced NOR-1 upregulation (Fig. 7D). As expected, the hypoxic induction of VEGF, a well-known gene regulated by HIF-1α, was also abrogated by these siRNA against HIF-1α (Fig. 7E).
FIG. 7.
HIF-1α is early upregulated by hypoxia and CoCl2, and its inhibition by siRNA prevents hypoxia-induced NOR-1 upregulation. Hypoxia (A) and CoCl2 (B) upregulated HIF-1α protein levels in a dose- and time-dependent manner. Western blots showing HIF-1α protein levels from HUVEC cultured under normoxia (Norm), exposed to hypoxia (5 to 1% O2) or increasing concentrations of CoCl2 for 4 h, or exposed to hypoxia (1% O2) or CoCl2 (1 mM) for increasing times. (C to E) HUVEC were transfected with an siRNA against a control silencer sequence (siControl) or with two different siRNA against HIF-1α (siHIF-1α1 or siHIF-1α2 [see Materials and Methods]) and were exposed to hypoxia or CoCl2. (C) Western blot showing the increase of HIF-1α protein levels in cells transfected with siControl and exposed to CoCl2 (1 mM) or hypoxia (1% O2) for 4 h, and the preventive effect exerted by siRNAs against HIF-1α. (D and E) mRNA levels of NOR-1 (D) and VEGF (E) from cells transfected (+) or not (−) with siControl, siHIF-11, or siHIF-12 and exposed to hypoxia or CoCl2 for 4 h. Data from three independent experiments performed in triplicate are shown. *, P < 0.05 versus cells transfected with siControl and exposed to normoxia; #, P < 0.05 versus cells transfected with siControl and exposed to hypoxia or CoCl2.
The PI3K/Akt pathway is key for the upregulation of HIF-1α and the subsequent induction of NOR-1 expression by hypoxia.
Inhibition of the PI3K/Akt pathway (by LY294002 or wortmannin) or inhibition of its downstream target mTOR (by rapamycin) significantly reduced HIF-1α protein levels (Fig. 8A) and concomitantly decreased NOR-1 mRNA levels induced by hypoxia (or CoCl2) (Fig. 8B). As expected, these compounds also reduced hypoxia-induced VEGF mRNA levels (Fig. 8C).
FIG. 8.
The PI3K/Akt pathway is involved in hypoxia-induced NOR-1 expression. HUVEC were cultured under normoxia (Norm) or exposed to hypoxia (1% O2) or CoCl2 (1 mM) for 4 h in the presence or absence (−) of LY294002 (LY; PIK3/Akt inhibitor), wortmannin (Wor; PIK3/Akt inhibitor), or rapamycin (Rap; mTOR inhibitor). (A) Representative Western blot showing the increase of HIF-1α protein levels induced by hypoxia and the significant reduction produced by LY294002, wortmannin, and rapamycin. Unchanged levels of β-actin are shown as a loading control. (B) NOR-1 mRNA levels from HUVEC cultured under normoxia or exposed to hypoxia or CoCl2 for 4 h in the presence of the inhibitors mentioned above. (C) VEGF mRNA levels from endothelial cells cultured as indicated for panel B. Data from three independent experiments performed in triplicate are shown. *, P < 0.05 versus cells exposed to normoxia; #, P < 0.05 versus cells exposed to hypoxia (or treated with CoCl2) in the absence of inhibitors.
Hypoxia induces NOR-1 promoter activity through a HRE.
In transient transfection experiments, hypoxia and CoCl2 increased the transcriptional activity of a luciferase reporter plasmid containing 1,703 bp of the NOR-1 promoter (pGL3-NOR/-1703) in both HUVEC (Fig. 9A) and HDMEC (data not shown). Furthermore, in cotransfection assays, an HIF-1α expression vector was able to increase the transcriptional activity of the reporter plasmid under normoxic conditions (Fig. 9B). By serial deletions, we located a putative HRE 683 bp upstream from the transcriptional start site (Fig. 9C). The mutation of this HRE by site-directed mutagenesis abolished hypoxia-induced NOR-1 promoter activity (Fig. 9D). By contrast, mutation of the three CRE sites located near the transcriptional start site significantly reduced basal promoter activity but did not affect hypoxia-induced NOR-1 transcriptional activity. Finally, ChIP assays were performed to confirm that HIF-1 directly binds to this putative HRE site. As shown in Fig. 10, HIF-1 specifically binds to that HRE site. Quantitative analysis by real-time PCR revealed that hypoxia and CoCl2 increased HIF-1 binding by 9.3- and 14.1-fold, respectively. This binding was significantly reduced by LY294002 (an inhibitor of PI3K/Akt), consistent with this signaling cascade being a key pathway in the upregulation of NOR-1 by hypoxia and in cell survival. In concert, these experiments demonstrate that HIF-1 binds to an HRE motif in the human NOR-1 promoter, resulting in the activation of NOR-1 transcription.
