Abstract
Chronic immune activation is a driver of human immunodeficiency virus type 1 (HIV-1) disease progression. Here, we describe that subjects with chronic hepatitis C virus (HCV)/HIV-1 coinfection display sharply elevated immune activation as determined by CD38 expression in T cells. This occurs, despite effective antiretroviral therapy, in both CD8 and CD4 T cells and is more pronounced than in the appropriate monoinfected control groups. Interestingly, the suppression of HCV by pegylated alpha interferon and ribavirin treatment reduces activation. High HCV loads and elevated levels of chronic immune activation may contribute to the high rates of viral disease progression observed in HCV/HIV-1-coinfected patients.
Hepatitis C virus (HCV) establishes chronic infection in a majority of infected humans. About 15 to 30% of patients with human immunodeficiency virus type 1 (HIV-1) infection are also infected with HCV (23, 26), and this patient group experiences an augmented rate of HCV disease progression, with increased liver-related morbidity, and higher HCV loads (2, 5, 6, 29). T-cell responses to HCV are generally poor in chronic infection, and this deficiency is exacerbated in HCV/HIV-1 coinfection. This is likely due in part to the HIV-mediated loss of CD4 helper T cells, which in turn impairs the function of HCV-specific CD8 T cells (15, 17). The spontaneous clearance of chronic HCV infection is rare in monoinfection and probably even more so in HIV-1-coinfected subjects, although a few such cases have been described (8, 10, 30). Interestingly, some studies suggest that HCV/HIV-1 coinfection is associated with an increased rate of HIV-1 disease progression (14, 22, 24).
HCV can be successfully treated with pegylated alpha interferon (peg-IFN-α) plus ribavirin. A sustained virological response, with eradication of the virus, is obtained in only 50 to 80% of patients with HCV monoinfection, and the rates are generally lower in HCV/HIV-1-coinfected subjects (19). Several viral and host factors influence the outcome of treatment with peg-IFN-α plus ribavirin, and HCV genotype, viral burden, age, and gender are among the most important. Cellular sensitivity to IFN-α and the ability to increase the expression of IFN-α-regulated genes are important for the response to treatment (20, 25, 27). Because of the key role of chronic immune activation in driving HIV-1 disease (7, 11, 16; for a review, see reference 9) and the interplay between the two viruses in the coinfected host, we were interested in analyzing the levels of T-cell immune activation in patients with chronic HCV on the background of an HIV-1 coinfection effectively controlled by highly active antiretroviral therapy (HAART).
The study subjects were from the Viral Dynamics and Immunology in HCV/HIV-1 Coinfection Study, including patients from the Karolinska University Hospital in Stockholm, Sweden (13), as well as the Hvidovre Hospital and Rigshospitalet in Copenhagen, Denmark, and the Aalborg and Aarhus university hospitals in Denmark (Table 1). Fourteen HCV/HIV-1-coinfected subjects who had not received prior HCV therapy were sampled at baseline, and 11 of these were further sampled during and after treatment with peg-IFN-α-2a (Pegasys, Roche AB, Sweden) and ribavirin (Copegus, Roche AB, Sweden). Coinfected subjects were on stable HAART for at least 1 year and had no active intravenous drug use for at least 6 months before the start of the study. Nine HIV-1-monoinfected subjects on stable HAART and 14 chronically HCV-monoinfected subjects were included. The 21 uninfected control subjects were healthy volunteers donating blood at the Stockholm blood bank. All study subjects were of Caucasian white ethnic origin. The study protocols were approved by local institutional review boards as well as the Swedish National Medical Products Agency, the Danish Medicinal Agency, and the Danish Data Protection Agency. Peripheral blood mononuclear cells were isolated from heparinized whole-blood samples by Lymphoprep gradient centrifugation (Axis-Shield, Oslo, Norway).
TABLE 1.
