Abstract
Activation of microglia and astroglia is seen in many neurodegenerative diseases including prion diseases. Activated glial cells produce cytokines as a protective response against certain pathogens and as part of the host inflammatory response to brain damage. In addition, cytokines might also exacerbate tissue damage initiated by other processes. In the present work using multiplex assays to analyze protein levels of 24 cytokines in scrapie agent-infected C57BL/10 mouse brains, we observed elevation of CCL2, CCL5, CXCL1, CXCL10, granulocyte-macrophage colony-stimulating factor (GM-CSF), gamma interferon (IFN-γ), interleukin 1α (IL-1α), IL-1β, IL-6, and IL-12p40. Scrapie agent-infected wild-type mice and transgenic mice expressing anchorless prion protein (PrP) had similar cytokine responses in spite of extensive differences in neuropathology. Therefore, these responses may be primarily a reaction to brain damage induced by prion infection rather than specific inducers of a particular type of pathology. To study the roles of astroglia and microglia in these cytokine responses, primary glial cultures were exposed to scrapie agent-infected brain homogenates. Microglia produced only IL-12p40 and CXCL10, whereas astroglia produced these cytokines plus CCL2, CCL3, CCL5, CXCL1, G-CSF, IL-1β, IL-6, IL-12p70, and IL-13. Glial cytokine responses from wild-type mice and transgenic mice expressing anchorless PrP differed only slightly, but glia from PrP-null mice produced only IL-12p40, indicating that PrP expression was required for scrapie agent induction of other cytokines detected. The difference in cytokine response between microglia and astroglia correlated with 20-fold-higher levels of PrP expression in astroglia versus microglia, suggesting that high-level PrP expression on astroglia might be important for induction of certain cytokines.
Prion diseases are fatal infectious neurodegenerative disorders that affect both humans and animals (43). These diseases include scrapie in sheep and goats, bovine spongiform encephalopathy in cattle, chronic wasting disease in cervids, and Creutzfeldt-Jakob disease in humans. A key event in the pathogenesis is the conformational change of a normal host protease-sensitive prion protein (PrPsen), also known as cellular PrP (PrPC) into an abnormal, partially protease-resistant, disease-associated form (PrPres), also known as PrP scrapie (PrPSc). The accumulation of PrPres in brain is associated with gray matter spongiform degeneration, neuronal death, and gliosis involving activation of both microglia and astroglia (45, 61). The specific roles of glia in prion disease pathogenesis are still not clear; however, glia that appear to be activated by morphological criteria are usually found in the same brain areas as PrPres (65).
Glia are important in controlling central nervous system (CNS) damage in many situations including wounds, ischemia, hemorrhage, autoimmunity, infections, and degenerative processes (3, 50, 57). Alternatively, activated glia may also contribute to progression of some CNS diseases including prion diseases (7, 23, 25, 27, 37, 53). Moreover, activated glia are known to produce cytokines including chemotactic cytokines (chemokines) as well as neurotrophic and neurotoxic factors (10, 49), all of which might contribute to the overall effect of glia on disease.
In previous studies, microglia and astroglia stimulated by aggregated PrP peptide p106-126 were neurotoxic (8, 36). PrP expression by glia was required for this effect, suggesting that cellular PrP might be involved in recognition of the aggregated PrP peptides (5, 22). The mechanism of this neurotoxic effect was not demonstrated, but other studies showed upregulation of cytokine expression in microglia or N9 cells stimulated by aggregated PrP peptides (41, 59, 62). Furthermore, studies of brain samples from prion agent-infected mice demonstrated upregulation of multiple cytokines and chemokines, including tumor necrosis factor (TNF), interleukin 1α (IL-1α), IL-1β, IL-6, transforming growth factor β, chemokine (C-X-C motif) ligand 10 (CXCL10), chemokine (C-C motif) ligand 2 (CCL2), CCL3, CCL4, CCL5, CCL6, CCL9, CCL12, CXCL9, and CXCL13 (1, 6, 12, 21, 28, 32, 33, 46, 47, 66, 67, 69). In contrast, another study of scrapie agent-infected mouse brain samples did not find elevation of mRNA for IL-1β, IL-6, gamma interferon (IFN-γ), or inducible nitric oxide synthase (63). However, these studies were done by quantitative analysis of mRNA levels or nonquantitatively by immunohistochemistry. Since increased mRNA does not always correlate with increased protein (40, 42), quantitative cytokine analysis at the protein level might give more accurate information.
The development of multiplex bead array assays now permits quantitative analysis of many individual proteins simultaneously from the same sample. This method has been used previously to study expression of cytokines and other proteins in brain tissue homogenates (11, 24, 26). Therefore, in the present study, we used this method to analyze cytokine levels from scrapie agent-infected brain samples and correlated these findings with those from scrapie agent-stimulated primary astroglial and microglial cell cultures. The roles of PrP expression in these in vivo and in vitro systems were also studied by comparing scrapie agent-infected wild-type mice, which develop classical prion disease spongiform pathology, versus scrapie agent-infected transgenic mice expressing anchorless PrP, which develop nonspongiform amyloid brain pathology. The cytokine responses were similar in these two models, suggesting that these cytokines may be primarily a response to brain damage rather than specific inducers of a particular type of pathology.
