Abstract
Human immunodeficiency virus type 1 (HIV-1) Vpr protein exists in three different forms: soluble, intracellular, and virion associated. Previous studies showed that virion-associated Vpr induces apoptosis in activated peripheral blood mononuclear cells (PBMCs) and Jurkat T cells, but these studies were conducted in the presence of other de novo-expressed HIV proteins that may have had additive proapoptotic effects. In this report, we show that virion-associated Vpr triggers apoptosis through caspases 3/7 and 9 in human T cells independently of other HIV de novo-expressed proteins. In contrast to a previous study, we also detected the activation of caspase 8, the initiator caspase of the death receptor pathway. However, activation of all caspases by virion-associated Vpr was independent of the Fas death receptor pathway. Further analyses showed that virion-associated Vpr enhanced caspase activation in Fas-mediated apoptosis in Jurkat T cells and human activated PBMCs. Thus, our results indicate for the first time that viral particles that contain virion-associated Vpr can cause apoptosis in the absence of other de novo-expressed viral factors and can act in synergy with the Fas receptor pathway, thereby enhancing the apoptotic process in T cells. These findings suggest that virion-associated Vpr can contribute to the depletion of CD4+ lymphocytes either directly or by enhancing Fas-mediated apoptosis during acute HIV-1 infection and in AIDS.
Vpr is a small accessory protein of 14 kDa encoded by the human immunodeficiency virus type 1 (HIV-1) genome that is well conserved in HIV-1, HIV-2, and simian immunodeficiency virus (SIV) (21, 68). It exists in three different forms: extracellular, when found in cerebrospinal fluids or plasma; intracellular, when expressed from the genome; and virion-associated, when packaged into the virion via interaction with Gag p6 (4, 36, 60). We and others have shown that Vpr is capable of mediating several different potential biological functions or effects in vivo. These pleiotropic functions include cell cycle arrest in the G2 plus M phase (24, 28, 52, 53), transactivation of the long terminal repeat, and immune suppression by downregulating the expression of NF-κB (3, 12). Vpr facilitates infection in nondividing cells, such as macrophages (7, 13, 69). In addition, it displays proapoptotic effects that may contribute to the decline of CD4+ T-cell counts during HIV-1 infection (2, 25, 43, 44, 62-64, 75).
Apoptosis can be initiated through two different pathways (intrinsic and extrinsic/death receptor), both of which result in several events, such as caspase activation, phosphatidylserine (PS) exposure on the outer leaflet of the plasma membrane, mitochondrial-membrane potential (ΔΨm) dissipation, DNA condensation, and degradation, that can be measured and therefore can be used as early or late markers of that death process (see references 14, 18, and 22 for reviews). The extrinsic pathway is activated through the binding of death ligands, such as FasL and TRAIL (tumor necrosis factor-related apoptosis-inducing ligand), to their death receptors (Fas and DR5 [death receptor 5], respectively), whereas the intrinsic pathway is initiated by intracellular insults, such as DNA damage. Each pathway is typically characterized by the activation of a specific initiator caspase—caspase 8 for the extrinsic pathway and caspase 9 for the intrinsic pathway—resulting in the activation of downstream effector caspases 3, 6, and 7 (see references 14 and 18 for reviews).
The proapoptotic property of each form of Vpr has been studied. It is now clear that de novo-expressed Vpr can induce apoptosis in various types of cells in vitro and in vivo (2, 44, 48, 64, 76). Recombinant Vpr was shown to induce apoptosis in neuronal cells (49, 51), thus mimicking the potential in vivo effects of the extracellular soluble form of Vpr found in the plasma and cerebrospinal fluid of HIV-infected patients (32). We and others have shown that virion-associated Vpr can trigger apoptosis in different types of cells (25, 43, 63).
Different forms of Vpr have been used to study the general mechanism of induction of apoptosis. In one proposed model, recombinant Vpr or its derived peptides were used to show that Vpr would interact directly via its C-terminal domain with the ANT (adenosine nucleotide translocator) protein of the mitochondrial inner membrane to open the permeability transition pore, allowing the release of apoptogenic factors in the cytosol (26, 27, 54). In this model, apoptosis would be triggered independently of caspases and cell cycle arrest. A recent report challenged this model. Indeed, the knockdown of the proapoptotic protein Bax and not that of the ANT protein drastically reduced the induction of caspase activation and apoptosis by de novo-expressed Vpr (2). In that model, Vpr leads to the activation of Bax, which promotes cytochrome c release from mitochondria into the cytosol, resulting in the activation of caspase 9 (see reference 14 for a review). This model is supported by a previous report by Muthumani et al., who investigated the pathway of apoptosis triggered by virion-associated Vpr. They detected caspase 9 activation and not that of caspase 8, the typical initiator caspase of the extrinsic/death receptor pathway. They concluded that Vpr activates the intrinsic pathway through caspase 9 (43).
Besides Vpr, several HIV-1 proteins can independently induce apoptosis directly or favor its onset. It has been shown that the Tat protein can activate apoptosis directly (33). It can also induce the downregulation of the antiapoptotic Bcl-2 (57). The protein Pro can cleave the same antiapoptotic protein (65). Studies have shown that, like Vpr, Vif can induce cell cycle arrest in the G2 plus M phase and interact with the same protein of the ubiquitin ligase complex known to interact with Vpr (15, 55, 70, 71). Therefore, the possibility that all these proteins could act together with virion-associated Vpr to promote cell death, as illustrated by the work of Sakai et al. (55), cannot be excluded. These authors observed that the elimination of both Vif and Vpr, and not that of the individual proteins, resulted in the abrogation of the cytopathic effects induced by the HIV-1 NL4-3 strain. Hence, the real contribution of virion-associated Vpr alone cannot be exactly assessed when other HIV proteins are expressed.
In this study, we hypothesized that particles, both defective and infectious, that incorporate Vpr can induce apoptosis by delivering the protein to their target cells. In order to mimic infection by defective viruses, we infected Jurkat T cells and human activated peripheral blood mononuclear cells (PBMCs) with viral particles that could not express any HIV protein de novo but contained virion-associated Vpr. We show that virion-associated Vpr can induce apoptosis independently of both de novo HIV-expressed proteins and the Fas death receptor pathway. Also, we show that virion-associated Vpr can enhance the activities of effector, as well as initiator, caspases in Jurkat T cells and human activated PBMCs treated with the anti-Fas antibody CH11, thereby resulting in an enhancement of apoptosis. Our results therefore indicate that in the absence of de novo-expressed HIV proteins, virion-associated Vpr can promote apoptosis directly, as well as in concert with normal physiological apoptotic signals.
MATERIALS AND METHODS
Cell culture.
HeLa cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen-Gibco, Carlsbad, CA) supplemented with 10% calf serum (Sigma, St. Louis, MO), l-glutamine, penicillin, and streptomycin. HEK 293T cells were maintained in Iscove's modified Dulbecco's medium (Sigma) supplemented with 10% fetal calf serum (Sigma), penicillin, and streptomycin. Jurkat cells were cultured in RPMI 1640 medium (Invitrogen-Gibco) supplemented with 10% fetal calf serum (Sigma), l-glutamine, penicillin, and streptomycin. PBMCs were prepared by Ficoll-Paque gradient purification from heparinized whole human blood from different healthy donors. The PBMCs were activated for 48 h in complete stimulating medium consisting of RPMI 1640 supplemented with 10% fetal calf serum, l-glutamine, penicillin, streptomycin, 5 μg/ml phytohemagglutinin (Sigma), and 10 U/ml interleukin 2 (Roche, Mannheim, Germany), after which the cells were pelleted and resuspended in complete growth medium (RPMI 1640 supplemented with 10% fetal calf serum, l-glutamine, penicillin, streptomycin, and 10 U/ml interleukin 2) and allowed to rest overnight. The next day, dead cells were removed by using a dead-cell removal kit (Miltenyi, Bergisch Gladbach, Germany), and the remaining live cells were allowed to rest overnight before being infected according to the protocol described below.
Preparation of viral stocks and infections.