Subject characteristics at baseline
| Characteristic | Value for groupa |
|||
|---|---|---|---|---|
| HCV/HIV-1 coinfected | HCV monoinfected | HIV monoinfected | Healthy control | |
| Subjects (n) | 14 | 11 | 9 | 21 |
| Sex [no. of patients (%)] | ||||
| Female | 3 (21) | 5 (50) | 2 (22) | 9 (43) |
| Male | 11 (79) | 6 (50) | 7 (78) | 12 (57) |
| Age (yr) (range) | 43 (21-53) | 52 (43-59) | 46 (29-67) | 45 (32-59) |
| CD4 absolute median (cells/μl) (range) | 499 (250-1370) | 729 (200-1746) | 605 (370-720) | 845 (441-1491) |
| CD4 median % (range) | 27 (12-45) | 44 (38-57) | 30 (21-41) | 46 (31-67) |
| CD8 absolute median (cells/μl) (range) | 910 (530-2440) | 666 (352-754) | 770 (520-1770) | 443 (195-776) |
| CD8 median % (range) | 50 (24-70) | 26 (15-39) | 46 (24-64) | NA |
| HIV ART [no. of patients (%)] | 14 (100) | NA | 9 (100) | NA |
| HIV load median (copies/ml) (range) | <50 (<50) | NA | <50 (<50) | NA |
| HCV genotype [no. of patients (%)] | ||||
| 1 | 10 (72) | 2 (18) | NA | NA |
| 2 | 2 (14) | 4 (36) | NA | NA |
| 3 | 2 (14) | 5 (46) | NA | NA |
| Serum HCV RNA median (IU/ml) (range) | 3.64 × 106 (0.068 × 106−20.0 × 106) | 0.57 × 106 (0.023 × 106-3.65 × 106) | NA | NA |
NA, not applicable.
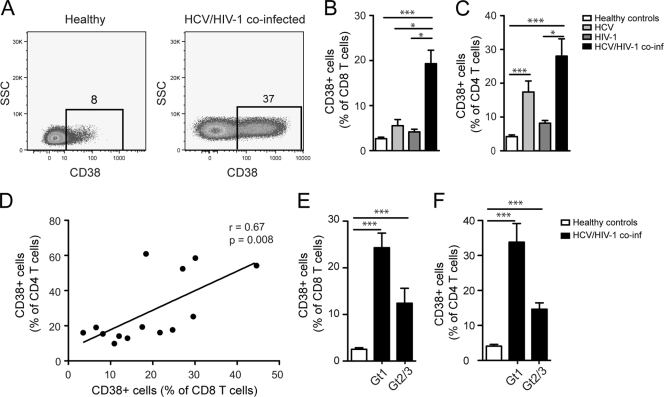
To address the role of immune activation in HCV/HIV-1 coinfection, we analyzed CD38 expression in the CD4 and CD8 T-cell compartments of subjects chronically coinfected with HCV and HIV-1, in comparison to that of monoinfected controls and healthy blood donors (Fig. 1A and Table 1). Anti-CD3 phycoerythrin-Cy7, anti-CD4 Pacific Blue, anti-CD8 peridinin chlorophyll protein, and anti-CD38 allophycocyanin were from BD Biosciences (San Diego, CA). Multicolor flow cytometry data were acquired on a CyAn ADP instrument (Beckman Coulter, Inc., Fullerton, CA) and analyzed using FlowJo software (Tree Star, OR) (12). These patient groups allowed the investigation of T cells in patients with uncontrolled HCV infection on the background of a drug-controlled HIV-1 infection. We observed considerably elevated CD38 expression in both CD8 and CD4 T cells in HCV/HIV-1-coinfected subjects in comparison to that in monoinfected subjects and healthy controls (Fig. 1B and C, respectively). In CD4 T cells, CD38 expression was significantly elevated also in HCV-monoinfected subjects (Fig. 1C). There was a direct correlation between CD38 expression in CD4 T cells and CD8 T cells, indicating a coordinated elevation of immune activation levels in both T-cell subsets (Fig. 1D). CD38 expression in CD8 and CD4 T cells tended to be more pronounced in HCV genotype 1 infection than genotype 2 or 3 infection (Fig. 1E and F). Together, these data indicate that HCV/HIV-1 coinfection is associated with high levels of chronic activation of both CD8 and CD4 T-cell compartments despite effective control of HIV-1 replication by HAART.
FIG. 1.
Elevated CD38 levels in CD4 and CD8 T cells in HCV/HIV-1-coinfected subjects despite effective HAART. (A) Representative dot plots of CD38 staining in CD8 T cells. (B) CD38 expression in CD8 T cells in coinfected patients (n = 14) in comparison to HCV (n = 11)- and HIV-1 (n = 9)-monoinfected subjects as well as to healthy donors (n = 21). (C) CD38 expression in CD4 T cells in the four study groups. (D) Spearman rank correlation between the CD38 expression in CD4 and CD8 T cells in coinfected subjects. CD38 expression in CD8 T cells (E) and CD4 T cells (F) in coinfected patients with HCV genotype 1 (Gt1) (n = 10) infection and HCV genotype 2 or 3 (Gt2/3) infection (n = 4) and healthy donors (n = 21). Means and standard errors are shown. Statistical analysis performed was one-way analysis of variance on ranks followed by Dunn's multiple comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. All statistical analyses were performed using GraphPad Prism software 5.0 (GraphPad Software, Inc., San Diego, CA).
