Abstract
Natural killer (NK) cells derived from the human female reproductive tract (FRT) are phenotypically and functionally distinct from those obtained from peripheral blood. Because the FRT is a primary site of human immunodeficiency virus type 1 (HIV-1) infection in women, we determined whether soluble factors secreted by uterine-derived NK (uNK) cells inhibit HIV-1 infection. Clonal populations of uNK cells were activated with interleukin-12 (IL-12) and IL-15, and conditioned media (CM) from these cultures evaluated for their ability to inhibit infection of cells by HIV-1IIIB, HIV-1NL4.3, and HIV-1HC4 (X4-tropic) or HIV-1BaL (R5-tropic) viruses. We found that soluble factors secreted by activated uNK cells significantly inhibited X4-tropic virus infection of TZM-bl cells, peripheral blood mononuclear cells, and primary human endometrial cells, but not infection by HIV-1BaL. In contrast, CM from peripheral blood NK (bNK) cells did not inhibit HIV-1 infection of cells. Analysis of factors secreted from uNK clones with anti-HIV-1 activity demonstrated significantly higher levels of CXCL12 compared to uNK clones without this activity, and the HIV inhibitory activity was neutralized by antibodies to CXCL12. Collectively, these data demonstrate that human uNK cells release chemokines with anti-HIV-1 activity for X4-tropic strains and this suggest that these chemokines may contribute to the inhibition of X4-tropic strain transmission across mucosal tissues.
Natural killer (NK) cells play a pivotal role in the innate immune defense against tumors and in the killing of virus-infected cells (45). In the peripheral blood, NK cells account for ∼10% of mononuclear cells and are subdivided into two groups based on CD56 and CD16 surface expression (35, 40). The CD56dim CD16+ subtype comprises the majority of blood NK (bNK) cells and are highly cytolytic, whereas the CD56bright CD16− subset accounts for <10% of bNK cells, are less cytolytic, and are primarily cytokine producers (11, 39). In the female reproductive tract (FRT), however, NK cells form a large population of the resident leukocytes, with an even higher proportion found during the second half (secretory phase) of the menstrual cycle (21, 23, 24, 31). The phenotype of NK cells in the FRT is more similar to the CD56bright CD16− bNK cell subset. However, in contrast to bNK cells, FRT NK cells are unique in that they express CD9 and CD69 and are much larger and granular than bNK cells (31). The differences between bNK cells and those in the FRT are perhaps largely due to the microenvironment in which they reside, including exposure to fluctuations in steroid sex hormones throughout the menstrual cycle, as well as to inflammatory cytokines and chemokines secreted by innate and acquired immune cells in response to microbial exposure within this site (33).
bNK cells play an important role in protection against human immunodeficiency virus (HIV). This is either directly, through antibody-dependent cell-mediated cytotoxicity (2, 18, 38, 43), or indirectly, through the production of chemokines; CC-chemokine ligand 3 (CCL3; also called MIP-1α), CCL4 (MIP1-β), and CCL5 (RANTES), which are natural ligands of CCR5 (10) and which block viruses that utilize the CCR5 coreceptor for entry (5, 6, 9, 13, 16, 26). NK cells also produce cytokines, including gamma interferon (IFN-γ), granulocyte/macrophage colony-stimulating factor, and tumor necrosis factor alpha which can suppress HIV replication by recruiting other effector cells (16). Studies have shown that bNK cells from uninfected HIV-exposed individuals produce significantly larger amounts of IFN-γ than HIV-infected individuals, indicating that IFN-γ could have some protective role (30, 37). The ability of uterine-derived NK (uNK) cells to inhibit HIV-1 infection is unknown.
Because the major route of HIV-1 transmission in women occurs across the mucosal surfaces of the FRT, we sought to determine whether uNK cells play a role in protection against HIV-1 infection. Mucosal NK cells have unique properties compared to bNK cells. Recently, a subset of mucosal NK cells found in tonsils and gut has been described that produce interleukin-22 (IL-22) and that may be important for protection from bacterial infection (8, 36). Thus, the unique microenvironment of different tissues may lead to differentiation of NK cells to express effector functions not found in bNK cells. This study demonstrates that uNK cells have the ability to inhibit HIV-1 infection of cells, and this may play an important role in protection of mucosal surfaces from infection by X4-tropic strains of HIV-1.
MATERIALS AND METHODS
Human subjects and cell isolation.
uNK cells were isolated from human endometrial tissue samples obtained from women aged between 32 to 55 years undergoing hysterectomy for benign gynecological indications, such as prolapse or fibroids, at the Dartmouth Hitchcock Medical Center, Lebanon, NH. Briefly, endometrial tissues were minced and digested with a cocktail of 0.01% DNase 1 and 0.1% type IV collagenase enzymes (Sigma-Aldrich, St. Louis, MO) at 37°C in Dulbecco's modified Eagle's medium-F-12 (Invitrogen, Carlsbad, CA) for 1 h. For primary endometrial cell isolation, the digested tissues were passed through a 100-μm cell strainer to facilitate dispersion into single-cell suspensions consisting of leukocytes, epithelial cells, and stromal fibroblasts. bNK cells were isolated from peripheral blood mononuclear cell (PBMC) samples obtained from the same patient or from healthy individuals. PBMCs were separated on Lymphoprep gradients according to protocols provided by the manufacturer (Axis Shield, Oslo, Norway). Institutional Review Board approval and informed consent were obtained before tissue and blood donation.
Generation of NK cell clones.
