Abstract
Human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infections impair plasmacytoid dendritic cell (PDC) and natural killer (NK) cell subset numbers and functions, though little is known about PDC-NK cell interactions during these infections. We evaluated PDC-dependent NK cell killing and gamma interferon (IFN-γ) and granzyme B production, using peripheral blood mononuclear cell (PBMC)-based and purified cell assays of samples from HCV- and HIV-infected subjects. CpG-enhanced PBMC killing and IFN-γ and granzyme B activity (dependent on PDC and NK cells) were impaired in viremic HIV infection. In purified PDC-NK cell culture experiments, CpG-enhanced, PDC-dependent NK cell activity was cell contact and IFN-α dependent, and this activity was impaired in viremic HIV infection but not in HCV infection. In heterologous PDC-NK cell assays, impaired PDC-NK cell killing activity was largely attributable to an NK cell defect, while impaired PDC-NK cell IFN-γ-producing activity was attributable to both PDC and NK cell defects. Additionally, the response of NK cells to direct IFN-α stimulation was defective in viremic HIV infection, and this defect was not attributable to diminished IFN-α receptor expression, though IFN-α receptor and NKP30 expression was closely associated with killer activity in viremic HIV infection but not in healthy controls. These data indicate that during uncontrolled HIV infection, PDC-dependent NK cell function is impaired, which is in large part attributable to defective IFN-α-induced NK cell activity and not to altered IFN-α receptor, NKP30, NKP44, NKP46, or NKG2D expression.
Immature dendritic cells (DC) are key innate mediators of the adaptive immune response. Myeloid DC (MDC) and plasmacytoid DC (PDC) have been identified as two main peripheral DC subsets (43). Numerical and functional defects in these populations have been described for both hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infections, with the impairments distinctly different in each infection (3, 12, 19, 26, 33, 48, 53, 56, 57). Natural killer (NK) cells are capable of cytotoxic, cytokine-expressing, and chemokine-expressing functions (31, 38). These lymphocytes are vital during the early stages of mouse hepatic viral infection (22, 42) and during human herpes virus infection (5, 7, 10). NK cell cytotoxic function has long been known to be reduced during chronic HIV infection and in subjects with AIDS (32, 44), and unfractionated cell assays of NK cell function indicate an impaired NK cell response to alpha interferon (IFN-α) (52). Additionally, genetic markers of NK cell phenotype are associated with disease progression rate (36). In contrast, HIV-exposed but uninfected subjects appear to have enhanced NK cell function (45), and after highly active antiretroviral therapy (HAART), NK cell numbers and function appear to normalize (1, 4). In the setting of HCV infection, genetic markers of NK cell phenotype appear to predict the outcome after acute exposure (28). During chronic HCV infection, some studies indicate reduced NK cell cytotoxicity (14, 40, 55), while more-recent studies indicate normal NK cell function and reduced peripheral NK cell numbers (18, 27, 39).
DC-NK cell bidirectional cross talk has recently been shown to play a key role in host defense (20, 30, 34, 35, 37). This cross talk can be facilitated by Toll-like receptor (TLR) signaling and results in NK cell activation, enhanced NK cell effector function, and DC maturation (15, 20, 21, 30, 49). In the setting of viremic HIV infection, recent unfractionated cell system data indicate impairment in PDC-dependent NK cell activity (11). These data may be explained by the previously described numerical defects in PDC or NK cells, though whether there are additional functional defects within these cell populations resulting in impaired interaction is not known.
In this study, we evaluated the effect of chronic HCV infection, viremic HIV infection, and HAART-controlled HIV infection on TLR ligand-activated PDC-dependent NK cell activity in unfractionated and purified cell populations, using direct ex vivo assays. Results indicate that in addition to numerical defects in peripheral PDC and NK cell subsets, there is functional impairment in the PDC-NK cell interaction during viremic HIV infection. This functional impairment is in large part due to reduced NK cell responsiveness to IFN-α and in part due to defective PDC function.
MATERIALS AND METHODS
Study subjects.
Chronic HCV-infected subjects (n = 15 for unfractionated cell assays, and n = 10 for purified cell assays) had detectable serum HCV antibodies for at least 6 months, had HCV RNA detectable by PCR, and were not previously treated for HCV infection. HIV-infected subjects had HIV antibodies detectable by both enzyme-linked immunosorbent assay (ELISA) and Western blotting. Viremic HIV subjects were not on antiretroviral therapy and had HIV detectable by PCR. Aviremic HIV subjects were virally suppressed (had plasma virus undetectable by PCR) as a result of HAART. Healthy control subjects were not infected with HCV or HIV. All study subjects provided written informed consent for venous blood sampling under approval of the institutional review boards for human studies at the Cleveland VA Medical Center and University Hospitals of Cleveland.
Cell isolation.
Peripheral blood mononuclear cells (PBMC) were prepared from fresh peripheral blood specimens, using Ficoll (Fisher Scientific, Hudson, NH). For purified cell assays, PDC were prepared from PBMC by BDCA4-positive bead selection (Miltenyi Biotech, Auburn, CA). For IFN-γ and granzyme B enzyme-linked immunospot (ELISPOT) analysis, NK cells were prepared by the negative selection method, in which CD19-, BDCA1-, and BDCA2-expressing cells (Miltenyi Biotech, Auburn, CA) were first removed, followed by the removal of CD3-, CD4-, and CD14-expressing cells (EasySep; StemCell Technologies, Vancouver, British Columbia, Canada). For heterologous assays, NK cells were prepared by negative selection (depletion of T cells, B cells, stem cells, DC, monocytes, granulocytes, and erythroid cells [Miltenyi Biotech, Auburn, CA]). NK cells used in PCR assays were prepared by negative selection (StemCell Technologies, Vancouver, British Columbia, Canada) (6).
Flow cytometric analysis.
Flow cytometric analysis was performed on a FACSCalibur (Becton Dickinson, San Jose, CA) flow cytometer with CellQuest software (Becton Dickinson) for studies with unfractionated PBMC, or on an LSR II flow cytometer (BD Biosciences, San Jose, CA) with CellQuest software (BD Biosciences) for studies of purified PDC-NK cell activity. PBMC were stained with isotype control, anti-CD3, anti-CD16, anti-CD56, anti-NKG2D, anti-NKP30, anti-NKP44, anti-NKP46 (Becton Dickinson, San Jose, CA), or anti-IFN-αR (R&D Systems, Minneapolis, MN) monoclonal antibodies for 20 min (at room temperature for stains containing NK cell subset markers, and at 4°C for IFN-α receptor stains) and were then washed in phosphate-buffered saline with 0.01% bovine serum albumin. Lymphocytes were identified by forward and side scatter, and NK cell subset frequencies were measured (CD3−CD56+, CD3−CD56dim, CD3−CD56bright, CD3−CD16+, CD3−CD16−CD56+, CD3−CD16+CD56−, CD3−CD16+CD56+, and CD3−CD16+ or CD56+). Proportions of each subset that expressed IFN-αR, NKG2D, NKP30, NKP44, or NKP46, as well as PDC frequency (lineage marker [CD3−, CD14−, CD16−, CD19−, CD20−, and CD56−] HLA-DR+CD123+) and level of expression (mean fluorescence intensity) for each subset, were also determined.
NK cell killing activity.
Unfractionated PBMC were cultured for 20 h in the presence and absence of CpG 2216 (5uM; Integrated DNA Technologies, Inc., Coralville, IA) at 37°C in 24-well culture plates. K562 target cells (ATCC, Manassas, VA) were stained with PKH26 (Sigma, St. Louis, MO) and added to the precultured PBMC at 3:1, 10:1, 30:1, and 50:1 effector-to-target (E:T) ratios and were cultured an additional 2 h at 37°C. Cells were then removed and stained for 15 min at room temperature with annexin V (BD Biosciences Pharmingen, San Diego, CA). Killing activity was measured by quantifying the proportion of PKH26-positive target cells that were annexin V positive by flow cytometric analysis using an LSR II flow cytometer (Becton Dickinson, San Jose, CA).
For purified cell assays, NK cell-to-K562 E:T ratios of 0:1, 3:1, 10:1, and 30:1 were performed with fixed numbers of K562 cells (104/well), and PDC, when included, were at a 1:20 ratio with NK cells (optimized for K562-killing activity). For heterologous assays, PDC and NK cells were obtained from different donors (either viremic HIV or control donors), and each PDC fraction was tested against at least one healthy control and one viremic HIV NK cell fraction, and each NK cell fraction was tested against at least one viremic HIV and one healthy control PDC fraction. Autologous activity assays were performed in parallel to heterologous assays for direct comparison. Assays were performed in granzyme B ELISPOT wells, supernatants were analyzed for IFN-γ by ELISA, and cells were analyzed for annexin V staining as described above.
