Abstract
To reliably infect a primate model for human immunodeficiency virus (HIV), ∼10,000-fold more virus must be delivered vaginally than intravenously. However, the vaginal mechanisms that help protect against HIV are poorly understood. Here, we report that human cervicovaginal mucus (CVM), obtained from donors with normal lactobacillus-dominated vaginal flora, efficiently traps HIV, causing it to diffuse more than 1,000-fold more slowly than it does in water. Lactobacilli acidify CVM to pH ∼4 by continuously producing lactic acid. At this acidic pH, we found that lactic acid, but not HCl, abolished the negative surface charge on HIV without lysing the virus membrane. In contrast, in CVM neutralized to pH 6 to 7, as occurs when semen temporarily neutralizes the vagina, HIV maintained its native surface charge and diffused only 15-fold more slowly than it would in water. Thus, methods that can maintain both a high lactic acid content and acidity for CVM during coitus may contribute to both vaginal and penile protection by trapping HIV before it can reach target cells. Our results reveal that CVM likely plays an important but currently unappreciated role in decreasing the rate of HIV sexual transmission.
Cervicovaginal mucus (CVM) and semen from human immunodeficiency virus (HIV)-infected individuals contain cell-free and cell-associated HIV (8, 25, 26). Both forms of virions are plausible mediators of infection, and both, to be infectious, must penetrate the mucus barrier that coats and adheres to vaginal and penile epithelia during coitus. To the extent that mucus can limit the amount of virus that contacts the epithelium, the mucus layer can reduce the probability of infection. Leukocytes can migrate through neutral mucus (24) but are rapidly immobilized and then killed by mild acidity (pH ≤ 6) (22); leukocytes do not survive in the acidic vagina. However, prior research has not revealed whether cell-free HIV can penetrate human CVM.
To directly determine whether cell-free HIV can diffuse through CVM, we used a HIV virus-like particle (VLP) that was fluorescently labeled internally by incorporation of a green fluorescent protein (GFP)-Vpr fusion (5). For biosafety considerations, the derivative was replication defective and pseudotyped with X4-tropic HIV envelope. We mixed the labeled HIV at minimal dilution (∼3% [vol/vol]) into fresh, undiluted CVM obtained from donors with normal lactobacillus-dominated vaginal flora and observed the translational movements of hundreds of individual HIV virions in each sample using high-resolution multiple-particle tracking (15, 29). CVM from women with healthy vaginal microflora is acidified to pH ∼4 by lactic acid produced continuously by anaerobic metabolism of the lactobacilli (4, 21). However, during coitus, vaginal secretions are temporarily neutralized by the alkaline pH of semen (9, 30). In addition, women with bacterial vaginosis (BV), a condition that leads to a more neutral vaginal pH of ∼5 to 6 (4), are at significantly increased risk of acquiring HIV infection (1, 28). Therefore, we also neutralized CVM samples to pH ∼6 to determine whether the pH may alter the ability of HIV to diffuse through CVM.
MATERIALS AND METHODS
Preparation of fluorescent HIV VLPs.
GFP-Vpr-labeled, pseudotyped HIV was produced by polyethyleneimine transfection of 293T cells with the proviral construct BrudeltaEnv, HXB2 (an X4-tropic envelope expression plasmid), and the plasmid peGFPC3 (Clontech Laboratories, Inc.) containing the entire Vpr coding region fused to the COOH terminus of eGFP (GFP-Vpr). The cells were washed at 16 to 24 h posttransfection, the medium was replaced, and the supernatant containing labeled virus was collected for two or three harvests. Virus was collected and concentrated by ultracentrifugation for 1 h at 32,000 rpm through a 30% sucrose pad and resuspended in Dulbecco's modified Eagle's medium at an eightfold concentration. All virus preparations were assayed for infectivity using Ghost indicator cells, and the GFP-Vpr incorporation was assessed by p24CA staining of virions bound to glass using a monoclonal antibody against p24 (AG3.0).
Western blot analysis of HIV after exposure to lactic acid.