All viruses were produced in HEK 293T cells by the calcium phosphate transfection method with the indicated amounts of the following vectors. For Vpr(+)cis virus production, cells were cotransfected with 12.5 μg of pHR′Vpr, which expresses Flag-tagged wild-type Vpr (30); 12.5 μg of pCMVΔR8.2ΔVpr; and 5 μg of pCMV VSV-G. For Vpr(−) virus production, cells were cotransfected with 12.5 μg of pHR′EGFP, 12.5 μg of pCMVΔR8.2ΔVpr, and 5 μg of pCMV VSV-G. For Vpr(+)trans virus production, cells were cotransfected with the same amounts of plasmid indicated above. In addition, Flag-tagged wild-type Vpr was supplied in trans from 0.7 μg of the expression vector BSVpr (28). Flag-tagged Vpr has been previously shown to induce apoptosis and cell cycle arrest in the G2 plus M phase (29, 46). For preparation of NL4-3 EGFP R− E− (NLEGFP) viruses, 21.4 μg of pNL4-3 EGFP R− E− and 8.6 μg of pCMV VSV-G were used. Viral stocks were collected 48 and 72 h posttransfection, concentrated over a 10% sucrose cushion, resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum, and stored at −80°C. The viruses were first normalized for p24gag content by p24 enzyme-linked immunosorbent assay and then titrated on Jurkat cells to determine the number of enhanced green fluorescent protein (EGFP) transduction units/ml. Typically, viral titers for Vpr(−), Vpr(+)trans, and NLEGFP viruses were 4 × 107, 7 × 107, and 7 × 108 EGFP transduction units/ml, respectively. For infection of HeLa cells for cell cycle analyses, 5 × 104 HeLa cells were seeded on the day before infection. The following day, the cells were infected with an equivalent number of EGFP transduction units of viral stocks in the presence of 8 μg/ml Polybrene for 2 hours, after which the medium was changed and the cells were further cultured for 48 h before being harvested for cell cycle analysis. For viral infection of Jurkat cells, the appropriate number of wells of a 96-well plate (Becton Dickinson, Franklin Lakes, NJ) were seeded with 0.4 × 106 cells per well. The cells were then infected with Vpr(−) and Vpr(+)trans viruses at a multiplicity of infection (MOI) of 0.5 in the presence of 8 μg/ml Polybrene for 2 hours, after which 60% of the medium was removed and replaced with fresh medium. Six hours postinfection, 50% of the medium was removed. The cells were counted and resuspended at a density of 0.2 × 106 cells/ml and cultured further for 44 h before analyses for PS externalization, caspase activation, and ΔΨm dissipation. As a reference, cells were also infected with NLEGFP viruses as described in the above protocol. The same protocol was followed to infect human activated PBMCs. The amount of virus used was such that less than 60% of activated PBMCs were infected, as assessed by EGFP expression.
Cell cycle analysis.
Forty-eight hours postinfection, HeLa cells were collected and fixed in 2 ml of cold 70% ethanol overnight. The next day, the cells were washed in phosphate-buffered saline (PBS) and stained with propidium iodide (100 μg/ml propidium iodide, 2% fetal calf serum, 0.1 mg/ml RNase, 0.02% sodium azide in PBS) at 37°C for 20 min (28). All stained cells were acquired on a Cytomics FC 500 flow cytometry system (Beckman Coulter, Fullerton, CA). A total of 10,000 events were collected for each sample. The cell cycle profile of each sample was analyzed with ModFit software (Verity Software House, Topsham, ME) to calculate the percentages of cells in the G1 and G2 plus M phases, and the G2-plus-M/G1 ratio was then calculated.
Induction and blockade of apoptosis.
As positive controls for the caspase assay and annexin V-phycoerythrin (A5PE) staining, cells were treated with either the Fas antibody CH11 (MBL International, Woburn, MA) at the indicated concentration or staurosporine (Sigma) at a concentration of 1 μM for 4 to 6 h, respectively. For the studies of the effect of Vpr on Fas-induced apoptosis, 46 h postinfection, Jurkat cells were either mock treated or treated for 4 and 8 h with 12.5 ng/ml of the Fas antibody CH11. Forty-seven hours postinfection, PBMCs were either mock treated or treated with the Fas antibody CH11 (2.5 μg/ml) for 1 and 3 h. Cells were then harvested and analyzed for caspase activation, PS exposure, and ΔΨm dissipation at the indicated time points. For inhibition of Fas-induced apoptosis, Jurkat cells were incubated with 2.5 μg/ml of the Fas antagonist antibody ZB4 (MBL International) just after infection. As a positive control for the neutralizing effect of the ZB4 antibody, mock-infected Jurkat cells were preincubated with 2.5 μg/ml of the ZB4 antibody for 6 h before addition of 12.5 ng/ml of the Fas antibody CH11. Caspase activity was then measured 2 days later.
Caspase activation assay.
Caspase 3/7, 8, and 9 activities were measured by using the Caspase-Glo bioluminescence assay (Promega, Madison, WI) specific for each caspase. Two days postinfection, 50 μl of a homogeneous cell suspension was aliquoted in a white-walled 96-well plate (Nunc, Fisher Scientific, Chino, CA) and incubated at room temperature for 20 min before 50 μl of room temperature equilibrated Caspase-Glo 3/7, 8, or 9 reagent was added and mixed. After incubation at room temperature for 1 h, the luminescence was measured using the Fluostar Optima Luminometer microplate reader (BMG Labtech Inc., San Diego, CA). Blank values were subtracted, and the increase in activity was calculated based on activity measured from mock-infected cells. Caspase activities for each sample were measured in triplicate.
Annexin V staining.
PS exposure was assessed by using the A5PE apoptosis kit (MBL International) as instructed by the manufacturer. Briefly, 2 days postinfection, 0.2 × 106 cells were collected and resuspended in 500 μl of annexin V binding buffer. The cells were then incubated with 5 μl of A5PE for 5 min at room temperature in the dark. All stained cells were then acquired ungated on a Cytomics FC 500 flow cytometry system (Beckman Coulter). Debris was electronically excluded. A total of 10,000 events were collected for each sample and then analyzed with FCS Express 3 software (Denovo Software, Los Angeles, CA) to generate FL1 versus FL2 dot plots. The population of interest was gated postacquisition to determine the percentage of cells that were EGFP+ A5PE+ or EGFP+ A5PE−.
Measurement of ΔΨm.
To measure the ΔΨm, we used the MitoProbe 1,1′,3,3,3′,3′-hexamethylindodicarbo-cyanine iodide [DiIC1(5)] assay kit (Molecular Probes, Eugene, OR) as instructed by the manufacturer. Briefly, 2 days postinfection, 0.4 × 106 cells were washed once with PBS and then resuspended in 400 μl of RPMI 1640 medium supplemented with 10% fetal calf serum, l-glutamine, penicillin, and streptomycin and labeled with 50 nM DiIC1(5) at 37°C in the dark for 30 min. As a positive control for the assay, cells were preincubated with 250 μM carbonyl cyanide m-chlorophenyl hydrazone at 37°C in the dark for 5 min before the addition of DiIC1(5). The labeled cells were then collected and resuspended in PBS. FL1 versus FL4 dot plots were generated by acquisition of 10,000 ungated events with the Cytomics FC 500 flow cytometry system (Beckman Coulter). Gating of the population of interest and postacquisition analyses were performed with the FCS Express 3 software to determine the percentage of cells that were EGFP+ with high ΔΨm (EGFP+ ΔΨmhigh) or EGFP+ with low ΔΨm (EGFP+ ΔΨmlow).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Equal amounts of Vpr(+)cis, Vpr(−), and Vpr(+)trans viral stocks (as determined by p24gag enzyme-linked immunosorbent assay) were loaded onto a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (Lonza, Rockland, ME). After electrophoresis, the proteins were transferred onto Immobilon transfer membranes (Millipore Inc., Bedford, MA). The membranes were saturated with 5% milk in phosphate-buffered saline containing 0.01% Tween 20 and either probed with a monoclonal anti-Flag tag antibody (F1804; Sigma) at a dilution of 1/1,500 or the anti-Gag monoclonal antibody AG3.0 (kindly provided by D. Vatakis, UCLA AIDS Institute) at a dilution of 1/2,000. Horseradish peroxidase-coupled goat secondary antibodies (Pierce; no. 31446) were used at a dilution of 1/8,000. Peroxidase activity was revealed by chemiluminescence (Amersham ECL Plus Western blotting detection system; Amersham Biosciences, Buckinghamshire, United Kingdom).
RESULTS
Vpr can be efficiently packaged in trans in virions and induces cell cycle arrest in the G2 plus M phase.
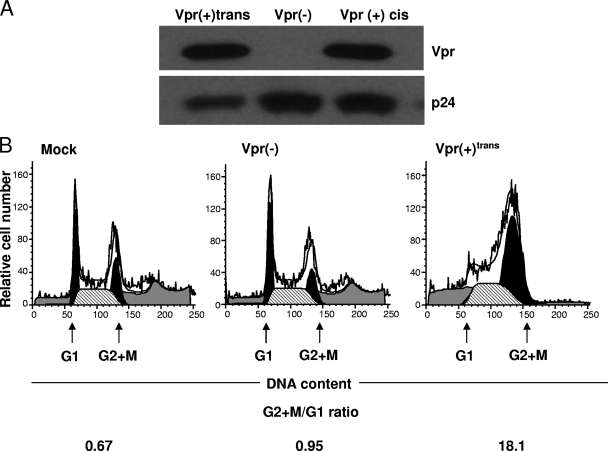
In order to study the effect of virion-associated Vpr, we generated vesicular stomatitis virus G protein-pseudotyped viruses with the pHR′EGFP lentiviral vector, which carries the EGFP cDNA only. These viruses contained or lacked Flag-tagged Vpr supplied in trans [Vpr(+)trans and Vpr(−) viruses, respectively]. These viral particles give us the advantages of (i) monitoring the level of infection of target cells through EGFP expression and (ii) studying the effects of virion-associated Vpr in the absence of de novo expression of Vpr or that of other accessory or regulatory genes. Indeed, the products of env and other genes of HIV-1 do exhibit proapoptotic activities (31, 33, 57, 65, 73). Figure 1A shows that Vpr(+)trans viruses were readily able to package Vpr. As reported earlier by our group and others for the ability of Vpr to induce cell cycle arrest in the G2 plus M phase in HeLa cells (24, 28, 52, 53), the DNA content of HeLa cells that were mock infected or infected with Vpr(−) or Vpr(+)trans virus was analyzed by flow cytometry after staining them with propidium iodide. The percentages of cells in the G2 plus M and the G1 phases were determined, and the G2-plus-M/G1 ratio was then calculated. The substantial increase in the ratio of the percentage of cells in the G2 plus M phase infected with Vpr(+)trans viruses over that of mock-infected cells or cells infected by Vpr(−) viruses [18.1, 0.67, and 0.95 for Vpr(+)trans, mock, and Vpr(−) virus infection, respectively] indicated that virion-associated Vpr in our viruses was indeed able to induce cell cycle arrest in the G2 plus M phase (Fig. 1B).
