Abstract
Atk can be activated by two independent phosphorylation events. Growth factor-dependent phosphorylation of threonine 308 (Akt-308) by phosphatidylinositol 3-kinase-dependent PDK1 leads to activation of mammalian target of rapamycin (mTor) complex 1 (TORC1) and stimulation of protein synthesis. Phosphorylation on serine 473 (Akt-473) is catalyzed by mTor in a second complex (TORC2), and Akt-473 phosphorylates Foxo3a to inhibit apoptosis. Accumulation of both phosphorylated forms of Akt is frequent in cancer, and TORC2 activity is required for progression to prostate cancer with Pten mutation. Here, we link Akt-473 to the Rb1 pathway and show that mTor is overexpressed with loss of the Rb1 family pathway. This leads to constitutive Akt-473 and, in turn, phosphorylation of Foxo3a and resistance to cell adhesion-dependent apoptosis (anoikis). Additionally, Akt-473 accumulation blocks c-Raf activation, thereby preventing downstream Erk activation. This block cannot be overcome by constitutively active Ras, and it also prevents induction of the Arf tumor suppressor by Ras. These studies link inactivation of the Rb1 pathway, a hallmark of cancer, to accumulation of Akt-473, resistance to anoikis, and a block in c-Raf/Erk activation.
Binding of growth factors to their cell surface receptors activates the Ras family of GTP-binding proteins, which in turn classically activates a cell proliferation signaling cascade, including c-Raf and Erk (27, 28). Mutations leading to constitutive Ras activation are among the most frequently found in cancer, highlighting the importance of this pathway in cancer initiation. Ras can also activate phosphatidylinositol 3-kinase (PI3K), which in turn leads to phosphorylation and activation of Akt (reviewed in reference 20). Akt activation can also stimulate cell proliferation by controlling the expression and subcellular localization of cell cycle regulators, such as cyclin D1 and p27Kip1, and it can regulate protein synthesis by controlling the activity of mammalian target of rapamycin (mTor) complex 1 (TORC1); inhibit apoptosis by controlling the subcellular localization of Foxo3a, which transactivates proapoptotic genes; and change cell shape and motility through regulation of the Rac-Rho-Ccd42 pathway (2). Constitutively active Akt is oncogenic, and activated Akt frequently accumulates in cancer cells (9, 22, 29, 38). As opposed to acting in concert, these two Ras-activated pathways appear to function separately or even antagonistically in at least some cells, because Akt can block activation of c-Raf, which in turn prevents Erk activation (20). Indeed, recent results suggest that the two pathways may function sequentially during tumor progression. While Ras-mediated activation of the MAP kinase/Erk pathway is important in tumor initiation, as tumorigenesis progresses, the major role of Ras activation may become the PI3K/Akt pathway (22).
Akt is phosphorylated by two different kinases. PDK1 is activated via PI3K in response to growth factors, and it phosphorylates Akt on threonine 308 in the A loop (Akt-308) (1). The Pten phosphatase is negatively related to PI3K activity, and mutations in Pten are common in cancer (4). Akt-308 phosphorylates TSC2, thereby blocking the GAP activity of TSC1/TSC2 toward Rheb, whose GTPase activity is required for TORC1 function. mTor, in the context of TORC1, phosphorylates and activates the AGC kinase family member S6 to regulate protein synthesis, thereby linking growth factor stimulation to protein synthesis (5, 14, 36). Mutations in Ras and Pten are mutually exclusive in some tumors, and lack of Erk activation is an adverse prognostic factor in melanoma (4, 19, 21, 26, 38). Taken together, these results suggest that activation of PI3K and, in turn, Akt is a critical function of Ras mutation in some tumors.
mTor forms a second complex (TORC2) with Sin1, Rictor, and mLST8 (24, 30), and mTor in the context of TORC2 phosphorylates Akt (which, like S6, is also an AGC family member) on serine 473 in the hydrophobic motif (11, 18, 32, 37). Knocking out components of TORC2 prevents Akt-473 without affecting Akt-308 (11, 18, 32, 37). Thus, Akt-473 and Akt-308 can be independently catalyzed. TORC2 also phosphorylates Akt and another AGC family member, protein kinase C, on the turn motif, and this phosphorylation is important for protein folding/stability (8, 17).
Akt-308 and Akt-473 appear to be directed at distinct cellular pathways. Akt-308 is essential for regulation of TORC1 and thus protein synthesis, whereas Akt-473 is important for phosphorylation of Foxo3a and prevention of its translocation to the nucleus, where it activates apoptotic genes (16, 18). Accordingly, Akt-473 is associated with resistance of cells to stress and to anoikis (survival in the absence of normal matrix contact) (10). Although Akt-473 has been associated with cytoskeletal changes, no obvious effect of loss of TORC2 components and thus Akt-473 on the actin cytoskeleton or on cell proliferation was seen in culture (18), suggesting that these activities may be linked to Akt-308. Both Akt-308 and Akt-473 frequently accumulate in cancer cells, consistent with increased proliferation and protein synthesis and increased motility and survival as cells lose their normal matrix contacts in the forming tumor and during metastasis (9, 29). Such accumulation of Akt-308 can be facilitated by mutations, such as Ras, leading to constitutive activation of growth factor signaling or to mutation or epigenetic silencing of the Pten phosphatase, which inhibits PI3K activity (4, 9). However, less is known about how TORC2 is regulated or why Akt-473 accumulates in cancer.