HIV virions (BaL strain), generated by transfection of 293T cells, were mixed with phosphate-buffered saline (PBS) (Cellgro, Manassas, VA) at pH 7.4, PBS acidified to pH 4 with HCl, or PBS with a final concentration of either 0.3% or 1.0% lactic acid (racemic mixture; Sigma, St. Louis, MO) at pH 4 or pH 7. Following treatment (a 60-min exposure at 37°C), the viral suspensions were layered onto a 20% sucrose cushion, pelleted by ultracentrifugation at 148,862 × g for 1 h, resuspended in Laemmli buffer (Bio-Rad, Hercules, CA) with β-mercaptoethanol, and boiled for 5 min. The concentrated viral lysates were resolved using a 10% polyacrylamide gel and transferred for Western blot analysis. ID6 antibody was used to identify the gp120 protein and AG3.0 antibody to identify the p24 protein (both monoclonal antibodies were provided by the NIH AIDS Reagent Repository). Viral proteins were visualized with secondary antibodies (IRDye800-conjugated goat anti-mouse immunoglobulin G [Li-Cor Biosciences, Lincoln, NE]) using a Li-Cor Odyssey Infrared Imaging System (Li-Cor Biotechnology, Lincoln, NE).
Collection of human CVM.
CVM samples were obtained by a self-sampling method (3, 15) under an Institutional Review Board-approved protocol. Briefly, the device was inserted into the vagina for 30 s, removed, and placed into a 50-ml centrifuge tube. Samples were centrifuged at 200 × g for 2 min to collect the secretions. All mucus samples studied were from donors between 18 and 30 years old. Women from all races and ethnicities were invited to donate. Gram-stained swab samples were examined to ensure that the CVM samples had normal, lactobacillus-dominated flora (Nugent score, 0 or 1). Only nonovulatory mucus was used in this study; ovulatory mucus was visually identified by examining the spinnbarkeit of the sample, which increases significantly during ovulation. All mucus samples were kept at 4°C prior to use (always within a few hours of collection).
Multiple-particle tracking of HIV in CVM.
HIV virions were added to ∼30 μl of CVM at a 3% (vol/vol) ratio, placed in a custom-made glass chamber (with or without pH adjustment), and incubated for 1 h prior to microscopy. For pH-neutralized CVM, <3% (vol/vol) 3 to 5N NaOH was added to the CVM and incubated for 1 h prior to the addition of virions. Mucus samples were gently mixed during neutralization to minimize any perturbations to the mucus structure. The initial and final pHs of all samples were confirmed by blotting a small mucus sample onto pH paper. The translational motions of fluorescent HIV virions were recorded using an electron-multiplying charge-coupled device camera (Cascade II: 512, Photometrics, Tucson, AZ) mounted on an inverted epifluorescence microscope (3-I Marianas; Zeiss, Thornwood, NY) equipped with a 100× oil immersion objective (numerical aperture, 1.3). Movies were captured with Slidebook 4.2 Advanced Imaging Software (Olympus Soft Imaging Corp., Lakewood, CO) at a temporal resolution of 66.7 ms for 20 s. The translational motions were analyzed using Metamorph software (Universal Imaging Corp., Downington, PA), as described previously (15, 29). Six independent experiments with CVM from different donors (n ≈ 100 virions per experiment) were performed for each condition. The tracking resolution was 10 nm, as determined by tracking particles immobilized with a strong adhesive (15, 29).
Laser Doppler anemometry.
The surface charges of HIV virions, suspended in 10 mM NaCl with and without HCl or 0.3% (wt/wt) lactic acid at pH 4.0 or 7.0, were measured in triplicate with a 633-nm He-Ne laser using a Nanosizer ZS90 (Malvern Instruments, Southborough, MA) according to the manufacturer's instructions.
RESULTS AND DISCUSSION
Acidic CVM traps HIV, but neutral CVM does not.