IFN-γ and granzyme B ELISPOT assays.
ELISPOT assays were used to measure PBMC and NK cell (in the presence or absence of autologous PDC) IFN-γ or granzyme B production in response to stimulation with interleukin-12 ([IL-12] 1 ng/ml), CpG (2216; 5 uM), IFN-α (1,000 units/ml), or IL-12, IL-15, and IL-18 combined (1 ng/ml, 5 ng/ml, and 1.25 μg/ml, respectively). Freshly isolated NK cells (1 × 105 cells/well unless otherwise stated) were plated in duplicate in the presence or absence of freshly isolated autologous PDC (2 × 104 cells/well) in the presence or absence of K562 cells (2 × 104 cells/well unless otherwise stated) onto 96-well IFN-γ or granzyme B ELISPOT plates and cultured for 20 h at 37°C. The cultures were developed and analyzed as previously described (2) for IFN-γ spot-forming frequency and as per the kit instructions for granzyme B (Becton Dickinson, San Jose, CA). In pilot assays, the PDC-to-NK cell ratio was optimized for TLR ligand-dependent, PDC-dependent NK cell IFN-γ activity. For blocking experiments, IL-12 neutralizing antibodies (10 μg/ml; Becton Dickinson, San Jose, CA), IL-12 isotype control antibodies (10 μg/ml; Becton Dickinson, San Jose, CA), IFN-α-blocking antibody (40 μg/ml; PBL Biomedical Laboratories, Piscataway, NJ), or IFN-α isotype control antibody was added in duplicate.
IL-12, IFN-γ, and IFN-α ELISA.
Freshly isolated PBMC (6 × 105 cells/well), NK cells (2 × 105 cells/well), and PDC (5 × 104 cells/well) were suspended in RPMI medium containing 5% human serum,1% l-glutamine, and 1% penicillin-streptomycin and plated in 96-well tissue culture plates. Cell cultures were incubated for 20 h at 37°C, supernatants were removed, and cytokine levels were measured by ELISA (for IL-12 production, R&D Systems, Minneapolis, MN, and for IFN-α production, PBL Biomedical Laboratories, Piscataway, NJ). PDC-NK cell coculture assay supernatants were also analyzed for IFN-γ production by ELISA.
Measurement of HIV-1 DNA by real-time PCR.
NK cells were analyzed for HIV-1 minus-strand strong stop (SS) DNA as previously described (51).
Statistical analysis.
We analyzed the data with SPSS for Windows, version 15.0 (SPSS Inc., Chicago, IL), and Stata/SE, version 9.2 (StataCorp, College Station, TX). We used conventional measures of central tendency and variability to describe the data. To compare continuous and categorical variables across groups, we used the Mann-Whitney U test or Kruskal-Wallis H test and Pearson's chi-square test, respectively. We compared responses before and after stimulation and those with and without target cells with Wilcoxon signed-rank test and assessed the associations between continuous variables by Spearman's rank correlation coefficient. All tests were two-sided, and a P value of ≤ 0.05 was considered significant.
RESULTS
Frequencies of PDC and NK cell subsets in peripheral blood of control, HCV-infected, viremic HIV-infected, and aviremic HIV-infected subjects.
Since NK cell and PDC numerical defects have been previously described for both HCV and HIV infections, to understand the contribution of numerical defects to unfractionated cell activity defects we first looked at the peripheral frequencies of these cells. We evaluated subjects with chronic HCV infection, viremic HIV infection (subjects not on HAART), and aviremic HIV infection (successful viral control with HAART). CD4 counts ranged from 364 to 603 and 236 to 923 cells/μl in viremic HIV-infected and aviremic HIV-infected subjects, respectively (Table 1). All HCV-infected subjects had HCV genotype 1 infections. Consistent with previous literature (3, 11, 48, 53, 56), PDC frequencies were decreased in HCV and viremic HIV subjects compared to those of the controls (0.13% versus 0.25%, P = 0.02, and 0.05% versus 0.25%, P = 0.001, respectively). In viremic HIV-infected subjects, CD3−CD16+CD56+ NK cell subset frequencies were decreased (2.67% versus 3.59%, P = 0.03), while CD3−CD16−CD56+ NK cell subset frequencies were increased compared to those of the controls (2.91% versus 1.15%, P = 0.04). In aviremic HIV-infected subjects, CD3− (CD16+ or CD56+) frequencies were increased compared with those of the controls (11.66% versus 7.52%, P = 0.02).
TABLE 1.
NK and PDC subset frequencies for control, HCV-infected, viremic HIV-infected, and aviremic HIV-infected subjectsa
| Lymphocyte | Median % frequency of lymphocyte gate (interquartile range) for indicated subjects: |
|||
|---|---|---|---|---|
| Controls | HCV-infectedb | Viremic HIV-infectedc,d | Aviremic HIV-infectedc,d | |
| CD3−56+ | 5.98 (4.19-8.34) | 6.34 (6.07-7.80) | 5.98 (3.97-7.69) | 8.64 (6.24-13.81) |
| CD3−56dim | 4.77 (3.55-7.52) | 5.30 (4.37-7.02) | 5.62 (3.62-7.52) | 7.39 (6.02-11.54) |
| CD3−56bright | 1.02 (0.62-1.29) | 1.06 (0.72-1.33) | 0.54 (0.41-1.34) | 1.16 (0.59-2.92) |
| CD3−16+ | 5.53 (4.26-7.10) | 6.94 (5.57-7.74) | 4.50 (2.65-8.71) | 9.12 (3.42-12.53) |
| CD3−16−56+ | 1.15 (0.90-2.93) | 1.60 (0.88-2.17) | 2.91 (2.00-5.46)e | 2.41 (1.47-7.66) |
| CD3−16+56− | 1.18 (0.93-5.02) | 2.08 (0.91-3.13) | 1.38 (0.57-3.56) | 1.47 (0.51-5.39) |
| CD3−16+56+ | 3.59 (3.11-6.03) | 4.74 (3.80-5.28) | 2.67 (1.41-4.21)f | 6.00 (2.91-9.06) |
| CD3−16+ or CD3−56+ | 7.52 (5.02-9.26) | 8.64 (7.35-10.06) | 8.67 (5.87-11.34) | 11.66 (10.60-23.23)g |
| PDC (Lin−DR+123+) | 0.25 (0.13-0.35) | 0.13 (0.10-0.19)h | 0.05 (0.02-0.10)i | 0.14 (0.05-0.22) |
There were 15 subjects per group. Lin, lineage marker; DR, HLA DR.
The median HCV level was 496,252 IU/ml, and the range was 19,882 to 2,622,260 IU/ml. The control, viremic HIV-infected, and aviremic HIV-infected subjects were not tested by quantitative PCR.
The median HIV level for viremic HIV-infected subjects (lower sensitivity level, 50 copies/ml) was 17,600 copies/ml, and the range was 60 to 510,000 copies/ml. For aviremic HIV-infected subjects, the median level was 50 copies/ml, and the range was 50 to 50 copies/ml. The control and HCV-infected subjects were not tested by PCR.
The median number of CD4 cells for viremic HIV-infected subjects was 493 cells/μl, and the range was 364 to 603 cells/μl. For aviremic HIV-infected subjects, the median number was 508 cells/μl, and the range was 236 to 923 cells/μl. The control and HCV-infected subjects were not tested.
P = 0.04 for viremic HIV-infected versus control subjects.
P = 0.03 for viremic HIV-infected versus control subjects.
P = 0.02 for aviremic HIV-infected versus control subjects.
P = 0.02 for HCV-infected versus control subjects.
P = 0.001 for viremic HIV-infected versus control subjects.
CpG-enhanced NK cell-associated PBMC killing activity is impaired in viremic HIV infection.
To measure PDC-NK cell interactions in the most direct ex vivo setting, we first evaluated freshly prepared PBMC for NK cell killing activity of K562 target cells in the presence and absence of the PDC-activating TLR9 ligand class A CpG 2216. We performed assays with increasing numbers of PBMC to achieve different E:T cell ratios. As shown in Fig. 1A, K562 target cell death was substantially enhanced by preculturing healthy control PBMC effectors for 20 h with CpG 2216. This CpG-enhanced activity was dependent on the presence of NK cells and PDC (Fig. 1B), which established this assay as one measuring PDC-dependent, NK cell-dependent, CpG-enhanced killing activity. In parallel, we also measured IFN-γ- and granzyme B-producing PBMC frequencies. These latter functional readouts of NK cell activity were also shown to be CpG enhanced and dependent on CD56-bearing NK cells and PDC (data not shown).