One target of the increase in cyclin-dependent kinase (cdk) activity resulting from Erk-dependent induction of genes such as the one encoding cyclin D1 is the Rb1 pathway, whose family members are hyperphosphorylated and inactivated by the resulting accumulation of cdk activity (13, 25). While Rb1 is mutated in some cancers (e.g., retinoblastoma, small-cell lung cancer, and osteosarcoma), more frequently the pathway, including all three Rb1 family members, is inactivated through hyperphosphorylation resulting from mutation or epigenetic silencing of a cdk inhibitor (13). Interestingly, mouse embryo fibroblasts (MEFs) mutated for only one of the three family members, Rb1, no longer require Erk signaling for proliferation in culture, but they do require Akt activity (6). Additionally, expression of the adenoviral oncoprotein E1a in cells blocks Erk activation, and this effect was eliminated with a point mutation in E1a that prevented its binding and by inhibition of the Rb1 family (3). These results suggest that even partial inactivation of the Rb1 pathway (e.g., mutation of Rb1) eliminates a requirement for Erk signaling, but further, the Rb1 pathway seems to be required for cells to activate Erk. As a model for Rb1 pathway inactivation in cancer, we examined the effects of mutation of the three Rb1 family members on Akt and Erk signaling pathways in MEFs.
MATERIALS AND METHODS
Cell lines and cell culture.
Four different primary cultures of triple-knockout (TKO) MEFs from different litters were used with similar results in the studies. TKO Ras cells were generated by infection of TKO cells with a V12 Ha-ras-expressing retrovirus, as previously described (33). TKO and TKO Ras cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Atlanta, GA) and maintained in a humidified atmosphere at 37°C and 5% CO2.
RNA isolation and analysis of gene expression by real-time PCR.
RNA isolation and real-time PCR were performed as described previously (23). The primers for real-time PCR are shown in Table S1 in the supplemental material.
Transfection assays.
A full-length mTor cDNA expression vector was obtained from OpenBiosystems, and the full-length mTor cDNA insert was confirmed by multiple restriction enzyme digestions. MEFs were seeded at 200,000 cells per well of a 12-well plate containing antibiotic-free DMEM (Cellgro) with 10% FBS (HyClone) and cultured overnight. The next day, the cultures were switched to DMEM without antibiotics and FBS and cotransfected with the mTOR expression vector and a green fluorescent protein (GFP) expression vector at a ratio of 5:1 for 6 h with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocol. The transfected cells were then cultured in DMEM containing 10% FBS and 1% antibiotic mixture (Cellgro) at 37°C and 10% CO2.
Immunostaining.
Cells were washed with phosphate-buffered saline (PBS), fixed with 4% formaldehyde for 10 min, and then washed again with PBS. The cells were then treated with methanol at −20°C for 10 min and blocked with 4% goat serum at room temperature for 1 hour. The cells were incubated with primary antibody overnight at 4°C. The following primary antibodies (see Table S1 in the supplemental material) were used: rabbit anti-phospho-Akt (Ser 473) polyclonal antibody (1:10; Cell Signaling Technology, Inc., Boston, MA), rabbit anti-phospho-Akt (Thr 308) polyclonal antibody (1:10; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-FKHRL1 (Foxo3a) polyclonal antibody (1:10; Santa Cruz Biotechnology, Inc.), and mTor (H-266) (1:10; Santa Cruz). The next day, the cells were washed with PBS, followed by incubation with secondary antibody (Alexa Fluor goat anti-rabbit immunoglobulin G; Invitrogen, Carlsbad, CA) for 1 h at room temperature. Images were captured with a Zeiss confocal microscope.
Western blot analysis.
Cell extracts were prepared by washing cells with cold PBS and adding 100 μl of lysis buffer (Pierce Biotechnology, Rockford, IL) with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO), followed by high-speed centrifugation. The protein concentration was assayed by the Bradford method (Bio-Rad Laboratories, Richmond, CA). Forty micrograms of protein from each sample was subjected to electrophoresis on a 4 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel (Bio-Rad Laboratories, Richmond, CA) and transferred to a nitrocellulose membrane (GE Healthcare Biosciences, Piscataway, NJ). The membranes were blocked for 1 h in 5% powdered milk and 0.1% Tween 20 and then incubated overnight with primary antibody, followed by a 1-h incubation with horseradish peroxidase-conjugated secondary antibody (Pierce Biotechnology, Rockford, IL), and detection was done with enhanced chemiluminescence (Perkin Elmer, Waltham, MA). The following antibodies were used: rabbit anti-phospho-Akt (Ser 473) polyclonal antibody (1:1,000; Cell Signaling Technology, Inc., Boston, MA), rabbit anti-phospho-Akt (Thr 308) polyclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-Akt polyclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc.), mouse anti-phospho-Erk (T202/Y204) monoclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc.), rabbit anti-Erk polyclonal antibody (1:1,000; Cell Signaling Technology, Inc., Boston, MA), rabbit anti-FRAP polyclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc.), rabbit anti-phospho-Raf-1 (Ser 259) polyclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc.), mouse anti-Raf-1 monoclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc.), rabbit anti-phospho-FKHRL1 (Foxo3a) (Thr 32) polyclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc.), rabbit anti-phospho-FKHRL1 (Ser 253) polyclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc.), and mouse anti-FKHRL1 monoclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc.).
Ras activation assay.
Ras activity was carried out by using the EZ-Detect Ras activation kit (Pierce Biotechnology, Rockford, IL) that uses a glutathione S-transferase (GST) fusion protein containing the Ras-binding domain of Raf.
Construction of a lentivirus expressing mTor shRNA.