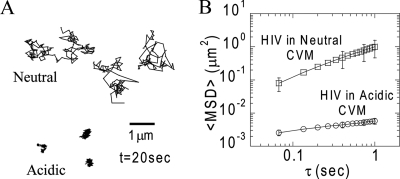
Fresh, acidic CVM efficiently trapped HIV, as indicated by the highly constrained, non-Brownian nature of the time-lapse traces of individual virions (Fig. 1A) and as shown in Movie S1 in the supplemental material. Polystyrene nanoparticles trapped permanently in mucus by strong polyvalent adhesive interactions with mucin fibers exhibit similar time-lapse traces (15). The rates of virion diffusion through mucus were quantified by measurement of time scale (τ)-dependent ensemble geometric mean square displacements (<MSD>) (Fig. 1B). The averaged values of the effective diffusivity (Deff) for HIV in acidic mucus samples from six different donors, calculated based on the formula <MSD> = 4Deff τ, were less than ∼10−3 μm2/s at a time scale of 1 s.
FIG. 1.
Transport of GFP-tagged HIV VLPs in CVM. (A) Sample 20-s trajectories of HIV VLPs, with mean square displacement values within 1 standard error of the ensemble mean square displacement in neutral and acidic CVM samples. (B) Arithmetic averages of ensemble geometric mean square displacement (<MSD>) as a function of τ. The data represent six experiments with CVM samples from different donors for acidic and neutral mucus (n ≈ 100 VLPs per experiment). The error bars indicate standard errors.
In contrast, in the same CVM samples neutralized to pH ∼6 with NaOH (at minimal dilution [<3%]) prior to the addition of virions, HIV diffused over large distances (Fig. 1A; see Movie S2 in the supplemental material), with an average Deff of 0.25 μm2/s. The diffusion constant for a particle of the same size as HIV (120-nm diameter) in water is ∼3.5 μm2/s; thus, while acidic CVM slows the diffusion of HIV by more than 1,000-fold, neutral CVM slows HIV diffusion by a factor of only ∼15. These results indicate that HIV is trapped in CVM acidified by lactobacilli but only somewhat slowed in neutralized CVM, such as CVM in contact with semen. Because CVM is rapidly and continuously acidified by lactic acid from lactobacilli, HIV may also become increasingly trapped in CVM over time.
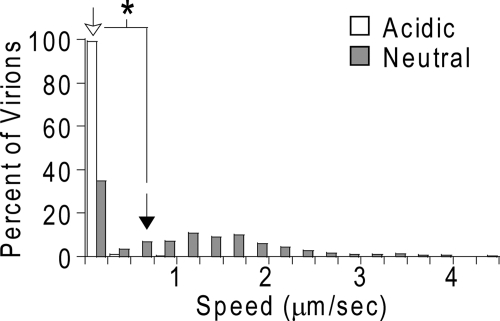
In a study based on tissue culture (19), Maher et al. observed HIV virions present in the neutral mucus overlying the epithelium and suggested that neutral mucus may trap HIV, contrary to our current findings. The speed distributions of individual HIV virions over a time interval of 1 s are shown in Fig. 2 for both acidic and neutralized CVM. The relatively high speeds exhibited by the majority of HIV virions in neutral CVM suggest that HIV accumulation in the neutral mucus in tissue culture is not due to HIV trapping, but rather, is caused by weak adhesive interactions that “partition” HIV into neutral mucus. The low-affinity interactions are not strong enough to permanently trap or immobilize HIV and instead only lower the rate of HIV diffusion, as is evident from images in the same study by Maher et al. showing that virions bound to and penetrated beneath the underlying epithelium. In contrast, every HIV particle in the present study was trapped in acidic CVM, as indicated in Fig. 2. This efficient trapping of HIV in acidic mucus most likely reduces the fraction of HIV particles that could penetrate the acidic mucus barrier and reach target cells located in the epithelium (20, 27).
FIG. 2.
Distributions of the average speeds of individual HIV VLPs, as a percentage of the total number of HIV VLPs, in acidic and neutral CVM at a time scale of 1 s. The geometric mean of the distributions is represented by an empty arrow for HIV VLPs in acidic CVM and by a filled arrow for HIV VLPs in neutral CVM. The asterisk represents statistical significance with a one-sided Student t test (P ≤ 0.05).
