Abstract
We evaluated the efficacy of rhesus theta-defensin 1 (RTD-1), a novel cyclic antimicrobial peptide, as a prophylactic antiviral in a mouse model of severe acute respiratory syndrome (SARS) coronavirus (CoV) lung disease. BALB/c mice exposed to a mouse-adapted strain of SARS-CoV demonstrated 100% survival and modest reductions in lung pathology without reductions in virus titer when treated with two intranasal doses of RTD-1, while mortality in untreated mice was ∼75%. RTD-1-treated, SARS-CoV-infected mice displayed altered lung tissue cytokine responses 2 and 4 days postinfection compared to those of untreated animals, suggesting that one possible mechanism of action for RTD-1 is immunomodulatory.
Severe acute respiratory syndrome (SARS) emerged as a global health threat in 2002 to 2003, infecting over 8,000 individuals and causing nearly 800 deaths (26). The causative agent, SARS coronavirus (CoV), appears to have originated in bats (19) and by passage through animals such as palm civet cats gained features supporting infection and replication in humans (30, 31). The respiratory tract is the major target of the virus, with viral mRNA or antigens detected in the epithelium of the airway, bronchioles, and alveoli (8, 17, 37). Lung pathological findings in patients succumbing to the infection within 10 days of illness onset include diffuse alveolar damage, epithelial cell desquamation, edema, and leukocyte infiltration (24, 26). Treatment options pursued during the SARS outbreak were primarily supportive, although some reports suggest that early anti-inflammatory therapy improved patient outcomes (3, 20, 40).
Recently, serial passage of SARS-CoV through rat or mouse lungs yielded a robust animal model of lung disease (21, 22, 28). After 15 passages in BALB/c mouse lung, virus adapted to the new host and caused clinically apparent respiratory disease. The mouse-adapted SARS-CoV (MA15 strain) causes a disease that is primarily localized to the lungs, but virus spreads to other organs, reminiscent of the systemic disease in SARS patients (28). This offers a model for screening novel antiviral agents.
RTD-1 pretreatment prevents lethal pulmonary infection in mice.
Rhesus theta-defensin 1 (RTD-1) is a unique cyclic antimicrobial peptide first identified in rhesus macaque leukocytes (35). It is produced by a novel posttranslational processing pathway involving the excision of two 9-amino-acid oligopeptides from a pair of propeptides that is further stabilized by three disulfide bonds. Interestingly, humans and New World monkeys express no theta-defensins (7, 23). Theta-defensins possess broad antimicrobial properties in vitro against bacteria, fungi, and viruses (25, 38, 39, 42). Moreover, they exhibit very low levels of toxicity in vitro (38) and in vivo (unpublished data), indicating that they may have utility as therapeutic agents.
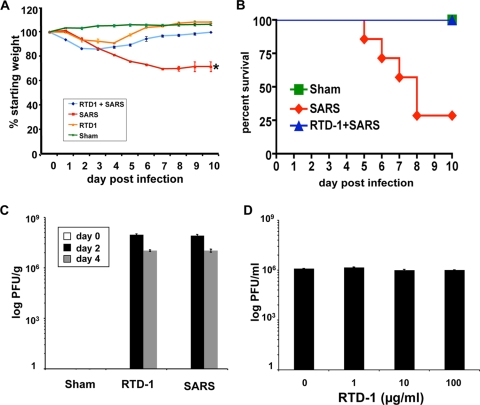
We inoculated groups of mice with 3 × 105 PFU of MA15 SARS-CoV (28), a dose previously shown to cause ∼75% mortality (J. Zhao, J. Zhao, N. Van Rooijen, and S. Perlman, submitted for publication). As shown in Fig. 1A, infected mice began to lose weight within 2 to 3 days of inoculation and continued to do so until they succumbed to the infection or recovered. The survival curves for sham-treated, SARS-CoV-infected, and RTD-1-treated mice are shown in Fig. 1B. In contrast to the natural course of infection in untreated mice, animals pretreated with intranasal RTD-1 15 min prior to infection followed by a single treatment 18 h later lost little weight and exhibited 100% survival. Animals receiving RTD-1 treatment alone exhibited modest, transient weight loss and survived, while sham-treated mice exhibited no weight loss.
FIG. 1.
