Abstract
Epstein-Barr virus (EBV; human herpesvirus 4) poses major clinical problems worldwide. Following primary infection, EBV enters a form of long-lived latency in B lymphocytes, expressing few viral genes, and it persists for the lifetime of the host with sporadic bursts of viral replication. The switch between latency and replication is governed by the action of a multifunctional viral protein Zta (also called BZLF1, ZEBRA, and Z). Using a global proteomic approach, we identified a host DNA damage repair protein that specifically interacts with Zta: 53BP1. 53BP1 is intimately connected with the ATM signal transduction pathway, which is activated during EBV replication. The interaction of 53BP1 with Zta requires the C-terminal ends of both proteins. A series of Zta mutants that show a wild-type ability to perform basic functions of Zta, such as dimer formation, interaction with DNA, and the transactivation of viral genes, were shown to have lost the ability to induce the viral lytic cycle. Each of these mutants also is compromised in the C-terminal region for interaction with 53BP1. In addition, the knockdown of 53BP1 expression reduced viral replication, suggesting that the association between Zta and 53BP1 is involved in the viral replication cycle.
The Epstein-Barr virus (EBV) life cycle is divided temporally into two phases, latency and the lytic cycle. Following the infection of epithelial cells of the oropharynx, EBV enters the lytic cycle, where the expression of approximately 80 genes and numerous rounds of genome replication occur, culminating in the production of infectious virions. The infection of B lymphocytes results in the establishment of viral latency with a restricted gene expression pattern; these cells sporadically enter the lytic cycle and reproduce infectious virus (27, 53).
The EBV gene BZLF1 has been associated specifically with the disruption of latency (reviewed in references 34 and 50). This gene encodes the protein Zta (ZEBRA, BZLF1, Z), which has an undisputed role in activating the viral lytic cycle. Not only is the enforced expression of Zta in cells harboring the latent virus able to induce the viral lytic cycle, but a mutant virus where BZLF1 has been inactivated also is unable to replicate the viral genome (10). Zta has homology to the bZIP family of transcription factors whose general structure includes a transactivation domain and a bZIP domain consisting of a basic DNA contact region and a coiled-coil dimerization motif, termed a leucine zipper (24, 49, 50). Zta has a more complex dimerization domain than other bZIP family members, consisting of a dimeric leucine zipper entwined with an adjacent carboxyl-terminal region (35, 38, 44, 50). Zta is multifunctional; through its basic region, it interacts with specific sequence DNA motifs (ZREs) that occur in the promoters of several viral and cellular genes (49) and in the viral origin of lytic replication (Ori-lyt) (46, 47). Through its bZIP domain, Zta interacts with cellular transcription factors such as p53, RAR, NF-κB, CBP, and C/EBPα (7), giving it the additional ability to affect transcription without directly contacting DNA. Zta also reprograms the host cell environment through its bZIP domain by perturbing cell cycle control (6, 7, 11, 29, 39, 42, 43) and altering the expression of cellular genes (6, 7, 11, 30, 36, 37, 42, 43).
During this investigation, a global tandem affinity purification (TAP) approach was used to identify host proteins that interact with Zta. This resulted in the identification of the nuclear protein 53BP1, a component of the ATM DNA damage response pathway, as a novel binding partner. It has been shown recently that signal transduction through the ATM pathway is activated during EBV replication (23), and it was suggested that replicating EBV genomes are recognized as damaged DNA. Interestingly, other DNA and RNA viruses activate DNA damage response pathways during their replication. Retroviruses and the murine gamma herpesvirus MHV68 are postulated to exploit this activation to aid replication (25, 48, 54, 57).
The relevance of the Zta-53BP1 interaction is investigated with respect to the lytic replication of EBV.
MATERIALS AND METHODS
Cloning.
An N-terminal TAP tag (provided by Tomoo Ogi and Alan Lehmann) containing protein A, the tobacco etch virus (TEV) protease cleavage site, and calmodulin binding peptide (41) was inserted into pEGFP (BD Biosciences) to replace the green fluorescent protein gene, generating CT212. The C-terminal half of Zta (amino acids 133 to 245) was cloned C terminally to the TAP tag, generating TAP-Tag Zta-bZIPCT. glutathione S-transferase (GST)-53BP1 fusion proteins were used as described by Iwabuchi et al. (19).
Vectors used for the in vitro translation of Zta and expression in eukaryotic cells have been described previously (16, 44, 51).
The full-length human p27KIP1 coding sequence was subcloned into pRSETA under the direction of the T7 promoter.
Full-length Zta was cloned into pCDNA3 (Invitrogen) using primers containing a histidine tag and EcoRI and BamHI restriction sites (5′-CTGCACACCGGGGATCCATGCATCATCATCATCATCATATGATGGACCCAATCGACTTCT-3′ and 5′-CTGCACACCGGGGAATTCTTAGAAATTTAAGAG ATCCTCGTGTAA 3′) to generate pCDNA3 HisZta.
TAP.
HEK 293 cells (14) expressing Tap Zta were selected with G418. Zta and associated proteins were purified using the method previously described by Puig et al. in a HEPES-based buffer (40). The purified Zta complex was eluted and analyzed by mass spectrometry by the University of Sussex Proteomics Centre.