Enzymatically isolated endometrial cells were cultured in 500 IU recombinant IL-2 (rIL-2)/ml (National Cancer Institute, Bethesda, MD) in complete medium (RPMI 1640 supplemented with 50 μM 2-mercaptoethanol [Sigma-Aldrich, St. Louis, MO], 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 mM sodium pyruvate [Mediatech, Manassas, VA], 0.1 mM nonessential amino acids [Invitrogen, Carlsbad, CA], and 5% human serum), for 2 to 3 days to allow for the recovery of cells following digestion. uNK and bNK cells were purified from endometrial single-cell suspensions and PBMCs, respectively, by negative selection using a human NK cell enrichment cocktail containing a combination of biospecific TAC-bound antibodies directed against CD3, CD4, CD19, CD66b, glycophorin A, and dextran (Stem Cell Technologies, Vancouver, BC, Canada), according to the manufacturer's instructions. The remaining (negatively selected) cells were highly enriched for NK cells as determined by flow cytometric analysis using antibodies to CD56. These cells were cloned using standard NK cell cloning procedures as previously described (7, 15). Briefly, sorted uNK cells were plated at 1 to 3 cells/well in U-bottom 96-well plates together with irradiated (100 Gy) feeder cells (5 × 104 allogeneic PBMCs together with 5 × 103 RPMI 8866 cells), supplemented with 1 μg/ml phytohemagglutinin (PHA), and 500 U rIL-2. After 10 to 14 days, the wells were examined for growth of clones. Cells from positive wells were expanded further and maintained in 500 U IL-2/ml for the remainder of their culture. In some cases, primary uNK cells were expanded in IL-2 for 5 to 8 days and analyzed for anti-HIV activity and cytokine production. These primary expanded uNK cells represent the uNK cells from these donors.
Antibodies and reagents.
The antibodies used in this study for flow cytometric analysis of NK clones included allophycocyanin-conjugated anti-CD3, rhodamine-phycoerythrin-conjugated anti-CD56, and mouse immunoglobulin G1 (IgG1) isotype control (Invitrogen, San Jose, CA); fluorescein isothiocyanate-conjugated anti-CD4, CD16, anti-CD69, anti-CD94, anti-NKB1, and anti-CD158b (BD Pharmingen, San Jose, CA); and CXCL12 and goat IgG (R&D Systems, Minneapolis, MN).
Flow cytometry.
To determine the purity of NK clones before use, NK clones were incubated with allophycocyanin-conjugated anti-CD3 and rhodamine-phycoerythrin-conjugated anti-CD56 antibodies. FACSCalibur and FACSCanto flow cytometers were used for analysis of cell surface expression and purity of NK clones.
Preparation of uNK and bNK CM.
uNK and bNK clones were cultured in quadruplicate wells at 105 cells/well in microtiter plates. Cells were stimulated by incubation with IL-12 (10 ng/ml) and IL-15 (100 ng/ml) or were left unstimulated. Cell-free conditioned media (CM) from these cultures were harvested after 72 h and stored at −20°C until further analysis.
Determination of anti-HIV activity.
To test the NK cell CM for anti-HIV-1 activity, the viruses used were HIV-1IIIB and HIV-1BaL (provided by Phalguni Gupta at University of Pittsburgh), HIV-1NL4-3 (provided by John Kappes, University of Alabama at Birmingham), and HIV-1HC4 (provided by Ruth Connor, Dartmouth Medical School). We used the TZM-bl indicator cell line (NIH AIDS Research & Reference Reagent Program, Bethesda, MD), which expresses CD4, CXCR4, and CCR5, and contains a β-galactosidase (β-Gal) reporter gene under the transcriptional control of the HIV-1 long terminal repeat (LTR) (44). TZM-bl cells were seeded at a density of 2.5 × 104 cells/well in a 96-well plate. After 24 h, 40 μl of conditioned medium from either the IL-12- plus IL-15-activated, or nonactivated, NK cells was added to quadruplicate wells containing TZM-bl cells for 1 h, followed by the addition of HIV-1 at a multiplicity of infection (MOI) of 1. After 48 h in culture, beta-glo (Promega, Madison, WI) was added to each well. The relative light units (RLU) were detected after 1 h of incubation using a luminometer (Turner Biosystems, Sunnyvale, CA). Inhibition that was <15% was not considered to be biologically relevant.
Infection of PBMCs and FRT cells.
PBMCs isolated from the hysterectomy tissue donor or from volunteer donors and FRT cells from the endometrium were cultured in complete medium with 1 μg/ml PHA. After 2 days in culture, cells were washed and cultured at 105 cells/well in fresh complete medium supplemented with 10 IU/ml IL-2, with or without uNK CM at a medium/uNK CM ratio of 3:1. After 1 h, the cells were infected with HIV-1IIIB for 2 h, washed, and returned to culture in the presence of complete medium supplemented with IL-2. Cell-free culture supernatants were collected from the PBMC and FRT cultures on days 3, 5, and 7 postinfection. These supernatants were then added to cultures of TZM-bl cells for 48 h, and the β-Gal expression was determined as described above.
ELISAs to detect cytokines in NK cell conditioned media.
CM from control and IL-12- plus IL-15-stimulated uNK and bNK cells were tested in triplicate for soluble IFN-γ, CXCL12, and IL-22 by enzyme-linked immunosorbent assay (ELISA), using human-specific Duoset ELISA development system kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Statistical analysis.
Data are expressed as the mean ± standard error of the mean (SEM). A one-way analysis of variance with Newman-Keuls post test or a Mann-Whitney nonparametric analysis was performed using GraphPad Prism 4 (La Jolla, CA). Statistical significance was defined as P < 0.05.
RESULTS
Human uNK cells secrete soluble factors that inhibit infection by HIV-1.