Treatment with RTD-1 protects mice against lethality of SARS-CoV infection. (A and B) BALB/c mice 6 to 8 weeks old were treated with sham control (40 μl phosphate-buffered saline, no virus), RTD-1 alone (125 μg [∼5 mg/kg] intranasal RTD-1 15 min prior to infection, followed by an identical dose 18 h later), SARS-CoV alone (3 × 105 PFU MA15 intranasally in 40 μl phosphate-buffered saline), or RTD-1 followed with SARS-CoV infection. Mice were monitored daily for weight loss (A) and survival (B) (n = 6 or 7/group). SARS-CoV-infected mice without RTD-1 treatment had a 30% survival rate and 25% decrease in weight in those that survived (*, P ≤ 0.05 by Student's t test for SARS alone versus all other groups). Data presented in panels A and B are representative of two independent experiments. (C) Lung tissue was harvested from mice, and viral titer levels were determined. (D) RTD-1 has no direct antiviral effect on SARS-CoV. Viral titers were determined using Vero cells and samples of 1 × 105 PFU SARS-CoV (Urbani) that were preincubated for 30 min at 37°C with RTD-1 in serum-free phosphate-buffered saline at the indicated concentrations prior to plaque assay in Vero cells. Results in panels A, C, and D are presented as means ± standard errors (A and B, n = 6 or 7; C and D, n = 3). All experiments were performed under biosafety level 3 containment. This study was approved by the University of Iowa Animal Care and Use Committee.
We assessed SARS-CoV titers in lung tissue 0, 2, and 4 days postinfection. As shown in Fig. 1C, RTD-1 treatment had no significant effect on the tissue virus titers at day 2 or 4 postinfection. In addition, incubation of the virus with RTD-1 showed no evidence of direct virus inactivation based on titer (Fig. 1D). RTD-1-treated animals also had levels of lung tissue N gene antigen expression and virus titers similar to those of sham control-treated animals, suggesting an immunomodulatory rather than directly antiviral mechanism of activity (data not shown).
In light of the weight loss seen following one or two intranasal doses of 5 mg/kg (of body weight) of RTD-1 in the absence of virus challenge (Fig. 1A and data not shown), we performed a broader dose-response assay (5, 2.4, 0.8, 0.3, 0.1, and 0.03 mg/kg) and also evaluated animals for pulmonary histopathologic changes at 2 and 4 days postadministration. Intranasal RTD-1 produced dose-dependent changes in tissue histopathology (data not shown). The 2.4-mg/kg dose caused significant lesions at both the 2- and 4-day time points. At 0.8 mg/kg, there was mild to moderate perivascular inflammation and necrotic debris in airway lumens with some resolution (to mild/scant perivascular inflammation) by 4 days postadministration. Doses of 0.3, 0.1, or 0.03 mg/kg caused very mild scattered perivascular inflammation and airway karyorrhectic/cellular debris. These changes decreased by day 4 to only mild/detectable scant perivascular leukocytes.
We next investigated the effects of intranasal doses of 2.5, 0.8, and 0.3 mg/kg (at 4 h before and 18 h after MA15 infection) on weight loss and survival. A 2.5-mg/kg dose was fully protective against a 3 × 105 PFU MA15 inoculum (data not shown). In contrast, the 0.8- and 0.3-mg/kg doses failed to protect the animals from morbidity. We also evaluated the efficacy of intravenously administered RTD-1 in modifying disease outcomes. Two doses of 5-mg/kg RTD-1 administered 4 h before and 18 h after MA15 exposure did not protect the animals from SARS-CoV-associated morbidity and mortality (data not shown).
RTD-1 treatment decreases pulmonary pathology during SARS-CoV infection.