Nickel pulldown and Western blotting.
pCDNA3 or pCDNA3 HisZta was transfected into Raji cells using Amaxa transfection technology according to the manufacturer's protocol. The solution V and program M-13 were used with 5 × 106 cells and 2 μg of DNA per electroporation. HEK 293 cells and HEK 293 cells harboring a recombinant EBV plasmid with a deletion of the BZLF-1 gene (designated HEK 293-BZLF1-KO) (10) were transfected using the Effectene transfection reagent (Qiagen) with 2.5 × 106 cells and 10 μg of DNA. Forty-eight hours after transfection, the cells were washed and lysed in 500 μl of TAP tag lysis buffer. After three freeze-thaw cycles, the cell lysate was centrifuged at 2,000 rpm for 5 min before the supernatant was transferred to 200 μl of His-select nickel affinity gel (Sigma). The cell lysate and nickel resin were incubated together at 4°C overnight. The resin was washed three times and analyzed on a 3 to 8% Tris-acetate NuPAGE gel in Tris-acetate buffer (for 53BP1) or a 12% Bis-Tris NuPAGE gel in morpholinepropanesulfonic acid (MOPS) buffer (for Zta) (Invitrogen). Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred onto nitrocellulose membranes and incubated with BZ1 antibody to Zta (60) or 53BP1 antibody (BD Biosciences). Rabbit anti-mouse secondary antibody (DakoCytomation) was incubated on the membrane, followed by incubation with horseradish peroxidase-linked protein A (GE Healthcare). Proteins then were detected by chemiluminescence.
Effect of knockdown of 53BP1 on EBV lytic cycle using siRNA.
LCL#3 lymphoblastoid cells were seeded at 2 × 105/ml in Opti-MEM (Invitrogen). Small interfering RNA (siRNA) duplexes (Invitrogen) were added to a final concentration of 10 or 20 nM and admixed with the SiPort-neofx transfection reagent (Ambion). siRNA was added at days 1 and 2. Cells were washed on day 3 and placed in fresh OPTIMEM, and a further dose of siRNA was added. Cells were harvested on day 4 for protein content, and the supernatant was harvested for secreted virus. The sequences of the siRNA molecules were the following: 53BP1, 5′UAUUACCGUCUCCUCGUUCTT and 5′ GAACGAGGAGACGGUAAUATT; control, 5′ GGUGCGCUCCUGGACGUAGCCTT and 5′GGCUACGUCCAGGAGCGCACCTT.
Protein expression was detected by Western blot analysis with a 53BP1-specific antibody (ABCAM) and a glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific antibody (Ambion).
In vitro transcription and translation.
RNA was synthesized using a RiboMAX Large Scale RNA Production System kit (Promega). Treatment with DNase was carried out to ensure no vector DNA was present and was used for in vitro translation using wheat germ extract (Promega). Translated protein was run on a 12% NuPAGE Novex Bis-Tris gel in MOPS buffer (Invitrogen) and detected by phosphorimaging (STORM; Amersham). Samples subsequently were diluted to ensure similar levels of labeled protein were present in each reaction.
In vitro association assays.
GST-53BP1 mutants were synthesized in BL21 pLysS bacteria (Invitrogen) as previously described (18, 19) and purified on glutathione Sepharose (GE Healthcare) in phosphate-buffered saline (PBS). Purified GST-53BP1 slurry was incubated with in vitro-translated protein diluted (10 μl in 360 μl) in binding buffer and incubated for 2 h at 4°C. The beads were washed and analyzed on a 12% NuPAGE Novex Bis-Tris gel in MOPS buffer (Invitrogen). Proteins were detected by phosphorimaging (STORM; Amersham).
Ability of Zta and mutants to activate EBV lytic cycle.
HEK 293-BZLF1-KO cells were transfected with pBABE vector, pBABEZta, or a pBABEZta mutant using Effectene in a 6-well plate format (45). Cells (1 × 105) were seeded 1 day prior to transfection, and 0.8 μg of DNA was used. For RNA analysis, cells were harvested after 48 h and total RNA was prepared. Gene expression was assayed using quantitative PCR (qPCR) primers specific for BMRF1 and the host housekeeping gene L32 as a control. For DNA, cells were harvested after 96 h and lysed, and genomic DNA was prepared using a Wizard genomic DNA purification kit (Promega) and then analyzed for EBV genome and human genome copy numbers as described previously (12).
RESULTS
Identification of Zta-interacting cellular proteins.
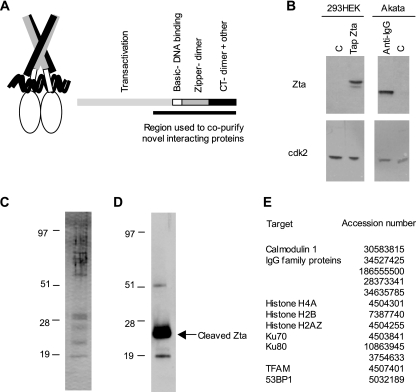
TAP was used to undertake a global proteomic search for host proteins that interact with Zta. The C-terminal 113 amino acids of Zta containing the basic, zipper, and carboxyl-terminal regions was fused to a protein A-calmodulin binding protein tag (called Tap Zta) containing a TEV protease cleavage motif and was expressed in epithelial cells (Fig. 1). Tap Zta protein was expressed at levels equivalent to those of Burkitt's lymphoma cells undergoing the EBV lytic cycle (Fig. 1B). Tap Zta and the proteins associated with it were purified using immunoglobulin affinity chromatography, released by TEV cleavage, and further purified using calmodulin affinity chromatography. Tap Zta-associated proteins were visualized by silver staining (Fig. 1C), and the location of the cleaved Tap Zta protein was identified by Western blotting (Fig. 1D). Host proteins that interact with Zta were identified using liquid chromatography-coupled mass spectrometry (Fig. 1E). Proteins expected to interact with one or another purification resin were identified (calmodulin and immunoglobulins). In addition, three histones, Ku70, Ku80, and mitochondrial transcription factor A, as well as 53BP1, also copurify with Tap Zta. A recent study by Wiedmer et al. also identified Ku70, Ku80, and histones as proteins that copurify with Zta (58). Interestingly, none of the previously reported proteins that functionally interact with Zta were isolated in either system, namely p53 (62), RAR, RXR (52), NF-κB, C/EBPα, CBP (1, 61), RACK1 (3), or TBP (33). Thus, the interaction of Zta with the cellular proteins listed in Fig. 1 appears to be either more stringent or more abundant in these cells than its interactions with some previously reported binding partners.
FIG. 1.