To generate a lentivirus expressing mTOR short hairpin RNA (shRNA), a primer containing an mTOR shRNA sequence (5′-CTGTCTAGACAAAAGAGATGGAGGAGATCACCCTCTCTTGAAGGGTGATCTCCTCCATCTCGGGGATCTGTGGTCTCATACA-3′) was used to amplify a 500-bp fragment containing the H1 promoter using the pSuper vector as a template. The resulting PCR product was digested with SpeI and XbaI and cloned back into the lentiviral vector digested with NheI. The control sequence was 5′-CAACAAGATGAAGAGCACCAATCTCTTGAATTGGTGCTCTTCATCTTGTTG-3′, which was BLAST searched against all mouse RNA sequences to ensure that it did not target an mRNA. We first cloned the shRNA oligomers into a cytomegalovirus-GFP lentiviral vector, where its expression was driven by the mouse U6 promoter. Briefly, the shRNA constructs were generated by synthesizing an 83-mer oligonucleotide containing (i) a 19-nucleotide sense strand and a 19-nucleotide antisense strand separated by a 9-nucleotide loop (TTCAAGAGA), (ii) a stretch of five adenines as a template for the Pol III promoter termination signal, (iii) 21 nucleotides complementary to the 3′ end of the Pol III U6 promoter, and (iv) a 5′ end containing a unique XbaI restriction site. The long oligonucleotide was used together with an sp6-binding oligonucleotide (5′-ATTTAGGTGACACTATAGAAT-3′) to PCR amplify a fragment containing the entire U6 promoter plus shRNA sequences; the resulting product was digested with XbaI and SpeI ligated into the NheI site of the lentivirus vector, and the insert was sequenced to ensure that no errors occurred during the PCR or cloning steps. The detailed procedure has been published elsewhere (32a, 34a). Lentiviral particles were produced by a four-plasmid transfection system as described previously (34a). Briefly, 293T cells were transfected with the lentiviral vector and packaging plasmids, and the supernatants, containing recombinant pseudolentiviral particles, were collected from culture dishes on the second and third days after transfection.
RESULTS
Rb1 family mutation leads to accumulation of Akt-473, but not Akt-308.
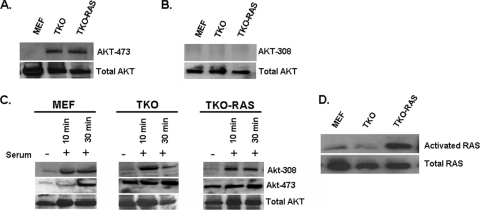
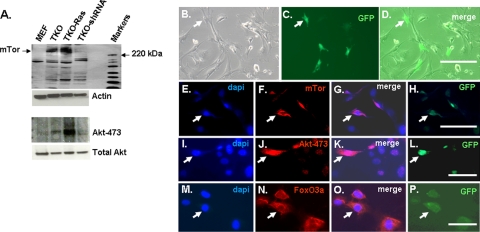
Akt phosphorylation was compared in wild-type MEFs and MEFs in which the three Rb1 family members were mutated (TKO MEFs) (7, 31). Akt-308 was not detected in either cell type; however, Akt-473 was present constitutively in the TKO MEFs, but it was not detected in wild-type MEFs (Fig. 1A and B). Next, we examined Rb1−/− MEFs and RbL1−/− RbL2−/− double-knockout MEFs (see Fig. S1 in the supplemental material). Neither of these cells significantly accumulated Akt-473, suggesting that mutation or inactivation of the Rb1 family is required for such accumulation. The cells were serum starved for 24 h, and then serum was added and Akt phosphorylation was followed. Serum stimulated Akt-308 in all of the cell types within 10 min, but by 24 h, Akt-308 was no longer detected (Fig. 1C and results not shown). Thus, activation and loss of Akt-308 appear to be unaffected by Rb1 family mutation. Addition of serum to serum-starved cells also led to the appearance of Akt-473 in the wild-type MEFs, and as with Akt-308, Akt-473 was no longer detectable in the wild-type cells by 24 h (Fig. 1C and results not shown). In contrast, serum starvation did not significantly diminish Akt-473 in the TKO MEFs, nor did Akt-473 increase further when the cells were re-treated with serum (Fig. 1C). Thus, Rb1 family mutation leads selectively to constitutive Akt-473.
FIG. 1.
Mutation of the Rb1 pathway leads to constitutive Akt-473. (A) Western blot showing the levels of Akt-473 in the indicated proliferating cells. (B) Western blot showing the levels of Akt-308 in proliferating cells. (C) Western blot showing that the level of Akt-473 becomes elevated and independent of serum with mutation of the Rb1 pathway and that Ras activation does not further affect the level of Akt-473 when the Rb1 pathway is mutated. (D) TKO MEFs were infected with a retrovirus expressing V12 Ras to create TKO RAS cells, and activated Ras was assessed by Western blotting after binding to Gst-Raf as described in Materials and Methods. Lanes not used in the original blot were spliced out.
Rb1 family mutation blocks Erk1/2 phosphorylation.