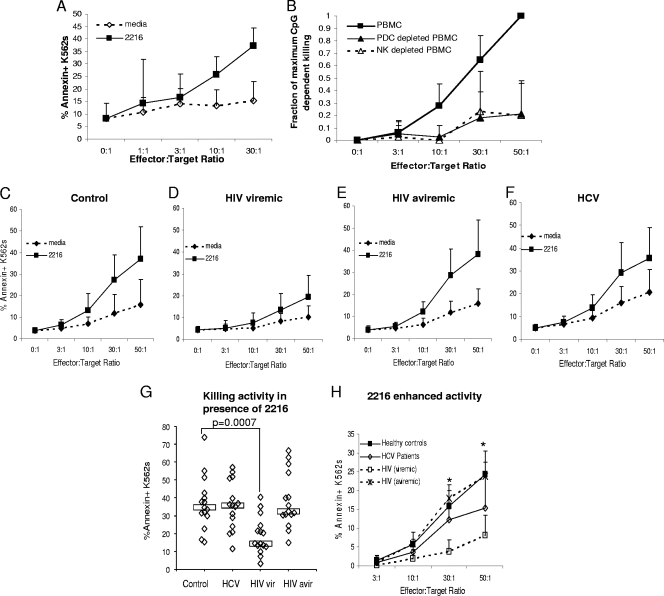
FIG. 1.
CpG-enhanced NK cell-associated PBMC killing is impaired in viremic HIV infection. (A) PBMC from five healthy control subjects were cultured in the absence or presence of CpG 2216 (22 h at 37°C). PKH26-labeled K562 target cells were added during the final 2 h of culture, and PKH26-gated annexin V-stained cells were quantified by flow cytometric analysis. Data are shown as means and standard deviations. (B) PBMC, PBMC depleted of PDC (BDCA4 depletion), and PBMC depleted of NK cells (CD56 depletion) were cultured in the presence or absence of CpG, and annexin V was measured. The proportion (fraction) of maximum CpG-enhanced killing is shown on the y axis for data representing six healthy control subjects. PBMC from healthy control (C), viremic HIV-infected (D), aviremic HIV-infected (E), and HCV-infected (F) subjects (n = 15 for each group) were assayed for CpG 2216-dependent killing activity. (G) Comparison of killing activity in the presence of CpG among groups at an E:T ratio of 50:1. vir, viremic; avir, aviremic. Boxes represent median values. (H) CpG-enhanced activity (activity in the presence of CpG minus that in the absence of CpG) is represented for each group. *, P < 0.05 for comparison of results for viremic HIV-infected subjects to those for healthy control subjects.
We next evaluated PBMC from each group of subjects for NK-associated PBMC killing activity dependent on CpG-activated PDC. As shown in Fig. 1C through F, CpG enhanced the PBMC killing activity in all subject groups, though the degree of enhancement was diminished in viremic HIV infections. Specifically, in the presence of CpG the PBMC killing activity was decreased in viremic HIV-infected subjects compared to that in the controls (P = 0.0007 at an E:T of 50:1) (Fig. 1G), while it was maintained in aviremic HIV and HCV infections (the latter indicates that viremia is likely a strong determinant of the observed defect). This difference between viremic HIV-infected and control subjects was also significant at E:T ratios of 10:1 and 30:1 (P = 0.04 and 0.0006, respectively). The degree of CpG enhancement was also impaired in viremic HIV-infected subjects (P = 0.04, 0.0004, and 0.0008 at E:T ratios of 10:1, 30:1, and 50:1) (Fig. 1H). PBMC killing activity in samples from HCV-infected subjects compared to that of the controls tended to be increased in the absence of CpG (P = 0.05) (Fig. 1F) but not to be as remarkably enhanced by the presence of CpG (P = 0.1) (Fig. 1H).
CpG-enhanced granzyme B and IFN-γ are impaired in viremic HIV infection.
In parallel to assays of killing activity, granzyme B and IFN-γ ELISPOT assays were also performed. Similar to findings for the killing activity defect, fewer CpG-stimulated PBMC from viremic HIV-infected subjects produced granzyme B compared to that from healthy controls (P = 0.02, P = 0.019, and P = 0.004 at E:T ratios of 10:1, 30:1, and 50:1) (Fig. 2A). Activity was also reduced in aviremic HIV-infected subject samples (50:1 E:T ratio, P = 0.02). When IFN-γ-secreting activity was measured, fewer frequencies of CpG-stimulated PBMC from viremic HIV-infected subjects produced IFN-γ compared to those from the controls (P = 0.02, P = 0.01, and P = 0.01 at E:T ratios of 3:1, 10:1, and 30:1) (Fig. 2B).
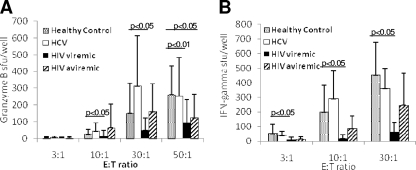
FIG. 2.
CpG-enhanced, NK cell-associated PBMC granzyme B and IFN-γ production are reduced in viremic HIV infection. PBMC from healthy control, HCV-infected, viremic HIV-infected, and aviremic HIV-infected subjects (the same PBMC samples analyzed for killing activity for Fig. 1) were assayed for CpG 2216-dependent granzyme B (A)- and IFN-γ (B)-producing cell activity at different PBMC effector-to-K562 target ratios (shown on the x axis) by ELISPOT assay. sfu, spot-forming units.
In viremic HIV-infected subjects, higher HIV plasma levels were associated with lower PBMC granzyme B production in the absence of CpG only at the highest E:T ratio (r = −0.60, P = 0.04). No other associations between IFN-γ production, granzyme B-secreting activity, or killing activity and HCV level, HIV level, or CD4 cell count in the HCV- or HIV-infected subject groups were observed.
NK cell IFN-γ production dependent on CpG-activated PDC is impaired in viremic HIV infection.
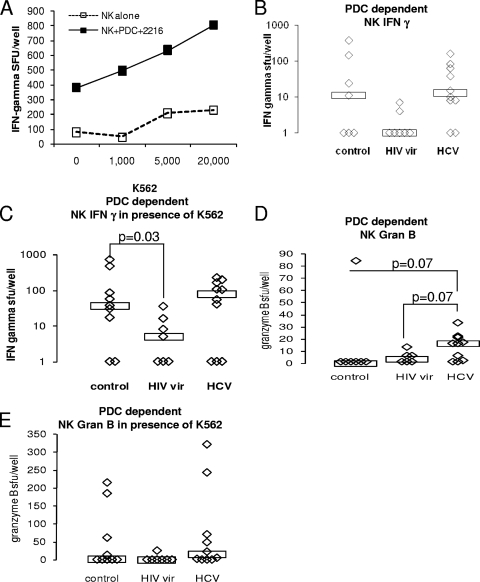
Because defects identified in the unfractionated system may be due to either numerical or functional defects in PDC or NK cells, we next focused on whether there are per cell functional defects of PDC or NK cells in the setting of viremic HIV infection. To characterize purified NK cell and PDC activities, we analyzed NK cell IFN-γ and granzyme B production in the presence and absence of PDC that were, or were not, activated with TLR ligand. Because the presence of K562 target cells facilitates NK cell activation and IFN-γ production (25), we first measured NK cell IFN-γ production in response to target cells in the absence of PDC. Overlapping groups of subjects were studied for this experiment. Levels of HCV in HCV-infected subjects ranged from 119,000 to 4,960,000 IU/ml. For viremic HIV-infected subjects, CD4 cell counts ranged from 196 to 855 cells/μl (median, 381), and HIV levels ranged from 252 to 591,000 copies/ml (median, 39,000). Consistent with previous findings by others (17, 32, 44), fewer NK cells from viremic HIV-infected subjects than from control subjects produced IFN-γ in response to K562 target cells (P = 0.002) (data not shown). This also tended to be the case for granzyme B (P = 0.08) (data not shown). These functions were not significantly different in HCV-infected subjects (P = 0.1 and 0.7 for IFN-γ and granzyme B, respectively) (data not shown).
We then measured PDC-dependent NK cell IFN-γ and granzyme B production in the presence and absence of K562 target cells. Assays were performed in the presence and absence of PDC-activating CpG 2216. In the absence of CpG, no PDC-mediated enhancement of NK cell IFN-γ-secreting activity was observed in the presence or absence of K562 target cells (data not shown). In contrast, in the presence of CpG-activated PDC, enhancement of NK cell IFN-γ-secreting activity was observed in the presence and absence of K562 target cells (Fig. 3A). In the absence of K562 target cells, NK cell IFN-γ-secreting activity dependent on CpG-activated PDC tended to be reduced in viremic HIV-infected subjects compared to that in control subjects (P = 0.1) (Fig. 3B), whereas in the presence of K562 target cells, PDC-dependent NK cell IFN-γ activity was significantly reduced in viremic HIV-infected subjects compared to that in the controls (Fig. 3C, P = 0.03). This activity appeared intact in HCV-infected subjects. Little PDC-dependent activity was observed for granzyme B-producing NK cell function (Fig. 3D and E). Granzyme B-producing activity was enhanced by PDC to a greater degree in HCV-infected subjects (P = 0.07) (Fig. 3D). No correlation between HCV level, HIV level, or CD4 cell count and functional activity was observed in these assays.