Eleven of the HCV/HIV-1-coinfected subjects started a 48-week HCV treatment with peg-IFN-α plus ribavirin and were sampled at weeks 0, 4, 12, and 72 (24 weeks posttreatment). Plasma HCV RNA levels were measured using the Cobas TaqMan HCV test with a limit of detection of 15 IU/ml (Roche Diagnostics, Branchburg, NJ). Baseline comparison between HCV-monoinfected and HCV/HIV-1-coinfected subjects revealed significantly higher HCV loads in the coinfected patients (Fig. 2A), confirming previous observations (2, 5, 6, 29). Upon the initiation of treatment with peg-IFN-α plus ribavirin, the viral loads were rapidly reduced (Fig. 2A). At week 12, HCV RNA was undetectable in a majority of the patients. We assessed T-cell activation by means of CD38 expression in T cells during treatment and observed a significant reduction in the frequency of activated CD8 and CD4 T cells (Fig. 2B and C). It is interesting to note that the initial response to treatment was different in the two T-cell compartments. Activation levels at week 4 increased slightly in the CD8 T-cell compartment, whereas the CD4 T cells displayed reduced CD38 expression already at this time point. Thus, immune activation as assessed by CD38 expression decreased in response to treatment with IFN-α plus ribavirin, and the CD4 and CD8 subsets differed in the initial response to treatment.
FIG. 2.

Reduction of T-cell activation upon IFN-α and ribavirin treatment. HCV/HIV-1-coinfected subjects (n = 14) had significantly higher HCV RNA levels (log10 IU/ml) at baseline (BL) than HCV-monoinfected subjects (n = 11). **, P < 0.01 as determined by the Mann-Whitney rank sum test. (A) Viral load decreased upon treatment (T) with peg-IFN-α and ribavirin. CD38 expression in CD8 (B) and CD4 (C) T cells in coinfected subjects (n = 11), measured at BL, during treatment (T) with peg-IFN-α and ribavirin, and at 24 weeks posttreatment (PT), corresponding to week 72 after treatment start. CD8 (D) and CD4 (E) T-cell counts in coinfected subjects measured at BL, during T with peg-IFN-α and ribavirin, and at 24 weeks PT. (B to E) Statistical analysis was performed using the repeated measures one-way analysis of variance on ranks followed by Dunn's multiple comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. All statistical analyses were performed using GraphPad Prism software 5.0 (GraphPad Software, Inc., San Diego, CA).
HCV/HIV-1 coinfection presents a formidable challenge to the human immune system. The present study indicates that HCV/HIV-1-coinfected patients have high levels of CD8 and CD4 T-cell immune activation, as indicated by CD38 expression, despite effective HAART-mediated suppression of HIV. This was associated with high HCV loads in the coinfected patients and the CD38 expression declined when HCV replication was suppressed by treatment with IFN-α plus ribavirin. This may suggest that the high levels of immune activation were driven by high HCV viral loads. It is well known that reversible T-cell lymphopenia is a side effect of treatment with IFN-α plus ribavirin. CD8 and CD4 T-cell counts rebounded after treatment (Fig. 2D and E), whereas the percentage of CD38+ T cells did not. However, it remains possible that the reduced frequency of CD38+ T cells upon treatment with IFN-α plus ribavirin could be due to the direct effects of treatment on T cells, which may cause them to enter tissues other than the blood or go into apoptosis. Further studies are needed to address these distinct but not mutually exclusive possibilities.