HIV is trapped by acidic CVM by mucoadhesion.
By fitting the <MSD> of HIV particles versus τ to the equation <MSD> = 4D0 τα, where D0 is the time-dependent diffusion constant, one can obtain an average value for α (the slope of the curve on a log-log scale) that provides insight into the extent of impediment to HIV motion (α = 1 for purely Brownian, unobstructed diffusion; α < 1 for hindered motion) (15). In neutral CVM, HIV diffused essentially unobstructed, with an average α of 0.91 (Fig. 1B). In contrast, the average α of HIV was 0.29 in acidic CVM (Fig. 1B), and no HIV particle exhibited freely diffusive behavior. The low α value for HIV in acidic CVM is comparable to that measured in control experiments for polystyrene particles that are permanently trapped in CVM; the α values for 100- and 200-nm polystyrene particles were 0.16 and 0.36, respectively (15). The highly hindered motions of these synthetic particles were likely due in part to the thermal motions of the mucin fibers to which the particles were bound. Thus, in acidic CVM, HIV exhibited hindered motions characteristic of particles firmly attached to mucin fibers. It is unlikely that the hindered motions of HIV can be attributed to steric hindrance from the mucus mesh, since we have recently shown that nonmucoadhesive synthetic particles up to 500 nm in diameter, more than three times larger than HIV, diffuse through acidic CVM at rates only fourfold lower than in water (15-17). Together, these observations suggest that HIV is trapped in the acidic CVM gel by mucoadhesion.
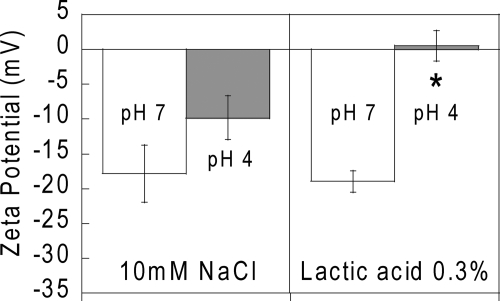
Lactic acid at pH 4 abolishes the negative surface charge of HIV.
To determine whether acidity alone may cause HIV to become immobilized in CVM or whether lactic acid may have a specific effect, we used laser Doppler anemometry to measure the surface charge (zeta potential) of HIV exposed to acidity alone (HCl, pH 4) and to a low but physiologically relevant lactic acid concentration of 0.3% at both pH 7 and pH 4. The results are shown in Fig. 3. In both pH 7 and pH 4 lactic acid-free solutions, the zeta potential of HIV was negative, as expected, since the viral envelope is derived from negatively charged mammalian cell membranes. HIV in pH 7 lactic acid also exhibited a negative surface charge similar to that in pH 7 lactic acid-free solutions. However, lactic acid at pH 4 abolished the negative surface charge. Since lactate ions are themselves negatively charged at pH 4, this major change in the HIV surface charge suggests that lactic acid at pH 4 may alter HIV surface protein structures and/or possibly inactivate the virus by disrupting the envelope membrane and exposing the capsid.
FIG. 3.
Zeta potentials of HIV VLPs incubated in 0.3% (wt/wt) lactic acid or 10 mM NaCl at pH 4.0 and 7.0.