FIG. 3.
NK cell IFN-γ production dependent on CpG-activated PDC is impaired in viremic HIV infection. (A) NK cell (105/well) IFN-γ-producing activity dependent on the presence of CpG and PDC (2 × 104 cells/well). Data are representative of healthy controls and are from one healthy control subject. (B to E) PDC and NK cells from 9 healthy control subjects, 7 viremic HIV-infected subjects, and 10 chronically HCV-infected subjects were prepared and assayed for CpG-activated PDC-dependent NK cell IFN-γ- and granzyme B-secreting activity. Boxes represent median values. (B) CpG-activated PDC-dependent NK cell IFN-γ-secreting activity (activity in the presence of PDC, CpG, and NK cells minus activity in the presence of CpG and NK cells). (C) CpG-activated PDC-dependent NK cell IFN-γ production in the presence of K562 cells (20 × 103/well). (D) CpG-activated PDC-dependent NK cell granzyme B-secreting activity. (E) CpG-activated PDC-dependent NK cell granzyme B production in the presence of K562 cells. vir, viremic; Gran B, granzyme B; SFU and sfu, spot-forming units.
CpG-dependent, PDC-NK cell killing activity defect is largely attributable to defective NK cell function, while IFN-γ-producing activity defect is attributable to both PDC and NK cell defects.
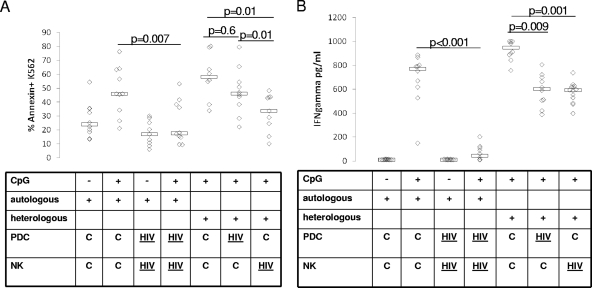
Since impaired PDC-NK cell activity may be due to defects in PDC or NK cells or a combination of the two, we next evaluated the contribution of PDC versus that of NK cells to this defect in a heterologous assay system. We compared autologous healthy control PDC-NK, autologous viremic HIV PDC-NK, PDCcontrol-NKcontrol heterologous, PDCcontrol-NKviremic HIV heterologous, and PDCviremic HIV-NKcontrol coculture activities in assays of K562 killing activity (superscript descriptors indicate sources of cells). Overlapping groups of subjects were studied for this experiment. For viremic HIV-infected subjects, CD4 counts ranged from 401 to 745 cells/μl (median, 516) and HIV levels ranged from 3,880 to 142,000 copies/ml (median, 26,900). As shown in Fig. 4, similar to data shown in Fig. 3 for IFN-γ-producing activity, in the autologous PDC-NK cell culture system there was decreased PDC-NK cell killing (P = 0.007) and IFN-γ (P < 0.001) activity in the presence of CpG for viremic HIV samples (Fig. 4A) compared to those for control subject samples (Fig. 4B). In the heterologous system, there was substantially decreased PDC-NK cell killing activity for NK cells derived from viremic HIV-infected individuals (P = 0.01). For PDC derived from viremic HIV-infected individuals, killing activity was nonsignificantly and less dramatically reduced (P = 0.6) (Fig. 4A). The latter activity significantly differed from that for NK cells derived from viremic HIV-infected individuals (P = 0.01), again indicating that for NK cells derived from viremic HIV-infected individuals there is a defect in activity. Therefore, while there may be a minor contribution of impaired PDC function to the impaired PDC-NK cell killing activity, we conclude that a main portion of the defect is attributable to impaired NK cell function. In contrast, for IFN-γ-producing activity, both PDC and NK cells appear to contribute equally to the defect (P = 0.009 and P = 0.001, respectively) (Fig. 4B). Granzyme B activity was also measured (data not shown), and the variability within each PDC-NK cell coculture was greater. The difference in PDC or NK cell activity between samples from HIV-infected subjects and those from healthy controls (P = 0.2 and P = 0.4, respectively) is not significant. No correlation between PDC-NK cell functional activity and HIV level or CD4 cell count was identified in these assays.
FIG. 4.
Autologous and heterologous PDC-NK cell killer function is impaired in viremic HIV infection, and this defect is attributable to a defect in both PDC and NK cells. (A) NK cells and PDC from healthy control ([C] n = 10) and viremic HIV-infected ([HIV] n = 10) subjects were prepared and cultured for 20 h at 37°C in the absence (−) or presence (+) of CpG. PKH-labeled K562 cells were added for the final 2 h of culture (103 cells/well), followed by staining with annexin V and analysis for the proportion of PKH-gated K562 cells expressing annexin V (y axis). The E:T ratios for these assays were 0:1, 3:1,10:1, and 30:1. Data for a 30:1 E:T ratio are shown in this figure, while data for other E:T ratios were similar. The NK-to-PDC ratio was kept constant at 20:1 for all E:T ratios. Both autologous and heterologous assays were performed with each sample, and comparisons of PDC-NK cell activity for samples from autologous healthy control subjects, autologous viremic HIV-infected subjects, and heterologous control and viremic HIV-infected subjects are shown. (B) Supernatants for cultures were analyzed for IFN-γ by ELISA.
PDC-NK cell interaction is cell contact and cytokine dependent.
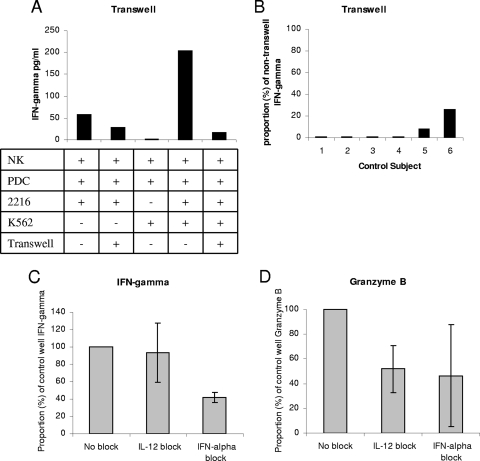
To explore the mechanism of impaired PDC-NK cell interaction in viremic HIV infection, we characterized the cell contact and soluble factor requirements for this system. PDC-mediated NK cell activation and NK cell killer activity have been shown to be IFN-α dependent (21, 50). Since our system is slightly different, we evaluated both cell contact and soluble factor (IFN-α and IL-12) dependence. An example of a transwell experiment is shown in Fig. 5A. The transwell insert separated PDC and NK cells. When K562 target cells were present, they were in the same compartment as the NK cells. For this example of healthy control subject NK cells, the presence of the transwell insert substantially reduced PDC-dependent NK cell IFN-γ activity in both the absence and the presence of K562 target cells. This experiment was repeated in the presence of K562 target cells another 5 times with samples from different healthy control subjects. At most, 20% of the activity present in the nontranswell experiment was observed when the transwell insert was present, indicating a strong cell contact requirement for this activity (Fig. 5B). Similar transwell assays were performed to evaluate killing readout, and CpG-enhanced PDC-dependent killing activity was also cell contact dependent (data not shown). Blocking-study assays indicated that NK cell IFN-γ activity dependent on CpG-activated PDC was partially dependent on IFN-α and not on IL-12 (Fig. 5C). In contrast, NK granzyme B production dependent on CpG-activated PDC appears to be partially dependent on both IL-12 and IFN-α (Fig. 5D). NK cell killing activity was IFN-α, but not IL-12, dependent (data not shown). Taken together, these data suggest that the activated PDC-enhanced NK cell IFN-γ and killing activity is both cell contact and IFN-α dependent, while requirements for the granzyme B-producing function appear slightly different.
FIG. 5.
PDC-NK cell interaction is cell contact and cytokine dependent. (A) Representative transwell experiment with healthy control cells. NK cell IFN-γ activity was measured by cytokine ELISA performed on cell culture supernatants. Cell cultures were performed in the presence (+) or absence (−) of CpG, K562 cells, and a transwell insert (between NK cells and PDC). (B) Transwell IFN-γ activity for cultures of PDC plus NK plus CpG plus K562 cells from six control subjects. (C and D) IL-12- or IFN-α-blocking data for cultures of NK plus PDC plus CpG plus K562 cells from three control subjects.