FIG. 1.
Vpr can be efficiently packaged in trans in virions and induces cell cycle arrest in the G2 plus M phase. (A) Ten nanograms of p24gag from concentrated virus preparations was analyzed for the presence of trans Flag-tagged Vpr in Vpr(+)trans viruses. The blots were probed with a mouse monoclonal antibody raised against the Flag tag. Vpr(+)cis viruses that expressed Flag-tagged Vpr in cis were used as positive controls. Vpr(−) viruses that did not contain virion-associated Vpr were used as negative controls. p24gag was used as a loading control. (B) HeLa cells (5 × 104) were seeded 24 h prior to infection and then mock infected or infected with an equivalent number of EGFP transduction units of Vpr(−) and Vpr(+)trans viral stocks. Two days postinfection, cells were harvested and stained with propidium iodide. The percentage of cells at the G1 or G2 plus M phase was determined after analysis of the cell cycle profiles with ModFit software, and the G2-plus-M/G1 ratio was then calculated. The left and right black peaks represent the relative numbers of cells in the G1 and G2 plus M phases, respectively. The results shown are representative of three independent experiments in which similar results were obtained.
Virion-associated Vpr induces apoptosis in T cells.
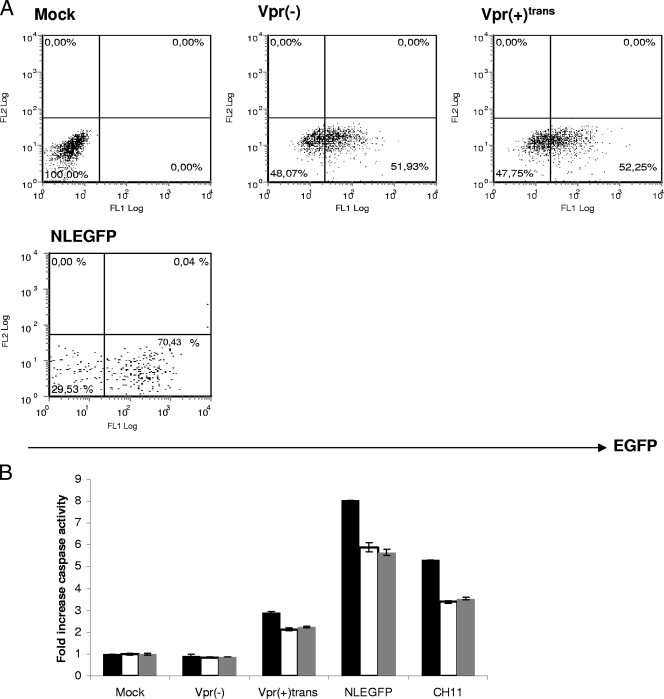
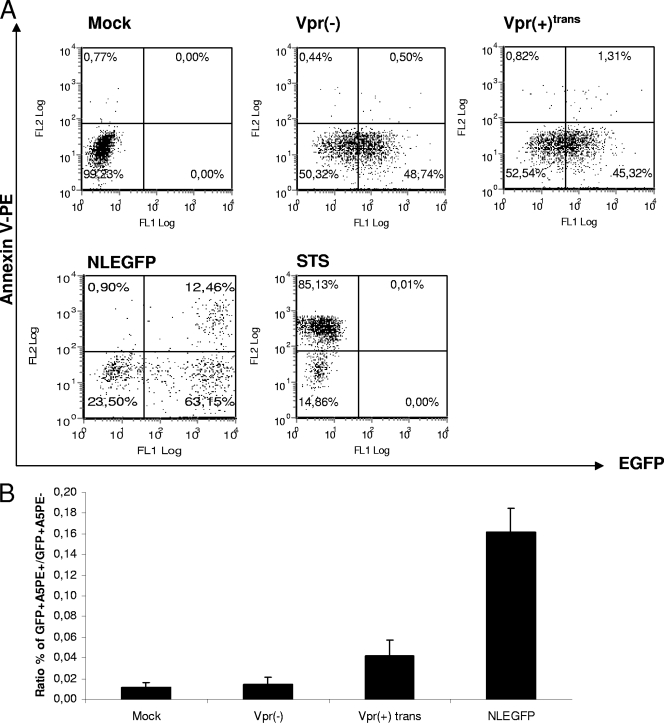
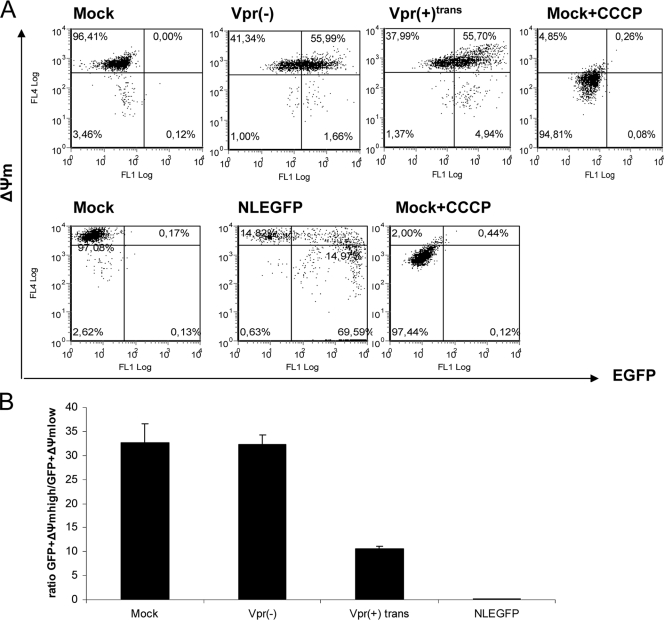
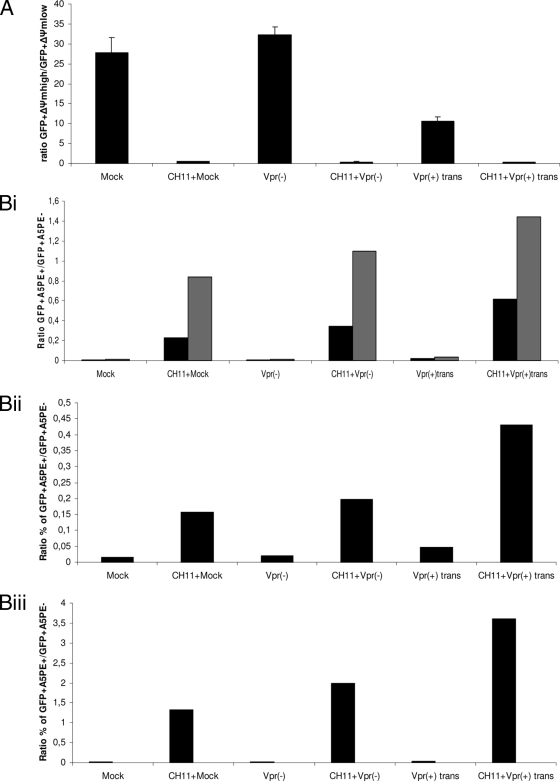
Caspases are activated in a specific manner during apoptosis and can be divided into initiator caspases (caspases 8 and 9) and effector caspases (caspases 3, 6, and 7) (74). A previous study showed that virion-associated Vpr has the ability to induce the intrinsic pathway of apoptosis in activated PBMCs by activating the initiator caspase 9 (43). However, the infection was carried out with viruses bearing the vector pNL4-3.HSA R− E−, which allows de novo expression of mouse CD24 and all HIV genes except vpr and env (24). We hypothesized that virion-associated Vpr could activate apoptosis through caspases independently of any de novo-expressed HIV protein. In order to verify this hypothesis, caspase activation was measured in infected cells 2 days postinfection. Similar percentages of infected cells were obtained for infection by Vpr(−) and Vpr(+)trans viruses, as determined through EGFP expression by flow cytometry (Fig. 2A). As a reference, Jurkat cells were infected with NLEGFP viruses. As expected, we did not observe any caspase activation in cells infected with Vpr(−) viruses compared to mock-infected cells (Fig. 2B). However, the presence of virion-associated Vpr did trigger the activation of effector caspase 3/7 and initiator caspase 9. In contrast to the data obtained by Muthumani et al. (43), we further detected caspase 8 activation. However, we noticed that the levels of caspase activation were lower than those of NLEGFP virus-infected cells or Fas-induced apoptosis by the antibody CH11, which triggers the extrinsic pathway (Fig. 2B). Previous studies have shown that in general, PS externalization, an early marker of apoptosis, occurs as a downstream event of caspase activation (45). We therefore tested whether virion-associated Vpr could trigger PS exposure. Two days postinfection, infected cells were stained with A5PE and analyzed by flow cytometry (Fig. 3A). We calculated the EGFP+ A5PE+/EGFP+ A5PE− ratio. A higher value of that ratio than of mock or Vpr(−) infection would indicate that apoptosis had been triggered. The presence of virion-associated Vpr led to at least a threefold increase in that ratio (Fig. 3B) compared to that of mock or Vpr(−) infection. In order to confirm that apoptosis was initiated, we determined whether there was any dissipation of the ΔΨm. Studies have shown that in certain apoptotic systems, ΔΨm dissipation may precede cytochrome c release and therefore caspase 9 activation (see references 22 and 39 for reviews). Cells were therefore loaded with DilC1(5), a lipophilic cationic molecule that accumulates primarily in mitochondria. A ΔΨm reduction translates into a reduction of fluorescence intensity when measured by flow cytometry. We determined the percentages of cells that were EGFP+ ΔΨmhigh and EGFP+ ΔΨmlow and calculated the EGFP+ ΔΨmhigh/EGFP+ ΔΨmlow ratio for each sample (Fig. 4A). A decrease in the value of that ratio compared to that of mock infection would indicate a decrease in the ΔΨm and confirm the induction of apoptosis. As shown in Fig. 4B, compared to Vpr(−) virus-infected cells, the EGFP+ ΔΨmhigh/EGFP+ ΔΨmlow ratio was 3.2-fold lower in Vpr(+)trans virus-infected cells [33 and 10.3 for Vpr(−) and Vpr(+)trans virus infection, respectively]. Taking the data together, we therefore concluded that, independently of de novo-expressed HIV proteins, virion-associated Vpr is capable of caspase activation, PS exposure, and mitochondrial depolarization and consequently of inducing apoptosis, albeit at a lower level than an infection with NLEGFP viruses. Of note, the extra 20% infected cells observed in NLEGFP infection cannot account for the strong apoptotic response observed compared to that of Vpr(+)trans virus-infected cells.