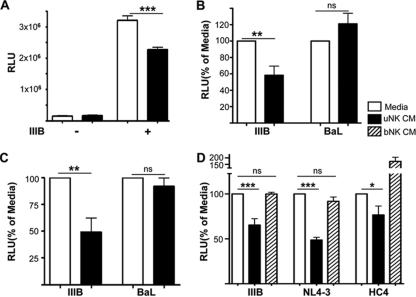
We determined the ability of CM from clonal populations of uNK cells to inhibit infection of TZM-bl cells with either HIV-1IIIB (X4) or HIV-1BaL (R5). Cell-free media from clonal populations of uNK cells significantly inhibited infection of TZM-bl cells by HIV-1IIIB (P < 0.001), whereas the same CM had no inhibitory activity against the HIV-1BaL (P > 0.05) (Fig. 1A and B). The data shown in Fig. 1B represent results from three different experiments using seven different uNK clones obtained from four different individuals. Similarly, CM from primary expanded uNK cells inhibited HIV-1IIIB, but not HIV-1Bal (Fig. 1C). To assess the ability of uNK CM to inhibit other X4-tropic strains, we compared the inhibitory activity of uNK CM against HIV-1NL4-3 and HIV-1HC4 (a primary X4 HIV-1 strain) and found that uNK cell clones were able to reduce the infection by all X4-tropic HIV-1 strains tested (Fig. 1D). In contrast to uNK cell CM, bNK cell CM did not mediate similar inhibition of HIV-1 infection. In fact in side-by-side comparisons, there was no inhibition of HIV infection by bNK cell CM. These data indicate that uNK cells, but not bNK cells, significantly inhibited infection by all three X4 HIV-1 strains tested.
FIG. 1.
Human uNK cells produce soluble factors with anti-HIV activity. (A) uNK cells were cultured with or without IL-12 plus IL-15 for 3 days. The resulting cell-free uNK CM was incubated with TZM-bl cells for 1 h and then infected with HIV-1IIIB at an MOI of 1. β-Gal activity was measured after 48 h. Results are means ± SEM of a representative experiment in which each condition was tested in quadruplicate. Media (open bars) and uNK CM (black bars), in the absence (−) or presence of HIV-1IIIB (IIIB), are shown. uNK CM significantly reduced HIV-1 infection in TZM-bl cells. ***, P < 0.001. (B) uNK CM from four different individuals in three different experiments were treated in quadruplicate as in panel A and infected with either HIV-1IIIB or HIV-1BaL (BaL). The data are normalized to RLU from the medium-plus-virus (HIV-1IIIB or HIV-1BaL) conditions and show that the uNK anti-HIV activity is specific for HIV-1IIIB. ***, P < 0.001 (Mann-Whitney test). Values represent means ± SEM (n = 7 different uNK clones). ns, not significant. The y axis shows RLU values, converted into percentages with media as 100%. (C) CM from primary NK cells were treated in quadruplicate as in panel B. Data are representative of two experiments with two different individuals. (D) TZM-bl cells were preincubated for 1 h with tissue culture media (open bars), bNK CM (hatched bars), or uNK CM (black bars). bNK and uNK CM were prepared from the blood and endometrial tissue of the same individual. One hour later, TZM-bl cells were infected with the indicated X4-tropic HIV-1 strains. After 48 h in culture, β-gal activity was measured. Data are expressed as the percentage of β-gal expression relative to the media control. Values represent means ± SEM, with n = 4 different clones. ***, P < 0.001 (one-way analysis of variance).
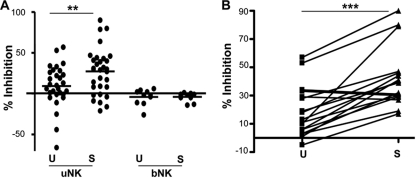
The anti-HIV activity of uNK cells but not bNK cells is increased by cytokine activation.
NK cell effector functions are known to be increased by cytokine activation (19), and IL-12 and IL-15, which are present within the human endometrium, stimulate uNK cells to produce cytokines (14). The anti-HIV activity of CM from both uNK and bNK cells was analyzed from both stimulated and unstimulated (control) NK cells. As shown in Fig. 2A, 64% of uNK clones showed significant inhibition of TZM-bl cell infection by HIV-1IIIB (P < 0.01). These data represent analysis of uNK CM of 33 different clones from 15 different individuals. Approximately 40% of CM from unstimulated uNK clones demonstrated inhibition of HIV-1IIIB infection, indicating that some uNK cells can constitutively inhibit HIV-1. There was no correlation found between the uNK anti-HIV activity and age of the donor (data not shown). To establish whether bNK cell CM had similar anti-HIV activity, bNK clones from normal healthy donors or from the blood of the hysterectomy patients were assayed for anti-HIV activity. When 10 bNK cell clones prepared from six individuals were tested, none of the bNK clones showed anti-HIV activity. Analysis of paired uNK clones from both cytokine-stimulated and unstimulated cells showed that the anti-HIV activity of uNK CM was significantly increased from stimulated uNK cells compared to unstimulated uNK cells (P < 0.001) (Fig. 2B). Thus, uNK cells are able to produce soluble factors that can inhibit HIV-1 infection and this activity is increased upon cytokine stimulation.
FIG. 2.
Anti-HIV-1 activity of CM from uNK cells is increased by cytokine activation. (A) CM from uNK and bNK clones collected after 3 days of stimulation with IL-12 and IL-15 (S) or in the absence of cytokine stimulation (U) were preincubated for 1 h with TZM-bl cells prior to the addition of HIV-1IIIB. The percentage of inhibition of HIV-1 infection was determined by comparing RLU values with those of the medium control. Each data point represents an individual clone, and the average of each group is shown. Stimulated uNK clones showed significantly higher inhibition than unstimulated uNK clones (**, P < 0.01; n = 31), and CM from bNK clones did not inhibit HIV-1IIIB (n = 10). These data represent uNK clones from 15 individuals and bNK clones from 6 individuals. (B) Comparison of anti-HIV activities between CM from paired IL-12- plus IL-15-stimulated and unstimulated uNK clones. These data show that stimulation of uNK cells resulted in an increase in HIV-1 inhibitory activity. ***, P < 0.001.