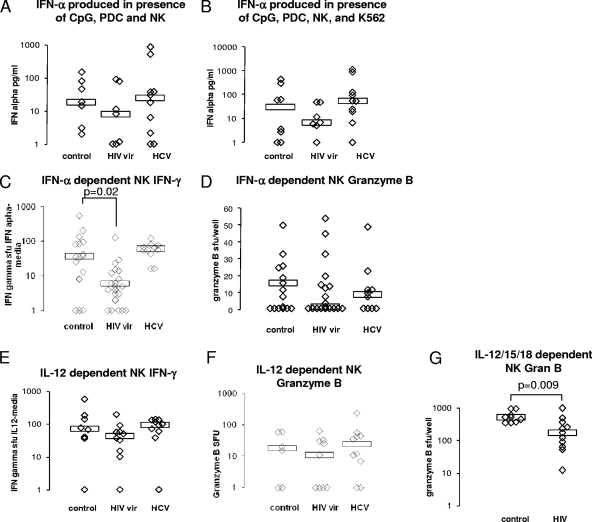
IFN-α-induced NK cell IFN-γ production is impaired in viremic HIV infection.
To further explore the mechanism of impaired PDC-mediated NK cell activity, we focused on IFN-α and IL-12 production in PDC-NK cell cocultures and on the ability of these cytokines to directly activate NK cells. Very little IL-12 was detectable in the supernatants of PDC-NK cocultures performed in the presence and absence of CpG or K562 target cells (data not shown). IFN-α production in the supernatants of these cocultures was nonsignificantly reduced for samples from viremic HIV-infected subjects when the cultures were performed in the absence and presence of K562 target cells (P = 0.2 and P = 0.7, respectively) (Fig. 6A and B). However, upon direct stimulation of NK cells, IFN-α-induced NK cell IFN-γ production was strikingly impaired in samples from viremic HIV-infected subjects (P = 0.02) (Fig. 6C), while IL-12-induced NK cell IFN-γ production appeared intact (Fig. 6E). A similar pattern of impaired IFN-α-induced NK cell granzyme B production was observed (Fig. 6D and F). When NK cell stimulus of combined IL-12, IL-15, and IL-18 was used, greater frequencies of NK cells produced IFN-γ than when stimulated with IL-12 alone (data not shown). This activity was above the IFN-γ detection limit for the assay system for nearly all data points (data not shown). IL-12/IL-15/IL-18-induced NK cell granzyme B production was impaired in viremic HIV infection (P = 0.009) (Fig. 6G). Taken together, these data suggest that in the setting of viremic HIV infection, there is a nonsignificant and modest impairment of IFN-α production in the PDC-NK cell coculture system, while at the same time, there is substantially impaired NK cell responsiveness to IFN-α and IL-12/IL-15/IL-18 but not to IL-12 alone.
FIG. 6.
IFN-α induced NK cell IFN-γ-secreting activity is impaired in viremic HIV infection. PDC and NK cells from 17 healthy controls, 24 viremic HIV-infected subjects, and 10 chronically HCV-infected subjects were prepared and assayed for IFN-α, IL-12, and IL-12/IL-15/IL-18-dependent NK cell IFN-γ- and granzyme B-secreting activity and PDC-NK cell coculture IFN-α-secreting activity. (A) CpG-treated PDC (2 × 104 cells) and NK cell (1 × 105cells) culture (20 h at 37°C) supernatant IFN-α as measured by ELISA. (B) Supernatant IFN-α from a culture (20 h at 37°C) of CpG-treated PDC and NK cells in the presence of K562 cells (2 × 104 cells) as measured by ELISA. (C) IFN-α-induced NK cell IFN-γ, as measured by ELISPOT assay, was calculated by subtracting the number of spot-forming units (SFU) produced in the absence of IFN-α from that produced in the presence of IFN-α. (D) IFN-α-induced NK cell granzyme B, as measured by ELISPOT assay, was calculated by subtracting the number of SFU produced in the absence of IFN-α from that produced in the presence of IFN-α. (E) IL-12-dependent NK cell IFN-γ was calculated by subtracting the number of SFU in the absence of IFN-α from that in the presence of IFN-α. (F) IL-12-dependent NK cell granzyme B was calculated by subtracting the number of SFU in the absence of IFN-α from that in the presence of IFN-α. (G) IL-12/IL-15/IL-18-induced NK cell granzyme B was calculated by subtracting the number of SFU in the absence of IFN-α from that in the presence of IFN-α. vir, viremic; Gran B, granzyme B.
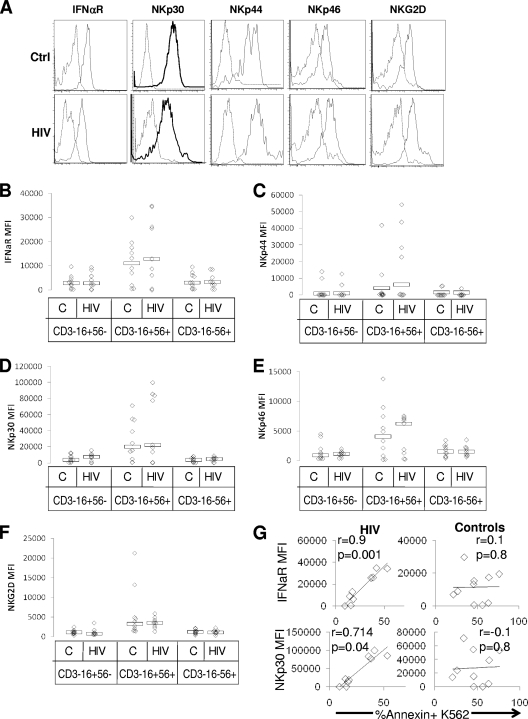
Impaired NK cell response to IFN-α is not the result of diminished IFN-α receptor expression, altered NKG2D, NKP30, NKP44, or NKP46 expression, or likely the result of direct infection, but killing activity is closely associated with IFN-α receptor expression and NKP30 expression in viremic HIV infection.
Since impaired NK cell response to IFN-α may be due directly to altered IFN-α expression, we measured IFN-α receptor levels on NK cell subsets. Since activation of NK cell receptors has been directly related to killing activity (16, 41, 47), and since NK cell-activating receptors have been described as reduced in expression during HIV infection (17), we also measured expression levels of NKG2D, NKP30, NKP44, and NKP46 in CD16+CD56+, CD16+CD56−, and CD16−D56+ NK cell subsets (an example of analysis on the CD16+CD56+ subset is shown in Fig. 7A). IFN-α receptor expression was similar on the CD16+CD56+, CD16+CD56− and CD16−CD56+ NK cell subsets (Fig. 7B), comparing samples from control and viremic HIV-infected subjects. Similarly, NKG2D, NKP30, NKP44, and NKP46 expression did not differ between HIV-infected and healthy control subjects on each of the NK cell subsets (Fig. 7C through F). However, in viremic HIV-infected, but not control, subjects, NK cell NKP30 and IFN-α receptor expression was associated with killing activity (the CD16+CD56+ NK cell subset is shown in Fig. 7G; data are similar for the CD16+CD56− and CD16−CD56+ subsets [data not shown]). This finding suggests a stronger dependence of killing activity on expression of these receptors in viremic HIV infection. Additionally, NKP30 expression was found to inversely correlate with HIV level, but with statistical significance only on the CD16+CD56− subset (r = −0.68 and −0.63 and P = 0.04 and 0.07 for NKP30 expression on the CD16+CD56− and CD16+CD56+ NK cell subsets, respectively [data not shown]). Together these data indicate that while altered IFN-α receptor, NKP30, NKP44, NKP46, or NKG2D expression does not account for overall-reduced NK cell-killer activity in HIV infection, the expression of NKP30 and IFN-α receptors is fundamentally related to killing activity in viremic HIV infection.
FIG. 7.
IFN-α receptor, NKG2D, NKP30, NKP44, and NKP46 expression are intact during viremic HIV infection, and both NKP30 and IFN-α receptor expression are associated with killer function in viremic HIV infection. PBMC from healthy control ([C] n = 10) and viremic HIV-infected ([HIV] n = 10) subjects were stained with CD3, CD16, CD56, IFN-α receptor, NKG2D, NKP30, NKP44, and NKP46 monoclonal antibodies versus an isotype control. CD3−CD16+CD56−-, CD3−CD16+CD56+-, and CD3−CD16−CD56+-gated cells were analyzed for IFN-α receptor, NKG2D, NKP30, NKP44, and NKP46 expression by six-color flow cytometry (performed in two separate stainings). (A) Histograms indicating expression of each receptor (solid line) versus that of an isotype control (dashed line) for representative healthy control (Ctrl) and viremic HIV (HIV)-infected subjects. Summative data for mean fluoresence intensity of receptor expression above isotype control is shown for each NK cell subset for the IFN-α receptor (B), NKP44 (C), NKP30 (D), NKP46 (E), and NKG2D (F). (G) Correlation between IFN-α receptor expression on the CD16+CD56+ NK cell subset and killing activity, as well as between NKP30 expression and killing activity, is shown for HIV-infected and healthy control samples.