It is not entirely clear how immune activation drives HIV disease progression and what is the primary driver of immune activation. One hypothesis is that destruction of the immune system in the gut in acute HIV-1 infection leads to the leakage of microbial products into circulation, promoting activation via innate pattern recognition (4). Another possibility has been suggested by studies of the sooty mangabey monkey, the natural host of simian immunodeficiency virus SIVsm. This virus is nonpathogenic in this monkey but much more pathogenic in the rhesus macaque. This was recently linked to differences in immune activation and the levels of type I IFN expression triggered by the virus (21), of which high levels of IFN were associated with pathogenic immune activation. To what extent these findings can be extended to human HIV infection remains undetermined. Our finding that treatment with IFN-α plus ribavirin reduces immune activation may seem to contradict a role for type I IFNs in pathogenic immune activation. However, it is important to remember that only one species of type I IFN, IFN-α-2a, is used for treatment. It remains possible that the precise species of IFN, the dose, and the mode of delivery might influence the effects on immune activation.
One might speculate that the direct recognition by Toll-like receptors of viral RNA is likely to be the basis of immune activation in HCV infection. The damage to the immune system inflicted by HIV-1 leads to impaired immune control of HCV with higher HCV loads, which may in turn feed the observed elevation in immune activation. Interestingly, however, it was recently noted that microbial translocation into the circulation can be more severe in HCV-coinfected subjects than in HIV-1-monoinfected subjects, and this could be one mechanism behind the accelerated HCV disease progression in HIV-1-infected patients (1). Whereas we observed that CD38 expression in T cells decreases when HCV replication is suppressed, the levels of CD38 remain higher than in normal donors. It is clearly possible that the excessive microbial translocation or impaired clearance of microbe-derived products from circulation contributes to this pattern.
HCV liver disease has become a leading cause of death in HCV/HIV-1-coinfected patients who have access to HAART, and HCV disease progresses faster in this group of patients (2, 5, 6, 29). Some studies suggest that this is also true for HIV-1 disease (14, 22, 24), although this is not observed in all cohorts (28). The high levels of immune activation may contribute to high rates of viral disease progression by impairing the ability of the immune system to respond. Interestingly, we recently observed that a subset of CD8 T cells expressing the Fc receptor CD16 is numerically elevated in chronically HCV-infected subjects and that these cells mediate NK cell-like cytolytic function (3). The expression of CD16 correlated with that of CD38, suggesting that the development of NK-like activity in T cells may be part of the immunopathogenesis of HCV disease. Also of note, Kovacs et al. reported high levels of CD38 expression in the CD8 T cells of HCV/HIV-1-coinfected women who were not on HAART (18). We observe here that high levels of activation do not require active HIV-1 replication, and activation levels are even higher in CD4 T cells. Taken together, the results of the present study suggest that chronic immune activation is a component in the pathogenesis of HCV infection and might be particularly important in the context of HIV-1 coinfection.
Acknowledgments
This work was supported by grants from the Swedish Research Council, the Swedish International Development Agency, the Swedish Foundation for Strategic Research, the Swedish Physicians Against AIDS Research Foundation, the Swedish National Board of Health and Welfare, Roche A/S Denmark, and Karolinska Institutet.
We thank Markus Moll for careful reading of the manuscript and nurses Elisabeth Rilegård, Ingrid Andrén, Lene Rosenoern, Lene Pors Jensen, and Bente Baadegaard, as well as technician Ann-Louise Soerensen, for help with patient samples.
We declare that we have no conflicting financial interests.
Footnotes
Published ahead of print on 26 August 2009.
