Abstract
Theiler's murine encephalomyelitis virus (TMEV)-induced immune-mediated demyelinating disease in susceptible mouse strains has been extensively investigated as a relevant model for human multiple sclerosis. Previous investigations of antiviral T-cell responses focus on immune responses to viral capsid proteins, while virtually nothing is reported on immune responses to nonstructural proteins. In this study, we have identified noncapsid regions recognized by CD4+ T cells from TMEV-infected mice using an overlapping peptide library. Interestingly, a greater number of CD4+ T cells recognizing an epitope (3D21-36) of the 3D viral RNA polymerase, in contrast to capsid epitopes, were detected in the CNS of TMEV-infected SJL mice, whereas only a minor population of CD4+ T cells from infected C57BL/6 mice recognized this region. The effects of preimmunization and tolerization with these epitopes on the development of demyelinating disease indicated that capsid-specific CD4+ T cells are protective during the early stages of viral infection, whereas 3D21-36-specific CD4+ T cells exacerbate disease development. Therefore, protective versus pathogenic CD4+ T-cell responses directed to TMEV appear to be epitope dependent, and the differences in CD4+ T-cell responses to these epitopes between susceptible and resistant mice may play an important role in the resistance or susceptibility to virally induced demyelinating disease.
Although the cause of human multiple sclerosis (MS) is unknown, one or multiple infectious agents may be involved in the initial infliction of tissue damage leading to autoimmunity. A possible viral association with MS is suggested by epidemiological studies (1, 8, 44), as well as the detection of viral antigens and virus-specific antibodies in the majority of MS patients (44). Intracerebral infection of the BeAn strain of Theiler's murine encephalomyelitis virus (TMEV) into susceptible mouse strains induces a progressive demyelinating disease that is similar to a form of MS (25). In addition, various immunological and genetic factors that affect the disease outcome in TMEV-infected mice closely parallel those associated with the development of MS (22). Furthermore, recent studies suggest that TMEV is an emerging human viral group (6, 24). Therefore, TMEV-induced demyelinating disease (TMEV-IDD) is an attractive and relevant infectious model for studying the underlying mechanisms of MS.
Previous immunological studies with susceptible SJL mice suggested that a Th1 response to viral capsid proteins is involved in the pathogenesis of demyelination (19, 36, 49, 50). The major population of Th cells specific for TMEV during the course of disease essentially recognizes three predominant viral epitopes (VP1233-250, VP274-86, and VP324-37), one each on the external capsid proteins (11, 48, 49). However, a recent study indicated that <4% of the CD4+ T cells in the central nervous systems (CNS) of TMEV-infected susceptible SJL/J (SJL) mice are reactive to viral capsid epitopes, whereas >40% are reactive in resistant C57BL/6 (B6) mice (31). Nevertheless, the level of overall CD4+ T-cell infiltrating the CNS is significantly higher in susceptible SJL mice, suggesting either that the majority of CD4+ T cells in SJL mice are nonfunctional or reactive to epitopes derived from noncapsid viral proteins.
In addition to CD4+ T-cell responses, CD8+ T cells also play important roles in the protection from and development of TMEV-IDD. Resistance to TMEV-IDD has been closely associated with the major histocompatibility complex (MHC) class I genetic locus (27, 40), suggesting that class I-restricted CD8+ T cells may also be involved in protection and/or pathogenesis. Furthermore, highly susceptible SJL mice show viral persistence in the CNS (4, 26, 42), whereas resistant B6 mice efficiently clear the virus (38). Since the MHC class I-restricted CD8+ T-cell population is mainly associated with viral clearance, this T-cell type appears to confer protection from TMEV-induced demyelination in resistant strains (9, 32, 34, 37, 41, 43). However, the role of CD8+ T cells in the development of clinical disease (i.e., waddling gait and eventual paralysis) remains unresolved (2, 32, 39). The majority of CNS-infiltrating CD8+ T cells (50 to 70%) from B6 mice recognize VP2121-130 (3, 7), while two minor populations (<10%) react with VP2165-173 and VP3110-120 capsid epitopes (28). Similarly, three capsid epitopes recognized by CNS-infiltrating CD8+ T cells of virus-infected SJL mice were identified (17); i.e., VP3159-166, VP3173-181, and VP111-20. However, the overall magnitude of a CD8+ T-cell response is threefold lower in susceptible SJL mice than in resistant B6 mice at the peak of virus-specific immune responses (29). Therefore, it is conceivable that a lower magnitude of virus-specific CD8+ T cells in SJL mice, especially during the early stages of viral infection, may be associated with susceptibility to TMEV-IDD.
Despite extensive investigations of virus-specific CD4+ and CD8+ T-cell responses toward capsid proteins in TMEV-infected resistant B6 and susceptible SJL mice, virtually nothing is known about T-cell responses to noncapsid viral proteins. Low levels of capsid-specific CD4+ T cells are detected in the CNS of TMEV-susceptible SJL mice. Therefore, the identification of noncapsid epitopes recognized by this T-cell population may provide an important clue for the role of CD4+ T cells in protection and/or the pathogenesis of demyelination. TMEV, like other picornaviruses, is encapsulated with capsid proteins, whereas noncapsid proteins are expressed only during viral replication in infected cells. Consequently, the majority of epitopes recognized by immune responses are located on their capsid proteins (46). However, it was previously shown that hosts infected with foot-and-mouth disease virus, a picornavirus, stimulate T cells that are reactive to noncapsid proteins, including RNA polymerase 3D (5, 10). In addition, T cells reactive to internal nucleoproteins or core proteins of the CNS-infiltrating influenza virus and mouse hepatitis virus, respectively, were shown to play important roles in the protection and pathogenesis of demyelination (21, 47). Therefore, investigations of the role of T cells specific for noncapsid TMEV epitopes in virus-infected susceptible mice would be informative in understanding the underlying pathogenic and/or protective mechanisms associated with antiviral T-cell responses.
In the present study, we generated a 20mer overlapping peptide library covering the noncapsid region of the TMEV genome encoded by P2 and P3 regions and identified the regions recognized by T cells from virus-infected susceptible SJL and resistant B6 mice. Our results indicate that an N-terminal region (3D21-36) of viral RNA polymerase bears a predominant epitope recognized by CD4+ T cells infiltrating the CNS of TMEV-infected SJL mice, which far exceeds the level of capsid-specific CD4+ T cells observed. Interestingly, a minor CD4+ T-cell population in TMEV-infected B6 mice recognizes the same region. Further studies indicate that 3D-specific CD4+ T cells are pathogenic, whereas capsid-specific CD4+ T cells are protective during the early stage of viral infection. Therefore, different levels of protective versus pathogenic CD4+ T-cell responses to TMEV between susceptible SJL and resistant B6 mice may critically affect their resistance or susceptibility to virally induced demyelinating disease.
MATERIALS AND METHODS
Animals.
Female SJL/J and C57BL/6 mice were purchased from the Charles River Laboratories (Charles River, MA) through the National Cancer Institute (Frederick, MD). All mice were housed at the Center for Comparative Medicine Facility of Northwestern University, and procedures approved by the Northwestern University Animal Care and Use Committee were used in the present study.
Synthetic peptides and antibodies.
All synthetic peptides were purchased from Genmed Synthesis (San Francisco, CA). All peptide stocks (2 mM) were dissolved in 8% dimethyl sulfoxide in phosphate-buffered saline (PBS). All antibodies used for flow cytometry were purchased from BD Pharmingen (San Diego, CA).
TMEV propagation and infection of mice with virus.