FIG. 2.
Virion-associated Vpr causes activation of caspases 3, 8, and 9. Jurkat cells were mock infected or infected with Vpr(+)trans or Vpr(−) viruses at an MOI of 0.5. (A) Fifty hours postinfection, the percentage of infected cells was determined by flow cytometry through EGFP expression. (B) Fifty hours postinfection, caspase 3/7, 8, and 9 activities were measured using the appropriate Caspase-Glo assay. Uninfected cells treated with 12.5 ng/ml of the Fas CH11 antibody for 4 to 6 h were used as a positive control for the assay. Blank values were subtracted, and the increase in activity was calculated based on activity measured from mock-infected cells. The black, white, and gray bars represent caspase 3/7, 8, and 9 activities, respectively. Data shown are representative of one of four independent experiments performed in triplicate. The results are shown as means ± standard deviations. Infection with NLEGFP viruses was used as a reference.
FIG. 3.
Virion-associated Vpr triggers PS exposure. (A) Jurkat cells were mock infected or infected with Vpr(+)trans or Vpr(−) viruses at an MOI of 0.5. Fifty hours postinfection, the cells were stained with A5PE and analyzed by flow cytometry to generate dot plots. The fluorescence intensities of EGFP and A5PE are represented on the x axis and the y axis, respectively. Uninfected cells treated with 1 μM staurosporine (STS) for 4 to 6 h were used as a positive control for the assay. The plots shown are representative of one of four independent experiments. NLEGFP virus infection and subsequent staining with A5PE were used as a reference. (B) The ratio of the percentage of EGFP+ A5PE+ cells to that of EGFP+ A5PE− cells was determined for each viral infection from the dot plots in panel A and was plotted on a bar graph. The results are shown as means plus standard deviations. Note that the ratio indicated for mock infection is the ratio of the percentage of A5PE+ cells to that of A5PE− cells, which was used as an internal control.
FIG. 4.
Virion-associated Vpr causes ΔΨm dissipation. (A) Jurkat cells were mock infected or infected with Vpr(+)trans or Vpr(−) viruses at an MOI of 0.5. Fifty hours postinfection, the ΔΨm was measured by using the MitoProbe DiIC1(5) assay kit. Infected cells (0.4 × 106) were labeled with 50 nM DiIC1(5) at 37°C in the dark for 30 min before analyses by flow cytometry to generate dot plots. The fluorescence intensities of EGFP and DilC1(5) are represented on the x axis and y axis, respectively. Uninfected cells treated with 250 μM of carbonyl cyanide m-chlorophenyl hydrazone before being labeled with DiIC1(5) were used as a positive control. NLEGFP virus infection and subsequent staining with DiIC1(5) were used as a reference. The plots shown are representative of one of four experiments. (B) The ratio of the percentage of EGFP+ ΔΨmhigh to that of EGFP+ ΔΨmlow cells was determined for each viral infection from the dot plots and was plotted on a bar graph. The results are shown as means plus standard deviations. Note that the ratio indicated for mock infection is the ratio of the percentage of ΔΨmhigh cells to that of ΔΨmlow cells.
Caspase activation induced by virion-associated Vpr is independent of the Fas receptor pathway.
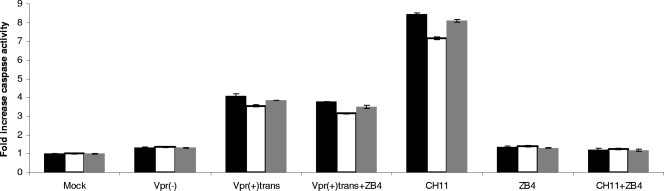
Intracellular insults, such as DNA damage induced by genotoxic drugs or UV radiation, favor the onset of the intrinsic pathway, which leads successively to cytochrome c release from mitochondria and initiator caspase 9 activation with the downstream activation of effector caspases 3, 6, and 7 (see reference 14 for a review). On the other hand, binding of death ligands to their respective receptors triggers the activation of the extrinsic, or death receptor, pathway. The prototypical receptor of that pathway is Fas. When FasL binds to its receptor Fas, there is formation of a protein complex called DISC, within which caspase 8 is activated. This in turn leads to the cleavage and activation of caspases 3, 6, and 7 (see reference 14 for a review). Because caspase 8, the initiator caspase of the extrinsic pathway, was activated, we hypothesized that virion-associated Vpr may trigger the extrinsic pathway through Fas. To verify this hypothesis, we evaluated whether blocking the Fas receptor with the ZB4 Fas antagonist antibody would inhibit caspase 8 activation and the downstream activation of caspases 9 and 3. As shown in Fig. 5, preincubation of Jurkat cells with the Fas antagonist ZB4 antibody, followed by a challenge with the agonist Fas antibody CH11, exhibited background levels of caspase activity similar to those observed in cells not treated with the CH11 antibody, showing that the ZB4 antibody did block the activation of the Fas death receptor pathway. However, cells treated with the CH11 antibody displayed typical caspase activation. Vpr(+)trans virus-infected cells were also incubated with the Fas antagonist ZB4 antibody just after infection, but no reduction of any caspase activity was observed compared to infected cells not treated with the ZB4 antibody (Fig. 5), indicating that the Fas receptor pathway is not implicated in caspase 8 activation, as well as that of caspases 3/7 and 9. We therefore concluded that the Fas death receptor pathway is not engaged by virion-associated Vpr.
FIG. 5.
Caspase activation induced by virion-associated Vpr is independent of the Fas receptor pathway. Jurkat cells were infected at an MOI of 0.5 with the indicated viruses for 2 hours. After the 2 h of infection, cells infected with Vpr(+)trans viruses were cultured for 48 h in the presence or absence of the Fas-neutralizing antibody ZB4 (2.5 μg/ml). Forty-eight hours postinfection, caspase 3/7, 8, and 9 activities were measured for each sample by using the appropriate Caspase-Glo assay. As a positive control for the neutralizing effect of the ZB4 antibody, uninfected cells were preincubated with the ZB4 antibody (2.5 μg/ml) for 6 h before the addition of the Fas-stimulating CH11 antibody (12.5 ng/ml). Caspase activities were then measured 42 h later. Blank values were subtracted, and the increase in activity was calculated based on activity measured from mock-infected cells. The black, white, and gray bars represent caspase 3/7, 8, and 9 activities, respectively. The results are shown as means plus standard deviations.
Virion-associated Vpr enhances Fas-induced apoptosis.