Cellular proteins that copurify with Zta in vivo. (A) On the left is a schematic showing the current model for the structure of Zta bound to DNA, and on the right it is shown in a linear form depicting the corresponding regions of Zta and the area used as bait for TAP. CT, carboxy-terminal region. (B) Extracts from Tap Zta-expressing HEK 293 cells, or vector only (C), and immunoglobulin G (IgG)-induced Zta in Akata cells, or nontreated cells (C), were fractionated on SDS-PAGE gels, and the relative expression of Tap Zta and Zta were determined by Western blotting. Western blotting of CDK2 was used to determine loading equivalence. The panels showing induced Zta and Tap Zta were taken from the same gel and were processed identically for both Zta and cdk2 staining. (C) Tap Zta and associated proteins were purified from HEK 293 cells using the tandem affinity approach, fractionated on an SDS-PAGE gel, and silver stained. (D) Tap Zta and associated proteins depicted in panel C were fractionated on an SDS-PAGE gel, and Tap Zta was identified by Western blotting with a specific antibody. (E) A list of proteins that copurified with the bZIPCT region of Zta and that were identified by mass spectrometry is shown, together with their accession numbers.
53BP1 interacts with Zta during viral replication in vivo.
The DNA damage response is activated during the replication of EBV (8, 23) and is required for the replication of a related gamma herpesvirus, MHV68 (54). It therefore is appealing to speculate that the observed interaction between Zta and 53BP1 contributes to EBV replication.
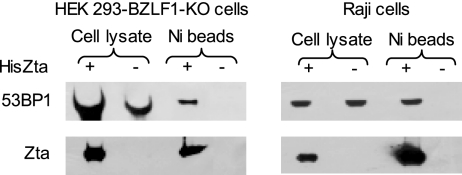
To question whether 53BP1 is a bona fide binding partner of Zta, in vivo protein association assays were carried out in two cell systems containing EBV that were permissive to lytic reactivation. HEK 293 cells harboring recombinant EBV with a deletion of the BZLF-1 gene (HEK 293-BZLF1-KO) (10) were used as an epithelial model, and an EBV-positive Burkitt's Lymphoma cell line (Raji), which is frequently used to analyze early events in the EBV lytic cycle, was utilized as a B-lymphocyte model. HEK 293-BZLF1-KO cells are readily transfected with lipofection-based reagents, whereas Raji cells are less tractable but were amenable to the transfection of expression vectors following electroporation. Both cell lines were transfected with an expression vector encoding a His-tagged version of Zta, pCDNA3HisZta, to induce lytic cycle reactivation. Single-step purification on nickel resin was used to isolate Zta and associated proteins and to question whether endogenous 53BP1 interacted with full-length Zta during viral replication. Western blot analysis of the purified proteins revealed that 53BP1 was present on the nickel resin from both HEK 293-BZLF1-KO and Raji cells following HisZta expression (Fig. 2), indicating that 53BP1 interacts with Zta in both epithelial cells and B lymphocytes. Lytic replication is known to stall in Raji cells prior to EBV genome replication (4, 64), and in our hands, HEK 293-BZLF1-KO cells do not replicate the EBV genome until 72 to 96 h after transfection with BZLF1 (data not shown). This suggests that the observed interaction between Zta and 53BP1 observed 48 h after the transfection of BZLF1 occurs during the early phase of the lytic cycle.
FIG. 2.
53BP1 association with Zta in vivo. HEK 293-BZLF1-KO cells were transfected with pCDNA3 alone or with pCDNA3 HisZta using Effectene transfection reagent. Raji cells were transfected with the same constructs using Amaxa transfection technology. After 48 h, cells were lysed and nickel agarose-associated proteins purified. Samples were analyzed by Western blotting for 53BP1 (top) and Zta (bottom), as indicated. Proteins then were detected by chemiluminescence.
The BRCA1 C-terminal (BRCT) repeat region of 53BP1 binds to Zta in vitro.
The interaction between Zta and 53BP1 may influence EBV replication. To question whether the interaction is relevant, we attempted to identify mutants of Zta that are compromised for their interaction with 53BP1 but competent for other functions. The first step toward this goal was to establish an in vitro assay to assess the interaction.
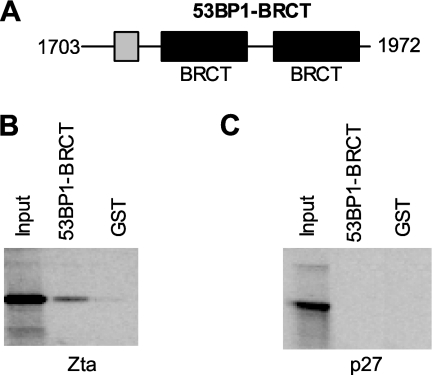
53BP1 is a large protein (1,972 amino acids); therefore, to decipher which region of 53BP1 was required to bind to Zta, an in vitro protein association assay was developed that used a series of overlapping GST-tagged 53BP1 mutants (19) and full-length Zta, which were generated using an in vitro translation system (51). These assays identified a region of 53BP1 contained within the 53BP1-BRCT construct that encompasses the nuclear localization signal and the tandem BRCT repeats (Fig. 3). The GST-53BP1-BRCT protein routinely associated with 5% of the input proteins in these assays. As both Zta and 53BP1 can bind DNA, the potential exists that the interaction that we observed between the two proteins in B cells is not due to direct interaction but rather to a common interaction with DNA. However, GST-53BP1-BRCT does not contain the 53BP1 DNA binding region of 53BP1, eliminating concern over this potential artifact. An unrelated protein, p27KIP1, did not bind to 53BP1 in the GST pulldown assay, confirming that the interaction between 53BP1-BRCT and Zta was specific. Further experiments with just the C-terminal 113 amino acids of Zta present in Tap Zta also confirmed the interaction (data not shown).
FIG. 3.