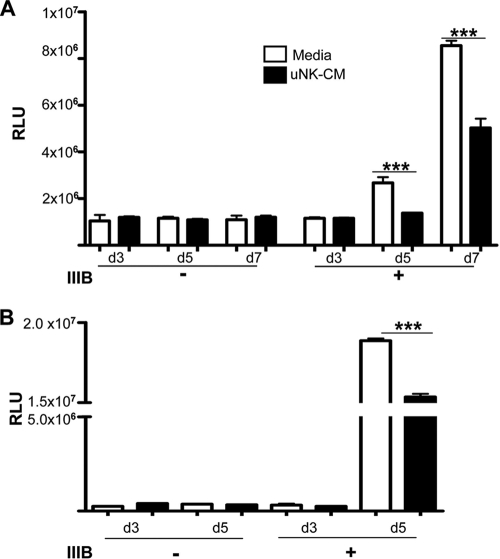
uNK cells secrete molecules that prevent infection of PBMCs and primary FRT cells by HIV-1.
The primary targets of HIV-1 in vivo are CD4+ T cells, monocytes and macrophages, and these leukocytes are present within PBMCs and primary human endometrium. To determine whether uNK cells secrete molecules that prevent HIV-1 infection of PBMCs or primary FRT cells, we tested the ability of CM from activated uNK clones to inhibit HIV-1IIIB infection of PHA-activated PBMCs and FRT cells. PBMCs and primary FRT cells were stimulated with PHA for 24 h to increase the efficiency of HIV infection. CM from uNK cell clones was added to these cells for 1 h, followed by incubation with HIV-1IIIB for an additional 2 h. At days 3, 5, and 7 postinfection, cell-free supernatants were collected and the presence of infectious virus was assessed using TZM-bl reporter cells. As shown by the data in Fig. 3, culturing the primary cells with CM from uNK clones resulted in a significant reduction in HIV-1 production by these primary cells compared to cells cultured with medium alone. These data show that uNK cells can produce soluble factors that can inhibit the infection of primary cells from the tissues where uNK cells reside.
FIG. 3.
CM from uNK cells inhibit infection of PBMC and primary FRT cells by HIV-1. (A) PHA-activated PBMCs were incubated with media or with CM from IL-12- plus IL-15-stimulated uNK cells (uNK CM), followed by infection with HIV-1 (IIIB +). Following infection, supernatant was collected from the PBMC cultures on days 3, 5, and 7 and added to TZM-bl cell cultures to quantify the amount of infectious HIV-1. After 48 h of incubation with TZM-bl cells, β-Gal activity was determined. Controls included supernatants from uninfected PBMCs (−). These data are representative of three independent experiments and are means ± SEM (n = 4). ***, P < 0.001. (B) PHA-activated endometrial cells from human uterine tissues were treated as in panel A, and cell-free supernatants collected on days 3 and 5 were tested for the presence of infectious HIV using TZM-bl reporter cells. Results are shown as means ± SEM (***, P < 0.001) and represent three independent experiments, and samples from different donors were used in each experiment.
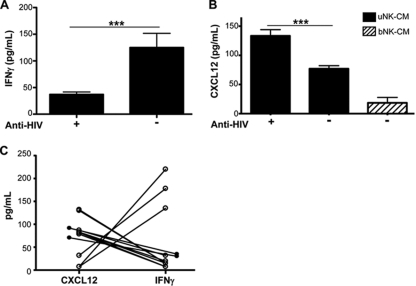
uNK cells that inhibited HIV-1 infection produced CXCL12 and not IFN-γ.
The ability of bNK cells to inhibit HIV-1 infection of target cells has been attributed to the production of chemokines and cytokines, including IFN-γ (3, 16, 46). To determine whether the anti-HIV activity of uNK cell secretions was due to IFN-γ, we measured IFN-γ amounts in CM from uNK cell clones and compared IFN-γ production in CM with the anti-HIV-1 activity of CM. As seen in Fig. 4A, CM with large amounts of IFN-γ negatively correlated with anti-HIV activity, and those that had small amounts of IFN-γ had significantly higher anti-HIV activity (P < 0.001). CXCR4 is a coreceptor required for infection by X4-tropic HIV-1, and CXCL12 (SDF-1α) is a chemokine that could block CXCR4 on CD4+ target cells (4). Because uNK cells preferentially inhibited X4-tropic HIV viruses and uNK cells have been found to secrete larger amounts of CXCL12 than bNK cells (41), we hypothesized that the inhibitory activity in the CM from activated uNK clones could be due to the secretion of CXCL12. We measured the amounts of CXCL12 in uNK CM and correlated these to anti-HIV activity. We observed that uNK CM that demonstrated anti-HIV activity had significantly larger amounts of CXCL12 than CM that showed no anti-HIV activity (P < 0.001) (Fig. 4B). CM from bNK clones, however, contained very small amounts of CXCL12, and these supernatants had no anti-HIV activity. Analysis of uNK samples, including primary expanded uNK cells from two individuals showed that uNK cells which secreted large amounts of CXCL12 produced little IFN-γ, and those that produced little CXCL12 secreted large amounts of IFN-γ (Fig. 4C). These data suggest that production of these cytokines by uNK cells may be from different functional uNK subsets (Fig. 4C).
FIG. 4.
uNK cells that inhibited HIV-1 infection produced CXCL12 and not IFN-γ. (A) Cell-free uNK CM from clones that demonstrated anti-HIV- activity (+) or lacking anti-HIV-1 activity (−) were analyzed for IFN-γ. There was a negative correlation between anti-HIV-1 activity and amounts of IFN-γ. ***, P < 0.001 (n > 6 uNK clones). (B) CM from uNK and bNK clones were analyzed for the presence of CXCL12. There was a positive correlation between CXCL12 production and anti-HIV-1 activity. ***, P < 0.001 (n = 8). Data are shown as means ± SEM. (C) Comparison between amounts of CXCL12 and IFN-γ in uNK CM. These data show that uNK CM with large amounts of CXCL12 had small amounts of IFN-γ and vice versa. Statistical analysis was performed using the Mann-Whitney U test.