The BeAn strain of TMEV was propagated in BHK cells grown in Dulbecco modified Eagle medium supplemented with 7.5% donor calf serum. For intracerebral infection, 30 μl of TMEV BeAn was injected into the right cerebral hemisphere of 6- to 8-week-old mice anesthetized with isoflurane. Intracerebral injection with 106 PFU consistently induces chronic gait abnormalities and neurologic signs in >90% of SJL/J mice. Clinical symptoms of disease were assessed weekly on the following grading scale: grade 0, no clinical signs; grade 1, mild waddling gait; grade 2, moderate waddling gait and hind-limb paresis; grade 3, severe hind-limb paralysis; grade 4, severe hind-limb paralysis and loss of righting reflex; and grade 5, death.
Isolation of CNS-infiltrating lymphocytes.
Mice were perfused through the left ventricle with 30 ml of sterile Hanks balanced salt solution (HBSS). Excised brains and spinal cords were forced through wire mesh and incubated at 37°C for 45 to 60 min in 250 μg of collagenase type 4 (Worthington Biochemical Corp., Lakewood, NJ)/ml. CNS-infiltrating lymphocytes were then enriched from the bottom one-third of continuous 100% Percoll (Pharmacia, Piscataway, NJ) gradient after centrifugation for 30 min at 27,000 × g.
Plaque assay.
After cardiac perfusion with cold HBSS, brains and spinal cords were removed. The tissues were homogenized in PBS as a 10% (wt/vol) solution by using a tissue homogenizer and clarified by low-speed centrifugation (600 × g). A standard plaque assay was performed on BHK-21 cell monolayers (38). Plaques in the BHK monolayer were visualized by staining with 0.1% crystal violet solution after fixing with methanol.
ELISPOT assay.
To enumerate gamma interferon (IFN-γ)-producing cells, enzyme-linked immunospot (ELISPOT) plates (Millipore, Bedford, MA) were precoated with 1 to 5 μg of anti-IFN-γ antibody/ml in 0.05 M carbonate buffer (pH 9.6). Plates were incubated with 2 × 104 CNS mononuclear cells plus 106 irradiated (3,000 rads) syngeneic spleen cells or 106 splenocytes from infected mice alone in 200 μl of HL-1 medium (BioWhittaker, Walkersville, MD) for 18 h at 37°C in the presence of 2 μM peptide. After a washing step, plates were incubated with biotin-conjugated anti-IFN-γ antibody (Endogen, Boston, MA) overnight. Spots were developed after incubation with streptavidin-horseradish peroxidase for 3 h using 3-amino-9-ethyl-carbazole (Sigma, St. Louis, MO) in 0.05 M sodium acetate buffer (45).
Proliferation assay.
T-cell proliferation was determined using splenocytes from control or virus-infected SJL mice. Single-cell suspensions of splenocytes (106/well) in RPMI medium supplemented with 5 × 10−5 M 2-mercaptoethanol and 0.5% normal syngeneic mouse serum were cultured for 72 h in the presence of 2 μM peptides. Approximately 18 h after the addition of 1 μCi of [3H]TdR (Amersham, Arlington Heights, IL)/well, the cells were harvested and [3H]TdR incorporation was determined in a liquid scintillation counter. The results were expressed as the Δcpm (i.e., the mean counts per minute of experimental stimulated cultures after subtraction of the background count with PBS) ± the standard error of the mean from triplicate cultures.
Cytokine assay.
The levels of IFN-γ (Pharmingen), interleukin-13 (IL-13), and IL-17 (R&D Systems, Inc., Minneapolis, MN) were assessed by using specific enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions. Supernatants of triplicate splenocyte cultures incubated with appropriate peptides for 3 days were measured for cytokine levels.
Intracellular cytokine staining.
Freshly isolated CNS mononuclear cells were cultured in 96-well round-bottom plates in the presence of relevant or control peptide and Golgi-Plug for 6 h at 37°C. Cells were then incubated in 50 μl of 2.4G2 hybridoma (American Type Culture Collection) supernatant for 20 min at 4°C to block the Fc receptors. Allophycocyanin-conjugated anti-CD8 (clone 53-6.7) or anti-CD4 (GK1.5) antibody diluted in 50 μl of 2.4G2 supernatant was added, and the cells were incubated for an additional 30 min at 4°C. After two washes, intracellular IFN-γ staining was performed according to the manufacturer's instructions (Pharmingen) using phycoerythrin-labeled rat monoclonal anti-IFN-γ antibody (XMG1.2) or isotype control (rat immunoglobulin G1). Cells were analyzed on a Becton Dickinson FACSCalibur flow cytometer. Live cells were gated based on light scatter properties.
Immunization of mice with epitope peptides.
Mice were subcutaneously immunized 7 days prior to intracerebral viral infection near the base of the tail with 25 μg of mixture of peptides representing three predominant capsid epitopes (VP1233-250, VP274-86, and VP324-37) or noncapsid epitopes (3D21-36, 3D6-23, and 3D412-430), which were emulsified 1:1 in complete Freund adjuvant (CFA; Difco, Detroit, MI).
Real-time PCR.
Total RNA isolated using TRIzol (Invitrogen) was reverse transcribed to cDNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen). The cDNAs were amplified with specific primer sets in iCycler SYBR green I Mastermix using iCycler (Bio-Rad). The sense and antisense primer sequences used for cytokines are as follows: IFN-γ, 5′-ACT GGC AAA AGG ATG GTG AC-3′ and 5′-TGA GCT CAT TGA ATG CTT GG-3′; IL-17A, 5′-CTC CAG AAG GCC CTC AGA CTA C-3′ and 5′-AGC TTT CCC TCC GCA TTG ACA CAG-3′; IL-22, 5′-TGC TCA ACT TCA CCC TGG AAG ACA-3′ and 5′-AGA AGG CAG GAA GGA GCA GTT CTT-3′; VP1, 5′-TGA CTA AGC AGG ACT ATG CCT TCC-3′ and 5′-CAA CGA GCC ACA TAT GCG GAT TAC-3′; and GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-AAC TTT GGC ATT GTG GAA GGG CTC-3′ and 5′-TGC CTG CTT CAC CAC CTT CTT GAT-3′. GAPDH expression was assessed as an internal reference for normalization. Relative gene expression levels were expressed as the fold increases versus the medium-treated control samples. Every real-time PCR was performed in triplicate.
Immune suppression by peptide-conjugated splenocytes.
To induce specific immune suppression, ECDI [1-ethyl-3-(3-dimethyl-aminopropyl)-carbodiimide HCl]-mediated peptide-conjugated splenocytes were prepared as previously described (12, 20, 36). After removal of red blood cells by treatment with Tris-NH4Cl, splenocytes from naive SJL mice (3 × 108 cells/ml) were conjugated with the mixtures of capsid or noncapsid peptides (1 mg/ml) for 1 h at 0°C in the presence of 30 mg of ECDI (Calbiochem, La Jolla, CA)/ml. After three washes with HBSS, 5 × 107 ECDI-conjugated splenocytes in 100 μl of PBS were injected intravenously. At 8 days after the cell transfer, recipient mice were infected with TMEV and analyzed at the indicated time points.
Statistical analyses.
Significant differences (two-tailed P value) between control and experimental animal groups were analyzed by unpaired Student t test using InStat Program (GraphPAD Software, San Diego, CA). Differences in the disease course between experimental groups were determined by paired two-tailed t test analysis, with the Welch correction. P values of < 0.05 were considered significant. For multigroup comparisons, one-way analysis of variance was used, followed by a Tukey-Kramer multiple-comparison test.
RESULTS
CD4+ T cells in TMEV-infected SJL and B6 mice recognize nonstructural epitopes.