53BP1 association with Zta in vitro. (A) Schematic showing known motifs in the BRCT region of 53BP1, a nuclear localization sequence (NLS; gray filled box), and two BRCA1 C-terminal domains (BRCT; black boxes). (B) In vitro associations between GST and GST-53BP1-BRCT and in vitro-translated Zta were determined in GST binding buffer. The left lane shows in vitro-translated protein added to the reaction mixture (marked Input). The remaining three lanes show any association Zta makes with the indicated GST proteins. (C) The same experiment as that described for panel B was carried out with in vitro-translated human p27KIP1 as a control.
The C-terminal region of Zta is responsible for 53BP1 binding.
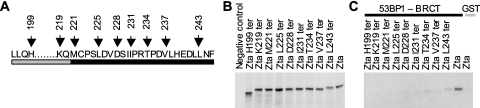
The in vivo and in vitro association assays established that the region of Zta responsible for the interaction with 53BP1 is located in the carboxy-terminal half of the protein, between amino acids 133 and 245. Subsequent studies to refine the region(s) of Zta required for the interaction with 53BP1 used a series of carboxyl-terminal termination mutants of Zta (Fig. 4) (44). These 35S-labeled truncated proteins were generated using an in vitro translation system and were applied to the in vitro association assay with GST-tagged 53BP1-BRCT. The purification of the 53BP1/Zta complexes revealed that the removal of the three extreme C-terminal amino acids of Zta (designated ZtaL243ter) was sufficient to considerably reduce Zta binding to 53BP1-BRCT (Fig. 4). The removal of a further five amino acids (ZtaV237ter), or more substantial deletions, completely abrogated the interaction. This demonstrates that the carboxyl-terminal region of Zta contributes to the interaction of Zta with 53BP1.
FIG. 4.
C-terminal region of Zta is important in 53BP1 binding in vitro. (A) Schematic diagram showing part of the Zta sequence with the location of the Zta termination mutants indicated above the zipper and C-terminal regions. The zipper region is shown by a gray box, and the carboxyl-terminal region is shown by the black box below the sequence. (B) The indicated in vitro-translated proteins were generated in wheat germ extract. Zta and its termination mutants were analyzed by SDS-PAGE on a 12% gel and quantitated after detection using phosphorimaging. (C) Equivalent quantities of in vitro-translated proteins, indicated above each lane, were incubated with GST-53BP1-BRCT-Sepharose or GST, as indicated, and the Zta that associates is shown.
The absolute conservation of the C terminus of Zta in all isolates of EBV that have been examined to date (15, 17, 21) suggests that this region of Zta is functionally relevant; however, interaction with 53BP1 is the first role that has been ascribed to it.
The interaction between 53BP1 and Zta is not required for basic DNA binding or transactivation functions of Zta.
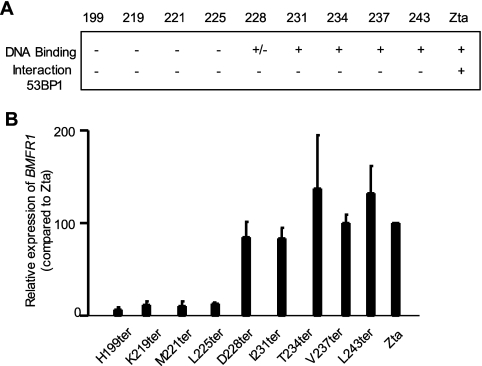
The identification of the Zta mutants that are compromised in their ability to interact with 53BP1 provides a potential route to address the importance of the 53BP1-Zta interaction in vivo, should they retain basic functions of Zta. The ability of the Zta termination series to form functional dimers and interact with DNA has been reported previously (44) and is summarized in Fig. 5. Importantly, four of the mutants from this series that are compromised for interaction with 53BP1 (ZtaL243ter, ZtaV237ter, Zta234ter, and Zta231ter) are able to form dimers and to interact with DNA (44). Here, we assessed the ability of the Zta mutants to transactivate the expression of a known target on the latent EBV genome in HEK 293-BZLF1-KO cells (Fig. 5B). All of the mutants that can bind DNA retain the ability to transactivate the expression of the viral gene BMRF-1 in this system. This reveals that those mutants of Zta that are compromised in their interaction with 53BP1 retain the characteristics of DNA binding and transactivation.
FIG. 5.
Zta mutants that are defective for binding to 53BP1 retain the ability to form dimers, bind DNA, and transactivate an EBV gene. (A) Schematic diagram summarizing the ability of the Zta termination mutant to form dimers, bind DNA, and activate the expression of BMRF1 from the endogenous EBV genome. (B) The indicated Zta mutants were expressed in HEK 293-BZLF1-KO cells, and 48 h later RNA was prepared and the amount of BMRF1 RNA relative to the expression of a control cellular gene and each Zta mutant was determined using a series of qPCR assays. The relative expression of BMRF1 is indicated, together with the standard errors from replicate experiments.
The ability to activate the viral lytic cycle correlates with the ability to interact with 53BP1.
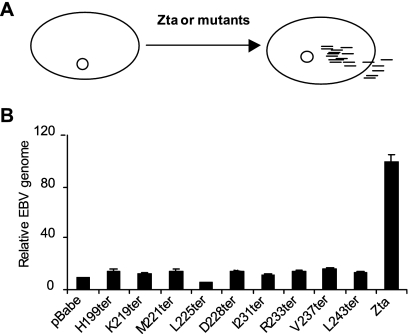
To assess whether the interaction between 53BP1 and Zta affected the ability of Zta to induce the viral lytic cycle, we questioned whether viral genome replication was affected by the termination mutants. The Zta mutant series was introduced into HEK 293-BZLF1-KO cells by transfection, and the EBV genome load within the cells was determined subsequently using qPCR (Fig. 6). A basal level of EBV DNA was detected in all cells due to the presence of latent EBV genomes, which is represented in the circular episomal form in Fig. 6. The expression of full-length Zta was anticipated to activate EBV lytic replication, resulting in an accumulation of EBV genomes within cells en route to their secretion as virions, as represented by linear EBV genomes. The expression of Zta successfully activated EBV genome replication, measured as an increase in intracellular genome accumulation. However, the deletion of the final three carboxyl-terminal amino acids of Zta was sufficient to abolish EBV genome replication in this system. The clear correlation between the compromised interaction of Zta mutants with 53BP1 and the loss of their ability to activate the EBV lytic cycle, as measured by lytic genome replication, demonstrates a role for the Zta interaction with 53BP1 for the EBV lytic cycle. The precise step in viral replication that requires this interaction remains to be determined.