FIG. 9.
HIF-1α regulates NOR-1 transcriptional activity through an HRE present in the NOR-1 promoter. (A) HUVEC were transfected with the luciferase reporter construct pGL3-NOR/-1703, and NOR-1 promoter activity was measured in cells cultured under normoxia (Norm) and in cells exposed to hypoxia (1% O2) or CoCl2 (1 mM) for 6 h. Data from three independent experiments performed in quadruplicate are shown. *, P < 0.05 versus cells exposed to normoxia. (B) NOR-1 promoter activity in cells transfected with the construct pGL-NOR/-1703 and cotransfected with the empty plasmid pcDNA3 or with a HIF-1α expression plasmid (pHIF-1α). *, P < 0.05 versus cells transfected with pcDNA3 (empty vector). (C) Schematic representation of the NOR-1 promoter (pGL-NOR/-1703) showing the putative HRE (white triangle; from −683 to −678) and three CRE sites (black triangles; from −83 to −42). The core consensus HRE binding site is indicated in bold, and changes in the HRE are boxed. (D) Hypoxia (black bars) increased NOR-1 promoter activity in cells transfected with the wild-type promoter construct but not in cells transfected with a construct with the putative HRE mutated (deleted white triangle) or with constructs (pNOR/-600 and pNOR/-300) that do not contain this element. Mutation of the three CRE sites in the construct pGL3-NOR/-1703 (deleted black triangles) did not prevent hypoxia-induced NOR-1 transcriptional activity. *, P < 0.05 versus cells transfected with the same construct and exposed to normoxia. Luc, luciferase.
FIG. 10.
Functional HIF-1 binding to the NOR-1 promoter in hypoxic cells. (A) The relative in vivo association of HIF-1 with the human NOR-1 promoter was analyzed by ChIP in HUVEC incubated under normoxia (Norm), hypoxia (1% O2), or CoCl2 (1 mM) for 4 h in the presence or in the absence (−) of the PI3K/Akt inhibitor LY294002 (LY). Sheared chromatin was immunoprecipitated with an anti-HIF-1α antibody or a nonspecific goat IgG. The enrichment of HIF-1α was quantified by real-time PCR using NOR-1 promoter-specific primers. Data were normalized to the total input DNA and are represented as means ± SEM of two independent experiments performed in duplicate. *, P < 0.05 versus cells exposed to normoxia; #, P < 0.05 versus cells exposed to hypoxia (or treated with CoCl2) in the absence of inhibitors. (B) Agarose gel electrophoresis of PCR products. Equal input DNA and control IgG (CT IgG) immunoprecipitations are shown.
Modulation of the antiapoptotic protein cIAP2 by hypoxia and by the attenuation/overexpression of NOR-1.
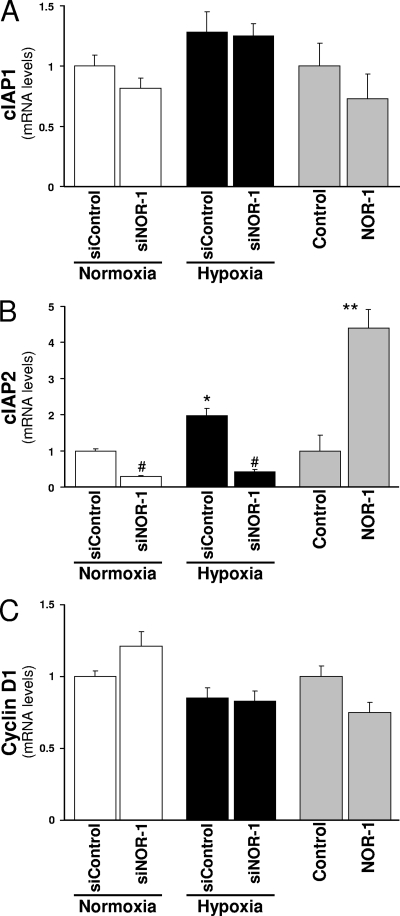
We subsequently examined the effect of hypoxia as well as NOR-1 attenuation/overexpression on the expression of a set of genes potentially involved in the prosurvival effects of hypoxia mediated by NOR-1. In particular, we focused on two families of proteins (cyclin D and IAP) involved in cell survival/apoptosis (6, 52) because it has been suggested that they could be regulated by NR4A receptors (40, 44). As shown in Fig. 11, cIAP2 was induced by hypoxia (approximately twofold over normoxia). Under these conditions, the attenuation of NOR-1 expression using siRNA reduced both basal and hypoxia-induced cIAP2 mRNA levels. Conversely, NOR-1 overexpression was able to upregulate cIAP2 expression. By contrast, neither hypoxia nor NOR-1 attenuation/overexpression modulated cIAP1 or cyclin D1 mRNA levels. Therefore, the cIAP2 gene could be a NOR-1 target gene involved in endothelial cell survival in response to hypoxia.