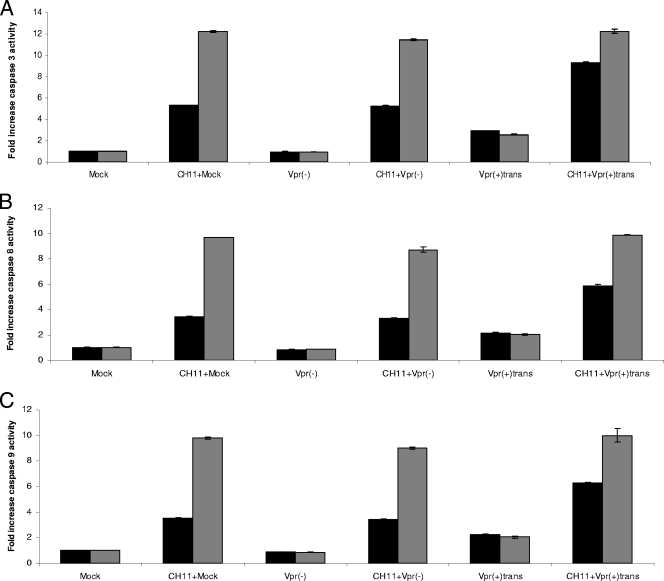
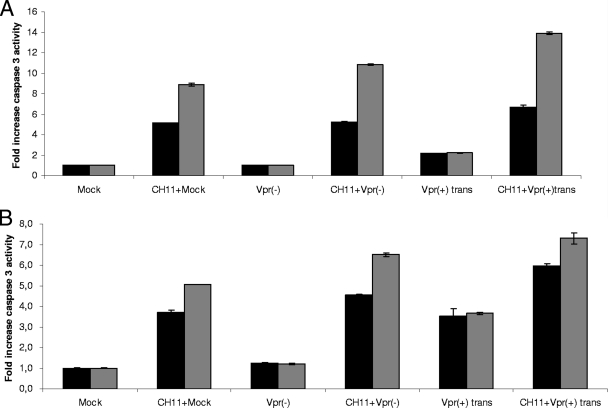
Several studies have reported elevated FasL/Fas levels in serum or on immune cells obtained from HIV-infected patients (5, 6, 8, 23, 41, 61). A recent study showed that blocking FasL with an antibody resulted in decreased B- and T-cell death, as well as lower viremia, in an SIV macaque infection model (56). The engagement of the Fas death receptor pathway could therefore contribute to the depletion of CD4+ T cells observed during HIV infection. As mentioned above, cells infected with Vpr(+)trans viruses displayed a weaker apoptotic response than those infected with NLEGFP viruses. Since we detected caspase 8, the initiator caspase of the death receptor pathway, being activated by virion-associated Vpr, we hypothesized that virion-associated Vpr might enhance the activation of that particular pathway in preinfected cells. To this end, caspase activities were measured in 2-day-postinfection cells treated with the Fas agonist antibody CH11 for 4 and 8 h (corresponding to 50 and 54 h postinfection, respectively). Our results showed that mock-infected and cells infected by Vpr(−) viruses presented similar levels of caspase 3/7 activity at 4 and 8 h after Fas-induced apoptosis, indicating that the infection itself did not have any enhancing effect on Fas-induced caspase 3/7 activity (Fig. 6A). However, cells infected with Vpr(+)trans viruses presented a 9.3-fold increase in caspase 3/7 activity compared to an average 5.2-fold increase for mock or Vpr(−) virus infections (Fig. 6A). No difference in caspase activity was observed after 8 h of CH11 treatment, suggesting that at that time point, the antibody was able to trigger caspase activity to its fullest independently of the presence of Vpr. We also measured the activities of both initiator caspases 8 and 9 (Fig. 6B and C). Vpr(−) virus- and mock-infected cells displayed similar caspase 8 and 9 activities when treated with the CH11 antibody for 4 h. However, these activities were enhanced in Vpr(+)trans virus-infected cells at 4 h post-CH11 treatment. At 8 h post-CH11 treatment, no difference in these activities was observed for all infections. Virion-associated Vpr thus enhances caspase activities early in Fas-induced apoptosis.
FIG. 6.
Virion-associated Vpr enhances caspase activation during Fas-induced apoptosis. Jurkat cells were mock infected or infected at an MOI of 0.5 with Vpr(−) and Vpr(+)trans viruses. Forty-six hours postinfection, the cells were treated with 12.5 ng/ml of the CH11 antibody for 4 or 8 h. Caspase 3/7 (A), 8 (B), and 9 (C) activities were measured for each sample at 4 and 8 h post-CH11 antibody treatment (corresponding to 50 and 54 h postinfection). Blank values were subtracted, and the increase in activity was calculated based on activity measured from mock-infected cells. The black and gray bars represent caspase activities at 50 and 54 h postinfection, respectively. The results are shown as means ± standard deviations.
To evaluate and confirm the enhancing effect of virion-associated Vpr on Fas-induced apoptosis, we also determined the extents of ΔΨm dissipation and PS externalization at 4 and 8 h post-CH11 treatment (corresponding to 50 and 54 h postinfection, respectively). When ΔΨm dissipation was measured 4 h post-CH11 treatment, no enhancing effect of Vpr was observed when the EGFP+ ΔΨmhigh/EGFP+ ΔΨmlow ratios for Vpr(−) and Vpr(+)trans virus-infected cells were compared. All the ratios had similar values at that time. This was due to the fact that ΔΨm dissipation is an early event in Fas-induced apoptosis, as was observed with mock-infected cells. Indeed, only 4 h post-CH11 treatment, mitochondria from mock-infected cells were extensively depolarized (Fig. 7A). The percentage of A5PE-positive cells was also determined by flow cytometry, and the EGFP+ A5PE+/EGFP+ A5PE− ratio was calculated for Vpr(−) and Vpr(+)trans virus infections, whereas the A5PE+/A5PE− ratio was calculated for mock-infected cells (Fig. 7Bi). When the A5PE+/A5PE− ratio of mock-infected cells (an internal control) was compared to the EGFP+ A5PE+/EGFP+ A5PE− ratio for Vpr(−) virus infection, similar values were obtained. On the other hand, the presence of Vpr gave a substantially higher EGFP+A5PE+/EGFP+ A5PE− ratio, showing the amplification effect on PS exposure by the higher caspase activities triggered by virion-associated Vpr. Unlike caspase activities, that amplification effect was maintained at a later time point (8 h post-CH11 treatment). We confirmed this enhancing effect of virion-associated Vpr on PS exposure in two other independent experiments at 4 h (Fig. 7Bii) and 8 h (Fig. 7Biii) post-CH11 treatment of infected cells. Taken together, these results show that virion-associated Vpr can enhance Fas-induced apoptosis.
FIG. 7.
Amplification of PS exposure during Fas-induced apoptosis confirms the enhancing effect of virion-associated Vpr. Jurkat cells were mock infected or infected at an MOI of 0.5 with Vpr(−) and Vpr(+)trans viruses. Forty-six hours postinfection, the cells were treated with 12.5 ng/ml of the CH11 antibody for 4 and 8 h. Measurement of the ΔΨm was performed at 4 h post-CH11 antibody treatment, and A5PE staining was performed at 4 and 8 h post-CH11 antibody treatment (corresponding to 50 and 54 h postinfection) for each sample. The ratio of the percentage of EGFP+ ΔΨmhigh cells to that of EGFP+ ΔΨmlow cells and that of the percentage of EGFP+ A5PE+ cells to that of EGFP+ A5PE− cells were determined for each viral infection at each indicated time point from dot plots (not shown) as for Fig. 4. The calculated ratios were then plotted on bar graphs. (A) The ratios of the percentage of EGFP+ ΔΨmhigh cells to that of EGFP+ ΔΨmlow cells after 4 h of CH11 treatment are shown on a bar graph. The results are shown as means plus standard deviations. (Bi) The ratios of the percentage of EGFP+ A5PE+ cells to that of EGFP+ A5PE− cells after 4 and 8 h of CH11 treatment are shown on a bar graph. The black and gray bars represent the calculated ratios at 50 and 54 h postinfection, respectively. (Bii and Biii) Results of two other independent experiments after 4 and 8 h of CH11 treatment, respectively. Note that the ratio indicated for mock infection is the ratio of the percentage of A5PE+ cells to that of A5PE− cells and that of Δψmlow cells to that of ΔΨmhigh cells for PS exposure assessment and ΔΨm measurement, respectively.
Virion-associated Vpr enhances the activation of caspase 3/7 in Fas-induced apoptosis of human activated PBMCs.