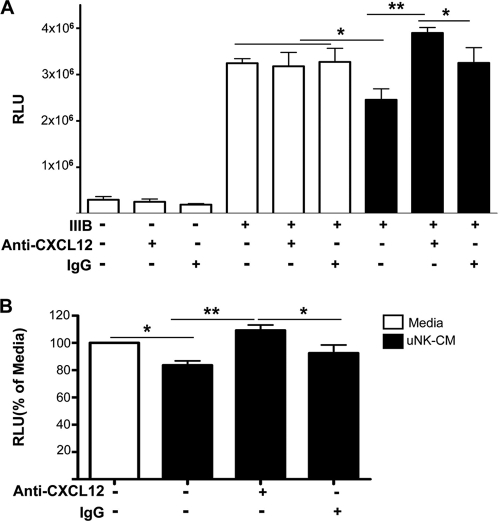
Neutralizing CXCL12 abolishes uNK cell-mediated HIV-1 inhibition.
The anti-HIV activity of CXCL12 is attributed to its ability to inhibit viral entry via CXCR4 (4, 32), and CXCL12 is produced by a number of cell types in humans, including NK cells in the FRT (1, 41). To establish whether the anti-HIV activity of uNK cells was due primarily to CXCL12 production, we neutralized CXCL12 with CXCL12-specific antibodies in the CM from cytokine-activated uNK cells and reassessed anti-HIV activity. The data demonstrate that neutralizing CXCL12 abrogated the anti-HIV activity of the uNK CM, whereas the use of an isotype control IgG had no effect (Fig. 5). TZM-bl cells treated directly with either the CXCL12 blocking antibody or the isotype control IgG demonstrated no activation of the HIV-1 LTR. Thus, one factor that can mediate anti-HIV activity from uNK cells is production of CXCL12.
FIG. 5.
Neutralizing CXCL12 abrogates uNK cell anti-HIV activity. (A) uNK clones were stimulated with IL-12 plus IL-15 for 3 days, and uNK CM (black bars) or culture medium (open bars) was preincubated with or without CXCL12 neutralizing antibodies or the isotype control (IgG). This mixture was then added to TZM-bl cells for 1 h before infecting the cells with HIV-1IIIB. β-Gal expression was determined after 48 h. (B) uNK CM from four different individuals were treated in four different experiments as in panel A, and the data were normalized to media (M). Values represent means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (n = 4). The y axis shows RLU values, converted into percentages with “media” as 100%. Statistical analysis was performed using the Mann-Whitney U test.
Anti-HIV activity is not confined to a known NK cell subset.
It has been reported that mucosal (tonsillar and gut) NK cells can be differentiated into IL-22-producing NK cells (NK-22 cells) following IL-23 stimulation and that these NK cells do not produce IFN-γ (8). Because we found that CXCL12-secreting uNK clones had anti-HIV activity while IFN-γ-expressing uNK clones did not, we tested whether the uNK cells with anti-HIV activity may be similar to these NK-22 cells. However, IL-22 was not found in CM from uNK cells cultured in the presence or absence of IL-23 or IL-12 and IL-15 (data not shown). These data were consistent with gene microarray data that showed that freshly isolated uNK cells did not express genes coding for IL-22, RORc, or NKp44 (data not shown), which are genes known to be expressed by NK-22 cells. We examined whether the anti-HIV activity of uNK cells could be defined by a particular subset of NK cells by determining the expression of CD4, CD8, CD16, CD25, CD38, CD57, CD69, CD94, CD158b, NKB1, NKG2D, NKp30, and NKp80 and compared these to the presence or absence of anti-HIV activity. As shown in Table 1, there was no correlation between the expression of these molecules and anti-HIV activity of the individual uNK clones.
TABLE 1.
Phenotype of uNK cells and anti-HIV-1 activity
| ID no.a | Expression ofb: |
Anti-HIV activity | ||||||
|---|---|---|---|---|---|---|---|---|
| CD4, CD11c, CD38, CD158b, or NKB1 | CD38 CD45 | CD8 | CD16 | NKG2D | NKp30 | NKp80 | ||
| 35-20 | − | ++ | − | − | ++ | ++ | + | No |
| 39-1 | − | ++ | − | − | + | ++ | ++ | No |
| 39-2 | − | ++ | − | − | + | ++ | ++ | Yes |
| 39-23 | − | ++ | − | − | ++ | ++ | ++ | Yes |
| 41-4 | − | ++ | − | − | ++ | ++ | ND | Yes |
| 41-6 | − | ++ | − | − | ++ | ++ | ND | No |
| 41-7 | − | ++ | − | − | + | ++ | ND | Yes |
| 47-6 | − | ++ | ++ | − | ++ | ++ | ND | No |
| 47-12 | − | ++ | − | − | + | ++ | ND | Yes |
| 47-32 | − | ++ | + | + | ++ | ++ | ND | No |
| 49-4 | − | ++ | − | − | ++ | ++ | ND | No |
| 49-23 | − | ++ | − | − | ++ | ++ | ND | No |
| 49-45 | − | ++ | + | ++ | ++ | ++ | ND | Yes |
| 50-39 | ND | ND | ++ | − | ++ | ++ | ND | Yes |
| 50-45 | ND | ND | − | ++ | ++ | ++ | ND | Yes |
| 50-50 | ND | ND | − | ++ | ++ | ++ | ND | No |
| 50-59 | ND | ND | + | + | ++ | ++ | ND | Yes |
The identification (ID) numbers shown represent uNK clones from six different individuals: 35, 39, 41, 47, 49, and 50.
−, +, and ++, absence, low, and high expression, respectively. ND, not done.