FIG. 11.
Hypoxia and NOR-1 attenuation/overexpression differentially modulate genes involved in cell survival. (A to C) HUVEC were transfected with either siRNA control silencer (siControl) or siNOR-11 against NOR-1 (siNOR-1) and exposed to normoxia or hypoxia (1% O2) for 24 h. mRNA levels of cIAP1, cIAP2, and cyclin D1 were determined by real-time PCR and were expressed relative to those of cells transfected with siControl and exposed to normoxia. Shaded bars, HUVEC were transfected with either a plasmid (pEGFP-NOR-1) that overexpresses NOR-1 (NOR-1) or the corresponding control vector (pIRES2-EGFP; Control). mRNA levels of cIAP1, cIAP2, and cyclin D1 were determined by real-time PCR 24 h later and were expressed relative to those of cells transfected with the control vector. Data represent means ± SEM (n = 6). *, P < 0.05 versus cells transfected with siControl and exposed to normoxia; #, P < 0.05 versus cells transfected with siControl and exposed to the same treatment (either normoxia or hypoxia); **, P < 0.05 versus cells transfected with the control vector (Control).
DISCUSSION
Experimental and clinical studies indicate that hypoxia plays a fundamental role in the pathogenesis of a variety of diseases, including cardiovascular, hematological, and pulmonary disorders and cancer (9, 47, 48). Areas of low pO2 are also present in atherosclerotic lesions as has been shown using hypoxia markers (7). In the vascular wall, immunoreactivity for HIF-1α parallels arterial intimal thickening (23), and pharmacological treatments that reduce intimal thickening attenuate the increase of HIF-1α observed in atherosclerotic lesions (57). Therefore, it has been suggested that hypoxia could be critical for driving vascular remodeling associated with the development of atherosclerotic lesions (17). The transcriptional response of mammalian cells to hypoxia is largely mediated by HIF-1 (48, 54). HIF-1 modulates a variety of genes that act in concert to facilitate the supply of oxygen and nutrients and promote survival and growth control. However, additional transcription factors cooperate in the complex response of cells to hypoxia in a HIF-dependent or -independent manner (1, 25). In the last years, new genes regulated by hypoxia/HIF have been identified (29); however, the regulatory network of transcription factors that cooperates in the response of vascular endothelial cells to hypoxia is not completely understood. In this work, we have investigated the role of the transcription factor NOR-1 in endothelial cell function under hypoxic conditions. We report here that NOR-1 is a downstream target of HIF-1α that could play an important role in the cell survival response induced by hypoxia.
To assess the potential role of NOR-1 in the response of endothelial cells to hypoxia, we overexpressed NOR-1 by a bicistronic recombinant plasmid or specifically inhibited NOR-1 expression with siRNA. We found that in cells exposed to hypoxia or CoCl2, the overexpression of NOR-1 decreased the rate of cells undergoing apoptosis, while the inhibition of NOR-1 increased this rate. Therefore, NOR-1 could be a transcription factor relevant in the survival response of endothelial cells to hypoxia. Furthermore, data from cells cultured in arresting medium under normoxia suggest that NOR-1 could be involved in cell survival not only in hypoxia/cobalt chloride-induced apoptosis but also in other circumstances, such as apoptosis induced by serum/growth-factor deprivation. Since NOR-1 is an early gene upregulated by different stimuli, including VEGF, we analyzed the effect of hypoxia on NOR-1 expression. We show that in vascular endothelial cells, hypoxia induces NOR-1. CoCl2 and the iron chelator DFO, two inhibitors of iron-depending enzymes that are widely used to analyze the cellular response to hypoxia, also upregulate the expression of NOR-1.
The cellular response to hypoxia is mediated, at least in part, by VEGF, which modulates gene expression, including NR4A receptors (26, 50, 61), and elicits an array of biologic activities (9, 15, 48). However, the incubation of endothelial cells with blocking antibodies against VEGF or SU5614 (a VEGF-R2 inhibitor) did not prevent hypoxia-induced NOR-1 expression, suggesting that NOR-1 is not induced by VEGF secreted by endothelial cells in response to hypoxia. Although we observed a progressive increase in VEGF mRNA levels in cells exposed to hypoxia during 1 to 8 h, it is likely that the autocrine secretion of VEGF during this time was not high enough to significantly upregulate NOR-1 expression. In fact, levels of secreted VEGF reported in HUVEC exposed to hypoxia or CoCl2 at these times (2, 38, 45) are lower than those required to early upregulate NOR-1 using exogenous recombinant VEGF-165 (50). In addition, under our experimental conditions, neither hypoxia nor CoCl2 significantly activates CREB, a key transcription factor involved in the early upregulation of NOR-1 expression by VEGF (26, 50) and other stimuli (12, 32, 33, 49), which is activated by longer exposures of HUVEC to hypoxia (37). Finally, the inhibition of CREB by siRNA did not affect hypoxia-induced NOR-1 expression. Therefore, hypoxia-induced NOR-1 expression is neither mediated by the autocrine secretion of VEGF by endothelial cells nor by CREB activation.