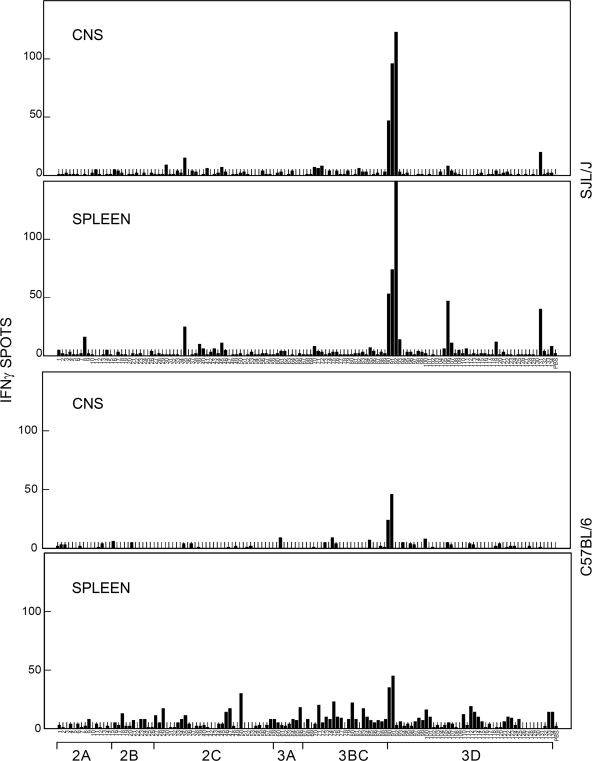
It was previously shown that the proportion of capsid-reactive CD4+ T cells in the CNS of TMEV-infected susceptible SJL mice is 10-fold lower than that of resistant B6 mice, despite the higher level of overall CD4+ T-cell infiltration (31). To examine the possibility that the majority of CD4+ T cells in the CNS of infected SJL mice recognize noncapsid protein epitopes, we generated a 10mer-overlapping 20mer peptide library covering the entire noncapsid viral proteins. The reactivity of CNS-infiltrating and splenic T cells of virus-infected SJL or B6 mice was determined by using ELISPOT assays based on their ability to produce IFN-γ (Fig. 1). The N-terminal region of viral 3D (RNA polymerase) protein was the prominent epitope recognized by T cells in the CNS and spleens of SJL mice. Splenic T cells recognized additional regions at lower degrees. In contrast to the high T-cell reactivity to the 3D epitope in SJL mice, a relatively low level of T cells of the CNS of B6 mice recognized this epitope. Splenic T cells from TMEV-infected B6 mice reacted in response to scattered regions at low levels. These results strongly suggest that the majority of T cells from SJL mice, as well as a minor T-cell population from B6 mice, recognize an identical N-terminal 3D region covered by three consecutive peptides.
FIG. 1.
Assessment of IFN-γ-producing cell numbers in the spleen and CNS of TMEV-infected SJL and B6 mice, in response to a 20mer peptide library covering P2 and P3 regions. CNS-infiltrating lymphocytes were isolated from mice at 7 days after TMEV infection. IFN-γ-producing cells from CNS-infiltrating lymphocytes were enumerated by ELISPOT assay after 18 h of incubation with 106 irradiated syngeneic spleen cells (3,000 rads) and peptides. IFN-γ-producing cells from the spleen were similarly assessed after stimulation with the peptides. The numbers of IFN-γ spots represent the numbers of IFN-γ-secreting cells from either 2 × 104 CNS-infiltrating mononuclear cells or 106 splenocytes pooled from three virus-infected mice. A representative result from two separate experiments is shown here. The background spots with PBS were 2 ± 1.
CD4+ T cells reactive to 3D21-36 are predominant in the CNS of TMEV-infected SJL mice.
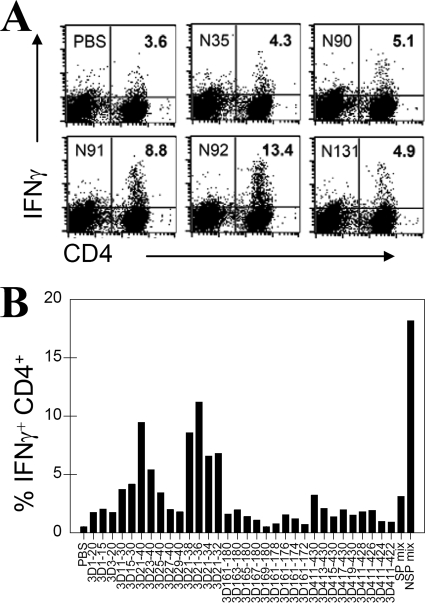
The predominance of CD4+ T cells reactive to the N92 peptide, representing the 3D21-40 region of RNA polymerase, was confirmed by using flow cytometry of IFN-γ-producing cells upon stimulation with the peptides and further identified as CD4+ T cells (Fig. 2A). The reactivity of CD4+ T cells toward 3D21-40 was most prominent, constituting as many as 13.4% of CNS-infiltrating CD4+ T cells at 8 days postinfection. To determine whether this region represents a single or multiple epitopes, additional smaller peptides within this region were used to stimulate CNS-infiltrating CD4+ T cells (Fig. 2B). Our results indicate that a single predominant epitope region (3D21-36) is responsible for the majority of CD4+ T-cell activation. Minor populations of CD4+ T cells were stimulated with 2C81-100, 3D1-15, 3D163-180, and 3D411-430 regions. Interestingly, the proportion of CNS-infiltrating CD4+ T cells reactive to noncapsid epitopes in TMEV-infected SJL mice was as many as sixfold greater than those reactive to the major capsid epitopes (VP1233-250, VP274-86, and VP324-37).
FIG. 2.
Further definition of the major epitopes in 3D protein. (A) CD4+ T cells producing IFN-γ upon stimulation with peptides were verified by flow cytometry after intracellular staining of pooled CNS mononuclear cells from five mice. The proportion of IFN-γ-producing CD4+ T cells is shown here. The level of IFN-γ producing CD8+ T cells was undetectable (not shown). (B) To further define the epitope regions of 3D recognized by CNS-infiltrating CD4+ T cells, 3D peptides truncated at their N and C termini were assessed for their ability to stimulate IFN-γ production. Activation of CD4+ T ells (from five mice) was analyzed by flow cytometry after intracellular staining of IFN-γ and CD4. The data are representative of three separate experiments. The backgrounds for all individual assays were <10% of the spots at the maximal stimulation.
The hierarchy of epitope-reactive CD4+ T cells in the periphery and CNS of SJL mice is maintained during the course of TMEV infection.