DISCUSSION
NK cells in blood can directly kill HIV-1-infected targets though cell-to-cell contact or through the secretion of CC chemokines that block CCR5 HIV-1 coreceptors (5, 26, 37). However, HIV-1 is predominantly transmitted heterosexually (34), and the primary entry site of virus is through the mucosa of the FRT. Moreover, NK cells present in the FRT are unique and distinct from those found in the peripheral blood (28, 31). We therefore examined whether uNK cells can mediate anti-HIV activity. In this study, we activated uNK cells with IL-12 and IL-15 as these are proinflammatory mediators which are present within the human endometrium, produced by macrophages during an initial phase of an infection, and have been shown to increase the cytolytic activity of NK cells (11, 25). Our study demonstrates that uNK cells secrete soluble mediators that exhibit anti-HIV activity for X4-tropic strains of HIV-1 and that this anti-HIV activity correlates with the amount of secreted CXCL12 but not IFN-γ.
Although the anti-HIV effects of bNK cells have been reported by others (26, 37, 43, 46), the ability of uNK cells from the FRT to secrete soluble mediators that inhibit HIV-1 infection has not been previously described. To the best of our knowledge, this study is the first showing that CM from uNK cells blocks HIV-1 infection of target cells. We found that bNK cell CM did not inhibit HIV infection under similar experimental conditions. In contrast, previous studies have shown that supernatants from NK cell cultures (both primary NK cells and NK cell lines YTS and NK 92) inhibited HIV activation in chronically HIV-infected immune cells (PBMCs) from HIV-infected subjects (46). In the present study, we used NK cell clones and uninfected TZM-bl cells, PBMCs, and primary endometrial cells to measure the inhibitory effects of HIV infection and found that CM from bNK cells lacked the ability to inhibit HIV-1 infection of target cells. These observed differences between uNK and bNK cells are likely due to the fact that bNK and uNK cells are phenotypically and functionally distinct NK cell subsets (15, 31). While the anti-HIV-1 activity shown in some uNK clones was modest, a limited number of NK cells were used in these experiments. Moreover, NK cells are present in large numbers in the human FRT (31); thus, we believe that the anti-HIV activity in vivo will be biologically relevant.
NK cells are present in the human FRT, where they play a critical role during implantation as well as in conferring protection against potential pathogens (14, 20, 22, 42). Our studies used uNK cells isolated from reproductive tissues from patients undergoing hysterectomy for benign conditions. To obtain sufficient numbers of fresh uNK cells for these studies, the uNK cells were cloned and expanded in the presence of IL-2. Although it is possible for the uNK cell clones to express effector functions that differ from primary uNK cells, we have found that uNK cell clones are quite similar to activated primary uNK cells in many effector functions (15). In this study, we demonstrated that a significant proportion of the uNK clones secrete soluble mediators that inhibit HIV infection, an activity that is enhanced upon cytokine activation and that is blocked by neutralizing antibody to CXCL12. The ability of anti-CXCL12 antibody to block the anti-HIV activity of uNK CM demonstrated that the anti-HIV activity was likely due to CXCL12. Furthermore, this inhibitory activity was effective against both laboratory-adapted as well as primary X4 HIV-1 strains, but was not effective at preventing an infection with an R5-tropic strain of HIV-1. Moreover, CM from cytokine-activated bNK cells did not have anti-HIV activity against any of the viruses tested. Other studies have shown that noncytolytic bNK cells have anti-HIV activity that is limited to R5, but not X4, HIV-1 strains (17, 26, 37).
CXCL12 is the natural ligand for the chemokine receptor CXCR4, and this chemokine can effectively block the entry of X4-, but not R5-tropic viruses. The blocking activity of this chemokine is consistent with our data in which we show that the anti-HIV activity was limited to uNK cells that produced larger amounts of CXCL12 and that the anti-HIV effect was restricted to X4 viruses. Marechal et al. reported that CXCL12 has dual role in HIV-1 in that it can block the entry of the X4 strain of HIV-1 by binding to CXCR4 and also can increase HIV replication through augmenting HIV LTR transcription (29). Thus, CXCL12 may have competing roles during infection so that a large amount of CXCL12 may block X4-tropic strains, but it may enhance replication of R5-tropic strains of HIV. In addition, there may be other factors present in uNK CM that contribute to inhibition of HIV-1 infection. The data show that CXCL12 is required, but we cannot exclude that other factors may be involved. R5-tropic viruses infect cells based on CCR5 and other cell surface molecules. The mechanisms that regulate how different R5-tropic viruses infect different cell types remain unclear. The data presented showed that uNK cells did not inhibit HIV-1BaL infection, but it cannot be concluded that uNK cells are unable to affect infection by other R5-tropic viruses. It is possible that uNK cells may prevent (or enhance) infection by other R5-tropic viruses by mechanisms as yet undiscovered.
HIV-1 that is sexually transmitted is typically R5 tropic, although the mechanism responsible for the selective transmission of R5 and the inhibition of X4 is not well characterized. Whether this is due in part to the susceptibility of X4-tropic strains to the host's mucosal immune cell defenses within mucosal tissue sites or to selective virulence of R5-tropic strains is not yet resolved. In contrast to reports by others (37, 46), our study showed that the presence of large amounts of IFN-γ did not correlate with anti-HIV activity, suggesting that the ability of uNK cells to inhibit HIV is independent of IFN-γ (26). It is likely that there is heterogeneity within the population of uNK cells, so that some uNK cell clones are able to mediate CXCL12 production, while others can produce IFN-γ. Thus, depending on their differentiation state, some uNK cells may produce CXCL12 and inhibit HIV-1, while others lack the ability to secrete this chemokine. Unique subsets of NK cells with different effector potential have been reported. Recently, a mucosal NK cell subset that expresses NKp44 was found in the tonsil and gut that produce IL-22 but not IFN-γ upon stimulation (8). Our findings in the present study that uNK cells did not produce IL-22 upon stimulation or express cell surface proteins or transcription factors associated with this unique mucosal NK cell subset suggest that uNK cells are unique with a different effector potential from those found at other mucosal sites. It has been shown that human bNK cells can be differentiated in vitro into cell subsets with different patterns of cytokine secretion: an NK1 subset that releases IFN-γ and an ΝΚ2 subset that secretes type 2 cytokines, including IL-5 and IL-13 (12, 27, 40).