FIG. 6.
C-terminal region of Zta is defective for EBV replication. (A) A schematic diagram represents the latent EBV genome as a circular episome and lytic replication induced by Zta generating linear molecules both within cells and outside cells packaged as virions. (B) Zta and the indicated Zta termination mutants were expressed in HEK 293-BZLF1-KO cells and harvested after 96 h. Cells were analyzed for EBV genome and human genome copy numbers. The graph shows the levels of EBV genome present (relative to those of the human genome) taken from duplicate experiments, with the standard errors shown.
Knockdown of 53BP1 expression reduces lytic replication.
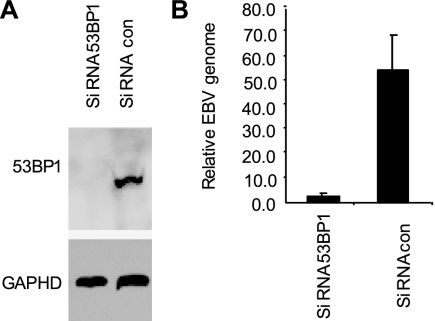
To further question the relevance of 53BP1 for EBV replication, we examined the effect on viral replication of knocking down 53BP1 expression using siRNA. LCL#3 cells were chosen for this study. A small proportion of these cells spontaneously initiate the viral lytic cycle at any one time. siRNA directed against 53BP1 or a control gene was introduced into LCL#3 cells for a 4-day period. This approach achieved a decrease in 53BP1 protein abundance in the cells (Fig. 7). Virion DNA released from the cells between days 3 and 4 was prepared, and the amount of viral DNA was quantitated using qPCR. A substantial reduction in EBV was found that correlated with the knockdown of 53BP1 expression in these cells (Fig. 7).
FIG. 7.
53BP1 contributes to EBV genome replication. siRNA duplexes directed against 53BP1 or a control (con) duplex (10 nM) were introduced into LCL#3 cells for 4 days. (A) The effect of each siRNA on 53BP1 protein expression and the expression of the protein encoded by the housekeeping gene GAPDH were monitored by the Western blot analysis of cell extracts with specific antibodies as indicated. (B) Supernatant from the cells was harvested, and the amount of EBV genome in virions was determined by the qPCR detection of the EBV genome.
DISCUSSION
During the EBV lytic cycle, 80 or so viral genes are expressed, and the EBV episomal DNA is replicated via long linear double-stranded DNA molecules that subsequently are cleaved into genome-length units during packaging into virions (55). Zta is critical for this process, acting as a transcriptional activator and a replication factor and by perturbing host signal transduction and cell cycle regulation (49). The interaction that we identify here between Zta and the host protein 53BP1 implies a direct connection with the DNA damage response. Using a combination of viral mutants and the knockdown of gene expression, we show that the interaction of Zta with 53BP1 is important for EBV lytic cycle replication.
This publication also demonstrates that the extreme carboxy-terminal end of Zta (L243-F245) is required for viral replication. No functions previously had been ascribed to this region, although a recent publication revealed a role for the interaction between position D236 in the C-terminal tail and the zipper for the viral lytic cycle (32).
53BP1 is intimately involved in the DNA damage response induced by exposure to ionizing radiation. It rapidly relocalizes within cells to form distinct foci on damaged DNA. It is phosphorylated by ATM and forms multicomponent complexes that include phosphorylated H2AX and components of the MRN complex (20, 26). The precise function of the complex is unknown; however, as 53BP1 has no known catalytic activity, the hypothesis that it acts as a scaffold to bring other components into close proximity provides a working model to account for its clear role in the DNA damage response.
The region of 53BP1 that associates with Zta contains two BRCT regions. Repeat units of this domain are present in many DNA repair and checkpoint-associated proteins (5). The domains can provide phosphorylation-dependent protein-docking functions that may be involved in the recruitment of proteins to nuclear foci following DNA damage (28). Intriguingly, the BRCT region of 53BP1 also is involved in a phosphorylation-independent interaction with p53 (9, 22). This is of interest, as both Zta and 53BP1 are able to interact with p53 independently (18, 63) to generate a local environment in which cross-regulation may be enhanced.
The involvement of 53BP1 in EBV lytic replication is particularly intriguing, as the replication of the EBV genome generates at least one exposed DNA end, which is the start of the newly replicating linear DNA. No protection mechanism is known to shield the newly replicating viral DNA, and potentially more exposed unprotected ends are generated following the cleavage of the replicated DNA to generate the unit-length viral genomes during packaging into virions. During such circumstances, it would be advantageous for EBV to manipulate any resultant DNA damage response.
During EBV replication, signal transduction through ATM is activated, resulting in the phosphorylation of H2AX, ATM, Chk2, and p53 and the recruitment of the MRN complex (8, 23). The potential danger to EBV of activating the ATM signal transduction pathway is the probability that signal transduction through ATM would prime attempts to repair the newly replicated ends of viral DNA, resulting in structures that either do not generate viable virus or promote programmed cell death. However, in EBV-positive cells, signal transduction falls short of the expected activation of downstream targets such as p21CIP1 (26). The relevance of the activation of the ATM pathway for EBV replication was questioned previously, using caffeine to inhibit signal transduction. In that study, the enforced expression of Zta was employed to activate viral replication in a marmoset cell line (23). Surprisingly, no effect of caffeine on viral replication was detected, suggesting that ATM activation was not required for viral replication.