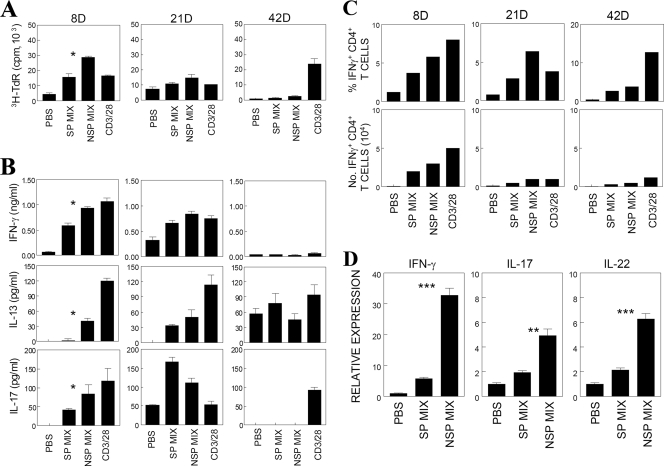
Response levels to capsid and noncapsid epitopes by peripheral and CNS-infiltrating CD4+ T cells in virus-infected SJL mice were further assessed (Fig. 3). Both proliferation and cytokine production (IFN-γ, IL-13, and IL-17) by splenic T cells were significantly higher in response to the noncapsid epitope than those to capsid epitopes on day 8 postinfection (Fig. 3A and B). The predominance of CD4+ T cells reactive to noncapsid epitopes was similarly maintained in the CNS of TMEV-infected SJL mice (Fig. 3C). To further determine the relative levels of CNS-infiltrating Th subtypes, Th1 and Th17 cytokine mRNAs induced upon stimulation with capsid or noncapsid epitopes for 6 h were assessed by using real-time PCR (Fig. 3D). The levels of both Th1 (IFN-γ) and Th17 cytokine (IL-17 and IL-22) mRNA expression were relatively higher after stimulation with noncapsid epitopes than after stimulation with capsid epitopes. These results indicate that CD4+ T-cell responses toward noncapsid proteins are significantly greater than those to capsid proteins in both the periphery and CNS of susceptible SJL mice during TMEV infection.
FIG. 3.
Peripheral and CNS CD4+ T-cell responses during the course of viral infection. (A) Proliferative responses of splenic T cells from mice at 8, 21, and 42 days postinfection (abbreviated as “8D”, etc., in this and subsequent figure panels) after stimulation for 3 days with PBS, capsid peptides (SP mix), noncapsid peptides (NSP mix), or anti-CD3/antiCD28 antibodies. SP mix: 2 μM concentrations of VP1233-250, VP274-86, and VP324-37. NSP mix: 2 μM concentrations of 3D6-23, 3D21-36, and 3D412-430. The results of a triplicate analysis of a single representative experiment out of three separate experiments is shown. (B) Cytokine levels (IFN-γ, IL-13, and IL-17) were measured from the supernatants of splenic cultures that were derived from mice at 8, 21, and 42 days postinfection. Cytokine levels in the supernatants were assessed with ELISA. The values given are the means ± the standard deviations of triplicate wells. (C) Enumeration of IFN-γ-producing CD4+ T cells in the CNS of mice during the course of TMEV infection. IFN-γ-producing cells were examined by flow cytometry after intracellular cytokine staining following stimulation with capsid peptides, noncapsid peptides, or anti-CD3/CD28 antibodies. A representative experimental result out of three similar experiments is shown. (D) Cytokine mRNA expression levels in CNS-infiltrating T cells specific to SP or NSP epitopes of TMEV were analyzed by quantitative PCR. CNS-infiltrating mononuclear cells isolated from TMEV-infected SJL mice at 8 days postinfection were stimulated with a PBS, an SP, or an NSP epitope mix (2 μM for each peptide) for 6 h without additional antigen-presenting cells. The relative cytokine expression is expressed as the fold induction after normalization to GAPDH mRNA. The results in panel D represent triplicate analysis of a single representative experiment. Similar patterns of cytokine gene expression were observed from two additional experiments.
SJL mice preimmunized with noncapsid peptides exacerbate the development of demyelinating disease.
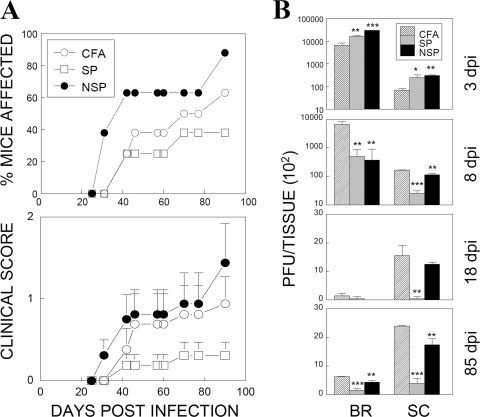
CNS-infiltrating CD4+ T cells from TMEV-infected SJL mice preferentially recognize noncapsid epitopes (Fig. 2), whereas those from resistant B6 mice recognize capsid epitopes (31). Therefore, we further examined the possibility that CD4+ T cells reactive to noncapsid epitopes early during viral infection are pathogenic, while those reactive to capsid epitopes are protective. This possibility was tested by comparing the disease course of SJL mice immunized with either CFA alone or CFA together with capsid or noncapsid peptides prior to TMEV infection (Fig. 4A). As previously shown (31), SJL mice preimmunized with a mixture of capsid epitopes resulted in a delayed and reduced development of demyelinating disease relative to control CFA-preimmunized mice (Fig. 4A). Surprisingly, mice preimmunized with noncapsid (3D) epitopes showed an exacerbated development of disease. Furthermore, viral persistence in the CNS of mice preimmunized with noncapsid epitopes was significantly greater than the persistence observed in mice preimmunized with capsid epitopes (Fig. 4B). In contrast, viral clearance was more efficient in the spinal cord of mice preimmunized with capsid epitopes compared to the brain. The virus titers were 10-fold lower in the spinal cords of capsid- versus noncapsid-immunized groups throughout the course of 8 to 85 days postinfection. Consistent with this finding, SJL mice that adoptively received capsid epitope-primed CD4+ T cells prior to viral infection showed a reduced viral load in the CNS compared to control mice that received sham-primed (CFA alone) CD4+ T cells (data not shown). These results strongly suggest that the presence of CD4+ T cells specific for capsid epitopes during early infection helps to clear virus from the CNS and ultimately provides protection against the development of demyelinating disease. However, CD4+ T cells reactive to noncapsid epitopes may not be able to provide such protection and instead contribute to the pathogenesis of demyelinating disease.
FIG. 4.
Altered TMEV-induced demyelinating disease courses in mice primed with capsid and noncapsid epitope peptides. (A) Frequency and severity of demyelinating disease development in mice preimmunized with CFA alone (n = 8), CFA plus capsid peptides (n = 8), or CFA plus noncapsid peptides (n = 8) at 7 days prior to TMEV infection. Statistical differences in disease levels between CFA and peptide-CFA groups over the course of a 42- to 90-day infection were analyzed by using a paired two-tailed t test. The incidence and severity of disease between CFA and SP-CFA groups were significantly different (P < 0.01 and P < 0.001, respectively). The differences between CFA and NSP-CFA groups were also significant (P < 0.001 and P = 0.01, respectively). To reflect the overall disease severity of each group, we included every mouse within the experimental group, regardless of disease development, in calculating disease severity scores. Error bars represent standard deviations of the group. (B) Levels of infectious virus recovered from the brains (BR) and spinal cords (SC) of experimental groups were determined by plaque assays over a course (3, 8, 18, and 85 days postinfection [dpi]) of a viral infection. Pooled CNS tissues from three to five mice per group were used due to the logistics of the plaque assays comparing different groups. The statistical significance of the differences between CFA and peptide-CFA groups were analyzed by using the Student t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Immunization with capsid epitopes induces higher peripheral immune responses than immunization with noncapsid epitopes.
To correlate the protective versus the pathogenic effects of preimmunization with respective capsid or noncapsid peptides, we examined splenic T-cell proliferative responses in the periphery after TMEV infection (Fig. 5). Although T-cell proliferation in mice immunized with CFA alone was somewhat elevated on days 21 and 42 postinfection compared to mice infected with TMEV without immunization, peptide-immunized mice showed higher levels of T-cell proliferation in response to the respective preimmunizing epitopes (Fig. 5A). The cytokine levels (IFN-γ, IL-13, and IL-17) produced during in vitro stimulation correlated with the levels of T-cell proliferation (Fig. 5B). Despite this correlation, IL-17 levels produced by T cells from noncapsid epitope-immunized mice were significantly higher throughout the viral infection compared to the levels produced by T cells from capsid-immunized mice. The significance of this difference is not clear at this time. However, it is interesting to speculate that high levels of the cytokine produced by noncapsid-reactive T cells may contribute to the pathogenesis of demyelination.