In summary, this report demonstrates that uNK cells can be induced to secrete soluble factors that can inhibit HIV-1. This anti-HIV activity is specific for X4-tropic strains, is increased by cytokine activation, and is mediated by CXCL12. These findings imply that uNK cells may play a role in the protection against HIV-1 within the FRT and that modulating uNK cells with inflammatory cytokines may augment this antiviral activity.
Acknowledgments
This study was supported by National Institutes of Health grants AI-071761(C.R.W.) and AI-51877 (C.R.W.) and by Fogarty FIC-5-D43-TW006807.
Footnotes
Published ahead of print on 19 August 2009.
REFERENCES
- 1.Amara, A., S. L. Gall, O. Schwartz, J. Salamero, M. Montes, P. Loetscher, M. Baggiolini, J. L. Virelizier, and F. Arenzana-Seisdedos. 1997. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandyopadhyay, S., U. Ziegner, D. E. Campbell, D. S. Miller, J. A. Hoxie, and S. E. Starr. 1990. Natural killer cell-mediated lysis of T cell lines chronically infected with HIV-1. Clin. Exp. Immunol. 79:430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biron, C. A. 1997. Natural killer cell regulation during viral infection. Biochem. Soc. Trans. 25:687-690. [DOI] [PubMed] [Google Scholar]
- 4.Bleul, C. C., M. Farzan, H. Choe, C. Parolin, I. Clark-Lewis, J. Sodroski, and T. A. Springer. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829-833. [DOI] [PubMed] [Google Scholar]
- 5.Bluman, E. M., K. J. Bartynski, B. R. Avalos, and M. A. Caligiuri. 1996. Human natural killer cells produce abundant macrophage inflammatory protein-1 alpha in response to monocyte-derived cytokines. J. Clin. Investig. 97:2722-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broder, C. C., and D. S. Dimitrov. 1996. HIV and the 7-transmembrane domain receptors. Pathobiology 64:171-179. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, K. S., and M. Colonna. 2000. Natural killer cell protocols: cellular and molecular methods, vol. 121. Humana Press, Totowa, NJ.
- 8.Cella, M., A. Fuchs, W. Vermi, F. Facchetti, K. Otero, J. K. Lennerz, J. M. Doherty, J. C. Mills, and M. Colonna. 2009. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457:722-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 11.Cooper, M. A., T. A. Fehniger, S. C. Turner, K. S. Chen, B. A. Ghaheri, T. Ghayur, W. E. Carson, and M. A. Caligiuri. 2001. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 97:3146-3151. [DOI] [PubMed] [Google Scholar]
- 12.Deniz, G., M. Akdis, E. Aktas, K. Blaser, and C. A. Akdis. 2002. Human NK1 and NK2 subsets determined by purification of IFN-gamma-secreting and IFN-gamma-nonsecreting NK cells. Eur. J. Immunol. 32:879-884. [DOI] [PubMed] [Google Scholar]
- 13.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson, M., S. K. Meadows, S. Basu, T. F. Mselle, C. R. Wira, and C. L. Sentman. 2006. TLRs mediate IFN-gamma production by human uterine NK cells in endometrium. J. Immunol. 176:6219-6224. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson, M., S. K. Meadows, C. R. Wira, and C. L. Sentman. 2004. Unique phenotype of human uterine NK cells and their regulation by endogenous TGF-beta. J. Leukoc. Biol. 76:667-675. [DOI] [PubMed] [Google Scholar]
- 16.Fauci, A. S., D. Mavilio, and S. Kottilil. 2005. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat. Rev. Immunol. 5:835-843. [DOI] [PubMed] [Google Scholar]
- 17.Fehniger, T. A., G. Herbein, H. Yu, M. I. Para, Z. P. Bernstein, W. A. O'Brien, and M. A. Caligiuri. 1998. Natural killer cells from HIV-1+ patients produce C-C chemokines and inhibit HIV-1 infection. J. Immunol. 161:6433-6438. [PubMed] [Google Scholar]
- 18.Fortis, C., P. Biswas, L. Soldini, F. Veglia, A. M. Careddu, F. Delfanti, B. Mantelli, M. Murone, A. Lazzarin, and G. Poli. 1999. Dual role of TNF-alpha in NK/LAK cell-mediated lysis of chronically HIV-infected U1 cells. Concomitant enhancement of HIV expression and sensitization of cell-mediated lysis. Eur. J. Immunol. 29:3654-3662. [DOI] [PubMed] [Google Scholar]
- 19.Giavedoni, L. D., M. C. Velasquillo, L. M. Parodi, G. B. Hubbard, and V. L. Hodara. 2000. Cytokine expression, natural killer cell activation, and phenotypic changes in lymphoid cells from rhesus macaques during acute infection with pathogenic simian immunodeficiency virus. J. Virol. 74:1648-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Givan, A. L., H. D. White, J. E. Stern, E. Colby, E. J. Gosselin, P. M. Guyre, and C. R. Wira. 1997. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am. J. Reprod. Immunol. 38:350-359. [DOI] [PubMed] [Google Scholar]
- 21.Hunt, J. S. 1994. Immunologically relevant cells in the uterus. Biol. Reprod. 50:461-466. [DOI] [PubMed] [Google Scholar]
- 22.King, A. 2000. Uterine leukocytes and decidualization. Hum. Reprod. Update 6:28-36. [DOI] [PubMed] [Google Scholar]
- 23.King, A., V. Wellings, L. Gardner, and Y. W. Loke. 1989. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum. Immunol. 24:195-205. [DOI] [PubMed] [Google Scholar]
- 24.Kitaya, K., T. Nakayama, N. Daikoku, S. Fushiki, and H. Honjo. 2004. Spatial and temporal expression of ligands for CXCR3 and CXCR4 in human endometrium. J. Clin. Endocrinol. Metab. 89:2470-2476. [DOI] [PubMed] [Google Scholar]