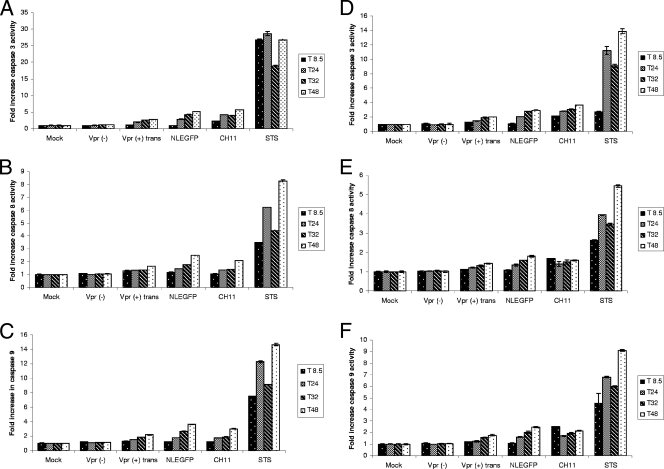
In order to confirm the activation of caspases by virion-associated Vpr in human primary cells, we infected human activated PBMCs from two donors with Vpr(−) and Vpr(+)trans viruses and performed a time course experiment during which the activities of caspases 3, 8, and 9 were monitored at several time points (8.5, 24, 32, and 48 h postinfection). We observed that virion-associated Vpr induced a gradual increase in the activities of all three caspases, whereas in mock- or Vpr(−) virus-infected cells, these activities remained stable (Fig. 8A to C). Similar results were observed in PBMCs from a second donor (Fig. 8D to F). We then assessed whether virion-associated Vpr could enhance the activity of the effector caspase 3/7 in Fas-induced apoptosis of these cells. We chose to measure caspase 3/7 activity, as it is an effector caspase which is activated downstream of caspases 8 and 9. Human activated PBMCs were infected, and 47 h postinfection, the cells were treated with the Fas antibody CH11 for 1 and 3 h (corresponding to 48 and 50 h postinfection), and caspase 3/7 activity was measured at these time points. We observed that at both time points, virion-associated Vpr enhanced caspase 3/7 activity compared to that obtained in mock- or Vpr(−) virus-infected cells (Fig. 9A). Similar results were observed in PBMCs from a second donor (Fig. 9B). These results therefore confirmed the enhancing capacity of virion-associated Vpr on caspase activation previously observed in Jurkat cells.
FIG. 8.
Virion-associated Vpr induces activation of caspases 3/7, 8, and 9 in human activated PBMCs. Activated human PBMCs from donors 1 (A to C) and 2 (D to F) were mock infected or infected with Vpr(+)trans or Vpr(−) viruses with equivalent numbers of infectious units. Caspase 3/7 (A and D), 8 (B and E), and 9 (C and F) activities were measured for each sample at 8.5 (T 8.5), 24 (T24), 32 (T32), and 48 (T48) h postinfection. Uninfected cells treated with 2.5 μg/ml of the Fas CH11 antibody or 1 μM of staurosporine (STS) for 2 to 6 h were used as positive controls for the assay at each time point. The results are shown as means plus standard deviations.
FIG. 9.
Virion-associated Vpr enhances effector caspase 3/7 activity in human activated PBMCs treated with anti-Fas antibody. Human activated PBMCs from donors 1 (A) and 2 (B) were mock infected or infected with Vpr(+)trans or Vpr(−) viruses with equivalent numbers of infectious units. Forty-seven hours postinfection, the cells were treated with 2.5 μg/ml of the Fas CH11 antibody for 1 and 3 h. Caspase 3/7 activity was measured for each sample at 1 and 3 h post-CH11 antibody treatment (corresponding to 48 and 50 h postinfection). Blank values were subtracted, and the increase in activity was calculated based on activity measured from mock-infected cells. The black and gray bars represent caspase 3/7 activities at 48 and 50 h postinfection, respectively. The results are shown as means ± standard deviations.
DISCUSSION
In this study, we showed that virion-associated Vpr can induce T-cell destruction in two ways. First, it can initiate apoptosis directly and independently of de novo-expressed HIV proteins and the Fas pathway. Second, we showed for the first time that it can enhance Fas-induced apoptosis of infected T cells, thereby providing an additional mechanism that might explain T-cell depletion kinetics in AIDS.
Our system allowed us to study the effects of virion-associated Vpr independently of other accessory or regulatory proteins that also exhibit proapoptotic functions. We found that virion-associated Vpr caused activation not only of effector caspase 3/7, but also of both initiator caspases 8 and 9. PS externalization and a drop in the ΔΨm also confirmed that cell death was initiated in the presence of virion-associated Vpr. We therefore conclude that virion-associated Vpr can initiate apoptosis in Jurkat T cells. In contrast to the report of Muthumani et al., in which virion-associated Vpr was shown to activate the intrinsic pathway with only caspases 9 and 3 being activated (43), here, we detected the activation of caspase 8, in addition to the aforementioned caspases. Importantly, the activation of caspases 3/7, 8, and 9 by virion-associated Vpr was confirmed in human activated PBMCs. Consistent with our results, two other studies reported that Vpr can also lead to caspase 8 cleavage and activation. In the first study, de novo-expressed Vpr led to the activation of caspase 8 in Jurkat cells, whereas the mutation R77Q in the Vpr coding sequence abolished that activation (37). In the other report, caspase 8 activity was observed when neurons were treated with recombinant Vpr (49).
Caspase 8 activation is classically detected in FasL- or other death ligand-induced cell death (1). Having detected the activity of caspase 8, we investigated whether the extrinsic pathway was involved through the Fas receptor. Blocking the Fas receptor with the Fas antagonist ZB4 antibody did not result in any decrease in caspase 3/7, 8, or 9 activity, showing that the Fas receptor is not involved, as reported previously (43). It appears, therefore, that virion-associated Vpr likely activates the intrinsic pathway, which is consistent with intracellular stress caused by Vpr, as shown by other groups (2, 11, 16, 66). Chemotherapeutic drugs, such as etoposide or doxorubicin, have been shown to induce caspase 8 activation and apoptosis in a Fas-independent manner (17, 19, 72). One study actually showed that caspase 8, which is usually an initiator caspase in the extrinsic pathway, can be activated downstream of caspase 3 (17). It is therefore possible that virion-associated Vpr may lead to the activation of caspase 8 downstream of caspase 3 in a similar manner.
Several studies implicate the Fas receptor pathway in T-cell depletion during HIV infection (6, 41, 42, 47). Several HIV proteins can independently upregulate the expression of components of the Fas/Fas L pathway in vitro (31, 33, 57, 73, 77). This is confirmed by other in vitro infection experiments or animal models of HIV infection in which it has been observed that HIV infection results in higher levels of Fas expression on T cells, as well as increased levels of FasL and sensitivity to apoptosis (8, 9, 23, 42, 56, 61). Also, treatment of SIV-infected macaques with an antibody raised against Fas L resulted not only in a decrease in T- and B-cell death, but also in a lower viremia set point, thus suggesting a role of FasL in T-cell depletion induced by SIVmac (56). We thus investigated whether virion-associated Vpr might enhance apoptosis of infected cells with the Fas agonist CH11 antibody. The results showed that cells that were preinfected with viruses containing virion-associated Vpr and then treated with the CH11 antibody for 4 h displayed higher levels of caspase 3, 8, and 9 activation than those observed in Vpr(−) virus- and mock-infected cells. At 8 h post-CH11 treatment, the Fas antibody had triggered maximum caspase activation, which was independent of virion-associated Vpr, as observed in Vpr(−) virus- and mock-infected cells. Thus, it appears that virion-associated Vpr hastens the apoptotic process. This premise is supported by the fact that although no caspase activities are enhanced by Vpr at that time point (8 h post-CH11 treatment), PS externalization (a downstream event of caspase activation), on the other hand, is still boosted substantially.
Our results support a model in which virion-associated Vpr can enhance Fas-induced apoptosis. One possible explanation is that virion-associated Vpr could amplify the mitochondrial pathway, most likely through caspase 8. It is well established that the Fas signaling pathways are different in different types of cells (58). In so-called type 2 cells, such as Jurkat cells (58) and activated PBMCs (59), FasL ligation does not directly trigger massive caspase 8 activation, which is then supposed to activate effector caspases 3, 6, and 7, as observed in so-called type 1 cells. Instead, in these cells, at the beginning, small amounts of caspase 8 are activated, which then cleave the BH3 (BCl-2 homology domain 3)-only protein Bid, a positive regulator of apoptosis (34, 38, 50, 58). The truncated form of Bid then activates the mitochondrial pathway to promote cytochrome c release in the cytosol, leading to caspase 9 and subsequently caspase 3/7 activation. Full activation of caspase 8 then occurs downstream of the mitochondrial pathway (see reference 50 for a review). Through caspase 8, virion-associated Vpr could therefore lead to higher activation of the mitochondrial pathway. Indeed, our results showed that there was an increase in both caspase 9 and 3 activities induced by virion-associated Vpr after CH11 treatment. This resulted in increased PS externalization. We therefore propose that virion-associated Vpr could amplify Fas-induced cell death, a process that could involve the amplification effect of caspase 8 through the mitochondrial pathway.