FIG. 5.
Analyses of peripheral immune responses in TMEV-infected mice primed with capsid and noncapsid peptides. (A) Splenic T-cell proliferative responses to capsid and noncapsid epitopes in mice unprimed or primed with CFA alone, capsid epitopes plus CFA, or noncapsid epitopes plus CFA were assessed at 8, 21, and 42 days (d) postinfection. (B) Cytokine levels (IFN-γ, IL-13, and IL-17) in the culture supernatants of splenocytes from primed mice at 8, 21, and 42 days postinfection were assessed by ELISA. Differences in the cytokine production between primed and unprimed mice were analyzed by using the Student t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Elevated T-cell responses corresponding to preimmunized epitopes are detected in the CNS of SJL mice.
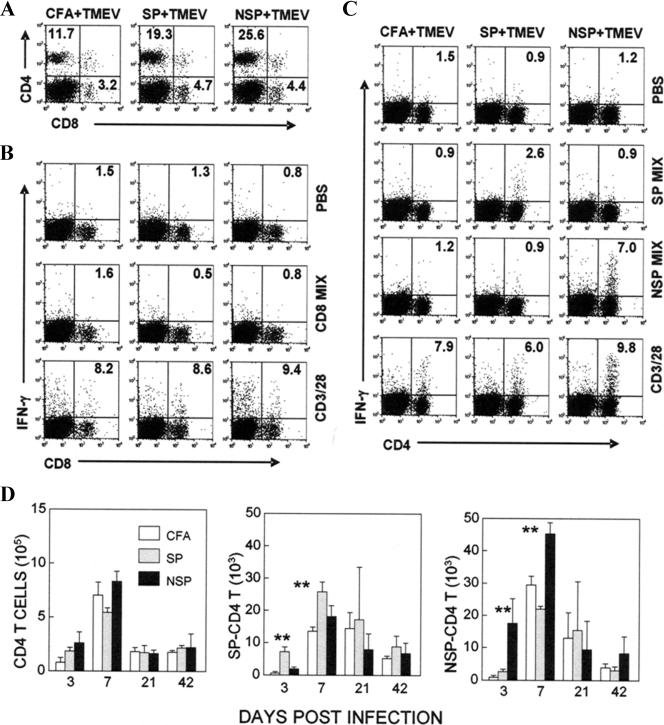
The levels of CNS-infiltrating T-cell responses were also examined in these mice (Fig. 6). During the early stage of viral infection (3 days), CD4+ T-cell infiltration to the CNS and proportion of CD4+ T cells specific for capsid epitopes were significantly elevated (>5-fold) in mice preimmunized with capsid epitopes compared to those of control CFA-immunized mice (Fig. 6A and C). Similarly, the level of CD4+ T cells specific to noncapsid epitopes was increased (>6-fold) in mice preimmunized with noncapsid epitope peptides (Fig. 6A and C). However, virus-specific CD8+ T cells were undetectable in these mice at this early stage of viral infection (Fig. 6B). These results indicate that such preimmunization induces epitope-specific immune responses, resulting in elevated levels of epitope-specific CD4+ T cells infiltrating the CNS at the early stage of viral infection. The initial accumulation of epitope-specific CD4+ T cells in the CNS was maintained (7 to 21 days postinfection) prior to the onset of demyelinating disease but no longer sustained after the development of disease (Fig. 6D). These results indicate that preimmunization with capsid or noncapsid epitope peptides induces elevated CD4+ T-cell responses to the corresponding epitopes in the CNS during the early stage of viral infection, similarly to those in the periphery.
FIG. 6.
Levels of CNS-infiltrating T-cell responses in mice primed with capsid and noncapsid peptides. (A) Levels of T-cell infiltration to the CNS of mice primed with mock, capsid, and noncapsid epitopes at 3 days postinfection are shown as a representative fluorescence-activated cell sorting profile. (B) Levels of IFN-γ-producing CD8+ T cells infiltrating the CNS at 3 days postinfection were analyzed after stimulation with a mixture of CD8 epitope peptides (VP3159-166, VP3173-181, and VP111-20) by flow cytometry in combination with intracellular staining. (C) Levels of IFN-γ-producing CD4+ T cells in the CNS were similarly determined after stimulation with PBS, capsid, or noncapsid peptides. (D) The levels of T-cell infiltration and epitope-specific T cells in the CNS during the course of viral infection were determined by intracellular IFN-γ staining. Differences in the cell numbers between primed and unprimed mice were analyzed by using the Student t test. **, P < 0.01.
Disease development is reduced in mice with low CD4+ T-cell responses to noncapsid epitopes.
If CD4+ T-cell responses to noncapsid epitopes play a major role in the pathogenesis of demyelinating disease, we speculated that SJL mice lacking anti-CD4+ T-cell responses to noncapsid epitopes might be resistant to the development of disease. To ascertain this possibility, syngeneic splenocytes conjugated to capsid or noncapsid epitope peptides were administered to SJL mice prior to viral infection to induce specific suppression in their CD4+ T-cell responses (Fig. 7), as previously described (12, 18). Mice receiving epitope-conjugated splenocytes showed a suppression of CD4+ T-cell responses, while no alterations were found in CD8+ T-cell responses (Fig. 7A). Disease incidence and development of clinical symptoms were very similar between mice receiving sham-conjugated and capsid-epitope-conjugated splenocytes (Fig. 7B). However, the development of demyelinating disease in mice receiving noncapsid epitope-conjugated splenocytes was significantly delayed and the clinical severity was significantly reduced, compared to other groups (Fig. 7B). To further correlate the altered disease development in these mice with histopathology, demyelination levels of spinal cords from mice receiving sham or epitope-conjugated splenocytes at 80 days postinfection were examined after staining with Luxol-fast blue (Fig. 7C). Myelin stripping was predominant within the white matter of spinal cords in mice receiving sham- or capsid-epitope-conjugated splenocytes, whereas only limited myelin damage was observed in the spinal cords of mice receiving noncapsid-epitope-conjugated splenocytes. These results strongly suggest that suppression of CD4+ T-cell responses to noncapsid epitopes inhibits demyelination in the CNS, leading to the curtailed development of demyelinating disease.
FIG. 7.
Effects of specific immune suppression induced with peptide-conjugated splenocytes on disease development. (A) CNS-infiltrating T cells specific for viral epitopes in mice treated with sham, SP or NSP-conjugated splenocytes at 7 days postinfection. (B) Courses of disease development in the recipient mice (n = 10 per group) of sham- or peptide-treated splenocytes. The statistical differences in disease levels between sham and peptide groups during 21 to 63 days postinfection were analyzed by using a paired two-tailed t test. The difference between groups receiving sham- and SP-treated splenocytes was not significant. Experimental groups receiving sham- or SP-treated splenocytes and NSP-treated splenocytes were significantly different (P < 0.001). (C) Representative histologic examination of spinal cords from each experimental group. Adjacent sections of spinal cords from mice at 80 days postinfection were stained with hematoxylin-eosin or Luxol-fast blue. Scale bar, 100 μm. (D) The levels of viral persistence in the CNS of each group were measured by using real-time PCR at 8, 21, and 80 days postinfection (DPI). Triplicate analysis of cDNAs prepared from pooled brains and spinal cords (three to five mice/group) was performed. Similar patterns were observed in a separate PCR and plaque assay experiment.