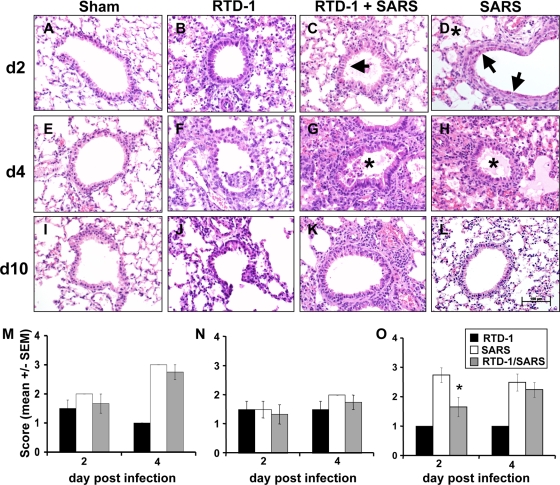
In humans and susceptible animals, SARS-CoV pulmonary pathology is characterized by perivascular cellular inflammation, necrotizing bronchiolitis, and alveolar edema (6, 15, 24, 26). We systematically evaluated lung histopathology at days 2, 4, and 10 postinfection in sham (no virus)-treated, RTD-1-treated, and untreated animals. As shown in Fig. 2, mice treated with RTD-1 alone developed a modest level of perivascular infiltrate that was largely resolved by 10 days. In contrast, animals receiving the MA15 virus alone developed alveolar edema, necrotizing bronchiolitis, and perivascular infiltrates that were resolving by 10 days postinfection in surviving animals. Mice receiving RTD-1 treatment and SARS-CoV exhibited pathological lesions similar to those in mice with SARS alone (Fig. 2); however, the overall severity of the pathology scores trended lower than that with SARS-CoV alone. As shown in Fig. 2O, the RTD-1-treated mice had significantly reduced severity of necrotizing bronchiolitis at 2 days postinfection. RTD-1 treatment alone induced some edema and necrotizing bronchiolitis that were similar to those in SARS-CoV infection without or with RTD-1 at 2 days postinfection but were significantly improved by 4 days postdelivery (Fig. 2).
FIG. 2.
(A to L) Pulmonary tissue histopathology in SARS-CoV (3 × 105 PFU MA15)-infected mice with or without RTD-1 (5 mg/kg, two doses) treatment. Hematoxylin- and eosin-stained (4 μM) tissue sections were examined 2, 4, and 10 days postinfection. See the text for additional details. Asterisks indicate alveolar edema; arrows indicate necrotizing bronchiolitis. n = 4 at each time point. Scale bar, 100 μm. (M to O) Pulmonary histopathology scores in SARS-CoV-infected mice treated with or without RTD-1. Tissues were harvested at 2 and 4 days and scored by a veterinary pathologist (D.K.M.) blinded to the treatment protocol, using a severity scale from 1 (absent/rare) to 3 (severe/multifocal). Data are presented for alveolar edema (M), perivascular cellular infiltrates (N), and necrotizing bronchiolitis (O). Results are presented as means ± standard errors (n = 4; *, P ≤ 0.05).
RTD-1 treatment modifies cytokine responses in infected lung tissue.
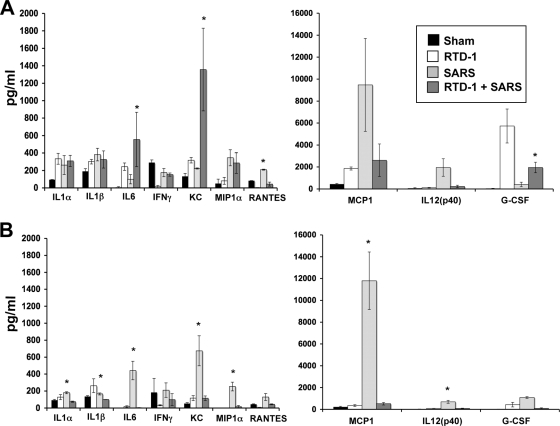
Among the hypotheses proposed to explain the morbidity and mortality associated with SARS is the notion that overly exuberant cytokine/chemokine responses or repressed innate immune responses contribute (11, 29, 40). We assessed cytokine responses in lung tissue homogenates at 2 and 4 days postinfection. At 2 days postinfection, interleukin-6 (IL-6), Keratinocyte chemoattractant, and granulocyte colony-stimulating factor were increased in SARS-CoV-infected mice treated with RTD-1 compared to mice infected with SARS-CoV alone. Mice treated with RTD-1 and infected with SARS-CoV displayed significant reductions in levels of RANTES at day 2 postinfection (Fig. 3A, top panels) and reductions in levels of IL-1α, IL-1β, IL-6, Keratinocyte chemoattractant, MIP1α, monocyte chemoattractant protein 1, and IL-12(p40) at day 4 postinfection (Fig. 3B, bottom panels) compared to those in SARS-CoV-infected mice that received no RTD-1. There were no significant changes in tissue gamma interferon (Fig. 3) or type I interferons (quantitative reverse transcription-PCR, data not shown). Thus, while RTD-1 had no significant direct inhibitory effect on MA15 virus titer in vivo or in vitro (Fig. 1C and D), it may alter disease outcome by modifying cytokine responses to infection.