Even though the percentage of infected cells is higher in NLEGFP than in Vpr(−) and Vpr(+)trans virus infections, as assessed by EGFP expression, that difference cannot account for the substantially higher level of caspase activation or PS externalization or the drop in the ΔΨm in NLEGFP virus-infected cells. The difference in the strengths of the apoptotic responses induced by the two viruses can be explained by the fact that NLEGFP viruses express HIV proteins, such as the Tat and Pro proteins, that cause cell death (33, 55, 57, 65). The higher levels of initiation of apoptosis may therefore be the result of the cumulative toxic effects of these proteins. Interestingly, besides exhibiting direct proapoptotic activities like virion-associated Vpr, certain of these proteins, such as Vpu, Nef, and Tat, display sensitizing properties to Fas-induced cell death through different mechanisms (10, 73, 77). The Nef and Tat proteins can cause upregulation of components of the Fas/FasL death pathway, whereas the mechanism behind increased susceptibility to Fas/FasL killing by Vpu has not been investigated.
Studies have revealed that the ratio of defective to productive viral particles may be quite high. Estimated to be in the range of 8 to 20 to 1 (67), these defective virions would still contain packaged Vpr and therefore could cause apoptosis in T cells. Outnumbering productive virions, these defective virions containing virion-associated Vpr may make a greater contribution to the induction of apoptosis directly or indirectly by enhancing Fas-induced cell death. Besides direct killing by productive infections, our results offer an additional mechanism that may explain the rapid kinetics of T-cell destruction that occurs during acute HIV infection, as observed in acute SIV infection animal models (35, 40). These particles may also explain the bystander killing observed in the lymph nodes of HIV-1-infected patients, where apoptosis is more pronounced in bystander cells than in infected cells (20). Also, during viral persistence, cell death may occur via these defective viral particles even if the levels of HIV RNA are below detection levels, leading to the slow and progressive destruction of T cells.
In conclusion, this work shows that virion-associated Vpr can initiate apoptosis directly and indirectly by enhancing cell death through the Fas receptor pathway, thereby contributing to T-cell destruction in HIV-1 infection.
Acknowledgments
We thank K. Morizono, D. Vatakis, and A. Balamurugan for critical reading of the manuscript; Ingrid Schmid for cell cycle profiles analyses with ModFit software; and the UCLA CFAR Virology Core Laboratory for p24gag testing and preparation of human PBMCs.
This work was supported by NIH grant CA070018.
Footnotes
Published ahead of print on 19 August 2009.
REFERENCES
- 1.Aggarwal, B. B. 2003. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3:745-756. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, J. L., J. L. DeHart, E. S. Zimmerman, O. Ardon, B. Kim, G. Jacquot, S. Benichou, and V. Planelles. 2006. HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT. PLoS Pathog. 2:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayyavoo, V., A. Mahboubi, S. Mahalingam, R. Ramalingam, S. Kudchodkar, W. V. Williams, D. R. Green, and D. B. Weiner. 1997. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat. Med. 3:1117-1123. [DOI] [PubMed] [Google Scholar]
- 4.Bachand, F., X. J. Yao, M. Hrimech, N. Rougeau, and E. A. Cohen. 1999. Incorporation of Vpr into human immunodeficiency virus type 1 requires a direct interaction with the p6 domain of the p55 gag precursor. J. Biol. Chem. 274:9083-9091. [DOI] [PubMed] [Google Scholar]
- 5.Badley, A. D., D. H. Dockrell, A. Algeciras, S. Ziesmer, A. Landay, M. M. Lederman, E. Connick, H. Kessler, D. Kuritzkes, D. H. Lynch, P. Roche, H. Yagita, and C. V. Paya. 1998. In vivo analysis of Fas/FasL interactions in HIV-infected patients. J. Clin. Investig. 102:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badley, A. D., J. A. McElhinny, P. J. Leibson, D. H. Lynch, M. R. Alderson, and C. V. Paya. 1996. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J. Virol. 70:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balliet, J. W., D. L. Kolson, G. Eiger, F. M. Kim, K. A. McGann, A. Srinivasan, and R. Collman. 1994. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology 200:623-631. [DOI] [PubMed] [Google Scholar]
- 8.Baumler, C. B., T. Bohler, I. Herr, A. Benner, P. H. Krammer, and K. M. Debatin. 1996. Activation of the CD95 (APO-1/Fas) system in T cells from human immunodeficiency virus type-1-infected children. Blood 88:1741-1746. [PubMed] [Google Scholar]
- 9.Boudet, F., H. Lecoeur, and M. L. Gougeon. 1996. Apoptosis associated with ex vivo down-regulation of Bcl-2 and up-regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J. Immunol. 156:2282-2293. [PubMed] [Google Scholar]
- 10.Casella, C. R., E. L. Rapaport, and T. H. Finkel. 1999. Vpu increases susceptibility of human immunodeficiency virus type 1-infected cells to fas killing. J. Virol. 73:92-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, F., F. Re, S. Sebastian, S. Sazer, and J. Luban. 2004. HIV-1 Vpr induces defects in mitosis, cytokinesis, nuclear structure, and centrosomes. Mol. Biol. Cell 15:1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen, E. A., E. F. Terwilliger, Y. Jalinoos, J. Proulx, J. G. Sodroski, and W. A. Haseltine. 1990. Identification of HIV-1 vpr product and function. J. Acquir. Immune Defic. Syndr. 3:11-18. [PubMed] [Google Scholar]
- 13.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 14.Danial, N. N., and S. J. Korsmeyer. 2004. Cell death: critical control points. Cell 116:205-219. [DOI] [PubMed] [Google Scholar]
- 15.DeHart, J. L., A. Bosque, R. S. Harris, and V. Planelles. 2008. Human immunodeficiency virus type 1 Vif induces cell cycle delay via recruitment of the same E3 ubiquitin ligase complex that targets APOBEC3 proteins for degradation. J. Virol. 82:9265-9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 17.Engels, I. H., A. Stepczynska, C. Stroh, K. Lauber, C. Berg, R. Schwenzer, H. Wajant, R. U. Janicke, A. G. Porter, C. Belka, M. Gregor, K. Schulze-Osthoff, and S. Wesselborg. 2000. Caspase-8/FLICE functions as an executioner caspase in anticancer drug-induced apoptosis. Oncogene 19:4563-4573. [DOI] [PubMed] [Google Scholar]
- 18.Fadeel, B., and S. Orrenius. 2005. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 258:479-517. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari, D., A. Stepczynska, M. Los, S. Wesselborg, and K. Schulze-Osthoff. 1998. Differential regulation and ATP requirement for caspase-8 and caspase-3 activation during CD95- and anticancer drug-induced apoptosis. J. Exp. Med. 188:979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 21.Fukasawa, M., T. Miura, A. Hasegawa, S. Morikawa, H. Tsujimoto, K. Miki, T. Kitamura, and M. Hayami. 1988. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature 333:457-461. [DOI] [PubMed] [Google Scholar]
- 22.Galluzzi, L., N. Zamzami, T. de La Motte Rouge, C. Lemaire, C. Brenner, and G. Kroemer. 2007. Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis 12:803-813. [DOI] [PubMed] [Google Scholar]
- 23.Grelli, S., S. Campagna, M. Lichtner, G. Ricci, S. Vella, V. Vullo, F. Montella, S. Di Fabio, C. Favalli, A. Mastino, and B. Macchi. 2000. Spontaneous and anti-Fas-induced apoptosis in lymphocytes from HIV-infected patients undergoing highly active anti-retroviral therapy. AIDS 14:939-949. [DOI] [PubMed] [Google Scholar]
- 24.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hrimech, M., X. J. Yao, F. Bachand, N. Rougeau, and E. A. Cohen. 1999. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J. Virol. 73:4101-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacotot, E., K. F. Ferri, C. El Hamel, C. Brenner, S. Druillennec, J. Hoebeke, P. Rustin, D. Metivier, C. Lenoir, M. Geuskens, H. L. Vieira, M. Loeffler, A. S. Belzacq, J. P. Briand, N. Zamzami, L. Edelman, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2001. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J. Exp. Med. 193:509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacotot, E., L. Ravagnan, M. Loeffler, K. F. Ferri, H. L. Vieira, N. Zamzami, P. Costantini, S. Druillennec, J. Hoebeke, J. P. Briand, T. Irinopoulou, E. Daugas, S. A. Susin, D. Cointe, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2000. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 191:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamata, M., N. Watanabe, Y. Nagaoka, and I. S. Chen. 2008. Human immunodeficiency virus type 1 Vpr binds to the N lobe of the Wee1 kinase domain and enhances kinase activity for CDC2. J. Virol. 82:5672-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamata, M., R. P. Wu, D. S. An, J. P. Saxe, R. Damoiseaux, M. E. Phelps, J. Huang, and I. S. Chen. 2006. Cell-based chemical genetic screen identifies damnacanthal as an inhibitor of HIV-1 Vpr induced cell death. Biochem. Biophys. Res. Commun. 348:1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, T. A., H. K. Avraham, Y. H. Koh, S. Jiang, I. W. Park, and S. Avraham. 2003. HIV-1 Tat-mediated apoptosis in human brain microvascular endothelial cells. J. Immunol. 170:2629-2637. [DOI] [PubMed] [Google Scholar]