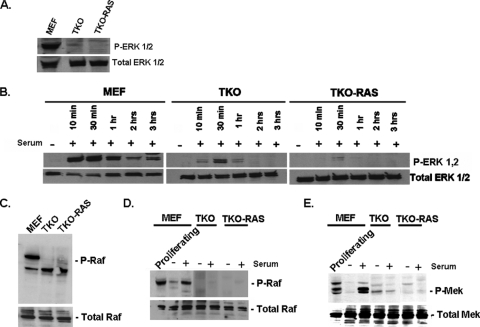
Next, we compared Erk activation in wild-type and TKO MEFs. While Erk1/2 phosphorylation was evident in proliferating wild-type MEFs, little phosphorylation was seen in the TKO MEFs (Fig. 2A). Erk phosphorylation was lost in the wild-type cells upon serum starvation, and phosphorylation was restored by 10 min following serum addition (Fig. 2B). This phosphorylation then returned to the basal level by 3 h. Only limited Erk phosphorylation was evident when serum-starved TKO cells were reexposed to serum (Fig. 2B). Next, we examined Rb1−/− MEFs, and we found relatively normal Erk activation in response to serum stimulation (see Fig. S1 in the supplemental material), suggesting that mutation of Rb1 alone is not sufficient for a block in Erk phosphorylation in MEFs.
FIG. 2.
Mutation of the Rb1 pathway inhibits activation of the c-Raf/Mek/Erk pathway, and Ras activation does not overcome this inhibition. (A) Western blot showing the levels of phosphorylated (P) Erk1/2 in proliferating cells. (B) Western blot analysis of the effects of serum starvation followed by readdition of serum on Erk phosphorylation. (C) Western blot showing the levels of phosphorylated c-Raf in proliferating cells. (D) Western blot showing the effects of serum starvation (−) and readdition of serum (+, 30 min after serum addition) on c-Raf phosphorylation. (E) The cells in panel D were assessed for phosphorylation of Mek. Lanes not used in the original blot were spliced out.
Activated V12 Ras cannot restore Erk activation in TKO MEFs.
TKO MEFs were infected with a retrovirus expressing activated V12 Ha-Ras (33) in an effort to force activation of Erk in the cells. This led to a significant increase in active Ras, as assessed by binding to GST-Raf (Fig. 1D). However, there was little evidence of Erk1/2 phosphorylation in the proliferating TKO Ras cells (Fig. 2A), nor was there evidence of Erk1/2 phosphorylation in the TKO Ras cells following serum stimulation of serum-starved cells (Fig. 2B). Thus, constitutive activation of Ras is unable to activate Erk in TKO MEFs. When TKO and TKO Ras MEFs were compared for Akt phosphorylation, no difference was evident (Fig. 1A to C), suggesting that constitutive activation of Ras in the TKO MEFs was not further affecting Akt phosphorylation.
c-Raf activation is blocked in TKO MEFs, and this activation cannot be restored by activated Ras.
It has been reported that Akt can phosphorylate and inactivate c-Raf, thereby blocking the downstream activation of Erk (20). Therefore, we examined c-Raf activation in normally proliferating MEFs and TKO MEFs. While there was a high basal level of c-Raf phosphorylation in wild-type MEFs, such phosphorylation was not detected in the TKO MEFs (Fig. 2C), nor was significant c-Raf phosphorylation evident in the TKO Ras MEFs (Fig. 2C). The cells were then serum starved, and serum was added back to the cells. In wild-type MEFs, serum starvation eliminated c-Raf activation, and this activation was restored following serum replacement (Fig. 2D). However, c-Raf phosphorylation was not detected in TKO or TKO-Ras MEFs when serum was replaced. Consistent with the lack of c-Raf activation in TKO and TKO-Ras MEFs, these cells also lacked significant Mek activation in normally proliferating cells or following readdition of serum to serum-starved cells (Fig. 2E). These results suggest that Erk activation is blocked at the level of c-Raf in cells when the Rb1 pathway is disrupted. This inability to activate c-Raf cannot be overcome by expression of constitutively active Ras, and it is correlated with the constitutive high level of Akt-473 in the cells.
Cell cycle genes are expressed in the absence of Erk signaling in MEFs lacking the Rb1 pathway, and Ras does not induce expression of Arf in these cells.
Erk signaling in response to Ras activation by growth factors provides an important proliferative signal. Because we found that Erk activation is blocked when the Rb1 pathway is disrupted, we used real-time PCR to compare expression levels of mRNAs for genes important for cell cycle progression in wild-type and TKO MEFs. With the high level of Akt-473 in the TKO MEFs, we suspected that genes dependent upon TCF-β-catenin may be activated due to inhibition of GSK-3-β by Akt (9). Two cell cycle genes classically activated by this pathway are c-myc and cyclin D1 (Ccnd1) (15, 34). Indeed, mRNAs for both of these genes were induced in the TKO MEFs (Fig. 3A). Furthermore, mRNAs for other cyclins and cdks associated with cell cycle progression were also induced (Fig. 3A). Arf is transcribed from the INK4 locus in response to constitutive Erk signaling (arising, for example, from Ras mutation), and it causes activation of p53 and thus tumor suppression by cell cycle arrest (senescence) and apoptosis (25). We found that expression of activated Ras did not induce Arf mRNA in the TKO MEFs (Fig. 3B). These results demonstrate that TKO MEFs induce important cell cycle-regulatory genes in the absence of Erk activation and that the Arf tumor suppressor is not induced along with these cell cycle genes.
FIG. 3.
mRNAs for cell cycle proteins are elevated when the Rb1 pathway is mutated in TKO MEFs, but Arf mRNA is not induced, nor is it induced in response to activated Ras. (A) Real-time PCR comparing mRNA levels in wild-type and TKO MEFs. (B) Real-time PCR for Arf mRNA compared in wild-type (WT), TKO, and TKO Ras MEFs. The error bars indicate standard deviations.