REFERENCES
- 1.Balagopal, A., F. H. Philp, J. Astemborski, T. M. Block, A. Mehta, R. Long, G. D. Kirk, S. H. Mehta, A. L. Cox, D. L. Thomas, and S. C. Ray. 2008. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology 135:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bica, I., B. McGovern, R. Dhar, D. Stone, K. McGowan, R. Scheib, and D. R. Snydman. 2001. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin. Infect. Dis. 32:492-497. [DOI] [PubMed] [Google Scholar]
- 3.Bjorkstrom, N. K., V. D. Gonzalez, K. J. Malmberg, K. Falconer, A. Alaeus, G. Nowak, C. Jorns, B. G. Ericzon, O. Weiland, J. K. Sandberg, and H. G. Ljunggren. 2008. Elevated numbers of Fc gamma RIIIA+ (CD16+) effector CD8 T cells with NK cell-like function in chronic hepatitis C virus infection. J. Immunol. 181:4219-4228. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365-1371. [DOI] [PubMed] [Google Scholar]
- 5.Daar, E. S., H. Lynn, S. Donfield, E. Gomperts, M. W. Hilgartner, W. K. Hoots, D. Chernoff, S. Arkin, W. Y. Wong, C. A. Winkler, and Hemophilia Growth and Development Study. 2001. Relation between HIV-1 and hepatitis C viral load in patients with hemophilia. J. Acquir. Immune Defic. Syndr. 26:466-472. [DOI] [PubMed] [Google Scholar]
- 6.Daar, E. S., H. Lynn, S. Donfield, E. Gomperts, S. J. O'Brien, M. W. Hilgartner, W. K. Hoots, D. Chernoff, S. Arkin, W. Y. Wong, C. A. Winkler, and Hemophilia Growth and Development Study. 2001. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J. Infect. Dis. 183:589-595. [DOI] [PubMed] [Google Scholar]
- 7.Deeks, S. G., C. M. Kitchen, L. Liu, H. Guo, R. Gascon, A. B. Narvaez, P. Hunt, J. N. Martin, J. O. Kahn, J. Levy, M. S. McGrath, and F. M. Hecht. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942-947. [DOI] [PubMed] [Google Scholar]
- 8.De Rosa, F. G., S. Audagnotto, O. Bargiacchi, S. Garazzino, D. Aguilar Marucco, L. Veronese, F. Canta, S. Bonora, A. Sinicco, and G. Di Perri. 2006. Resolution of HCV infection after highly active antiretroviral therapy in a HIV-HCV coinfected patient. J. Infect. 53:e215-e218. [DOI] [PubMed] [Google Scholar]
- 9.Douek, D. C., M. Roederer, and R. A. Koup. 2009. Emerging concepts in the immunopathogenesis of AIDS. Annu. Rev. Med. 60:471-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falconer, K., V. D. Gonzalez, O. Reichard, J. K. Sandberg, and A. Alaeus. 2008. Spontaneous HCV clearance in HCV/HIV-1 coinfection associated with normalized CD4 counts, low level of chronic immune activation and high level of T cell function. J. Clin. Virol. 41:160-163. [DOI] [PubMed] [Google Scholar]
- 11.Giorgi, J. V., L. E. Hultin, J. A. McKeating, T. D. Johnson, B. Owens, L. P. Jacobson, R. Shih, J. Lewis, D. J. Wiley, J. P. Phair, S. M. Wolinsky, and R. Detels. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859-870. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez, V. D., N. K. Bjorkstrom, K. J. Malmberg, M. Moll, C. Kuylenstierna, J. Michaelsson, H. G. Ljunggren, and J. K. Sandberg. 2008. Application of nine-color flow cytometry for detailed studies of the phenotypic complexity and functional heterogeneity of human lymphocyte subsets. J. Immunol. Methods 330:64-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez, V. D., K. Falconer, J. Michaelsson, M. Moll, O. Reichard, A. Alaeus, and J. K. Sandberg. 2008. Expansion of CD56- NK cells in chronic HCV/HIV-1 co-infection: reversion by antiviral treatment with pegylated IFNalpha and ribavirin. Clin. Immunol. 128:46-56. [DOI] [PubMed] [Google Scholar]
- 14.Greub, G., B. Ledergerber, M. Battegay, P. Grob, L. Perrin, H. Furrer, P. Burgisser, P. Erb, K. Boggian, J. C. Piffaretti, B. Hirschel, P. Janin, P. Francioli, M. Flepp, and A. Telenti. 2000. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet 356:1800-1805. [DOI] [PubMed] [Google Scholar]
- 15.Harcourt, G., E. Gomperts, S. Donfield, and P. Klenerman. 2006. Diminished frequency of hepatitis C virus specific interferon gamma secreting CD4+ T cells in human immunodeficiency virus/hepatitis C virus coinfected patients. Gut 55:1484-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazenberg, M. D., S. A. Otto, B. H. van Benthem, M. T. Roos, R. A. Coutinho, J. M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 17:1881-1888. [DOI] [PubMed] [Google Scholar]