Hypoxia upregulates NOR-1 through pathways dependent on intracellular calcium but independent of PKC, MAPK1/2, or p38 MAPK activation (pathways commonly involved in NOR-1 induction by growth factors and cytokines) (12, 32, 33, 39, 49, 50). Recent studies indicate that intracellular calcium and the Ca2+-calmodulin protein kinase cascade are crucial in the cellular response to hypoxia (60). Interestingly, calcium mobilization is key in the upregulation of NOR-1 by hypoxia and other stimuli (32, 41), and it has been shown that the Ca2+-calmodulin-dependent protein kinase cascade also regulates the transcriptional activity of NOR-1 (21). In agreement with previous studies (24), we show that intracellular calcium is needed for the hypoxia-induced activation of PI3K/Akt, a pathway involved in HIF-1α upregulation (34) and cell survival (3). In this regard, the reduction of HIF-1α protein levels by siRNA or by inhibitors of PI3k/Akt or mTOR significantly counteracts hypoxia-induced NOR-1 upregulation. Finally, in transient transfection assays, the activity of the NOR-1 promoter was increased by hypoxia (and CoCl2) and by cotransfection with a HIF-1α expression plasmid. This effect was mediated by an HRE present in the NOR-1 promoter, as shown by site-directed mutagenesis and serial deletions and by ChIP assays. Therefore, NOR-1 is upregulated by hypoxia in a HIF-1-dependent manner via a mechanism involving calcium and the PI3K/Akt/mTOR pathway. On the basis of previous results (33, 50) and those presented here, the mechanism proposed for NOR-1 upregulation in endothelial cells by VEGF, thrombin, and hypoxia is summarized in Fig. 12.
FIG. 12.
Schematic representation of pathways involved in NOR-1 upregulation by thrombin and VEGF (light lines) and hypoxia (bold lines) in endothelial cells. Thrombin and VEGF induce NOR-1 expression through a mechanism dependent on PAR-1 (protease-activated receptor-1) and VEGF receptor-2 (VEGFR-2/KDR), respectively, and multiple signaling pathways including cytosolic Ca2+, the activation of PKC, and MAPK pathways (both ERK1/2 and p38 MAPK) that lead to the downstream activation of CREB. VEGFR-2 can also activate Ras/Raf/MEK/ERK signaling. An increase in intracellular Ca2+ activates calcium/calmodulin-dependent kinases (Ca/CM). The potential cross talk among signaling pathways is indicated by dashed arrows. p90RSK, p38 MAPK, and Ca/CM are kinases potentially involved in CREB phosphorylation in Ser-133 (36). The indicated cross talk mechanisms and kinases potentially involved in CREB phosphorylation have not been specifically analyzed in relation with NOR upregulation. Hypoxia increases intracellular Ca2+ concentration, which activates the PI3K/Akt pathway and its downstream target mTOR. The PI3K/Akt/mTOR pathway could mediate the upregulation of HIF-1α protein levels by decreasing HIF-1α degradation and/or increasing HIF-1α synthesis (19, 20). Agents that inhibit NOR-1 expression interfering signaling pathways are indicated (boxed). Arrows and T-bars indicate activation and inhibition, respectively. ATAP-2, anti-PAR-1 blocking antibody; DAG, diacylglycerol; IP3, inositol-1,4,5-triphosphate; PLC, phospholipase C; PTX, pertussis toxin.