- 25.Kitaya, K., T. Nakayama, T. Okubo, H. Kuroboshi, S. Fushiki, and H. Honjo. 2003. Expression of macrophage inflammatory protein-1beta in human endometrium: its role in endometrial recruitment of natural killer cells. J. Clin. Endocrinol. Metab. 88:1809-1814. [DOI] [PubMed] [Google Scholar]
- 26.Kottilil, S., T. W. Chun, S. Moir, S. Liu, M. McLaughlin, C. W. Hallahan, F. Maldarelli, L. Corey, and A. S. Fauci. 2003. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J. Infect. Dis. 187:1038-1045. [DOI] [PubMed] [Google Scholar]
- 27.Loza, M. J., and B. Perussia. 2004. The IL-12 signature: NK cell terminal CD56+high stage and effector functions. J. Immunol. 172:88-96. [DOI] [PubMed] [Google Scholar]
- 28.Manaster, I., S. Mizrahi, D. Goldman-Wohl, H. Y. Sela, N. Stern-Ginossar, D. Lankry, R. Gruda, A. Hurwitz, Y. Bdolah, R. Haimov-Kochman, S. Yagel, and O. Mandelboim. 2008. Endometrial NK cells are special immature cells that await pregnancy. J. Immunol. 181:1869-1876. [DOI] [PubMed] [Google Scholar]
- 29.Marechal, V., F. Arenzana-Seisdedos, J.-M. Heard, and O. Schwartz. 1999. Opposite effects of SDF-1 on human immunodeficiency virus type 1 replication. J. Virol. 73:3608-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montoya, C. J., P. A. Velilla, C. Chougnet, A. L. Landay, and M. T. Rugeles. 2006. Increased IFN-gamma production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin. Immunol. 120:138-146. [DOI] [PubMed] [Google Scholar]
- 31.Mselle, T. F., S. K. Meadows, M. Eriksson, J. M. Smith, L. Shen, C. R. Wira, and C. L. Sentman. 2007. Unique characteristics of NK cells throughout the human female reproductive tract. Clin. Immunol. 124:69-76. [DOI] [PubMed] [Google Scholar]
- 32.Oberlin, E., A. Amara, F. Bachelerie, C. Bessia, J. L. Virelizier, F. Arenzana-Seisdedos, O. Schwartz, J. M. Heard, I. Clark-Lewis, D. F. Legler, M. Loetscher, M. Baggiolini, and B. Moser. 1996. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382:833-835. [DOI] [PubMed] [Google Scholar]
- 33.Ochiel, D. O., J. V. Fahey, M. Ghosh, S. N. Haddad, and C. R. Wira. 2008. Innate immunity in the female reproductive tract: role of sex hormones in regulating uterine epithelial cell protection against pathogens. Curr. Women's Health Rev. 4:102-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piot, P., M. Bartos, P. D. Ghys, N. Walker, and B. Schwartlander. 2001. The global impact of HIV/AIDS. Nature 410:968-973. [DOI] [PubMed] [Google Scholar]
- 35.Robertson, M. J., and J. Ritz. 1990. Biology and clinical relevance of human natural killer cells. Blood 76:2421-2438. [PubMed] [Google Scholar]
- 36.Satoh-Takayama, N., C. A. J. Vosshenrich, S. Lesjean-Pottier, S. Sawa, M. Lochner, F. Rattis, J.-J. Mention, K. Thiam, N. Cerf-Bensussan, O. Mandelboim, G. Eberl, and J. P. Di Santo. 2008. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 6:958-970. [DOI] [PubMed] [Google Scholar]
- 37.Scott-Algara, D., L. X. Truong, P. Versmisse, A. David, T. T. Luong, N. V. Nguyen, I. Theodorou, F. Barre-Sinoussi, and G. Pancino. 2003. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 171:5663-5667. [DOI] [PubMed] [Google Scholar]
- 38.Sirianni, M. C., G. De Sanctis, B. Macchi, S. Soddu, F. Ensoli, F. Aiuti, and L. Fontana. 1988. Natural killer activity from normal peripheral blood lymphocytes against a human T lymphotropic retrovirus type III (HTLV-III)-infected cell line. Diagn. Clin. Immunol. 5:297-303. [PubMed] [Google Scholar]
- 39.Timonen, T. 1997. Natural killer cells: endothelial interactions, migration, and target cell recognition. J. Leukoc. Biol. 62:693-701. [DOI] [PubMed] [Google Scholar]
- 40.Trinchieri, G. 1989. Biology of natural killer cells. Adv. Immunol. 47:187-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vacca, P., C. Cantoni, C. Prato, E. Fulcheri, A. Moretta, L. Moretta, and M. C. Mingari. 2008. Regulatory role of NKp44, NKp46, DNAM-1 and NKG2D receptors in the interaction between NK cells and trophoblast cells. Evidence for divergent functional profiles of decidual versus peripheral NK cells. Int. Immunol. 20:1395-1405. [DOI] [PubMed] [Google Scholar]
- 42.van den Heuvel, M. J., X. Xie, C. Tayade, C. Peralta, Y. Fang, S. Leonard, V. A. Paffaro, Jr., A. K. Sheikhi, C. Murrant, and B. A. Croy. 2005. A review of trafficking and activation of uterine natural killer cells. Am. J. Reprod. Immunol. 54:322-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward, J. P., M. I. Bonaparte, and E. Barker. 2004. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS 18:1769-1779. [DOI] [PubMed] [Google Scholar]
- 44.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama, W. M., S. Kim, and A. R. French. 2004. The dynamic life of natural killer cells. Annu. Rev. Immunol. 22:405-429. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, T., Y. Li, Y. J. Wang, X. Wang, M. Young, S. D. Douglas, and W. Z. Ho. 2007. Natural killer cell inhibits human immunodeficiency virus replication in chronically infected immune cells. Antivir. Res. 73:132-139. [DOI] [PubMed] [Google Scholar]