FIG. 3.
RTD-1 treatment alters pulmonary cytokine responses to SARS-CoV. Lung tissues were harvested at days 2 (A) and 4 (B) postinfection, and cytokine responses were evaluated using the Bio-Plex cytokine assay (Bio-Plex Cytokine 23-Plex kit; Bio-Rad Laboratories) to identify changes in cytokine protein expression in lung homogenates from baseline and days 2 and 4 postinfection. The results are shown for sham treatment (normal saline alone), RTD-1 alone (5 mg/kg, two doses), SARS-CoV (3 × 105 PFU MA15) with RTD-1 treatment, and SARS-CoV alone. Note differences in y-axis scales between left and right panels. See the text for interpretation. Results are presented as means ± standard errors (n = 3; *, P ≤ 0.05 by Student's t test for SARS-CoV alone versus SARS-CoV plus RTD-1).
Animals receiving RTD-1 (2.5 or 5 mg/kg) prior to exposure to MA15 were protected from severe disease and mortality. We next asked whether RTD-1-protected animals surviving a lethal inoculum of MA15 gained protective immunity against future exposure. Animals that had received RTD-1 pretreatment (2.5 mg/kg intranasally) were reexposed to MA15 SARS-CoV (3 × 105 PFU inoculum) 1 month after initial exposure. All animals previously protected with intranasal RTD-1 survived reexposure to MA15 without weight loss or signs of morbidity (data not shown). In contrast, naïve mice exhibited severe disease morbidity when exposed to virus.
The realization that SARS-CoV or other zoonotically transmitted coronaviruses may infect humans or cause severe disease in the future has fueled interest in the development of antivirals. Several agents have been investigated for prophylaxis and treatment in vitro and in various animal models (reviewed in references 9 and 32), including vaccines (5, 13, 14), passive immunization (33, 36), alpha interferon (1, 4, 20), RNA interference (10, 18), protease inhibitors (41), recombinant angiotensin converting enzyme 2 (16), angiotensin converting enzyme inhibitors (16), carbohydrate binding agents (43), ribavirin (27, 32), and corticosteroids (3, 20).
Our studies show that respiratory tract prophylaxis with RTD-1 prevented death and significantly modified the pulmonary disease course. The antiviral mechanism(s) of RTD-1 is of considerable interest. Multiple reports suggest that the pulmonary defense response to SARS-CoV infection is robust but may be detrimental to the host (2, 3, 12, 29, 34). Not unexpectedly, inflammatory cytokine and chemokine levels that regulate the inflammatory response are elevated during SARS-CoV infection, although there is some variability in the specific mediators shown to be elevated. It appears that the pulmonary defense response against SARS-CoV is complex but under some circumstances may contribute to respiratory dysfunction and poor outcome.
There are several possible mechanisms by which RTD-1 modifies the lung disease course. While the peptide alone appears to directly induce some dose-dependent airway inflammation, in the presence of SARS-CoV, RTD-1-treated animals show blunted proinflammatory cytokine responses in lung tissue 2 and 4 days postinfection and have a marked reduction in mortality. One possible mechanism is that RTD-1 serves as an inducer (or accelerator) of antiviral responses. Another possibility is that RTD-1 reduces viral bronchiolitis and/or diffuse alveolar damage. Finally, perhaps RTD-1 reduces pulmonary inflammation, thereby diminishing systemic dissemination of the pathogen. It remains to be determined whether there is a mechanistic relationship between the mild inflammation induced by RTD-1 in uninfected mice and the protective effect of the peptide observed in virus-challenged animals. This will require a more comprehensive analysis of cellular and soluble inflammatory responses. Moreover, since RTD-1 does not appear to have direct antiviral effects, its potential for modulating viral spread is likely to depend on the ability of the peptide to limit viral proliferation and shedding at the level of the individual animal. Further investigation of this novel agent is warranted.
Acknowledgments
We acknowledge the support of NIH PO1 AI060699 (P.B.M.) and the Roy J. Carver Trust (P.B.M.) and NIH R37 AI022931 and NIH R01AI058129 (M.E.S.).