NOR-1, as well as the other two closely related members of the NR4A subfamily, Nur77 and Nurr1, has been involved in different cell processes including apoptosis, cell differentiation and proliferation, and inflammation (10, 31, 43, 44, 46). Although these nuclear receptors are highly homologous and could play redundant functions in some systems (10), they exhibit significant differences, among them their ability to dimerize and to bind to response elements (62). NOR-1 null mice were not viable, suggesting that the role of NOR-1 could not be undertaken by the other members (14). Interestingly, recent results suggest that they could play opposite roles. Indeed, NOR-1 inhibition or the genetic ablation of NOR-1 reduces the proliferation of vascular cells (12, 32, 33, 39, 49, 50). In contrast, opposed results have been published regarding Nur77 and vascular cell proliferation. Nur77 overexpression has been associated with either the inhibition (4, 39) or promotion of vascular cell proliferation (61). Similarly, Nur77, crucially involved in apoptosis in immature thymocytes and T-cell hybridomas (27), has also been described as a survival factor in tumor necrosis factor signaling in mouse embryonic fibroblasts (55). Concerning modulation by hypoxia, the upregulation of Nurr1 and Nur77 (18), and recently NOR-1 (46), after cerebral ischemia has been described. Nur77, the best-studied NR4A receptor, is induced by hypoxia/HIF-1 in different cancer cell lines (11, 58, 59). Interestingly, HIF-1α is stabilized by interaction with the N-terminal transactivation domain of Nur77 (58), and the activation of Nur77 by 6-mercaptopurine enhances the transcriptional activity of HIF-1α, resulting in new vessel formation (59). Preliminary results indicate that the inhibition of NOR-1 did not significantly affect angiotube formation (unpublished results). Therefore, NOR-1 and Nur77 might be essential in the response of endothelial cells to hypoxia, albeit probably playing nonequivalent roles. Our data are in agreement with a recent report showing that NOR-1 deficiency compromises smooth muscle cell proliferation survival in response to serum deprivation (40). Finally, regarding the molecular mechanism by which NOR-1 preserves endothelial cell survival, we show that while the attenuation of NOR-1 expression reduced both basal and hypoxia-induced cIAP2 mRNA levels, NOR-1 overexpression upregulated cIAP2. By contrast, other genes (cyclin D1 and cIAP1 genes) involved in cell survival (6, 52) that have been previously suggested to be targets of NR4A receptors (40, 44) were not significantly induced by hypoxia and remained essentially unaltered after NOR-1 inhibition or overexpression. cIAP2 is a hypoxia-induced prosurvival factor (16) that belongs to a family of genetically conserved proteins characterized by the presence of one to three baculovirus IAP repeat motifs and that have been shown to be potent suppressors of cell apoptosis (52). Interestingly, cIAP2 but not cIAP1 possesses a full conserved consensus motif for NBRE in its proximal promoter (located at positions −326 to −319). Further studies are needed, however, to exhaustively analyze the functionality of this putative site and, most importantly, to identify other NOR-1 target genes that working in concert contribute to the antiapoptotic effect of this nuclear receptor in endothelial cells.
In summary, our results indicate that in endothelial cells, NOR-1 could be regarded as a survival transcription factor under basal conditions and a downstream effector of HIF signaling involved in the survival response elicited by hypoxic stress. The induction of different sets of transcription factors should promote the modulation of different sets of structural genes leading to differential cell responses. Nowadays, there is no information about genes controlled by dissimilar combinations of NR4A genes, and the relative contribution of these receptors to the transcriptional response of endothelial cells to hypoxia is still poorly understood. Further studies are needed to determine how NOR-1 and NOR family members cooperate with HIF-1 and other hypoxia-regulated transcription factors to depict the complex network of factors involved in the adaptive response of mammalian cells to hypoxia.
Acknowledgments
We thank Naganari Ohkura for generously providing the pNORα/-1703 construct and L. Eric Huang for the pCDNA3-HIF-1α expression vector. We also thank Esther Peña for her help with confocal microscopy.
This work was supported by the Ministerio de Educación y Ciencia (SAF2006-07378 and SAF2006-1009), Red Temática de Investigación Cardiovascular (RECAVA; RD06/0014/0027), PI061480 from the Ministerio de Sanidad-Instituto de Salud Carlos III, and Fundación Española de Arteriosclerosis-Sociedad Española de Arteriosclerosis (FEA-SEA 2006). M.G. is a recipient of a research fellowship (FI) from Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR) de la Generalitat de Catalunya.
Footnotes
Published ahead of print on 31 August 2009.