We further determined viral persistence levels in the CNS of each group at 8, 21, and 80 days postinfection by using real-time PCR in order to correlate them with the development of demyelinating disease (Fig. 7D). Viral persistence in the CNS of mice receiving noncapsid-conjugated splenocytes was significantly lower than that of mice receiving sham- or capsid-conjugated splenocytes at the onset of disease development (21 days postinfection). However, viral persistence in mice receiving capsid-conjugated splenocytes was uniquely elevated at the late stage of viral infection (80 days postinfection), suggesting that CD4+ T-cell responses to capsid epitopes are critical in controlling prolonged viral persistence in the CNS. Taken together, our results indicate that early CD4+ T-cell responses to capsid epitopes are protective, while those directed to noncapsid epitopes promote the pathogenesis of demyelinating disease in susceptible SJL mice.
DISCUSSION
Recent studies demonstrated that the level of viral capsid-specific CD4+ T cells in the CNS of TMEV-infected susceptible SJL mice is significantly (∼10-fold) lower than that in resistant B6 mice (31). However, the number of overall CD4+ T cells infiltrating the CNS is significantly higher in susceptible SJL mice, suggesting that CD4+ T cells in the CNS are either anergized or reactive to noncapsid viral proteins. To examine this possibility, we assessed the reactivity of CNS-infiltrating T cells to noncapsid epitopes in TMEV-infected SJL and B6 mice. In the present study, we have shown that the majority of antiviral CD4+ T cells accumulating in the CNS of TMEV-infected SJL mice recognize an epitope region (3D21-36) of RNA polymerase, in contrast to the overall capsid-reactive CD4+ T cells (Fig. 1 to 3). Interestingly, only a minor CD4+ T-cell population, equivalent to a fraction of capsid-reactive CD4+ T cells, is reactive to the same 3D region in resistant B6 mice. This finding is not surprising since CD4+ T cells from mice with different MHC can recognize the same core epitope region (15, 30). Thus, the main difference in viral epitope-reactive CD4+ T cells between resistant B6 and susceptible SJL mice appears to be their relative proportion of capsid versus noncapsid reactive CD4+ T cells. These results pose an interesting possibility that the high level of noncapsid-reactive CD4+ T cells in susceptible mice may function as a major pathogenic factor in the development of TMEV-induced demyelinating disease.
Further studies with epitope-primed susceptible SJL mice indicated that noncapsid-specific CD4+ T cells do not provide efficient protection, but rather promote the pathogenesis of demyelinating disease (Fig. 4). In contrast, capsid-specific CD4+ T cells provide the protection during the early stage of viral infection. Although noncapsid epitope-primed mice showed a minimal reduction of viral persistence in the CNS, capsid epitope-primed mice exhibited a significant reduction, which corresponded to their protective effects against the disease development. This protective role of capsid-specific CD4+ T cells during early stages of TMEV-infection is consistent with previous studies (31). In addition, mice adoptively receiving capsid epitope-primed CD4+ T cells, but not noncapsid epitope-primed CD4+ T cells, displayed reduced viral loads in the CNS (results not shown). Thus, the inferior ability of predominant noncapsid-reactive CD4+ T cells to clear the virus from the CNS in susceptible SJL mice may permit early establishment of viral persistence, which is a critical susceptibility factor in developing demyelinating disease.
However, exacerbation of demyelinating disease in noncapsid epitope-primed mice during early stages of viral infection is unexpected, in light of the partial protection against foot-and-mouse disease virus, a member of picornavirus, after vaccination with recombinant vaccinia virus containing 3D protein (10). The exacerbation of disease in these mice may not be entirely attributable to ineffective viral clearance by noncapsid-specific CD4+ T cells, since virus levels in the CNS of these mice do not exceed the level of sham-primed control mice (Fig. 4). In addition, mice suppressed in their CD4+ T-cell responses to noncapsid epitopes showed a delayed development of demyelinating disease and reduced severity compared to sham-suppressed mice or mice suppressed in their capsid-reactive CD4+ T cells (Fig. 7). These results suggest that noncapsid-reactive CD4+ T cells play a greater pathogenic role in the development of TMEV-induced demyelinating disease compared to capsid-reactive CD4+ T cells. We have recently demonstrated that treatment of SJL mice with anti-IL-17 antibody inhibits the development of TMEV-induced demyelinating disease (13), strongly suggesting that Th17 cells play a pathogenic role. However, it would be very difficult to discern how much is contributed by capsid-specific Th17 cells versus noncapsid specific Th17 cells, since these cells coexist after viral infection. Th1 and Th17 cytokine gene expression by the CNS infiltrating cells upon stimulation with capsid or noncapsid epitopes showed that significantly higher levels of both Th1 (IFN-γ) and Th17 cytokine genes (IL-17 and IL-22) are activated in response to noncapsid epitopes compared to those in response to capsid epitopes (Fig. 3D). Therefore, higher levels of Th17 cells are likely to reflect the higher numbers of noncapsid specific CD4+ T cells in the CNS (Fig. 1 and 2). Consequently, the presence of higher levels of noncapsid-specific Th17 cells may exert a greater pathogenic role compared to capsid-specific Th17 cells.
It is not yet clear whether the location of epitopes or the magnitude of CD4+ T-cell responses to individual epitopes determines the observed protective or pathogenic function. It is also conceivable that the epitope location may be associated with the level of CD4+ T-cell responses. It has previously been shown that capsid-specific CD4+ T cells display differential pathogenic roles depending on epitopes recognized within the capsid region after establishment of viral persistence (50). Furthermore, a spontaneously arising nonpathogenic virus, which bears a single amino acid substitution within one of the CD4+ T-cell capsid epitopes, results in CD4+ T-cell responses exhibiting an altered cytokine profile (23, 35). Therefore, both the location of epitopes on capsid or noncapsid proteins and the skewed T-cell cytokine production in response to these epitopes may affect the pathogenesis of demyelinating disease.
The location of epitopes reactive to CD4+ T cells may play a pivotal role in the protection versus pathogenesis of demyelinating disease due to differences in the type of antigen presenting cells. We speculate that the most prominent mechanism for differential protection induced by capsid versus noncapsid epitopes may reflect differences in epitope processing that arise via viral infection (for noncapsid epitopes) or both infection and endocytic processing pathways (for capsid epitopes). These differences may generate higher levels of pathogenic Th17 cells reactive to noncapsid proteins, as recently shown (13). Viral particles containing only capsid proteins are released and spread during viremia at the early peak infection, whereas limited cell types directly supporting viral replication will produce noncapsid proteins. Acutely infected glial cells are expected to express both viral capsid and noncapsid proteins. Professional antigen presenting cells are also permissive to TMEV infection (14, 35). Therefore, professional antigen presenting cells in the lymphoid tissues, such as dendritic cells and macrophages, will be able to present both capsid and noncapsid epitopes to CD4+ T cells during acute infection. Since viral infection compromises the T-cell stimulating function of antigen-presenting cells (14, 35), these professional antigen-presenting cells may have a limited role in virus-specific T-cell stimulation. However, noncapsid epitopes may be primarily presented locally via virus-infected glial cells (16, 33, 42, 51). Since these noncapsid epitope-specific CD4+ T cells appear to be less protective (Fig. 4), the predominant presence of this T-cell population in the CNS may be unable to exert sufficient protection and, perhaps, more vigorously facilitates the pathogenesis of demyelinating disease.