- 32.Levy, D. N., Y. Refaeli, R. R. MacGregor, and D. B. Weiner. 1994. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 91:10873-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 34.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 35.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 36.Lu, Y. L., R. P. Bennett, J. W. Wills, R. Gorelick, and L. Ratner. 1995. A leucine triplet repeat sequence (LXX)4 in p6gag is important for Vpr incorporation into human immunodeficiency virus type 1 particles. J. Virol. 69:6873-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lum, J. J., O. J. Cohen, Z. Nie, J. G. Weaver, T. S. Gomez, X. J. Yao, D. Lynch, A. A. Pilon, N. Hawley, J. E. Kim, Z. Chen, M. Montpetit, J. Sanchez-Dardon, E. A. Cohen, and A. D. Badley. 2003. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Investig. 111:1547-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 39.Ly, J. D., D. R. Grubb, and A. Lawen. 2003. The mitochondrial membrane potential (ΔΨm) in apoptosis; an update. Apoptosis 8:115-128. [DOI] [PubMed] [Google Scholar]
- 40.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 41.McCloskey, T. W., N. Oyaizu, M. Kaplan, and S. Pahwa. 1995. Expression of the Fas antigen in patients infected with human immunodeficiency virus. Cytometry 22:111-114. [DOI] [PubMed] [Google Scholar]
- 42.Mitra, D., M. Steiner, D. H. Lynch, L. Staiano-Coico, and J. Laurence. 1996. HIV-1 upregulates Fas ligand expression in CD4+ T cells in vitro and in vivo: association with Fas-mediated apoptosis and modulation by aurintricarboxylic acid. Immunology 87:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muthumani, K., D. S. Hwang, B. M. Desai, D. Zhang, N. Dayes, D. R. Green, and D. B. Weiner. 2002. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J. Biol. Chem. 277:37820-37831. [DOI] [PubMed] [Google Scholar]
- 44.Muthumani, K., D. Zhang, D. S. Hwang, S. Kudchodkar, N. S. Dayes, B. M. Desai, A. S. Malik, J. S. Yang, M. A. Chattergoon, H. C. Maguire, Jr., and D. B. Weiner. 2002. Adenovirus encoding HIV-1 Vpr activates caspase 9 and induces apoptotic cell death in both p53 positive and negative human tumor cell lines. Oncogene 21:4613-4625. [DOI] [PubMed] [Google Scholar]
- 45.Naito, M., K. Nagashima, T. Mashima, and T. Tsuruo. 1997. Phosphatidylserine externalization is a downstream event of interleukin-1 beta-converting enzyme family protease activation during apoptosis. Blood 89:2060-2066. [PubMed] [Google Scholar]
- 46.Nishizawa, M., M. Kamata, T. Mojin, Y. Nakai, and Y. Aida. 2000. Induction of apoptosis by the Vpr protein of human immunodeficiency virus type 1 occurs independently of G2 arrest of the cell cycle. Virology 276:16-26. [DOI] [PubMed] [Google Scholar]
- 47.Oyaizu, N., Y. Adachi, F. Hashimoto, T. W. McCloskey, N. Hosaka, N. Kayagaki, H. Yagita, and S. Pahwa. 1997. Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+ T cells apoptosis: a possible mechanism of bystander cell death in HIV infection. J. Immunol. 158:2456-2463. [PubMed] [Google Scholar]
- 48.Patel, C. A., M. Mukhtar, S. Harley, J. Kulkosky, and R. J. Pomerantz. 2002. Lentiviral expression of HIV-1 Vpr induces apoptosis in human neurons. J. Neurovirol. 8:86-99. [DOI] [PubMed] [Google Scholar]
- 49.Patel, C. A., M. Mukhtar, and R. J. Pomerantz. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J. Virol. 74:9717-9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peter, M. E., and P. H. Krammer. 2003. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26-35. [DOI] [PubMed] [Google Scholar]
- 51.Piller, S. C., P. Jans, P. W. Gage, and D. A. Jans. 1998. Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology. Proc. Natl. Acad. Sci. USA 95:4595-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Planelles, V., J. B. Jowett, Q. X. Li, Y. Xie, B. Hahn, and I. S. Chen. 1996. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J. Virol. 70:2516-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roumier, T., H. L. Vieira, M. Castedo, K. F. Ferri, P. Boya, K. Andreau, S. Druillennec, N. Joza, J. M. Penninger, B. Roques, and G. Kroemer. 2002. The C-terminal moiety of HIV-1 Vpr induces cell death via a caspase-independent mitochondrial pathway. Cell Death Differ. 9:1212-1219. [DOI] [PubMed] [Google Scholar]
- 55.Sakai, K., J. Dimas, and M. J. Lenardo. 2006. The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest. Proc. Natl. Acad. Sci. USA 103:3369-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salvato, M. S., C. C. Yin, H. Yagita, T. Maeda, K. Okumura, I. Tikhonov, and C. D. Pauza. 2007. Attenuated disease in SIV-infected macaques treated with a monoclonal antibody against FasL. Clin. Dev. Immunol. 2007:93462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sastry, K. J., M. C. Marin, P. N. Nehete, K. McConnell, A. K. el-Naggar, and T. J. McDonnell. 1996. Expression of human immunodeficiency virus type I tat results in down-regulation of bcl-2 and induction of apoptosis in hematopoietic cells. Oncogene 13:487-493. [PubMed] [Google Scholar]
- 58.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scaffidi, C., I. Schmitz, P. H. Krammer, and M. E. Peter. 1999. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274:1541-1548. [DOI] [PubMed] [Google Scholar]
- 60.Selig, L., J. C. Pages, V. Tanchou, S. Preveral, C. Berlioz-Torrent, L. X. Liu, L. Erdtmann, J. Darlix, R. Benarous, and S. Benichou. 1999. Interaction with the p6 domain of the gag precursor mediates incorporation into virions of Vpr and Vpx proteins from primate lentiviruses. J. Virol. 73:592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silvestris, F., P. Cafforio, M. A. Frassanito, M. Tucci, A. Romito, S. Nagata, and F. Dammacco. 1996. Overexpression of Fas antigen on T cells in advanced HIV-1 infection: differential ligation constantly induces apoptosis. AIDS 10:131-141. [DOI] [PubMed] [Google Scholar]
- 62.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 71:5579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stewart, S. A., B. Poon, J. B. Jowett, Y. Xie, and I. S. Chen. 1999. Lentiviral delivery of HIV-1 Vpr protein induces apoptosis in transformed cells. Proc. Natl. Acad. Sci. USA 96:12039-12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Chen. 2000. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strack, P. R., M. W. Frey, C. J. Rizzo, B. Cordova, H. J. George, R. Meade, S. P. Ho, J. Corman, R. Tritch, and B. D. Korant. 1996. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc. Natl. Acad. Sci. USA 93:9571-9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tachiwana, H., M. Shimura, C. Nakai-Murakami, K. Tokunaga, Y. Takizawa, T. Sata, H. Kurumizaka, and Y. Ishizaka. 2006. HIV-1 Vpr induces DNA double-strand breaks. Cancer Res. 66:627-631. [DOI] [PubMed] [Google Scholar]
- 67.Thomas, J. A., D. E. Ott, and R. J. Gorelick. 2007. Efficiency of human immunodeficiency virus type 1 postentry infection processes: evidence against disproportionate numbers of defective virions. J. Virol. 81:4367-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tristem, M., C. Marshall, A. Karpas, and F. Hill. 1992. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 11:3405-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang, J., J. M. Shackelford, C. R. Casella, D. K. Shivers, E. L. Rapaport, B. Liu, X. F. Yu, and T. H. Finkel. 2007. The Vif accessory protein alters the cell cycle of human immunodeficiency virus type 1 infected cells. Virology 359:243-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wen, X., K. M. Duus, T. D. Friedrich, and C. M. de Noronha. 2007. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J. Biol. Chem. 282:27046-27057. [DOI] [PubMed] [Google Scholar]
- 72.Wesselborg, S., I. H. Engels, E. Rossmann, M. Los, and K. Schulze-Osthoff. 1999. Anticancer drugs induce caspase-8/FLICE activation and apoptosis in the absence of CD95 receptor/ligand interaction. Blood 93:3053-3063. [PubMed] [Google Scholar]
- 73.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 74.Yan, N., and Y. Shi. 2005. Mechanisms of apoptosis through structural biology. Annu. Rev. Cell Dev. Biol. 21:35-56. [DOI] [PubMed] [Google Scholar]
- 75.Yao, X. J., A. J. Mouland, R. A. Subbramanian, J. Forget, N. Rougeau, D. Bergeron, and E. A. Cohen. 1998. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 72:4686-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yasuda, J., T. Miyao, M. Kamata, Y. Aida, and Y. Iwakura. 2001. T cell apoptosis causes peripheral T cell depletion in mice transgenic for the HIV-1 vpr gene. Virology 285:181-192. [DOI] [PubMed] [Google Scholar]
- 77.Zauli, G., D. Gibellini, P. Secchiero, H. Dutartre, D. Olive, S. Capitani, and Y. Collette. 1999. Human immunodeficiency virus type 1 Nef protein sensitizes CD4+ T lymphoid cells to apoptosis via functional upregulation of the CD95/CD95 ligand pathway. Blood 93:1000-1010. [PubMed] [Google Scholar]