REFERENCES
- 1.Alfranca, A., M. D. Gutiérrez, A. Vara, J. Aragonés, F. Vidal, and M. O. Landázuri. 2002. c-Jun and hypoxia-inducible factor 1 functionally cooperate in hypoxia-induced gene transcription. Mol. Cell. Biol. 22:12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali, M. A., H. Choy, A. A. Habib, and D. Saha. 2007. SNS-032 prevents tumor cell-induced angiogenesis by inhibiting vascular endothelial growth factor. Neoplasia 9:370-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Tejado, M., S. Naranjo-Suarez, C. Jiménez, A. C. Carrera, M. O. Landázuri, and L. del Peso. 2001. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J. Biol. Chem. 276:22368-22374. [DOI] [PubMed] [Google Scholar]
- 4.Arkenbout, E. K., M. van Bragt, E. Eldering, C. van Bree, J. M. Grimbergen, P. H. Quax, H. Pannekoek, and C. J. de Vries. 2003. TR3 orphan receptor is expressed in vascular endothelial cells and mediates cell cycle arrest. Arterioscler. Thromb. Vasc. Biol. 23:1535-1540. [DOI] [PubMed] [Google Scholar]
- 5.Arkenbout, E. K., V. de Waard, M. van Bragt, T. A. van Achterberg, J. M. Grimbergen, B. Pichon, H. Pannekoek, and C. J. de Vries. 2002. Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation 106:1530-1535. [DOI] [PubMed] [Google Scholar]
- 6.Biliran, H., Jr., Y. Wang, S. Banerjee, H. Xu, H. Heng, A. Thakur, A. Bollig, F. H. Sarkar, and J. D. Liao. 2005. Overexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell line. Clin. Cancer Res. 11:6075-6086. [DOI] [PubMed] [Google Scholar]
- 7.Björnheden, T., M. Levin, M. Evaldsson, and O. Wiklund. 1999. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler. Thromb. Vasc. Biol. 19:870-876. [DOI] [PubMed] [Google Scholar]
- 8.Calvani, M., A. Rapisarda, B. Uranchimeg, R. H. Shoemaker, and G. Melillo. 2006. Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood 107:2705-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmeliet, P. 2003. Angiogenesis in health and disease. Nat. Med. 9:653-660. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, L. E., F. K. Chan, D. Cado, and A. Winoto. 1997. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. EMBO J. 16:1865-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, J. W., S. C. Park, G. H. Kang, J. O. Liu, and H. D. Youn. 2004. Nur77 activated by hypoxia-inducible factor-1alpha overproduces proopiomelanocortin in von Hippel-Lindau-mutated renal cell carcinoma. Cancer Res. 64:35-39. [DOI] [PubMed] [Google Scholar]
- 12.Crespo, J., J. Martínez-González, J. Rius, and L. Badimon. 2005. Simvastatin inhibits NOR-1 expression induced by hyperlipemia by interfering with CREB activation. Cardiovasc. Res. 67:333-341. [DOI] [PubMed] [Google Scholar]
- 13.Das, M., O. Djahanbakhch, B. Hacihanefioglu, E. Saridogan, M. Ikram, L. Ghali, M. Raveendran, and A. Storey. 2008. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 93:881-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeYoung, R. A., J. C. Baker, D. Cado, and A. Winoto. 2003. The orphan steroid receptor Nur77 family member Nor-1 is essential for early mouse embryogenesis. J. Biol. Chem. 278:47104-47109. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara, N., H. P. Gerber, and J. LeCouter. 2003. The biology of VEGF and its receptors. Nat. Med. 9:669-676. [DOI] [PubMed] [Google Scholar]
- 16.Greijer, A. E., and E. van der Wall. 2004. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J. Clin. Pathol. 57:1009-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hänze, J., N. Weissmann, F. Grimminger, W. Seeger, and F. Rose. 2007. Cellular and molecular mechanisms of hypoxia-inducible factor driven vascular remodeling. Thromb. Haemost. 97:774-787. [PubMed] [Google Scholar]
- 18.Honkaniemi, J., and F. R. Sharp. 1996. Global ischemia induces immediate-early genes encoding zinc finger transcription factors. J. Cereb. Blood Flow Metab. 16:557-565. [DOI] [PubMed] [Google Scholar]
- 19.Hudson, C. C., M. Liu, G. G. Chiang, D. M. Otterness, D. C. Loomis, F. Kaper, A. J. Giaccia, and R. T. Abraham. 2002. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22:7004-7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui, A. S., A. L. Bauer, J. B. Striet, P. O. Schnell, and M. F. Czyzyk-Krzeska. 2006. Calcium signaling stimulates translation of HIF-alpha during hypoxia. FASEB J. 20:466-475. [DOI] [PubMed] [Google Scholar]
- 21.Inuzuka, H., H. Tokumitsu, N. Ohkura, and R. Kobayashi. 2002. Transcriptional regulation of nuclear orphan receptor, NOR-1, by Ca(2+)/calmodulin-dependent protein kinase cascade. FEBS Lett. 522:88-92. [DOI] [PubMed] [Google Scholar]