The presence of HIV DNA within NK cells has been previously described (54), suggesting that these cells may be directly infected by HIV. To address whether the presence of HIV within NK cells may directly contribute to the functional defect observed here, we evaluated for the presence of HIV-1 minus-strand SS DNA by real-time PCR. HIV SS DNA was identified in three (73, 157, and 858 copies/million NK cells) of four separate viremic HIV-infected subject NK cell fractions (>99% purity). We have previously described an in vitro system of NK cell HIV infection (6). In the latter system, HIV p24 expression was found in 10 to 20% of NK cells. In such a system, a level of 15% of NK cells expressing p24 was found to be equivalent to one copy of HIV SS DNA/10 NK cells (on the same order of magnitude). When taking into consideration the sensitivity of the HIV SS DNA assay, this finding indicates that 0 to 858 copies of HIV SS DNA/million NK cells is consistent with <0.1% of the NK cells containing HIV DNA. Direct HIV infection is therefore unlikely to be responsible for the observed 3- to 10-fold activity defect.
DISCUSSION
The data shown here provide evidence for substantial impairment in the PDC-mediated NK effector function in the setting of viremic HIV infection but only modest impairment in the setting of HAART-suppressed, aviremic HIV infection. The latter indicates that viremia is likely the cause, not the consequence, of impaired PDC-NK cell function. However, this does not exclude the possibility that impaired PDC-NK cell function may contribute to suboptimal control of HIV in the infected host. No remarkable defects were observed in the setting of chronic HCV infection other than a numerical PDC defect that has been described previously (3, 26, 53, 56). In viremic HIV infection, killer, IFN-γ-producing, and granzyme B-producing activities were impaired in the unfractionated cell system, with the impairment likely due to a combination of numerical and functional defects in PDC and NK cells. Results from the purified cell system reveal a per cell functional defect in the PDC-NK cell interaction resulting in reduced NK cell killing activity and IFN-γ-producing activity. Heterologous system assays localized the majority of the defect to the NK cell for killing activity, while for IFN-γ-producing activity, defects in both PDC and NK cells appear equally responsible. Because we observed a defect in NK cell responsiveness to IFN-α, shown here to be necessary for full PDC-mediated NK cell activity, the overall PDC-NK cell defect is at least in part due to impairment in NK cell response to IFN-α. Though IFN-α receptor expression and NKP30 expression were found to be closely associated with killing activity in viremic HIV infection (but not in healthy controls), alterations in cell surface expression of the IFN-α receptor or NKP30 do not account for the observed overall functional defects. The latter selective association of NKP30 and IFN-α expression with killing activity in viremic HIV-infected but not control subject samples may indicate reduced NKP30 or IFN-α signaling activity in viremic HIV infection that can be partially overcome by higher levels of receptor expression or, alternatively, that receptors other than the IFN-α receptor or NKP30 also facilitated NK cell killing activity in the healthy controls but are defective in viremic HIV infection (indicated by increasing dependence on IFN-α and NKP30 expression in the case of viremic HIV infection).
Impaired PDC-NK cell interactions may be due to impaired cell contact between PDC and NK cells or impaired NK cell responsiveness to soluble factors involved in the PDC-NK cell interaction. With regard to the latter, as stated above, we determined that PDC-mediated NK cell activity is partially dependent on IFN-α (a finding consistent with previous literature [21, 50]) and that in viremic HIV infection, there is an impairment in responsiveness when NK cells are directly stimulated with IFN-α, a finding that extends upon those of previous studies that evaluated unfractionated cell assays of NK cell activity (52). This indicates that at least a portion of the PDC-NK cell defect is due to impaired NK cell responsiveness to IFN-α. However, as shown in Fig. 5, the PDC-mediated NK cell activity is cell contact dependent. Therefore, impairments in physical interactions or synapse formation may account for a portion of the defect. In fact, for the IFN-γ-producing function, a portion of the defect is attributable to PDC function. Whether the latter is due to cell contact factors or defective PDC IFN-α-producing activity is unclear, as in coculture assays there was a trend toward a significant reduction in IFN-α-producing activity. While cell contact factors for PDC-NK cell interactions have not been well described, MDC-NK or monocyte-derived MDC-NK cell interactions in both mouse and human systems are facilitated by synapse-localized IL-12, IL-15, and IL-18 (8, 9, 29, 34, 46). Additional factors that can modulate cell contact-dependent activity include interactions between inhibitory or activating NK cell receptors and their ligands (13, 15). With regard to the latter, DNAM-1 and NKP30 can facilitate PDC-NK cell interactions (15) and could potentially play a role in the defective PDC-NK cell interaction in HIV infection described here. However, while NKP30 expression was closely associated with killing activity, direct analysis of NKG2D, NKP30, NKP44, and NKP46 expression levels did not reveal diminished expression levels on viremic HIV-infected-subject NK cells.
The impaired NK cell response to IFN-α may be due to reduced IFN-α receptor expression, impaired IFN-α signaling, or reduced frequencies of a particular NK cell subset responsive to IFN-α. These possibilities may be the results of direct HIV infection of NK cells or indirect effects of HIV infection. Our data do not support an alteration in IFN-α receptor expression as an explanation. In regard to IFN-α signaling, downregulation of IFN-α signaling has been described in the setting of HCV infection, with HCV protein expression inhibiting Jak-Stat signaling (24). The status of Jak-Stat signaling in the setting of viremic HIV infection appears impaired in the monocyte compartment (23), though whether this extends to the NK cell compartment is not known. In regard to NK cell subset distribution skewing as an explanation for reduced IFN-α responsiveness, little is known about specific NK cell subsets responsive to IFN-α, so this remains a possibility. Certainly, as shown in Table 1, the frequencies of CD3−CD16+CD56+ cells were reduced 25% in the setting of viremic HIV infection. However, the activity defect observed in Fig. 6 suggests a defect on the order of sixfold, arguing for something beyond a NK cell subset numerical defect as an explanation. Additionally, when the killing activity was measured using purified NK cells, the frequencies of NK cells that were either CD3−16+56−, CD3−16+56+, or CD3−16−56+ were simultaneously measured, and none of these subset frequencies correlated with the killing activity observed in either the control or HIV viremic groups (data not shown). This finding highlights the fact that the activity observed should not be attributed to one subset using these definitions alone. Regarding direct HIV infection versus the indirect effect of HIV infection, the low frequencies of NK cells found to contain HIV SS DNA indicate that direct infection of NK cells is an unlikely explanation for the functional defect observed.
Numerous immune defects exist in the setting of viremic HIV infection. Recently, Chehimi et al. described reduced NK cell activity in viremic HIV infection in an unfractionated assay (11). This activity was found to be dependent upon PDC. Furthermore, this activity rapidly normalized with treatment-induced virologic suppression, suggesting a reversal of a functional, and not a numerical, defect. Our data are consistent with these previous findings, extending an understanding of this defect to include killer activity, IFN-γ secretion, and granzyme B secretion. We further extended a mechanistic understanding of this defect, attributing a large portion of the defect to impaired NK cell response to IFN-α and a portion of the defect to PDC dysfunction. We further established a likely role for NKP30 in mediating this PDC-NK cell-killing activity in viremic HIV infection.
Notably, expression of NKP30, NKP44, and NKP46 was not found to be reduced on NK cells analyzed in this study (shown as the mean fluorescence expression above the isotype control, though results are similar when analyzing proportions of cells expressing each receptor [data not shown]), which appears to contrast with the findings of De Maria et al. for NKP30 and NKP46 expression (17). One difference between these two studies is the use of unfractionated cells for flow cytometric analysis (gating on CD16- or CD56-expressing NK cell subsets in this study), compared to analyzing NKP expression on negatively selected bulk NK cells in the study by De Maria et al. However, there seem to be few other differences in study subject population inclusion criteria or in sample size. Notably, there appears to be increased NKP30 expression in the CD16+CD56+ compartment of a subset of viremic HIV-infected subject samples analyzed in this study (Fig. 7), making for substantial heterogeneity in expression of this receptor within this group. Factors that contribute to this heterogeneity are currently unclear, but for the experiment shown in Fig. 7, there is an inverse association between HIV level and NKP30 expression within the CD16+CD56− NK cell subset, indicating that HIV level may be one factor that contributes to NKP30 expression heterogeneity in HIV infection (the latter is consistent with the overall findings of De Maria et al.[17]).