Taken together, our present study indicates that capsid-specific CD4+ T cells provide an effective protective function, whereas noncapsid-reactive CD4+ T cells play a pathogenic role during the early stage of viral infection. Levels of protective versus pathogenic CD4+ T-cell responses to TMEV epitopes are different between susceptible SJL and resistant B6 mice, and this difference may critically affect the resistance or susceptibility to virally induced demyelinating disease. However, the mechanism of preferential CD4+ T-cell responses to noncapsid epitopes over capsid epitopes is unclear at this time. Since only virus-infected cells produce noncapsid proteins during viral replication, differences in the CNS viral load of resistant and susceptible mice may dictate the level of noncapsid proteins produced (i.e., low in resistant C57BL/6 and high in susceptible SJL mice). Further investigation of the mechanisms underlying the differential level and function of CD4+ T cells reactive to capsid versus noncapsid epitopes may elucidate the relationship between antiviral immunity and their involvement of protection and/or pathogenesis of demyelinating disease.
Acknowledgments
This study was supported by grants NS23349 and NS33008 from the National Institutes of Health.
Footnotes
Published ahead of print on 12 August 2009.
REFERENCES
- 1.Allen, I., and B. Brankin. 1993. Pathogenesis of multiple sclerosis: the immune diathesis and the role of viruses. J. Neuropathol. Exp. Neurol. 52:95-105. [DOI] [PubMed] [Google Scholar]
- 2.Begolka, W. S., L. M. Haynes, J. K. Olson, J. Padilla, K. L. Neville, M. Dal Canto, J. Palma, B. S. Kim, and S. D. Miller. 2001. CD8-deficient SJL mice display enhanced susceptibility to Theiler's virus infection and increased demyelinating pathology. J. Neurovirol. 7:409-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borson, N. D., C. Paul, X. Lin, W. K. Nevala, M. A. Strausbauch, M. Rodriguez, and P. J. Wettstein. 1997. Brain-infiltrating cytolytic T lymphocytes specific for Theiler's virus recognize H2Db molecules complexed with a viral VP2 peptide lacking a consensus anchor residue. J. Virol. 71:5244-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bureau, J. F., X. Montagutelli, F. Bihl, S. Lefebvre, J. L. Guenet, and M. Brahic. 1993. Mapping loci influencing the persistence of Theiler's virus in the murine central nervous system. Nat. Genet. 5:87-91. [DOI] [PubMed] [Google Scholar]
- 5.Cedillo-Barron, L., M. Foster-Cuevas, A. Cook, B. Gutierrez-Castaneda, S. Kollnberger, F. Lefevre, and R. M. Parkhouse. 2003. Immunogenicity of plasmids encoding T and B cell epitopes of foot-and-mouth disease virus (FMDV) in swine. Vaccine 21:4261-4269. [DOI] [PubMed] [Google Scholar]
- 6.Chiu, C. Y., A. L. Greninger, K. Kanada, T. Kwok, K. F. Fischer, C. Runckel, J. K. Louie, C. A. Glaser, S. Yagi, D. P. Schnurr, T. D. Haggerty, J. Parsonnet, D. Ganem, and J. L. DeRisi. 2008. Identification of cardioviruses related to Theiler's murine encephalomyelitis virus in human infections. Proc. Natl. Acad. Sci. USA 105:14124-14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dethlefs, S., N. Escriou, M. Brahic, S. van der Werf, and E. L. Larsson-Sciard. 1997. Theiler's virus and Mengo virus induce cross-reactive cytotoxic T lymphocytes restricted to the same immunodominant VP2 epitope in C57BL/6 mice. J. Virol. 71:5361-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhib-Jalbut, S., and D. E. McFarlin. 1990. Immunology of multiple sclerosis. Ann. Allergy. 64:433-444. [PubMed] [Google Scholar]
- 9.Fiette, L., C. Aubert, M. Brahic, and C. P. Rossi. 1993. Theiler's virus infection of β2-microglobulin-deficient mice. J. Virol. 67:589-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Briones, M. M., E. Blanco, C. Chiva, D. Andreu, V. Ley, and F. Sobrino. 2004. Immunogenicity and T-cell recognition in swine of foot-and-mouth disease virus polymerase 3D. Virology 322:264-275. [DOI] [PubMed] [Google Scholar]
- 11.Gerety, S. J., W. J. Karpus, A. R. Cubbon, R. G. Goswami, M. K. Rundell, J. D. Peterson, and S. D. Miller. 1994. Class II-restricted T-cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T-cell epitope in susceptible SJL/J. mice. J. Immunol. 152:908-918. [PubMed] [Google Scholar]
- 12.Getts, M. T., B. S. Kim, and S. D. Miller. 2007. Differential outcome of tolerance induction in naive versus activated Theiler's virus epitope-specific CD8+ cytotoxic T cells. J. Virol. 81:6584-6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou, W., H. S. Kang, and B. S. Kim. 2009. Th17 cells enhance viral persistence and inhibit T-cell cytotoxicity in a model of chronic virus infection. J. Exp. Med. 206:313-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou, W., E. Y. So, and B. S. Kim. 2007. Role of dendritic cells in differential susceptibility to viral demyelinating disease. PLoS Pathog. 3:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang, Y. S., J. A. Mikszta, and B. S. Kim. 1994. T-cell epitope recognition involved in the low-responsiveness to a region of hen egg lysozyme (46-61) in C57BL/6 mice. Mol. Immunol. 31:803-812. [DOI] [PubMed] [Google Scholar]
- 16.Jin, Y. H., M. Mohindru, M. H. Kang, A. C. Fuller, B. Kang, D. Gallo, and B. S. Kim. 2007. Differential virus replication, cytokine production, and antigen-presenting function by microglia from susceptible and resistant mice infected with Theiler's virus. J. Virol. 81:11690-11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang, B. S., M. A. Lyman, and B. S. Kim. 2002. The majority of infiltrating CD8+ T cells in the central nervous system of susceptible SJL/J. mice infected with Theiler's virus are virus specific and fully functional. J. Virol. 76:6577-6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karpus, W. J., N. W. Lukacs, B. L. McRae, R. M. Strieter, S. L. Kunkel, and S. D. Miller. 1995. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J. Immunol. 155:5003-5010. [PubMed] [Google Scholar]
- 19.Karpus, W. J., J. D. Peterson, and S. D. Miller. 1994. Anergy in vivo: downregulation of antigen-specific CD4+ Th1 but not Th2 cytokine responses. Int. Immunol. 6:721-730. [DOI] [PubMed] [Google Scholar]
- 20.Karpus, W. J., J. G. Pope, J. D. Peterson, M. C. Dal Canto, and S. D. Miller. 1995. Inhibition of Theiler's virus-mediated demyelination by peripheral immune tolerance induction. J. Immunol. 155:947-957. [PubMed] [Google Scholar]
- 21.Kedzierska, K., E. B. Day, J. Pi, S. B. Heard, P. C. Doherty, S. J. Turner, and S. Perlman. 2006. Quantification of repertoire diversity of influenza-specific epitopes with predominant public or private TCR usage. J. Immunol. 177:6705-6712. [DOI] [PubMed] [Google Scholar]
- 22.Kim, B. S., J. P. Palma, A. Inoue, and C. S. Koh. 2000. Pathogenic immunity in Theiler's virus-induced demyelinating disease: a viral model for multiple sclerosis. Arch. Immunol. Ther. Exp. 48:373-379. [PubMed] [Google Scholar]