We thank Hong Peng Jia for critical comments on the manuscript.
M.E.S. has a financial interest in an entity that has licensed theta-defensins for commercial development.
Footnotes
Published ahead of print on 26 August 2009.
REFERENCES
- 1.Antonelli, G., C. Scagnolari, E. Vicenzi, and M. Clementi. 2003. Treatment of SARS with human interferons. Lancet 362:1158-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baas, T., A. Roberts, T. H. Teal, L. Vogel, J. Chen, T. M. Tumpey, M. G. Katze, and K. Subbarao. 2008. Genomic analysis reveals age-dependent innate immune responses to severe acute respiratory syndrome coronavirus. J. Virol. 82:9465-9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, R. C., X. P. Tang, S. Y. Tan, B. L. Liang, Z. Y. Wan, J. Q. Fang, and N. Zhong. 2006. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 129:1441-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinatl, J., B. Morgenstern, G. Bauer, P. Chandra, H. Rabenau, and H. W. Doerr. 2003. Treatment of SARS with human interferons. Lancet 362:293-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deming, D., T. Sheahan, M. Heise, B. Yount, N. Davis, A. Sims, M. Suthar, J. Harkema, A. Whitmore, R. Pickles, A. West, E. Donaldson, K. Curtis, R. Johnston, and R. Baric. 2006. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 3:e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franks, T. J., P. Y. Chong, P. Chui, J. R. Galvin, R. M. Lourens, A. H. Reid, E. Selbs, C. P. McEvoy, C. D. Hayden, J. Fukuoka, J. K. Taubenberger, and W. D. Travis. 2003. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum. Pathol. 34:743-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia, A. E., G. Osapay, P. A. Tran, J. Yuan, and M. E. Selsted. 2008. Isolation, synthesis, and antimicrobial activities of naturally occurring theta-defensin isoforms from baboon leukocytes. Infect. Immun. 76:5883-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu, J., E. Gong, B. Zhang, J. Zheng, Z. Gao, Y. Zhong, W. Zou, J. Zhan, S. Wang, Z. Xie, H. Zhuang, B. Wu, H. Zhong, H. Shao, W. Fang, D. Gao, F. Pei, X. Li, Z. He, D. Xu, X. Shi, V. M. Anderson, and A. S. Leong. 2005. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 202:415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haagmans, B. L., and A. D. Osterhaus. 2006. Coronaviruses and their therapy. Antivir. Res. 71:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He, M. L., B. Zheng, Y. Peng, J. S. Peiris, L. L. Poon, K. Y. Yuen, M. C. Lin, H. F. Kung, and Y. Guan. 2003. Inhibition of SARS-associated coronavirus infection and replication by RNA interference. JAMA 290:2665-2666. [DOI] [PubMed] [Google Scholar]
- 11.Huang, K. J., I. J. Su, M. Theron, Y. C. Wu, S. K. Lai, C. C. Liu, and H. Y. Lei. 2005. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 75:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, Y., J. Xu, C. Zhou, Z. Wu, S. Zhong, J. Liu, W. Luo, T. Chen, Q. Qin, and P. Deng. 2005. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 171:850-857. [DOI] [PubMed] [Google Scholar]
- 13.Kapadia, S. U., J. K. Rose, E. Lamirande, L. Vogel, K. Subbarao, and A. Roberts. 2005. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 340:174-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobinger, G. P., J. M. Figueredo, T. Rowe, Y. Zhi, G. Gao, J. C. Sanmiguel, P. Bell, N. A. Wivel, L. A. Zitzow, D. B. Flieder, R. J. Hogan, and J. M. Wilson. 2007. Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine 25:5220-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, and L. J. Anderson. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 16.Kuba, K., Y. Imai, S. Rao, H. Gao, F. Guo, B. Guan, Y. Huan, P. Yang, Y. Zhang, W. Deng, L. Bao, B. Zhang, G. Liu, Z. Wang, M. Chappell, Y. Liu, D. Zheng, A. Leibbrandt, T. Wada, A. S. Slutsky, D. Liu, C. Qin, C. Jiang, and J. M. Penninger. 2005. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 11:875-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuiken, T., R. A. Fouchier, M. Schutten, G. F. Rimmelzwaan, G. van Amerongen, D. van Riel, J. D. Laman, T. de Jong, G. van Doornum, W. Lim, A. E. Ling, P. K. Chan, J. S. Tam, M. C. Zambon, R. Gopal, C. Drosten, S. van der Werf, N. Escriou, J. C. Manuguerra, K. Stohr, J. S. Peiris, and A. D. Osterhaus. 2003. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, B. J., Q. Tang, D. Cheng, C. Qin, F. Y. Xie, Q. Wei, J. Xu, Y. Liu, B. J. Zheng, M. C. Woodle, N. Zhong, and P. Y. Lu. 2005. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in rhesus macaque. Nat. Med. 11:944-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, W., Z. Shi, M. Yu, W. Ren, C. Smith, J. H. Epstein, H. Wang, G. Crameri, Z. Hu, H. Zhang, J. Zhang, J. McEachern, H. Field, P. Daszak, B. T. Eaton, S. Zhang, and L. F. Wang. 2005. Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676-679. [DOI] [PubMed] [Google Scholar]
- 20.Loutfy, M. R., L. M. Blatt, K. A. Siminovitch, S. Ward, B. Wolff, H. Lho, D. H. Pham, H. Deif, E. A. LaMere, M. Chang, K. C. Kain, G. A. Farcas, P. Ferguson, M. Latchford, G. Levy, J. W. Dennis, E. K. Lai, and E. N. Fish. 2003. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA 290:3222-3228. [DOI] [PubMed] [Google Scholar]
- 21.Nagata, N., N. Iwata, H. Hasegawa, S. Fukushi, A. Harashima, Y. Sato, M. Saijo, F. Taguchi, S. Morikawa, and T. Sata. 2008. Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am. J. Pathol. 172:1625-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata, N., N. Iwata, H. Hasegawa, S. Fukushi, M. Yokoyama, A. Harashima, Y. Sato, M. Saijo, S. Morikawa, and T. Sata. 2007. Participation of both host and virus factors in induction of severe acute respiratory syndrome (SARS) in F344 rats infected with SARS coronavirus. J. Virol. 81:1848-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen, T. X., A. M. Cole, and R. I. Lehrer. 2003. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides 24:1647-1654. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls, J. M., L. L. Poon, K. C. Lee, W. F. Ng, S. T. Lai, C. Y. Leung, C. M. Chu, P. K. Hui, K. L. Mak, W. Lim, K. W. Yan, K. H. Chan, N. C. Tsang, Y. Guan, K. Y. Yuen, and J. S. Peiris. 2003. Lung pathology of fatal severe acute respiratory syndrome. Lancet 361:1773-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen, S. M., D. L. Rudolph, W. Wang, A. M. Cole, A. J. Waring, R. B. Lal, and R. I. Lehrer. 2004. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res. Hum. Retrovir. 20:1157-1165. [DOI] [PubMed] [Google Scholar]
- 26.Peiris, J. S., K. Y. Yuen, A. D. Osterhaus, and K. Stohr. 2003. The severe acute respiratory syndrome. N. Engl. J. Med. 349:2431-2441. [DOI] [PubMed] [Google Scholar]
- 27.Poutanen, S. M., D. E. Low, B. Henry, S. Finkelstein, D. Rose, K. Green, R. Tellier, R. Draker, D. Adachi, M. Ayers, A. K. Chan, D. M. Skowronski, I. Salit, A. E. Simor, A. S. Slutsky, P. W. Doyle, M. Krajden, M. Petric, R. C. Brunham, and A. J. McGeer. 2003. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 348:1995-2005. [DOI] [PubMed] [Google Scholar]
- 28.Roberts, A., D. Deming, C. D. Paddock, A. Cheng, B. Yount, L. Vogel, B. D. Herman, T. Sheahan, M. Heise, G. L. Genrich, S. R. Zaki, R. Baric, and K. Subbarao. 2007. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 3:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockx, B., T. Baas, G. A. Zornetzer, B. Haagmans, T. Sheahan, M. Frieman, M. D. Dyer, T. H. Teal, S. Proll, J. van den Brand, R. Baric, and M. G. Katze. 2009. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 83:7062-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockx, B., T. Sheahan, E. Donaldson, J. Harkema, A. Sims, M. Heise, R. Pickles, M. Cameron, D. Kelvin, and R. Baric. 