The complexity of the interaction of gammaherpesviruses with the DNA damage response increased with the discovery that H2AX, a key component of the DNA damage response pathway, plays an important role in the replication of the related gammaherpesvirus MHV68 (54). H2AX is directly phosphorylated by MHV68 protein kinase (vPK) and is required for viral replication in some cell lineages (54, 59). Interestingly, the EBV homologue of vPK, BGLF4, coassociates with Zta (2) and may provide a link between H2AX and EBV replication: it is found in viral replication compartments (56), it is essential for viral replication (13), and it is able to directly phosphorylate H2AX (54). Thus, H2AX is an additional candidate on this pathway that may modulate EBV replication, but its potential is unproven.
Our conclusions that 53BP1 expression and 53BP1-Zta interaction aids or may even be required for viral replication suggest that the activation of ATM that occurs is detrimental to virus replication, and that the interaction between Zta and 53BP1 reduces or negates this. This may be complemented by the ability of Zta to neutralize the function of p53, a downstream effector of ATM that is required to drive apoptosis (30, 31, 63).
Acknowledgments
We thank Tomoo Ogi and Alan Lehmann (University of Sussex) for the TAP vector, Lucas Bowler (University of Sussex Proteomics Centre) for undertaking mass spectrometry, Henri-Jacques Delecluse for HEK 293-BZLF1-KO cells, and Mirjam Meyer for molecular biology assistance.
This project was funded by grants from the Medical Research Council, Biotechnology and Biological Sciences Research Council, and the Association for International Cancer Research United Kingdom.
Footnotes
Published ahead of print on 5 August 2009.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Adamson, A. L., and S. Kenney. 1999. The Epstein-Barr virus BZLF1 protein interacts physically and functionally with the histone acetylase CREB-binding protein. J. Virol. 73:6551-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai, R., A. Kato, K. Kato, M. Kanamori-Koyama, K. Sugimoto, T. Sairenji, Y. Nishiyama, and Y. Kawaguchi. 2006. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J. Virol. 80:5125-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann, M., O. Gires, W. Kolch, H. Mischak, R. Zeidler, D. Pich, and W. Hammerschmidt. 2000. The PKC targeting protein RACK1 interacts with the Epstein-Barr virus activator protein BZLF1. Eur. J. Biochem. 267:3891-3901. [DOI] [PubMed] [Google Scholar]
- 4.Biggin, M., M. Bodescot, M. Perricaudet, and P. Farrell. 1987. Epstein-Barr virus gene expression in P3HR1-superinfected Raji cells. J. Virol. 61:3120-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bork, P., K. Hofmann, P. Bucher, A. F. Neuwald, S. F. Altschul, and E. V. Koonin. 1997. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 11:68-76. [PubMed] [Google Scholar]
- 6.Cayrol, C., and E. Flemington. 1996. G0/G1 growth arrest mediated by a region encompassing the basic leucine zipper (bZIP) domain of the Epstein-Barr virus transactivator Zta. J. Biol. Chem. 271:31799-31802. [DOI] [PubMed] [Google Scholar]
- 7.Cayrol, C., and E. K. Flemington. 1996. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 15:2748-2759. [PMC free article] [PubMed] [Google Scholar]
- 8.Daikoku, T., A. Kudoh, M. Fujita, Y. Sugaya, H. Isomura, N. Shirata, and T. Tsurumi. 2005. Architecture of replication compartments formed during Epstein-Barr virus lytic replication. J. Virol. 79:3409-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derbyshire, D. J., B. P. Basu, L. C. Serpell, W. S. Joo, T. Date, K. Iwabuchi, and A. J. Doherty. 2002. Crystal structure of human 53BP1 BRCT domains bound to p53 tumour suppressor. EMBO J. 21:3863-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flemington, E. K. 2001. Herpesvirus lytic replication and the cell cycle: arresting new developments. J. Virol. 75:4475-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher, A., A. A. Armstrong, J. MacKenzie, L. Shield, G. Khan, A. Lake, S. Proctor, P. Taylor, G. B. Clements, and R. F. Jarrett. 1999. Detection of Epstein-Barr virus (EBV) genomes in the serum of patients with EBV-associated Hodgkin's disease. Int. J. Cancer 84:442-448. [DOI] [PubMed] [Google Scholar]
- 13.Gershburg, E., S. Raffa, M. R. Torrisi, and J. S. Pagano. 2007. Epstein-Barr virus-encoded protein kinase (BGLF4) is involved in production of infectious virus. J. Virol. 81:5407-5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 15.Grunewald, V., M. Bonnet, S. Boutin, T. Yip, H. Louzir, M. Levrero, J. M. Seigneurin, M. Raphael, R. Touitou, D. Martel-Renoir, C. Cochet, A. Durandy, P. Andre, W. Lau, Y. Zeng, and I. Joab. 1998. Amino-acid change in the Epstein-Barr virus ZEBRA protein in undifferentiated nasopharyngeal carcinomas from Europe and North Africa. Int. J. Cancer 75:497-503. [DOI] [PubMed] [Google Scholar]
- 16.Hicks, M. R., S. S. Al-Mehairi, and A. J. Sinclair. 2003. The zipper region of Epstein-Barr virus bZIP transcription factor Zta is necessary but not sufficient to direct DNA binding. J. Virol. 77:8173-8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hicks, M. R., S. Balesaria, C. Medina-Palazon, M. J. Pandya, D. N. Woolfson, and A. J. Sinclair. 2001. Biophysical analysis of natural variants of the multimerization region of Epstein-Barr virus lytic-switch protein BZLF1. J. Virol. 75:5381-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwabuchi, K., P. L. Bartel, B. Li, R. Marraccino, and S. Fields. 1994. Two cellular proteins that bind to wild-type but not mutant p53. Proc. Natl. Acad. Sci. USA 91:6098-6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwabuchi, K., B. P. Basu, B. Kysela, T. Kurihara, M. Shibata, D. Guan, Y. Cao, T. Hamada, K. Imamura, P. A. Jeggo, T. Date, and A. J. Doherty. 2003. Potential role for 53BP1 in DNA end-joining repair through direct interaction with DNA. J. Biol. Chem. 278:36487-36495. [DOI] [PubMed] [Google Scholar]