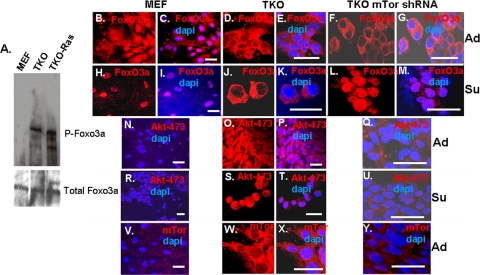
Accumulation of Akt-473 in TKO MEFs is associated with phosphorylation of Foxo3a and its cytoplasmic localization and with resistance to anoikis.
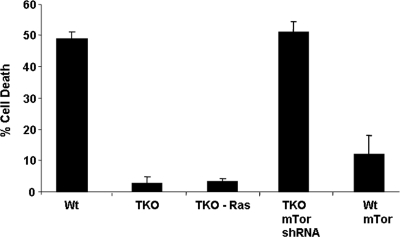
While Akt-308 is thought to act primarily to regulate TORC1 and thus protein synthesis, Akt-473 appears to be responsible for mediating resistance to apoptosis from stress triggered by chemotherapy drugs or loss of matrix contact (anoikis) (10, 18). Such inhibition of apoptosis is accomplished, at least in part, through phosphorylation of the proapoptotic transcription factor Foxo3a, which sequesters the transcription factor in the cytoplasm, where it is inactive (16). Accordingly, while we did not detect phosphorylated Foxo3a in wild-type MEFs, it was phosphorylated in TKO and TKO Ras MEFs (Fig. 4A). Consistent with this finding, Foxo3a was cytoplasmic in TKO MEFs, whereas a significant fraction of the protein was nuclear in wild-type MEFs (Fig. 4B to E). When wild-type MEFs were placed in suspension culture for 6 h, Foxo3a translocated to the nucleus (Fig. 4H and I), and most of the cells underwent anoikis (Fig. 5). In contrast, Foxo3a remained cytoplasmic when TKO MEFs were placed in suspension for 6 h (Fig. 4J and K), and relatively little anoikis was observed (Fig. 5).
FIG. 4.
Effects of cell adhesion and mTOR shRNA knockdown on the levels and subcellular localization of Akt-473, Foxo3a, and mTor in wild-type and TKO MEFs. (A) Phosphorylation of Foxo3a was monitored in the indicated cell types by Western blotting using a phosphospecific antibody. (B to Y) Cell types are shown above the panels. DAPI (4′,6′-diamidino-2-phenylindole) nuclear staining is blue, and immunostaining is shown in red. TKO cells with mTor shRNA knockdown are described in the legends to Fig. 7 and 8. “Su” indicates that cells were placed in suspension culture for 6 h prior to being immunostained; “Ad” indicates cells in adherent culture. Bars, 25 μm.
FIG. 5.
Mutation of the Rb1 pathway provides resistance to anoikis, shRNA knockdown of mTor restores anoikis, and mTor overexpression in wild-type (Wt) MEFs confers resistance to anoikis. Cells were trypsinized from tissue culture plates and placed in suspension culture for 18 h. Trypan blue exclusion was used to assess cell viability. The error bars indicate standard deviations.
Consistent with the cytoplasmic localization of Foxo3a and previous Western blot data (Fig. 1A), immunostaining for Akt-473 was evident in TKO MEFs, but not in wild-type MEFs (Fig. 4N to P), and, this expression of Akt-473 did not appear to be dependent upon the cell density (see Fig. S2 in the supplemental material). Akt-473 was primarily cytoplasmic in the TKO MEFs, although some nuclear immunostaining was evident. Akt-473 was still not detected when wild-type MEFs were placed in suspension, and interestingly, most of the Akt-473 translocated to the nucleus when TKO MEFs were placed in suspension (Fig. 4R to T). Previous studies demonstrated accumulation of phosphorylated Akt in the nuclei of cancer cells and that this nuclear Akt is required for phosphorylation of Foxo3a and thus for its translocation to the cytoplasm (35). Therefore, nuclear Akt-473 in TKO MEFs in suspension is consistent with exclusion of Foxo3a from the nucleus and survival under these conditions.
mTor expression is elevated in TKO MEFs.
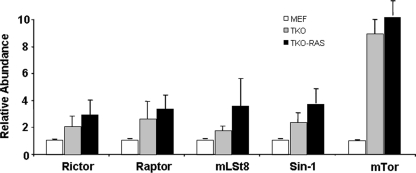
Next, we investigated why Akt-473 may be elevated in TKO MEFs. Akt is phosphorylated on serine 473 by mTor in the context of TORC2. Therefore, we compared the expression levels of mRNAs for components of TORC2 in wild-type and TKO MEFs. While only modest changes were seen in mRNAs for mLAT8, Rictor, and Sin1, mTor mRNA was elevated in TKO and TKO Ras MEFs (Fig. 6). Western blot analysis confirmed that the level of mTor protein was also elevated (see Fig. 8A). Consistent with these findings by real-time PCR and Western blotting, abundant mTor was evident in the cytoplasm of TKO MEFs, whereas mTor was barely detected by immunostaining in the wild-type MEFs (Fig. 4V to X).
FIG. 6.
Rb1 pathway mutation leads to induction of mTor mRNA. Real-time PCR was used to compare the levels of TORC2 mRNAs in wild-type (MEF), TKO, and TKO Ras MEFs. The error bars indicate standard deviations.
FIG. 8.