2007. Synthetic reconstruction of zoonotic and early human severe acute respiratory syndrome coronavirus isolates that produce fatal disease in aged mice. J. Virol. 81:7410-7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song, H. D., C. C. Tu, G. W. Zhang, S. Y. Wang, K. Zheng, L. C. Lei, Q. X. Chen, Y. W. Gao, H. Q. Zhou, H. Xiang, H. J. Zheng, S. W. Chern, F. Cheng, C. M. Pan, H. Xuan, S. J. Chen, H. M. Luo, D. H. Zhou, Y. F. Liu, J. F. He, P. Z. Qin, L. H. Li, Y. Q. Ren, W. J. Liang, Y. D. Yu, L. Anderson, M. Wang, R. H. Xu, X. W. Wu, H. Y. Zheng, J. D. Chen, G. Liang, Y. Gao, M. Liao, L. Fang, L. Y. Jiang, H. Li, F. Chen, B. Di, L. J. He, J. Y. Lin, S. Tong, X. Kong, L. Du, P. Hao, H. Tang, A. Bernini, X. J. Yu, O. Spiga, Z. M. Guo, H. Y. Pan, W. Z. He, J. C. Manuguerra, A. Fontanet, A. Danchin, N. Niccolai, Y. X. Li, C. I. Wu, and G. P. Zhao. 2005. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl. Acad. Sci. USA 102:2430-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockman, L. J., R. Bellamy, and P. Garner. 2006. SARS: systematic review of treatment effects. PLoS Med. 3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subbarao, K., J. McAuliffe, L. Vogel, G. Fahle, S. Fischer, K. Tatti, M. Packard, W. J. Shieh, S. Zaki, and B. Murphy. 2004. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 78:3572-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang, N. L., P. K. Chan, C. K. Wong, K. F. To, A. K. Wu, Y. M. Sung, D. S. Hui, J. J. Sung, and C. W. Lam. 2005. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin. Chem. 51:2333-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang, Y. Q., J. Yuan, G. Osapay, K. Osapay, D. Tran, C. J. Miller, A. J. Ouellette, and M. E. Selsted. 1999. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 286:498-502. [DOI] [PubMed] [Google Scholar]
- 36.ter Meulen, J., A. B. Bakker, E. N. van den Brink, G. J. Weverling, B. E. Martina, B. L. Haagmans, T. Kuiken, J. de Kruif, W. Preiser, W. Spaan, H. R. Gelderblom, J. Goudsmit, and A. D. Osterhaus. 2004. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet 363:2139-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.To, K., J. H. Tong, P. K. Chan, F. W. Au, S. S. Chim, K. A. Chan, J. L. Cheung, E. Y. Liu, G. M. Tse, A. W. Lo, Y. Dennis Lo, and H. Ng. 2004. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J. Pathol. 202:157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran, D., P. Tran, K. Roberts, G. Osapay, J. Schaal, A. Ouellette, and M. E. Selsted. 2008. Microbicidal properties and cytocidal selectivity of rhesus macaque theta defensins. Antimicrob. Agents Chemother. 52:944-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, W., S. M. Owen, D. L. Rudolph, A. M. Cole, T. Hong, A. J. Waring, R. B. Lal, and R. I. Lehrer. 2004. Activity of alpha- and theta-defensins against primary isolates of HIV-1. J. Immunol. 173:515-520. [DOI] [PubMed] [Google Scholar]
- 40.Wong, C. K., C. W. Lam, A. K. Wu, W. K. Ip, N. L. Lee, I. H. Chan, L. C. Lit, D. S. Hui, M. H. Chan, S. S. Chung, and J. J. Sung. 2004. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 136:95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang, H., W. Xie, X. Xue, K. Yang, J. Ma, W. Liang, Q. Zhao, Z. Zhou, D. Pei, J. Ziebuhr, R. Hilgenfeld, K. Y. Yuen, L. Wong, G. Gao, S. Chen, Z. Chen, D. Ma, M. Bartlam, and Z. Rao. 2005. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 3:e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasin, B., W. Wang, M. Pang, N. Cheshenko, T. Hong, A. J. Waring, B. C. Herold, E. A. Wagar, and R. I. Lehrer. 2004. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78:5147-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziolkowska, N. E., B. R. O'Keefe, T. Mori, C. Zhu, B. Giomarelli, F. Vojdani, K. E. Palmer, J. B. McMahon, and A. Wlodawer. 2006. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure 14:1127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]