- 20.Jeggo, P. A., and M. Lobrich. 2006. Contribution of DNA repair and cell cycle checkpoint arrest to the maintenance of genomic stability. DNA Repair (Amsterdam) 5:1192-1198. [DOI] [PubMed] [Google Scholar]
- 21.Ji, K. M., C. L. Li, G. Meng, A. D. Han, and X. L. Wu. 2008. New BZLF1 sequence variations in EBV-associated undifferentiated nasopharyngeal carcinoma in southern China. Arch. Virol. 153:1949-1953. [DOI] [PubMed] [Google Scholar]
- 22.Joo, W. S., P. D. Jeffrey, S. B. Cantor, M. S. Finnin, D. M. Livingston, and N. P. Pavletich. 2002. Structure of the 53BP1 BRCT region bound to p53 and its comparison to the Brca1 BRCT structure. Genes Dev. 16:583-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudoh, A., M. Fujita, L. Zhang, N. Shirata, T. Daikoku, Y. Sugaya, H. Isomura, Y. Nishiyama, and T. Tsurumi. 2005. Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J. Biol. Chem. 280:8156-8163. [DOI] [PubMed] [Google Scholar]
- 24.Landschulz, W. H., P. F. Johnson, and S. L. McKnight. 1988. The leucine zipper protein: a hypothetical structure common to a new class of DNA binding proteins. Science 240:1759-1764. [DOI] [PubMed] [Google Scholar]
- 25.Lilley, C. E., R. A. Schwartz, and M. D. Weitzman. 2007. Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol. 15:119-126. [DOI] [PubMed] [Google Scholar]
- 26.Löbrich, M., and P. A. Jeggo. 2005. The two edges of the ATM sword: co-operation between repair and checkpoint functions. Radiother. Oncol. 76:112-118. [DOI] [PubMed] [Google Scholar]
- 27.Macsween, K. F., and D. H. Crawford. 2003. Epstein-Barr virus—recent advances. Lancet Infect. Dis. 3:131-140. [DOI] [PubMed] [Google Scholar]
- 28.Manke, I. A., D. M. Lowery, A. Nguyen, and M. B. Yaffe. 2003. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science 302:636-639. [DOI] [PubMed] [Google Scholar]
- 29.Mauser, A., E. Holley-Guthrie, D. Simpson, W. Kaufmann, and S. Kenney. 2002. The Epstein-Barr virus immediate-early protein BZLF1 induces both a G2 and a mitotic block. J. Virol. 76:10030-10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauser, A., E. Holley-Guthrie, A. Zanation, W. Yarborough, W. Kaufmann, A. Klingelhutz, W. T. Seaman, and S. Kenney. 2002. The Epstein-Barr virus immediate-early protein BZLF1 induces expression of E2F-1 and other proteins involved in cell cycle progression in primary keratinocytes and gastric carcinoma cells. J. Virol. 76:12543-12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauser, A., S. Saito, E. Appella, C. W. Anderson, W. T. Seaman, and S. Kenney. 2002. The Epstein-Barr Virus immediate-early protein BZLF1 regulates p53 function through multiple mechanisms. J. Virol. 76:12503-12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald, C. M., C. Petosa, and P. J. Farrell. 2009. Interaction of Epstein-Barr virus BZLF1 C-terminal tail structure and core zipper is required for DNA replication but not for promoter transactivation. J. Virol. 83:3397-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikaélian, I., E. Drouet, V. Marechal, G. Denoyel, J. C. Nicolas, and A. Sergeant. 1993. The DNA-binding domain of two bZIP transcription factors, the Epstein-Barr virus switch gene product EB1 and Jun, is a bipartite nuclear targeting sequence. J. Virol. 67:734-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, G., A. El-Guindy, J. Countryman, J. Ye, and L. Gradoville. 2007. Lytic cycle switches of oncogenic human gammaherpesviruses. Adv. Cancer Res. 97:81-109. [DOI] [PubMed] [Google Scholar]
- 35.Morand, P., M. Budayova-Spano, M. Perrissin, C. W. Muller, and C. Petosa. 2006. Expression, purification, crystallization and preliminary X-ray analysis of a C-terminal fragment of the Epstein-Barr virus ZEBRA protein. Acta Crystallogr. F Struct. Biol. Cryst. Commun. 62:210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison, T. E., and S. C. Kenney. 2004. BZLF1, an Epstein-Barr virus immediate-early protein, induces p65 nuclear translocation while inhibiting p65 transcriptional function. Virology 328:219-232. [DOI] [PubMed] [Google Scholar]
- 37.Morrison, T. E., A. Mauser, A. Klingelhutz, and S. C. Kenney. 2004. Epstein-Barr virus immediate-early protein BZLF1 inhibits tumor necrosis factor alpha-induced signaling and apoptosis by downregulating tumor necrosis factor receptor 1. J. Virol. 78:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petosa, C., P. Morand, F. Baudin, M. Moulin, J. B. Artero, and C. W. Muller. 2006. Structural basis of lytic cycle activation by the Epstein-Barr virus ZEBRA protein. Mol. Cell 21:565-572. [DOI] [PubMed] [Google Scholar]
- 39.Prang, N. S., M. W. Hornef, M. Jager, H. J. Wagner, H. Wolf, and F. M. Schwarzmann. 1997. Lytic replication of Epstein-Barr virus in the peripheral blood: analysis of viral gene expression in B lymphocytes during infectious mononucleosis and in the normal carrier state. Blood 89:1665-1677. [PubMed] [Google Scholar]
- 40.Puig, O., F. Caspary, G. Rigaut, B. Rutz, E. Bouveret, E. Bragado-Nilsson, M. Wilm, and B. Seraphin. 2001. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24:218-229. [DOI] [PubMed] [Google Scholar]