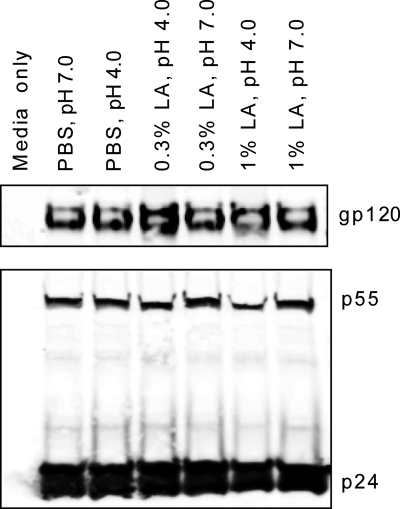
To determine whether the acidic pH of CVM or the presence of lactic acid might be disrupting the virions, we used Western blot analysis to examine the capsid (p24, the mature product of the Gag polyprotein) and envelope (gp120, the CD4 binding glycoprotein) content of infectious HIV-BaL exposed to either 0.3% or 1.0% lactic acid in PBS for 1 h at both pH 4.0 and 7.0. Neutral and pH 4.0 PBS were included as controls. As shown in Fig. 4, no changes in the p24 and gp120 contents in the viral particles were observed after exposure to lactic acid at either acidic or neutral pH, suggesting that the virus remained intact after exposure to the lactic acid-rich and low-pH environments associated with CVM from women with healthy vaginal microflora. Whatever surface alteration lactic acid causes at pH 4, it allows stronger adhesive interactions to take place between HIV and the negatively charged gel-forming mucins, thereby trapping HIV. It remains unclear whether the alteration by lactic acid varies for different envelope isolates, since we studied only a single CXCR4 (T-cell-tropic) isolate here. Future studies will compare the effects of lactic acid on different envelope isolates of HIV (e.g., multiple X4 and R5 isolates) to determine whether lactic acid may be a useful natural protective agent to include in vaginal microbicides. The fact that CVM acidified with lactic acid traps HIV also suggests that other types of sexually transmitted viruses might also be trapped.
FIG. 4.
Western blot detection of HIV type 1 envelope and capsid proteins in virions. Infectious viruses were exposed for 1 h to 0.3% or 1% lactic acid (LA) solution in PBS at pH 4.0 or pH 7.0. The virions were then concentrated by ultracentrifugation through a 20% sucrose cushion and analyzed by Western blotting using anti-gp120 (ID6) and anti-p24 (AG3.0) antibodies.
The distinct surface charge and transport behavior of HIV due to differences in pH and lactic acid content may have important implications for women with BV (and their sexual partners), since their CVM secretions are typically more pH neutral. BV has been identified as an important factor in the spread of the HIV pandemic (1, 28). Several mechanisms have been proposed to explain how BV might make women more susceptible, and perhaps also more infectious, for HIV transmission (2, 10, 13), but the actual mechanisms have yet to be identified. CVM from women with BV is thin and watery, with significantly decreased viscoelasticity (18, 23), as well as a more neutral pH and a lower lactic acid concentration (4). Based on our findings in neutralized CVM, it is likely that BV increases the otherwise low efficiency of HIV transmission by allowing HIV to diffuse rapidly through the watery and less acidic CVM from women with BV, thereby increasing the fraction of virions that reach the underlying epithelial surface (including female-to-male transmission).
Our findings also suggest that HIV has evolved to diffuse most rapidly in healthy human CVM buffered by semen, as occurs during heterosexual transmission. The high speed of virions in neutral CVM may potentially support the observation that HIV transmission is not reduced in macaques by douching within 30 to 60 min with HIV-inactivating agents (12, 31), which suggests that HIV might quickly traverse the pH-neutral mucus layer of female macaques and establish focal infections within susceptible resident cells in the reproductive tissues.
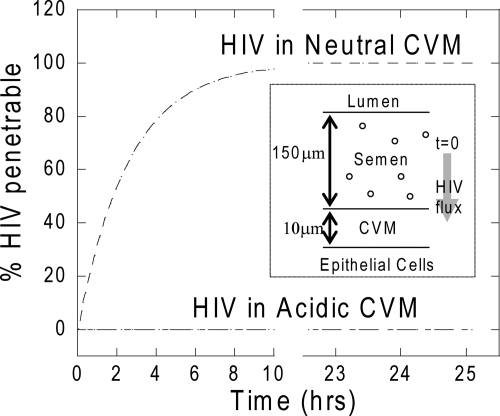
Despite the relatively high speed of HIV in neutralized human CVM, HIV is still slowed ∼15-fold in CVM compared to water. This reduction in HIV flux may enhance protection by other factors in mucus, such as SLPI (11) and defensins (7), and may partially account for the low efficiency of heterosexual HIV transmission (less than one transmission is likely to occur in 200 to 2,000 coital acts) (6, 32). Over time, further trapping of HIV in the CVM-semen mixture, due to reacidification of CVM by lactobacilli, may further facilitate complete HIV inactivation before infection can occur. Moreover, most HIV particles shed into CVM appear to be inactivated or otherwise noninfectious, since HIV is usually not detectable by culture even in vaginal secretions that contain high levels of HIV RNA detected by PCR (14). Nevertheless, our results suggest that methods capable of maintaining both a high lactic acid content and acidity for CVM during and after coitus may strongly limit the fraction of HIV particles capable of penetrating the CVM layer (Fig. 5) and therefore reduce the rate of HIV transmission.