While CpG-enhanced PBMC-killer activity tended to be decreased in HCV-infected subject samples (Fig. 1H), no specific significant per cell defect in NK cell or PDC-mediated NK cell function was observed in the setting of chronic HCV infection. In fact, PDC-mediated NK granzyme B secretion was enhanced (Fig. 3D). These data are consistent with more-recent literature describing intact NK cell function in the setting of HCV infection (18, 27, 39), extending here to PDC-mediated NK cell function.
Overall, the data presented here indicate impaired PDC-mediated NK cell function in the setting of viremic HIV infection. This impairment is likely due in large part to impaired NK cell responsiveness to IFN-α, a cytokine required for efficient PDC-NK cell interaction. For IFN-γ-producing activity and potentially for killing activity as well, the impairment is also due in part to defective PDC function. In the unfractionated system, this defect may also be due in part to reduced PDC and NK cell subset frequencies. Given that genetic markers of NK cell phenotype are associated with disease progression rate, the impairment described here may have significant implications for the HIV-infected host's ability to respond to both HIV and opportunistic pathogens.
Acknowledgments
This work was supported by VA Advanced Research Career Development and VA Merit grants, NIH grant R01 DK068361, NIH grant R21AI67094, and CWRU Center for AIDS Research Core facilities grant AI 36219. N.L.Y. was supported by NIAAA NRSA grant 1F31AA017853.
One of the authors, M. Tary-Lehmann, is part owner of Cellular Technologies Limited, where ELISPOT image analyzers are produced. This equipment was utilized for analysis of some of the data in this manuscript. However, the company is not mentioned in the manuscript, and only a reference to prior literature is given for the methodology for analysis. Therefore, the authors do not have any commercial or other associations that present a conflict of interest.
Footnotes
Published ahead of print on 19 August 2009.
REFERENCES
- 1.Alter, G., J. M. Malenfant, R. M. Delabre, N. C. Burgett, X. G. Yu, M. Lichterfeld, J. Zaunders, and M. Altfeld. 2004. Increased natural killer cell activity in viremic HIV-1 infection. J. Immunol. 173:5305-5311. [DOI] [PubMed] [Google Scholar]
- 2.Anthony, D. D., A. B. Post, H. Valdez, D. L. Peterson, M. Murphy, and P. S. Heeger. 2001. ELISPOT analysis of hepatitis C virus protein-specific IFN-gamma-producing peripheral blood lymphocytes in infected humans with and without cirrhosis. Clin. Immunol. 99:232-240. [DOI] [PubMed] [Google Scholar]
- 3.Anthony, D. D., N. L. Yonkers, A. B. Post, R. Asaad, F. P. Heinzel, M. M. Lederman, P. V. Lehmann, and H. Valdez. 2004. Selective impairments in dendritic cell associated function distinguish HCV and HIV infection. J. Immunol. 172:4907-4916. [DOI] [PubMed] [Google Scholar]
- 4.Azzoni, L., E. Papasavvas, J. Chehimi, J. R. Kostman, K. Mounzer, J. Ondercin, B. Perussia, and L. J. Montaner. 2002. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J. Immunol. 168:5764-5770. [DOI] [PubMed] [Google Scholar]
- 5.Ballas, Z. K., J. M. Turner, D. A. Turner, E. A. Goetzman, and J. D. Kemp. 1990. A patient with simultaneous absence of “classical” natural killer cells (CD3−, CD16+, and NKH1+) and expansion of CD3+, CD4−, CD8−, NKH1+ subset. J. Allergy Clin. Immunol. 85:453-459. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein, H. B., G. Wang, M. C. Plasterer, J. A. Zack, P. Ramasastry, S. M. Mumenthaler, and C. M. Kitchen. 2009. CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function. Virology 387:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron, C. A., K. S. Byron, and J. L. Sullivan. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320:1731-1735. [DOI] [PubMed] [Google Scholar]
- 8.Borg, C., A. Jalil, D. Laderach, K. Maruyama, H. Wakasugi, S. Charrier, B. Ryffel, A. Cambi, C. Figdor, W. Vainchenker, A. Galy, A. Caignard, and L. Zitvogel. 2004. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood 104:3267-3275. [DOI] [PubMed] [Google Scholar]
- 9.Brilot, F., T. Strowig, S. M. Roberts, F. Arrey, and C. Munz. 2007. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. J. Clin. Investig. 117:3316-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauda, R., V. Laghi, M. Tumbarello, L. Ortona, and R. J. Whitley. 1989. Immunological alterations associated with recurrent herpes simplex genitalis. Clin. Immunol. Immunopathol. 51:294-302. [DOI] [PubMed] [Google Scholar]
- 11.Chehimi, J., L. Azzoni, M. Farabaugh, S. A. Creer, C. Tomescu, A. Hancock, A. Mackiewicz, L. D'Alessandro, S. Ghanekar, A. S. Foulkes, K. Mounzer, J. Kostman, and L. J. Montaner. 2007. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J. Immunol. 179:2642-2650. [DOI] [PubMed] [Google Scholar]
- 12.Chehimi, J., D. E. Campbell, L. Azzoni, D. Bacheller, E. Papasavvas, G. Jerandi, K. Mounzer, J. Kostman, G. Trinchieri, and L. J. Montaner. 2002. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 168:4796-4801. [DOI] [PubMed] [Google Scholar]
- 13.Colmenero, P., A. L. Zhang, T. Qian, L. Lu, H. Cantor, K. Soderstrom, and E. G. Engleman. 2007. Qa-1b-dependent modulation of dendritic cell and NK cell cross-talk in vivo. J. Immunol. 179:4608-4615. [DOI] [PubMed] [Google Scholar]
- 14.Corado, J., F. Toro, H. Rivera, N. E. Bianco, L. Deibis, and J. B. De Sanctis. 1997. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin. Exp. Immunol. 109:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Della Chiesa, M., C. Romagnani, A. Thiel, L. Moretta, and A. Moretta. 2006. Multidirectional interactions are bridging human NK cells with plasmacytoid and monocyte-derived dendritic cells during innate immune responses. Blood 108:3851-3858. [DOI] [PubMed] [Google Scholar]
- 16.De Maria, A., R. Biassoni, M. Fogli, M. Rizzi, C. Cantoni, P. Costa, R. Conte, D. Mavilio, B. Ensoli, A. Cafaro, A. Moretta, and L. Moretta. 2001. Identification, molecular cloning and functional characterization of NKp46 and NKp30 natural cytotoxicity receptors in Macaca fascicularis NK cells. Eur. J. Immunol. 31:3546-3556. [DOI] [PubMed] [Google Scholar]
- 17.De Maria, A., M. Fogli, P. Costa, G. Murdaca, F. Puppo, D. Mavilio, A. Moretta, and L. Moretta. 2003. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur. J. Immunol. 33:2410-2418. [DOI] [PubMed] [Google Scholar]
- 18.Düesberg, U., A. M. Schneiders, D. Flieger, G. Inchauspe, T. Sauerbruch, and U. Spengler. 2001. Natural cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) is not impaired in patients suffering from chronic hepatitis C. J. Hepatol. 35:650-657. [DOI] [PubMed] [Google Scholar]
- 19.Feldman, S., D. Stein, S. Amrute, T. Denny, Z. Garcia, P. Kloser, Y. Sun, N. Megjugorac, and P. Fitzgerald-Bocarsly. 2001. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101:201-210. [DOI] [PubMed] [Google Scholar]
- 20.Gerosa, F., B. Baldani-Guerra, C. Nisii, V. Marchesini, G. Carra, and G. Trinchieri. 2002. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerosa, F., A. Gobbi, P. Zorzi, S. Burg, F. Briere, G. Carra, and G. Trinchieri. 2005. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J. Immunol. 174:727-734. [DOI] [PubMed] [Google Scholar]
- 22.Habu, S., K. Akamatsu, N. Tamaoki, and K. Okumura. 1984. In vivo significance of NK cell on resistance against virus (HSV-1) infections in mice. J. Immunol. 133:2743-2747. [PubMed] [Google Scholar]
- 23.Hardy, G. A., S. F. Sieg, B. Rodriguez, W. Jiang, R. Asaad, M. M. Lederman, and C. V. Harding. 2009. Desensitization to type-I interferon in HIV-1 infection correlates with markers of immune activation and disease progression. Blood 113:5497-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heim, M. H., D. Moradpour, and H. E. Blum. 1999. Expression of hepatitis C virus proteins inhibits signal transduction through the Jak-STAT pathway. J. Virol. 73:8469-8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jinushi, M., T. Takehara, T. Tatsumi, T. Kanto, T. Miyagi, T. Suzuki, Y. Kanazawa, N. Hiramatsu, and N. Hayashi. 2004. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J. Immunol. 173:6072-6081. [DOI] [PubMed] [Google Scholar]