- 17.Kim, A. Y., G. M. Lauer, K. Ouchi, M. M. Addo, M. Lucas, J. Schulze zur Wiesch, J. Timm, M. Boczanowski, J. E. Duncan, A. G. Wurcel, D. Casson, R. T. Chung, R. Draenert, P. Klenerman, and B. D. Walker. 2005. The magnitude and breadth of hepatitis C virus-specific CD8+ T cells depend on absolute CD4+ T-cell count in individuals coinfected with HIV-1. Blood 105:1170-1178. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs, A., L. Al-Harthi, S. Christensen, W. Mack, M. Cohen, and A. Landay. 2008. CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J. Infect. Dis. 197:1402-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koziel, M. J., and M. G. Peters. 2007. Viral hepatitis in HIV infection. N. Engl. J. Med. 356:1445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lempicki, R. A., M. A. Polis, J. Yang, M. McLaughlin, C. Koratich, D. W. Huang, B. Fullmer, L. Wu, C. A. Rehm, H. Masur, H. C. Lane, K. E. Sherman, A. S. Fauci, and S. Kottilil. 2006. Gene expression profiles in hepatitis C virus (HCV) and HIV coinfection: class prediction analyses before treatment predict the outcome of anti-HCV therapy among HIV-coinfected persons. J. Infect. Dis. 193:1172-1177. [DOI] [PubMed] [Google Scholar]
- 21.Mandl, J. N., A. P. Barry, T. H. Vanderford, N. Kozyr, R. Chavan, S. Klucking, F. J. Barrat, R. L. Coffman, S. I. Staprans, and M. B. Feinberg. 2008. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 14:1077-1087. [DOI] [PubMed] [Google Scholar]
- 22.Merchante, N., J. A. Giron-Gonzalez, M. Gonzalez-Serrano, J. Torre-Cisneros, J. A. Garcia-Garcia, A. Arizcorreta, J. Ruiz-Morales, P. Cano-Lliteras, F. Lozano, C. Martinez-Sierra, J. Macias, and J. A. Pineda. 2006. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS 20:49-57. [DOI] [PubMed] [Google Scholar]
- 23.Monforte, A., D. Abrams, C. Pradier, R. Weber, P. Reiss, F. Bonnet, O. Kirk, M. Law, S. De Wit, N. Friis-Møller, A. N. Phillips, C. A. Sabin, J. D. Lundgren, and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group. 2008. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS 22:2143-2153.18832878 [Google Scholar]
- 24.Piroth, L., M. Grappin, L. Cuzin, Y. Mouton, O. Bouchard, F. Raffi, D. Rey, D. Peyramond, F. Gourdon, C. Drobacheff, M. L. Lombart, F. Lucht, J. M. Besnier, L. Bernard, P. Chavanet, and H. Portier. 2000. Hepatitis C virus co-infection is a negative prognostic factor for clinical evolution in human immunodeficiency virus-positive patients. J. Viral Hepat. 7:302-308. [DOI] [PubMed] [Google Scholar]
- 25.Sarasin-Filipowicz, M., E. J. Oakeley, F. H. Duong, V. Christen, L. Terracciano, W. Filipowicz, and M. H. Heim. 2008. Interferon signaling and treatment outcome in chronic hepatitis C. Proc. Natl. Acad. Sci. USA 105:7034-7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherman, K. E., S. D. Rouster, R. T. Chung, and N. Rajicic. 2002. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US Adult AIDS Clinical Trials Group. Clin. Infect. Dis. 34:831-837. [DOI] [PubMed] [Google Scholar]
- 27.Shirakawa, H., A. Matsumoto, S. Joshita, M. Komatsu, N. Tanaka, T. Umemura, T. Ichijo, K. Yoshizawa, K. Kiyosawa, E. Tanaka, and Nagano Interferon Treatment Research Group. 2008. Pretreatment prediction of virological response to peginterferon plus ribavirin therapy in chronic hepatitis C patients using viral and host factors. Hepatology 48:1753-1760. [DOI] [PubMed] [Google Scholar]
- 28.Sulkowski, M. S., R. D. Moore, S. H. Mehta, R. E. Chaisson, and D. L. Thomas. 2002. Hepatitis C and progression of HIV disease. JAMA 288:199-206. [DOI] [PubMed] [Google Scholar]
- 29.Thomas, D. L., J. Astemborski, D. Vlahov, S. A. Strathdee, S. C. Ray, K. E. Nelson, N. Galai, K. R. Nolt, O. Laeyendecker, and J. A. Todd. 2000. Determinants of the quantity of hepatitis C virus RNA. J. Infect. Dis. 181:844-851. [DOI] [PubMed] [Google Scholar]
- 30.Zeitoun, J. D., V. Mallet, M. L. Chaix, J. P. Viard, S. Blanche, and S. Pol. 2007. Stable recovery from HCV in HIV-HCV co-infection under antiretroviral therapy. J. Clin. Virol. 40:71-73. [DOI] [PubMed] [Google Scholar]