- 23.Kim, B. S., R. L. Yauch, Y. Y. Bahk, J. A. Kang, M. C. Dal Canto, and C. K. Hall. 1998. A spontaneous low-pathogenic variant of Theiler's virus contains an amino acid substitution within the predominant VP1(233-250) T-cell epitope. J. Virol. 72:1020-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang, Z., A. S. Kumar, M. S. Jones, N. J. Knowles, and H. L. Lipton. 2008. Phylogenetic analysis of the species theilovirus: emerging murine and human pathogens. J. Virol. 82:11545-11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipton, H. L., and M. C. Dal Canto. 1976. Chronic neurologic disease in Theiler's virus infection of SJL/J. mice. J. Neurol. Sci. 30:201-207. [DOI] [PubMed] [Google Scholar]
- 26.Lipton, H. L., J. Kratochvil, P. Sethi, and M. C. Dal Canto. 1984. Theiler's virus antigen detected in mouse spinal cord 2 1/2 years after infection. Neurology 34:1117-1119. [DOI] [PubMed] [Google Scholar]
- 27.Lipton, H. L., and R. Melvold. 1984. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J. Immunol. 132:1821-1825. [PubMed] [Google Scholar]
- 28.Lyman, M. A., H. G. Lee, B. S. Kang, H. K. Kang, and B. S. Kim. 2002. Capsid-specific cytotoxic T lymphocytes recognize three distinct H-2Db-restricted regions of the BeAn strain of Theiler's virus and exhibit different cytokine profiles. J. Virol. 76:3125-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyman, M. A., J. Myoung, M. Mohindru, and B. S. Kim. 2004. Quantitative, not qualitative, differences in CD8+ T-cell responses to Theiler's murine encephalomyelitis virus between resistant C57BL/6 and susceptible SJL/J. mice. Eur. J. Immunol. 34:2730-2739. [DOI] [PubMed] [Google Scholar]
- 30.Mikszta, J. A., Y. S. Jang, and B. S. Kim. 1997. Role of a C-terminal residue of an immunodominant epitope in T-cell activation and repertoire diversity. J. Immunol. 158:127-135. [PubMed] [Google Scholar]
- 31.Mohindru, M., B. Kang, and B. S. Kim. 2006. Initial capsid-specific CD4+ T-cell responses protect against Theiler's murine encephalomyelitisvirus-induced demyelinating disease. Eur. J. Immunol. 36:2106-2115. [DOI] [PubMed] [Google Scholar]
- 32.Murray, P. D., D. B. McGavern, X. Lin, M. K. Njenga, J. Leibowitz, L. R. Pease, and M. Rodriguez. 1998. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J. Neurosci. 18:7306-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palma, J. P., and B. S. Kim. 2001. Induction of selected chemokines in glial cells infected with Theiler's virus. J. Neuroimmunol. 117:166-170. [DOI] [PubMed] [Google Scholar]
- 34.Palma, J. P., H. G. Lee, M. Mohindru, B. S. Kang, M. Dal Canto, S. D. Miller, and B. S. Kim. 2001. Enhanced susceptibility to Theiler's virus-induced demyelinating disease in perforin-deficient mice. J. Neuroimmunol. 116:125-135. [DOI] [PubMed] [Google Scholar]
- 35.Palma, J. P., R. L. Yauch, H. K. Kang, H. G. Lee, and B. S. Kim. 2002. Preferential induction of IL-10 in APC correlates with a switch from Th1 to Th2 response following infection with a low pathogenic variant of Theiler's virus. J. Immunol. 168:4221-4230. [DOI] [PubMed] [Google Scholar]
- 36.Peterson, J. D., W. J. Karpus, R. J. Clatch, and S. D. Miller. 1993. Split tolerance of Th1 and Th2 cells in tolerance to Theiler's murine encephalomyelitis virus. Eur. J. Immunol. 23:46-55. [DOI] [PubMed] [Google Scholar]
- 37.Pullen, L. C., S. D. Miller, M. C. Dal Canto, and B. S. Kim. 1993. Class I-deficient resistant mice intracerebrally inoculated with Theiler's virus show an increased T-cell response to viral antigens and susceptibility to demyelination. Eur. J. Immunol. 23:2287-2293. [DOI] [PubMed] [Google Scholar]
- 38.Pullen, L. C., S. H. Park, S. D. Miller, M. C. Dal Canto, and B. S. Kim. 1995. Treatment with bacterial LPS renders genetically resistant C57BL/6 mice susceptible to Theiler's virus-induced demyelinating disease. J. Immunol. 155:4497-4503. [PubMed] [Google Scholar]
- 39.Rivera-Quinones, C., D. McGavern, J. D. Schmelzer, S. F. Hunter, P. A. Low, and M. Rodriguez. 1998. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat. Med. 4:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez, M., and C. S. David. 1985. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J. Immunol. 135:2145-2148. [PubMed] [Google Scholar]
- 41.Rodriguez, M., A. J. Dunkel, R. L. Thiemann, J. Leibowitz, M. Zijlstra, and R. Jaenisch. 1993. Abrogation of resistance to Theiler's virus-induced demyelination in H-2b mice deficient in β2-microglobulin. J. Immunol. 151:266-276. [PubMed] [Google Scholar]
- 42.Rodriguez, M., J. L. Leibowitz, and P. W. Lampert. 1983. Persistent infection of oligodendrocytes in Theiler's virus-induced encephalomyelitis. Ann. Neurol. 13:426-433. [DOI] [PubMed] [Google Scholar]
- 43.Rossi, C. P., A. McAllister, M. Tanguy, D. Kagi, and M. Brahic. 1998. Theiler's virus infection of perforin-deficient mice. J. Virol. 72:4515-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soldan, S. S., R. Berti, N. Salem, P. Secchiero, L. Flamand, P. A. Calabresi, M. B. Brennan, H. W. Maloni, H. F. McFarland, H. C. Lin, M. Patnaik, and S. Jacobson. 1997. Association of human herpesvirus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 3:1394-1397. [DOI] [PubMed] [Google Scholar]
- 45.Targoni, O. S., and P. V. Lehmann. 1998. Endogenous myelin basic protein inactivates the high avidity T-cell repertoire. J. Exp. Med. 187:2055-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Usherwood, E. J., I. C. Johnston, L. J. Lovelidge, P. Tonks, and A. A. Nash. 1995. Lymphocyte recognition elements on the VP1 protein of Theiler's virus. Immunology 85:190-197. [PMC free article] [PubMed] [Google Scholar]
- 47.Wege, H., A. Schliephake, H. Korner, E. Flory, and H. Wege. 1993. Coronavirus induced encephalomyelitis: an immunodominant CD4+-T cell site on the nucleocapsid protein contributes to protection. Adv. Exp. Med. Biol. 342:413-418. [DOI] [PubMed] [Google Scholar]
- 48.Yauch, R. L., K. Kerekes, K. Saujani, and B. S. Kim. 1995. Identification of a major T-cell epitope within VP3 amino acid residues 24 to 37 of Theiler's virus in demyelination-susceptible SJL/J. mice. J. Virol. 69:7315-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yauch, R. L., and B. S. Kim. 1994. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J. mice infected with Theiler's virus is located within VP1(233-244). J. Immunol. 153:4508-4519. [PubMed] [Google Scholar]
- 50.Yauch, R. L., J. P. Palma, H. Yahikozawa, C. S. Koh, and B. S. Kim. 1998. Role of individual T-cell epitopes of Theiler's virus in the pathogenesis of demyelination correlates with the ability to induce a Th1 response. J. Virol. 72:6169-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng, L., M. A. Calenoff, and M. C. Dal Canto. 2001. Astrocytes, not microglia, are the main cells responsible for viral persistence in Theiler's murine encephalomyelitis virus infection leading to demyelination. J. Neuroimmunol. 118:256-267. [DOI] [PubMed] [Google Scholar]