- 22.Kim, Y., S. Hong, M. R. Noh, S. Y. Kim, P. W. Huh, S. H. Park, W. Sun, and H. Kim. 2006. Induction of neuron-derived orphan receptor-1 in the dentate gyrus of the hippocampal formation following transient global ischemia in the rat. Mol. Cells 22:8-12. [PubMed] [Google Scholar]
- 23.Kuwahara, F., H. Kai, K. Tokuda, R. Shibata, K. Kusaba, N. Tahara, H. Niiyama, T. Nagata, and T. Imaizumi. 2002. Hypoxia-inducible factor-1alpha/vascular endothelial growth factor pathway for adventitial vasa vasorum formation in hypertensive rat aorta. Hypertension 39:46-50. [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. H., M. Y. Lee, J. H. Lee, and H. J. Han. 2008. A potential mechanism for short time exposure to hypoxia-induced DNA synthesis in primary cultured chicken hepatocytes: correlation between Ca(2+)/PKC/MAPKs and PI3K/Akt/mTOR. J. Cell. Biochem. 104:1598-1611. [DOI] [PubMed] [Google Scholar]
- 25.Liao, H., M. C. Hyman, D. A. Lawrence, and D. J. Pinsky. 2007. Molecular regulation of the PAI-1 gene by hypoxia: contributions of Egr-1, HIF-1α, and C/EBPα. FASEB J. 21:935-949. [DOI] [PubMed] [Google Scholar]
- 26.Liu, D., H. Jia, D. I. Holmes, A. Stannard, and I. Zachary. 2003. Vascular endothelial growth factor-regulated gene expression in endothelial cells: KDR-mediated induction of Egr3 and the related nuclear receptors Nur77, Nurr1, and Nor1. Arterioscler. Thromb. Vasc. Biol. 23:2002-2007. [DOI] [PubMed] [Google Scholar]
- 27.Liu, Z. G., S. W. Smith, K. A. McLaughlin, L. M. Schwartz, and B. A. Osborne. 1994. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene Nur77. Nature 367:281-284. [DOI] [PubMed] [Google Scholar]
- 28.Maltais, A., C. Filion, and Y. Labelle. 2002. The AF2 domain of the orphan nuclear receptor TEC is essential for the transcriptional activity of the oncogenic fusion protein EWS/TEC. Cancer Lett. 183:87-94. [DOI] [PubMed] [Google Scholar]
- 29.Manalo, D. J., A. Rowan, T. Lavoie, L. Natarajan, B. D. Kelly, S. Q. Ye, J. G. García, and G. L. Semenza. 2005. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105:659-669. [DOI] [PubMed] [Google Scholar]
- 30.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schütz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, and R. M. Evans. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-González, J., and L. Badimon. 2005. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc. Res. 65:609-618. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-González, J., J. Rius, A. Castelló, C. Cases-Langhoff, and L. Badimon. 2003. Neuron-derived orphan receptor-1 (NOR-1) modulates vascular smooth muscle cell proliferation. Circ. Res. 92:96-103. [DOI] [PubMed] [Google Scholar]
- 33.Martorell, L., J. Martínez-González, J. Crespo, O. Calvayrac, and L. Badimon. 2007. Neuron-derived orphan receptor-1 (NOR-1) is induced by thrombin and mediates vascular endothelial cell growth. J. Thromb. Haemost. 5:1766-1773. [DOI] [PubMed] [Google Scholar]
- 34.Mayerhofer, M., P. Valent, W. R. Sperr, J. D. Griffin, and C. Sillaber. 2002. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood 100:3767-3775. [DOI] [PubMed] [Google Scholar]
- 35.Mayo, L. D., K. M. Kessler, R. Pincheira, R. S. Warren, and D. B. Donner. 2001. Vascular endothelial cell growth factor activates CRE-binding protein by signaling through the KDR receptor tyrosine kinase. J. Biol. Chem. 276:25184-25189. [DOI] [PubMed] [Google Scholar]
- 36.Mayr, B., and M. Montminy. 2001. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2:599-609. [DOI] [PubMed] [Google Scholar]
- 37.Min, J., Y. M. Jin, J. S. Moon, M. S. Sung, S. A. Jo, and I. Jo. 2006. Hypoxia-induced endothelial NO synthase gene transcriptional activation is mediated through the tax-responsive element in endothelial cells. Hypertension 47:1189-1196. [DOI] [PubMed] [Google Scholar]
- 38.Nagamatsu, T., T. Fujii, M. Kusumi, L. Zou, T. Yamashita, Y. Osuga, M. Momoeda, S. Kozuma, and Y. Taketani. 2004. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 145:4838-4845. [DOI] [PubMed] [Google Scholar]
- 39.Nomiyama, T., T. Nakamachi, F. Gizard, E. B. Heywood, K. L. Jones, N. Ohkura, R. Kawamori, O. M. Conneely, and D. Bruemmer. 2006. The NR4A orphan nuclear receptor NOR1 is induced by platelet-derived growth factor and mediates vascular smooth muscle cell proliferation. J. Biol. Chem. 281:33467-33476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomiyama, T., Y. Zhao, F. Gizard, H. M. Findeisen, E. B. Heywood, K. L. Jones, O. M. Conneely, and D. Bruemmer. 2009. Deficiency of the NR4A neuron-derived orphan receptor-1 attenuates neointima formation after vascular injury. Circulation 119:577-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohkubo, T., N. Ohkura, K. Maruyama, K. Sasaki, K. Nagasaki, H. Hanzawa, T. Tsukada, and K. Yamaguchi. 2000. Early induction of the orphan nuclear receptor NOR-1 during cell death of the human breast cancer cell line MCF-7. Mol. Cell. Endocrinol. 162:151-156. [DOI] [PubMed] [Google Scholar]