FIG. 5.
Estimated fraction of the initial HIV dose present in a semen layer that could penetrate across a layer of neutral or acidic CVM and reach the underlying epithelium over time. The speeds of individual HIV particles were obtained from particle-tracking results, and the fraction of particles that reached the epithelial cell layer was estimated using Fick's law. (Inset) Schematic of the model, in which a layer of HIV-containing semen sits on top of a layer of unstirred, “firmly adherent” CVM overlying the cervicovaginal epithelium.
Supplementary Material
Acknowledgments
This study was supported in part by NIH grants to J.H. (R21AI079740 and P01 HL51811), R.C. (5U01AI066726 and R21AI079740), S.S. (T32AI060523), and T.J.H. (AI067854) and by an NSF graduate research fellowship (Y.-Y.W.).
We thank Thomas R. Moench for helping initiate this collaborative study and for helpful comments. We also thank German Drazer for helpful discussions.
Footnotes
Published ahead of print on 19 August 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Atashili, J., C. Poole, P. M. Ndumbe, A. A. Adimora, and J. S. Smith. 2008. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 22:1493-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbes, C., and S. Boris. 1999. Potential role of lactobacilli as prophylactic agents against genital pathogens. AIDS Patient Care STDS 13:747-751. [DOI] [PubMed] [Google Scholar]
- 3.Boskey, E. R., T. R. Moench, P. S. Hees, and R. A. Cone. 2003. A self-sampling method to obtain large volumes of undiluted cervicovaginal secretions. Sex. Transm. Dis. 30:107-109. [DOI] [PubMed] [Google Scholar]
- 4.Boskey, E. R., K. M. Telsch, K. J. Whaley, T. R. Moench, and R. A. Cone. 1999. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 67:5170-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell, E. M., O. Perez, M. Melar, and T. J. Hope. 2007. Labeling HIV-1 virions with two fluorescent proteins allows identification of virions that have productively entered the target cell. Virology 360:286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, M. S. 2007. Preventing sexual transmission of HIV. Clin. Infect. Dis. 45(Suppl 4):S287-S292. [DOI] [PubMed] [Google Scholar]
- 7.Cole, A. M., and A. L. Cole. 2008. Antimicrobial polypeptides are key anti-HIV-1 effector molecules of cervicovaginal host defense. Am. J. Reprod. Immunol. 59:27-34. [DOI] [PubMed] [Google Scholar]
- 8.Coombs, R. W., P. S. Reichelderfer, and A. L. Landay. 2003. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS 17:455-480. [DOI] [PubMed] [Google Scholar]
- 9.Fox, C. A., S. J. Meldrum, and B. W. Watson. 1973. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J. Reprod. Fertil. 33:69-75. [DOI] [PubMed] [Google Scholar]
- 10.Hillier, S. L. 1998. The vaginal microbial ecosystem and resistance to HIV. AIDS Res. Hum. Retrovir. 14(Suppl 1):S17-S21. [PubMed] [Google Scholar]
- 11.Hocini, H., P. Becquart, H. Bouhlal, H. Adle-Biassette, M. D. Kazatchkine, and L. Belec. 2000. Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clin. Diagn. Lab. Immunol. 7:515-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klebanoff, S. J., and R. W. Coombs. 1991. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J. Exp. Med. 174:289-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacs, A., S. S. Wasserman, D. Burns, D. J. Wright, J. Cohn, A. Landay, K. Weber, M. Cohen, A. Levine, H. Minkoff, P. Miotti, J. Palefsky, M. Young, and P. Reichelderfer. 2001. Determinants of HIV-1 shedding in the genital tract of women. Lancet 358:1593-1601. [DOI] [PubMed] [Google Scholar]