- 41.Rigaut, G., A. Shevchenko, B. Rutz, M. Wilm, M. Mann, and B. Seraphin. 1999. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17:1030-1032. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez, A., M. Armstrong, D. Dwyer, and E. Flemington. 1999. Genetic dissection of cell growth arrest functions mediated by the Epstein-Barr virus lytic gene product, Zta. J. Virol. 73:9029-9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez, A., E. J. Jung, Q. Yin, C. Cayrol, and E. K. Flemington. 2001. Role of c-myc regulation in Zta-mediated induction of the cyclin-dependent kinase inhibitors p21 and p27 and cell growth arrest. Virology 284:159-169. [DOI] [PubMed] [Google Scholar]
- 44.Schelcher, C., S. Al Mehairi, E. Verrall, Q. Hope, K. Flower, B. Bromley, D. N. Woolfson, M. J. West, and A. J. Sinclair. 2007. Atypical bZIP domain of viral transcription factor contributes to stability of dimer formation and transcriptional function. J. Virol. 81:7149-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schelcher, C., S. Valencia, H. J. Delecluse, M. Hicks, and A. J. Sinclair. 2005. Mutation of a single amino acid residue in the basic region of the Epstein-Barr virus (EBV) lytic cycle switch protein Zta (BZLF1) prevents reactivation of EBV from latency. J. Virol. 79:13822-13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schepers, A., D. Pich, and W. Hammerschmidt. 1996. Activation of oriLyt, the lytic origin of DNA replication of Epstein-Barr virus, by BZLF1. Virology 220:367-376. [DOI] [PubMed] [Google Scholar]
- 47.Schepers, A., D. Pich, and W. Hammerschmidt. 1993. A transcription factor with homology to the AP-1 family links RNA transcription and DNA replication in the lytic cycle of Epstein-Barr virus. EMBO J. 12:3921-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair, A., S. Yarranton, and C. Schelcher. 2006. DNA-damage response pathways triggered by viral replication. Expert Rev. Mol. Med. 8:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinclair, A. J. 2003. bZIP proteins of human gamma herpesviruses. J. Gen. Virol. 84:1941-1949. [DOI] [PubMed] [Google Scholar]
- 50.Sinclair, A. J. 2006. Unexpected structure of Epstein-Barr virus lytic cycle activator Zta. Trends Microbiol. 14:289-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinclair, A. J., M. Brimmell, F. Shanahan, and P. J. Farrell. 1991. Pathways of activation of the Epstein-Barr virus productive cycle. J. Virol. 65:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sista, N. D., C. Barry, K. Sampson, and J. Pagano. 1995. Physical and functional interaction of the Epstein-Barr virus BZLF1 transactivator with the retinoic acid receptors RAR alpha and RXR alpha. Nucleic Acids Res. 23:1729-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talbot, S. J., and D. H. Crawford. 2004. Viruses and tumours—an update. Eur. J. Cancer 40:1998-2005. [DOI] [PubMed] [Google Scholar]
- 54.Tarakanova, V. L., V. Leung-Pineda, S. Hwang, C. W. Yang, K. Matatall, M. Basson, R. Sun, H. Piwnica-Worms, B. P. Sleckman, and H. W. T. Virgin. 2007. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe 1:275-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsurumi, T., M. Fujita, and A. Kudoh. 2005. Latent and lytic Epstein-Barr virus replication strategies. Rev. Med. Virol. 15:3-15. [DOI] [PubMed] [Google Scholar]
- 56.Wang, J. T., P. W. Yang, C. P. Lee, C. H. Han, C. H. Tsai, and M. R. Chen. 2005. Detection of Epstein-Barr virus BGLF4 protein kinase in virus replication compartments and virus particles. J. Gen. Virol. 86:3215-3225. [DOI] [PubMed] [Google Scholar]
- 57.Weitzman, M. D., C. T. Carson, R. A. Schwartz, and C. E. Lilley. 2004. Interactions of viruses with the cellular DNA repair machinery. DNA Repair (Amsterdam) 3:1165-1173. [DOI] [PubMed] [Google Scholar]
- 58.Wiedmer, A., P. Wang, J. Zhou, A. J. Rennekamp, V. Tiranti, M. Zeviani, and P. M. Lieberman. 2008. Epstein-Barr virus immediate-early protein Zta co-opts mitochondrial single-stranded DNA binding protein to promote viral and inhibit mitochondrial DNA replication. J. Virol. 82:4647-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie, A., and R. Scully. 2007. Hijacking the DNA damage response to enhance viral replication: gamma-herpesvirus 68 orf36 phosphorylates histone H2AX. Mol. Cell 27:178-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young, L. S., R. Lau, M. Rowe, G. Niedobitek, G. Packham, F. Shanahan, D. T. Rowe, D. Greenspan, J. S. Greenspan, A. B. Rickinson, et al. 1991. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J. Virol. 65:2868-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zerby, D., C. J. Chen, E. Poon, D. Lee, R. Shiekhattar, and P. M. Lieberman. 1999. The amino-terminal C/H1 domain of CREB binding protein mediates Zta transcriptional activation of latent Epstein-Barr virus. Mol. Cell. Biol. 19:1617-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, L., J. L. Chiu, and J. C. Lin. 1998. Activation of human herpesvirus 8 (HHV-8) thymidine kinase (TK) TATAA-less promoter by HHV-8 ORF50 gene product is SP1 dependent. DNA Cell Biol. 17:735-742. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, Q., D. Gutsch, and S. Kenney. 1994. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol. Cell. Biol. 14:1929-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.zur Hausen, H., G. W. Bornkamm, R. Schmidt, and E. Hecker. 1979. Tumor initiators and promoters in the induction of Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 76:782-785. [DOI] [PMC free article] [PubMed] [Google Scholar]