- 42.Ohkura, N., M. Hijikuro, A. Yamamoto, and K. Miki. 1994. Molecular cloning of a novel thyroid/steroid receptor superfamily gene from cultured rat neuronal cells. Biochem. Biophys. Res. Commun. 205:1959-1965. [DOI] [PubMed] [Google Scholar]
- 43.Pei, L., A. Castrillo, M. Chen, A. Hoffmann, and P. Tontonoz. 2005. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J. Biol. Chem. 280:29256-29262. [DOI] [PubMed] [Google Scholar]
- 44.Pei, L., A. Castrillo, and P. Tontonoz. 2006. Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77. Mol. Endocrinol. 20:786-794. [DOI] [PubMed] [Google Scholar]
- 45.Pichiule, P., J. C. Chavez, and J. C. LaManna. 2004. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J. Biol. Chem. 279:12171-12180. [DOI] [PubMed] [Google Scholar]
- 46.Ponnio, T., and O. M. Conneely. 2004. NOR-1 regulates hippocampal axon guidance, pyramidal cell survival, and seizure susceptibility. Mol. Cell. Biol. 24:9070-9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pouysségur, J., F. Dayan, and N. M. Mazure. 2006. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441:437-443. [DOI] [PubMed] [Google Scholar]
- 48.Pugh, C. W., and P. J. Ratcliffe. 2003. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 9:677-684. [DOI] [PubMed] [Google Scholar]
- 49.Rius, J., J. Martinez-Gonzalez, J. Crespo, and L. Badimon. 2004. Involvement of neuron-derived orphan receptor-1 (NOR-1) in LDL-induced mitogenic stimulus in vascular smooth muscle cells: role of CREB. Arterioscler. Thromb. Vasc. Biol. 24:697-702. [DOI] [PubMed] [Google Scholar]
- 50.Rius, J., J. Martinez-Gonzalez, J. Crespo, and L. Badimon. 2006. NOR-1 is involved in VEGF-induced endothelial cell growth. Atherosclerosis 184:276-282. [DOI] [PubMed] [Google Scholar]
- 51.Safran, M., and W. G. Kaelin, Jr. 2003. HIF hydroxylation and the mammalian oxygen-sensing pathway. J. Clin. Investig. 111:779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salvesen, G. S., and C. S. Duckett. 2002. IAP proteins: blocking the road to death's door. Nat. Rev. Mol. Cell Biol. 3:401-410. [DOI] [PubMed] [Google Scholar]
- 53.Semenza, G. L. 2009. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24:97-106. [DOI] [PubMed] [Google Scholar]
- 54.Semenza, G. L. 2000. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J. Clin. Investig. 106:809-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki, S., N. Suzuki, C. Mirtsos, T. Horacek, E. Lye, S. K. Noh, A. Ho, D. Bouchard, T. W. Mak, and W. C. Yeh. 2003. Nur77 as a survival factor in tumor necrosis factor signaling. Proc. Natl. Acad. Sci. USA 100:8276-8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, Z., G. Benoit, J. Liu, S. Prasad, P. Aarnisalo, X. Liu, H. Xu, N. P. Walker, and T. Perlmann. 2003. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 423:555-560. [DOI] [PubMed] [Google Scholar]
- 57.Wilson, S. H., J. Herrmann, L. O. Lerman, D. R. Holmes, Jr., C. Napoli, E. L. Ritman, and A. Lerman. 2002. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation 105:415-418. [DOI] [PubMed] [Google Scholar]
- 58.Yoo, Y. G., M. G. Yeo, D. K. Kim, H. Park, and M. O. Lee. 2004. Novel function of orphan nuclear receptor Nur77 in stabilizing hypoxia-inducible factor-1alpha. J. Biol. Chem. 279:53365-53373. [DOI] [PubMed] [Google Scholar]
- 59.Yoo, Y. G., T. Y. Na, W. K. Yang, H. J. Kim, I. K. Lee, G. Kong, J. H. Chung, and M. O. Lee. 2007. 6-Mercaptopurine, an activator of Nur77, enhances transcriptional activity of HIF-1alpha resulting in new vessel formation. Oncogene 26:3823-3834. [DOI] [PubMed] [Google Scholar]
- 60.Yuan, G., J. Nanduri, C. R. Bhasker, G. L. Semenza, and N. R. Prabhakar. 2005. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J. Biol. Chem. 280:4321-4328. [DOI] [PubMed] [Google Scholar]
- 61.Zeng, H., L. Qin, D. Zhao, X. Tan, E. J. Manseau, M. Van Hoang, D. R. Senger, L. F. Brown, J. A. Nagy, and H. F. Dvorak. 2006. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J. Exp. Med. 203:719-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zetterstrom, R. H., L. Solomin, T. Mitsiadis, L. Olson, and T. Perlmann. 1996. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol. Endocrinol. 10:1656-1666. [DOI] [PubMed] [Google Scholar]