Knocking down mTor in TKO MEFs leads to loss of Akt-473 and restores anoikis, whereas overexpression of mTor in wild-type MEFs leads to Akt-473, cytoplasmic retention of Foxo3a in suspension culture, and resistance to anoikis. (A) Western blot demonstrating induction of mTor in TKO and TKO-Ras MEFs and that knockdown of mTor following infection of TKO MEFs with mTor shRNA lentivirus (Fig. 7G) leads to loss of Akt-473. (B to P) Wild-type MEFs were transfected with an mTor-GFP expression vector and immunostained for mTor and Akt-473. (M to P) Foxo3a cytoplasmic retention in cells after 6 h in suspension. Bars, 30 μm. Arrows indicate the same location.
shRNA knockdown of mTor in TKO MEFs diminished Akt-473 and Foxo3a phosphorylation and cytoplasmic retention and restored anoikis.
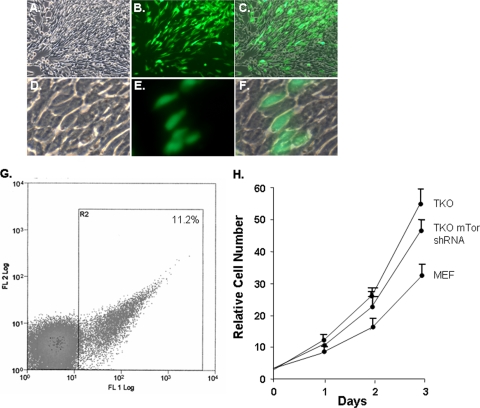
shRNA lentiviral infection of TKO MEFs was used to knock down expression of mTor (Fig. 7A to F). The lentiviral vector also expresses GFP, so the extent of infection could be evaluated. Only 11% of the cells expressed GFP; thus, to enrich for infected cells, the cells were sorted for GFP expression (Fig. 7G). Western blot analysis demonstrated that expression of mTor was knocked down in the infected TKO MEFs to a level similar to that seen in wild-type MEFs (Fig. 8A), and consistent with this knockdown, immunostaining for mTor was also diminished (Fig. 4Y). Infection of the cells with an empty lentiviral vector expressing GFP or with a vector containing a scrambled shRNA sequence had no effect on mTor expression or Akt-473 (results not shown).
FIG. 7.
Knockdown of mTor using lentiviral shRNA does not significantly alter cell morphology or proliferation. (A) Low-power phase-contrast image of TKO MEFs infected with mTor shRNA lentivirus. (B) GFP immunofluorescence in panel A, showing infected cells. (C) Merge of panels A and B. (D) Higher-power phase image of TKO MEFs infected with mTor shRNA lentivirus. (E) GFP immunofluorescence in panel D. (F) Merge of panels D and E. (G) Sorting for mTor shRNA lentivirus-infected TKO MEFs based on GFP expression. (H) Analysis of cell proliferation in the indicated cells. The error bars indicate standard deviations.
Akt-473 was reduced to background with mTor knockdown in the TKO MEFs (Fig. 4Q and U and 8A). Consistent with this loss of Akt-473, Foxo3a efficiently translocated to the nucleus when mTor knockdown TKO MEFs were placed in suspension (Fig. 4F, G, L, and M). Accordingly, anoikis was restored in the infected cells (similar to wild-type MEFs) (Fig. 5). Proliferation of the TKO MEFs expressing mTor shRNA was slightly less than that of control TKO MEFs but greater than that of wild-type MEFs (Fig. 7H). Additionally, there was no obvious change in morphology with mTor knockdown (Fig. 7A to F).
Overexpression of mTor in wild-type MEFs leads to Akt-473, cytoplasmic retention of Foxo3a, and resistance to anoikis.
A vector expressing full-length mTor and GFP was transiently transfected into wild-type MEFs. Approximately 20% of the cells were GFP+ and overexpressed mTor (Fig. 8B to H). These GFP+ cells also immunostained for Akt-473 (Fig. 8I to L). The transfected cells were also relatively resistant to anoikis when placed in suspension culture for 18 h (Fig. 5), and they sequestered Foxo3a in the cytoplasm (Fig. 8M to P).
DISCUSSION
Here, we showed that mutation of the Rb1 pathway in MEFs leads to increased expression of mTor and that this is associated with Atk-473, retention of Foxo3a in the cytoplasm when cells are placed in suspension, and resistance to anoikis. Knockdown of mTor reversed this phenotype, and overexpression of mTor in wild-type MEFs induced the phenotype. Thus, a major phenotypic change resulting from overexpression of mTor and accumulation of Akt-473 in Rb1 pathway mutant MEFs appears to be resistance to anoikis. It has been shown that mTORC2 activity is not essential for normal prostate epithelial cells, but its activity is required for prostate cancer formation when Pten is mutated (12). Mutation of Pten led to an increase in Akt-473 and to phosphorylation of Foxo and its exclusion from the nucleus. Taken together, the results suggest that the antiapoptotic activity of mTORC2 is the critical downstream target that is activated in cancers where Pten is mutated. We did not observe a change in Pten expression with Rb1 pathway mutation in MEFs (results not shown), but loss of the Rb1 pathway appears to lead to the analogous accumulation of Akt-473 and phosphorylation of Foxo seen with Pten mutation.
mTor overexpression and Akt-473 accumulation in MEFs seem to require mutation of all three Rb1 family members. While mutation of Rb1 is common in only a few rare tumors, such as retinoblastoma, it is notable that the family is inactivated by hyperphosphorylation in essentially all other tumors. This is thought to be due to the fact that other family members compensate when Rb1 is mutated in most cell types. Therefore, the loss of Rb1 family members in TKO MEFs is in this way analogous to the inactivation of the three family members that occurs in most tumors. Chromatin immunoprecipitation assays attempting to detect Rb1 or E2F family members at the mTor promoter have thus far been negative (results not shown). This may mean that Rb1 family-dependent repression of mTor is an indirect effect, or perhaps Rb1 family E2F binding sequences may be far upstream of the gene or within the gene in regions we have not examined.