- 15.Lai, S. K., D. E. O'Hanlon, S. Harrold, S. T. Man, Y. Y. Wang, R. Cone, and J. Hanes. 2007. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA 104:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai, S. K., Y. Y. Wang, R. Cone, D. Wirtz, and J. Hanes. 2009. Altering mucus rheology to “solidify” human mucus at the nanoscale. PLoS One 4:e4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai, S. K., Y. Y. Wang, and J. Hanes. 2009. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 61:158-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai, S. K., Y. Y. Wang, D. Wirtz, and J. Hanes. 2009. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 61:86-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maher, D., X. Wu, T. Schacker, J. Horbul, and P. Southern. 2005. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc. Natl. Acad. Sci. USA 102:11504-11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, C. J., and R. J. Shattock. 2003. Target cells in vaginal HIV transmission. Microbes Infect. 5:59-67. [DOI] [PubMed] [Google Scholar]
- 21.Moller, B. R., and P. Kaspersen. 1991. The acidity of the vagina, p. 63-67. In B. Horowitz and P. A. Mare (ed.), Vaginitis and vaginosis. Wiley-Liss, New York, NY.
- 22.Olmsted, S. S., K. V. Khanna, E. M. Ng, S. T. Whitten, O. N. Johnson III, R. B. Markham, R. A. Cone, and T. R. Moench. 2005. Low pH immobilizes and kills human leukocytes and prevents transmission of cell-associated HIV in a mouse model. BMC Infect. Dis. 5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olmsted, S. S., L. A. Meyn, L. C. Rohan, and S. L. Hillier. 2003. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sex. Transm. Dis. 30:257-261. [DOI] [PubMed] [Google Scholar]
- 24.Parkhurst, M. R., and W. M. Saltzman. 1994. Leukocytes migrate through three-dimensional gels of midcycle cervical mucus. Cell Immunol. 156:77-94. [DOI] [PubMed] [Google Scholar]
- 25.Pilcher, C. D., H. C. Tien, J. J. Eron, Jr., P. L. Vernazza, S. Y. Leu, P. W. Stewart, L. E. Goh, and M. S. Cohen. 2004. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J. Infect. Dis. 189:1785-1792. [DOI] [PubMed] [Google Scholar]
- 26.Quayle, A. J., C. Xu, K. H. Mayer, and D. J. Anderson. 1997. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J. Infect. Dis. 176:960-968. [DOI] [PubMed] [Google Scholar]
- 27.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25-34. [DOI] [PubMed] [Google Scholar]
- 28.Sobel, J. D. 2005. What's new in bacterial vaginosis and trichomoniasis? Infect. Dis. Clin. N. Am. 19:387-406. [DOI] [PubMed] [Google Scholar]
- 29.Suh, J., M. Dawson, and J. Hanes. 2005. Real-time multiple-particle tracking: applications to drug and gene delivery. Adv. Drug Deliv. Rev. 57:63-78. [DOI] [PubMed] [Google Scholar]
- 30.Tevi-Benissan, C., L. Belec, M. Levy, V. Schneider-Fauveau, A. Si Mohamed, M. C. Hallouin, M. Matta, and G. Gresenguet. 1997. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin. Diagn. Lab. Immunol. 4:367-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veazey, R. S., R. J. Shattock, M. Pope, J. C. Kirijan, J. Jones, Q. Hu, T. Ketas, P. A. Marx, P. J. Klasse, D. R. Burton, and J. P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343-346. [DOI] [PubMed] [Google Scholar]
- 32.Wawer, M. J., R. H. Gray, N. K. Sewankambo, D. Serwadda, X. Li, O. Laeyendecker, N. Kiwanuka, G. Kigozi, M. Kiddugavu, T. Lutalo, F. Nalugoda, F. Wabwire-Mangen, M. P. Meehan, and T. C. Quinn. 2005. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 191:1403-1409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.