- 26.Kanto, T., M. Inoue, H. Miyatake, A. Sato, M. Sakakibara, T. Yakushijin, C. Oki, I. Itose, N. Hiramatsu, T. Takehara, A. Kasahara, and N. Hayashi. 2004. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J. Infect. Dis. 190:1919-1926. [DOI] [PubMed] [Google Scholar]
- 27.Kawarabayashi, N., S. Seki, K. Hatsuse, T. Ohkawa, Y. Koike, T. Aihara, Y. Habu, R. Nakagawa, K. Ami, H. Hiraide, and H. Mochizuki. 2000. Decrease of CD56+ T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology 32:962-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khakoo, S. I., C. L. Thio, M. P. Martin, C. R. Brooks, X. Gao, J. Astemborski, J. Cheng, J. J. Goedert, D. Vlahov, M. Hilgartner, S. Cox, A. M. Little, G. J. Alexander, M. E. Cramp, S. J. O'Brien, W. M. Rosenberg, D. L. Thomas, and M. Carrington. 2004. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305:872-874. [DOI] [PubMed] [Google Scholar]
- 29.Koka, R., P. Burkett, M. Chien, S. Chai, D. L. Boone, and A. Ma. 2004. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J. Immunol. 173:3594-3598. [DOI] [PubMed] [Google Scholar]
- 30.Krug, A., A. R. French, W. Barchet, J. A. Fischer, A. Dzionek, J. T. Pingel, M. M. Orihuela, S. Akira, W. M. Yokoyama, and M. Colonna. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21:107-119. [DOI] [PubMed] [Google Scholar]
- 31.Lanier, L. L., H. Spits, and J. H. Phillips. 1992. The developmental relationship between NK cells and T cells. Immunol. Today 13:392-395. [DOI] [PubMed] [Google Scholar]
- 32.Lederman, M. M., O. D. Ratnoff, J. J. Scillian, P. K. Jones, and B. Schacter. 1983. Impaired cell-mediated immunity in patients with classic hemophilia. N. Engl. J. Med. 308:79-83. [DOI] [PubMed] [Google Scholar]
- 33.Longman, R. S., A. H. Talal, I. M. Jacobson, C. M. Rice, and M. L. Albert. 2005. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J. Infect. Dis. 192:497-503. [DOI] [PubMed] [Google Scholar]
- 34.Lucas, M., W. Schachterle, K. Oberle, P. Aichele, and A. Diefenbach. 2007. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26:503-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mailliard, R. B., Y. I. Son, R. Redlinger, P. T. Coates, A. Giermasz, P. A. Morel, W. J. Storkus, and P. Kalinski. 2003. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J. Immunol. 171:2366-2373. [DOI] [PubMed] [Google Scholar]
- 36.Martin, M. P., X. Gao, J. H. Lee, G. W. Nelson, R. Detels, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, J. Trowsdale, M. Wilson, S. J. O'Brien, and M. Carrington. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31:429-434. [DOI] [PubMed] [Google Scholar]
- 37.Mocikat, R., H. Braumuller, A. Gumy, O. Egeter, H. Ziegler, U. Reusch, A. Bubeck, J. Louis, R. Mailhammer, G. Riethmuller, U. Koszinowski, and M. Rocken. 2003. Natural killer cells activated by MHC class Ilow targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 19:561-569. [DOI] [PubMed] [Google Scholar]
- 38.Moretta, L., E. Ciccone, A. Poggi, M. C. Mingari, and A. Moretta. 1994. Ontogeny, specific functions and receptors of human natural killer cells. Immunol. Lett. 40:83-88. [DOI] [PubMed] [Google Scholar]
- 39.Morishima, C., D. M. Paschal, C. C. Wang, C. S. Yoshihara, B. L. Wood, A. E. Yeo, S. S. Emerson, M. C. Shuhart, and D. R. Gretch. 2006. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology 43:573-580. [DOI] [PubMed] [Google Scholar]
- 40.Pár, G., D. Rukavina, E. Podack, M. Horanyi, J. Szekeres-Bartho, G. Hegedus, M. Paal, L. Szereday, G. Mozsik, and A. Par. 2002. Decrease in CD3-negative-CD8dim+ and Vδ2/Vγ9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J. Hepatol. 37:514. [DOI] [PubMed] [Google Scholar]
- 41.Pende, D., S. Parolini, A. Pessino, S. Sivori, R. Augugliaro, L. Morelli, E. Marcenaro, L. Accame, A. Malaspina, R. Biassoni, C. Bottino, L. Moretta, and A. Moretta. 1999. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 190:1505-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rager-Zisman, B., P. C. Quan, M. Rosner, J. R. Moller, and B. R. Bloom. 1987. Role of NK cells in protection of mice against herpes simplex virus-1 infection. J. Immunol. 138:884-888. [PubMed] [Google Scholar]
- 43.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y. J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183-1186. [DOI] [PubMed] [Google Scholar]
- 44.Rook, A. H., H. Masur, H. C. Lane, W. Frederick, T. Kasahara, A. M. Macher, J. Y. Djeu, J. F. Manischewitz, L. Jackson, A. S. Fauci, and G. V. Quinnan, Jr. 1983. Interleukin-2 enhances the depressed natural killer and cytomegalovirus-specific cytotoxic activities of lymphocytes from patients with the acquired immune deficiency syndrome. J. Clin. Investig. 72:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott-Algara, D., L. X. Truong, P. Versmisse, A. David, T. T. Luong, N. V. Nguyen, I. Theodorou, F. Barre-Sinoussi, and G. Pancino. 2003. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 171:5663-5667. [DOI] [PubMed] [Google Scholar]
- 46.Semino, C., G. Angelini, A. Poggi, and A. Rubartelli. 2005. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood 106:609-616. [DOI] [PubMed] [Google Scholar]
- 47.Sivori, S., D. Pende, C. Bottino, E. Marcenaro, A. Pessino, R. Biassoni, L. Moretta, and A. Moretta. 1999. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur. J. Immunol. 29:1656-1666. [DOI] [PubMed] [Google Scholar]
- 48.Soumelis, V., I. Scott, F. Gheyas, D. Bouhour, G. Cozon, L. Cotte, L. Huang, J. A. Levy, and Y. J. Liu. 2001. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98:906-912. [DOI] [PubMed] [Google Scholar]
- 49.Strowig, T., F. Brilot, and C. Munz. 2008. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J. Immunol. 180:7785-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomescu, C., J. Chehimi, V. C. Maino, and L. J. Montaner. 2007. NK cell lysis of HIV-1-infected autologous CD4 primary T cells: requirement for IFN-mediated NK activation by plasmacytoid dendritic cells. J. Immunol. 179:2097-2104. [DOI] [PubMed] [Google Scholar]
- 51.Toossi, Z., H. Mayanja-Kizza, J. Baseke, P. Peters, M. Wu, A. Abraha, H. Aung, A. Okwera, C. Hirsch, and E. Arts. 2005. Inhibition of human immunodeficiency virus-1 (HIV-1) by beta-chemokine analogues in mononuclear cells from HIV-1-infected patients with active tuberculosis. Clin. Exp. Immunol. 142:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ullum, H., P. C. Gotzsche, J. Victor, E. Dickmeiss, P. Skinhoj, and B. K. Pedersen. 1995. Defective natural immunity: an early manifestation of human immunodeficiency virus infection. J. Exp. Med. 182:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulsenheimer, A., J. T. Gerlach, M. C. Jung, N. Gruener, M. Wachtler, M. Backmund, T. Santantonio, W. Schraut, M. H. Heeg, C. A. Schirren, R. Zachoval, G. R. Pape, and H. M. Diepolder. 2005. Plasmacytoid dendritic cells in acute and chronic hepatitis C virus infection. Hepatology 41:643-651. [DOI] [PubMed] [Google Scholar]
- 54.Valentin, A., M. Rosati, D. J. Patenaude, A. Hatzakis, L. G. Kostrikis, M. Lazanas, K. M. Wyvill, R. Yarchoan, and G. N. Pavlakis. 2002. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 99:7015-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voiculescu, C. L., M. Balasoiu, A. Turculeanu, C. Radu, C. Avramescu, and E. Radu. 1996. Different patterns of some systemic immunological cell markers in HIV only, and HIV/hepatitis C-infected children. Pediatr. AIDS HIV Infect. 7:31-36. [PubMed] [Google Scholar]
- 56.Wertheimer, A. M., A. Bakke, and H. R. Rosen. 2004. Direct enumeration and functional assessment of circulating dendritic cells in patients with liver disease. Hepatology 40:335-345. [DOI] [PubMed] [Google Scholar]
- 57.Yonkers, N. L., B. Rodriguez, K. A. Milkovich, R. Asaad, M. M. Lederman, P. S. Heeger, and D. D. Anthony. 2007. TLR ligand-dependent activation of naive CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection. J. Immunol. 178:4436-4444. [DOI] [PubMed] [Google Scholar]