Recent results suggest that while Ras-mediated activation of the MAP kinase pathway is important in tumor initiation, as tumorigenesis progresses, the major role of Ras activation becomes the PI3K/Akt pathway (22). Indeed, in skin tumors, it appears that mutations of the PI3K-inhibitory gene Pten and Ras are mutually exclusive, implying their participation in a common pathway (19, 26). Furthermore, inhibition of Erk activation is an adverse prognostic factor in cutaneous melanoma. One explanation for this switch to PI3K/Akt seems to be the inhibition of c-Raf, which results from accumulation of phosphorylated Akt, at least in some cells (20). Previously, it was demonstrated that Rb1 null MEFs are no longer dependent upon Erk activation for proliferation (6), and here we extended these observations and showed that Rb1 pathway mutation blocks Erk activation. Thus, mutation or inactivation of the Rb1 pathway may be a mechanism to switch Ras signaling from Raf/Erk to Akt as tumors progress. Despite the lack of Erk activation, key cell cycle control genes were induced in the TKO MEFs, but Arf was not induced by Ras. While pulses of Erk activation are tolerated, constitutive Erk activation (e.g., in response to activating Ras mutation) induces Arf from the INK4a locus (25). Arf causes accumulation of activated p53, leading to tumor suppression via cell cycle arrest and apoptosis. The ability of a cancer cell to constitutively activate Akt and thereby block Erk activation may then provide a means to circumvent tumor suppression resulting from induction of Arf.
Supplementary Material
Acknowledgments
We thank T. Jacks, J. Sage, and G. Lenoe for the kind gifts of TKO, double-knockout, Rb1 null, and wild-type littermate MEFs.
These studies were supported by grants from the NIH (RR018733 and EY015636).
Footnotes
Published ahead of print on 24 August 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGf-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaskar, P. T., and N. Hay. 2007. The two TORCs and Akt. Dev. Cell 12:487-502. [DOI] [PubMed] [Google Scholar]
- 3.Callejas-Valera, J. L., J. Guinea-Viniegra, C. Ramirez-Castillejo, J. A. Recio, E. Galan-Moya, N. Martinez, J. M. Rojas, S. Ramon y Cajal, and R. Sanchez-Prieto. 2008. E1a gene expression blocks the Erk1/2 signaling pathway by promoting nuclear localization and MKP up-regulation: implication in v-H-ras-induced senescence. J. Biol. Chem. 283:13450-13458. [DOI] [PubMed] [Google Scholar]
- 4.Carracedo, A., and P. P. Pandolfi. 2008. The Pten-Pi3K pathway: of feedbacks and cross-talks. Oncogene 27:5527-5541. [DOI] [PubMed] [Google Scholar]
- 5.Collins, B. J., M. Deak, J. S. Authur, L. J. Armit, and D. R. Alessi. 2003. In vivo role of the pif-binding docking site of PDK1 defined by knock-in mutation. EMBO J. 22:4202-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Abaco, G. M., S. Hooper, H. Paterson, and C. J. Marshall. 2002. Loss of rb overrides the requirement for erk activity for cell proliferation. J. Cell Sci. 115:4607-4616. [DOI] [PubMed] [Google Scholar]
- 7.Dannenberg, J.-H., A. van Rossum, L. Schuijff, and H. te Riele. 2000. Ablation of the retinoblastoma gene family deregulates G1 control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 14:3051-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facchinetti, V., W. Ouyang, K. Wei, N. Soto, A. Lazochak, C. Gould, C. Lowry, A. C. Newton, Y. Mao, R. Q. Miao, W. C. Sessa, J. Oin, P. Zhang, B. Su, and E. Jacinto. 2008. The mammalian target of rapamycin complex-2 controls folding and stability of Akt and protein kinase c. EMBO J. 27:1932-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke, T. F. 2008. PI3K/Akt: getting it right matters. Oncogene 27:6473-6488. [DOI] [PubMed] [Google Scholar]
- 10.Frisch, S. M., and R. A. Screaton. 2001. Anoikis mechanisms. Curr. Opin. Cell Biol. 13:555-562. [DOI] [PubMed] [Google Scholar]
- 11.Guertin, D. A., D. M. Stevens, C. C. Thoreen, A. A. Burds, N. Y. Kalaany, J. Moffat, M. Brown, K. J. Fitzgerald, and D. M. Sabatini. 2006. Ablation in mice of the mTor components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-Foxo and PKCα, but not S6K1. Dev. Cell 11:859-871. [DOI] [PubMed] [Google Scholar]
- 12.Guertin, D. A., D. M. Stevens, M. Saitoh, S. Kinkel, K. Crosby, J. H. Sheen, D. J. Mullholland, M. A. Magnuson, H. Wu, and D. M. Sabatini. 2009. mTor complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell 15:148-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbour, J. W., and D. C. Dean. 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14:2393-2409. [DOI] [PubMed] [Google Scholar]
- 14.Hay, N., and N. Sonnenberg. 2004. Upstream and downstream of mTor. Genes Dev. 18:1926-1945. [DOI] [PubMed] [Google Scholar]
- 15.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-myc as a target of the APC pathway. Science 281:1509-1512. [DOI] [PubMed] [Google Scholar]
- 16.Huang, H., and D. J. Tindall. 2007. Dynamic Foxo transcription factors. J. Cell Sci. 120:2479-2487. [DOI] [PubMed] [Google Scholar]
- 17.Ikenoue, T., K. Inoki, Q. Yang, X. Zhou, and K.-L. Guan. 2008. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signaling. EMBO J. 27:1919-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacinto, E., V. Facchinetti, D. Liu, N. Soto, S. Wei, S. Y. Jung, Q. Huang, J. Qin, and B. Su. 2006. Sin1/Mip1 maintains rector-mTor complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127:125-137. [DOI] [PubMed] [Google Scholar]
- 19.Jovanovic, B., D. Krockel, D. Linden, B. Nilsson, S. Egyhazi, and J. Hansson. 2008. Lack of cytoplasmic Erk activation is an independent adverse prognostic factor in primary cutaneous melanoma. J. Investig. Dermatol. 128:2696-2704. [DOI] [PubMed] [Google Scholar]
- 20.Jun, T., O. Gjoerup, and T. M. Roberts. 1999. Tangled webs: evidence of cross-talk between c-Raf1 and Akt. Sci. STKE 13:1-5. [DOI] [PubMed] [Google Scholar]
- 21.Lackey, J., J. Barnett. L. Davidson, I. H. Batty, N. R. Leslie, and C. P. Downes. 2007. Loss of Pten selectively desensitizes upstream IGF1 and insulin signaling. Oncogene 26:7132-7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim, K. H., and C. M. Counter. 2005. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/akt pathway during tumor maintenance. Cancer Cell 5:381-392. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Y., S. El-Naggar, D. S. Darling, Y. Higashi, and D. C. Dean. 2008. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development 135:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loewith, R., E. Jacinto, S. Wullscleger, A. Lorberg, J. L. Crespo, D. Bonenfant, W. Oppliger, P. Jenoe, and M. N. Hall. 2002. Two mTor complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10:457-468. [DOI] [PubMed] [Google Scholar]
- 25.Lowe, S. W., and C. J. Sherr. 2003. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13:77-83. [DOI] [PubMed] [Google Scholar]
- 26.Mao, J.-H., M. D. To, J. Perez-Losada, D. Wu, R. Del Rosario, and A. Balmain. 2004. Mutually exclusive mutations of the pten and Ras pathways in skin tumor progression. Genes Dev. 18:1800-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall, C. J. 1996. Ras effectors. Curr. Opin. Cell Biol. 8:197-204. [DOI] [PubMed] [Google Scholar]
- 28.Mitin, N., K. L. Rossman, and C. J. Der. 2005. Signaling interplay in Ras superfamily function. Curr. Biol. 15:563-574. [DOI] [PubMed] [Google Scholar]
- 29.Qiao, M., S. Sheng, and A. B. Pardee. 2008. Metastasis and Akt activation. Cell Cycle 7:2991-2996. [DOI] [PubMed] [Google Scholar]
- 30.Sabatini, D. M. 2006. mTor and cancer; insights into a complex relationship. Nat. Rev. Cancer. 6:729-734. [DOI] [PubMed] [Google Scholar]
- 31.Sage, J., G. J. Mulligan, L. D. Attardi, A. Miller, S. Chen, B. Williams, E. Theodorou, and T. Jacks. 2000. Targeted disruption of the three Rb-related genes leads to loss of G1 control and immortilization. Genes Dev. 14:3037-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiota, C., J. T. Woo, J. Lindner, K. D. Shelton, and M. A. Magnuson. 2006. Multiallelic disruption of the rictor gene in mice reveals that mTor complex 2 is essential for fetal growth and viability. Dev. Cell 11:583-589. [DOI] [PubMed] [Google Scholar]
- 32a.Singer, O., R. A. Marr, E. Rockenstein, L. Crews, N. G. Coufal, F. H. Gage, I. M. Verma, and E. Masliah. 2005. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat. Neurosci. 8:1343-1349. [DOI] [PubMed] [Google Scholar]
- 33.Telang, S., A. Yalcin, A. L. Clem, R. Bucala, A. N. Lane, J. W. Eaton, and J. Chesney. 2006. Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene 25:7225-7234. [DOI] [PubMed] [Google Scholar]
- 34.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin d1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 34a.Tiscornia, G., O. Singer, and I. M. Verma. 2006. Design and cloning of lentiviral vectors expressing small interfering RNAs. Nat. Protoc. 1:234-240. [DOI] [PubMed] [Google Scholar]
- 35.Trotman, L. C., A. Alumonti, P. P. Scaglioni, J. A. Koutcher, C. Cordon-Cardo, and P. P. Pandolfi. 2006. Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wullschleger, S., R. Loewith, and M. N. Hall. 2006. TOR signaling in growth and metabolism. Cell 124:471-484. [DOI] [PubMed] [Google Scholar]
- 37.Yang, Q., K. Inoki, T. Ikenoue, and K.-L. Guan. 2006. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 20:2828-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao, D., C. L. Alexander, J. A. Quinn, M. J. Porter, H. Wu, and D. A. Greenhalgh. 2006. Pten loss promotes rasHa-mediated papillomatogenesis via dual up-regulation of Akt activity and cell cycle deregulation but malignant conversion proceeds via Pten-associated pathways. Cancer Res. 66:1302-1312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.