MATERIALS AND METHODS
Mouse lines.
In vivo scrapie agent infection experiments and in vitro primary glial cultures were performed using the following three mouse strains: C57BL10/SnJ (also referred to as wild-type mice, as they express the wild-type PrP gene [Prnp]), C57BL10/SnJ-PrP−/− (made at Rocky Mountain Laboratories by serial backcrossing of 129/Ola-PrP−/− mice [35] with C57BL10/SnJ mice), and transgenic mice expressing anchorless mouse PrP (lines 23 and 44) on C57BL10/SnJ-PrP−/− background, lacking normal glycosylphosphatidylinositol (GPI)-anchored PrP (15). These two separate founder lines were used to show that the effects seen were due to the presence of the transgene and not to the site of transgene integration. Mice homozygous for the transgene were used in the current experiments. All mice were maintained at Rocky Mountain Laboratories and handled according to protocols approved by the Rocky Mountain Laboratories Animal Care and Use Committee and all applicable federal guidelines.
Preparation of brain homogenate.
Three- to 4-week-old mice were inoculated intracerebrally with scrapie agent-infected brain homogenate containing the 22L scrapie strain as described previously (44, 60). Wild-type mice were euthanized at the time of clinical signs, around 150 to 160 days postinoculation (dpi). Transgenic anchorless PrP-expressing mice were euthanized at various times between 150 and 350 dpi. PrP-null mice were euthanized at 500 dpi. Infected brains and uninfected brains were homogenized using a Mini-BeadBeater (BioSpec Products) for 20 s in a tube with 2 to 4 mm of glass beads and cold culture medium (Dulbecco modified Eagle medium with nutrient mixture F12 [DMEM-F12] [Gibco, Carlsbad, CA], 10% fetal bovine serum [FBS] [HyClone, Logan, UT], penicillin-streptomycin [P-S] [Gibco, Carlsbad, CA], and l-glutamine [Gibco, Carlsbad, CA]) to produce a 20% (wt/vol) brain homogenate. Brain homogenates were sonicated for 1 min, vortexed aggressively for 30 s, and frozen in aliquots at −80°C for future use.
Primary glial cell cultures.
Glial cells were obtained from cerebral cortices of 1- to 3-day-old mice. Meninges were removed by teasing with forceps, and meninx-free cortices were dissociated by pipetting in phosphate-buffered saline (Gibco, Carlsbad, CA) containing 0.25% trypsin (Invitrogen, Carlsbad, CA) for 10 min at 37°C and then diluted 1:1 in phosphate-buffered saline (Gibco, Carlsbad, CA) supplemented with 10% FBS (HyClone, Logan, UT). Dissociated cells were centrifuged at 200 × g for 5 min. Pellets were resuspended in DMEM-F12 supplemented with 0.5 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN), N2 supplement (Invitrogen, Carlsbad, CA), 10% FBS, P-S, and 2 mM L-glutamine for microglial cells and the same medium without GM-CSF and N2 supplement for astrocytes. The cells were triturated with a Pasteur pipette, pelleted, and resuspended in medium; cells from three brains were plated in each T-75 plastic culture flask. Cells were grown for 10 days at 37°C under 5% CO2; the medium was changed after 2 days.
To harvest microglia, flasks were rotated at 200 rpm using an orbital shaker for 2 h at 37°C. Cell suspensions were centrifuged at 200 × g, resuspended in culture medium, and plated in 96-well plates containing poly-d-lysine (R&D Systems, Minneapolis, MN) at a density of 5 × 104 cells/well in a volume of 200 μl.
Astrocytes were retrieved from the shaken culture by trypsinization. Cell suspensions were centrifuged at 200 × g for 5 min, resuspended in DMEM-F12 supplemented with 10% FBS, P-S, and 2 mM l-glutamine and plated in 96-well plates containing poly-d-lysine at a density of 5 × 104 cells/well in a volume of 200 μl.
One hour after plating, the medium was changed to remove cellular debris, and 24 h later, the cells were exposed to scrapie agent-infected brain homogenates (see below). The proportion of microglial cells in microglia culture and astrocytes in astroglia culture was estimated at 99% based on indirect immunofluorescence detection with an antibody specific for ionized calcium binding adaptor 1 (Iba-1) (Wako Chemicals, Richmond, VA) for microglial cells and an antibody specific for glial fibrillary acidic protein (GFAP) (Dako, Carpinteria, CA) for astrocytes (data not shown).
Stimulation of glial cells with scrapie agent-infected and healthy uninfected brain homogenates.
For glial cells, 24 h after the cells were plated, the cultures were overlaid with fresh medium containing 1% brain homogenate (10 μl of 20% homogenate in 200 μl total medium) from mice clinically infected with the 22L strain of scrapie agent or the equivalent dilution of healthy uninfected brain homogenate. For negative and positive controls, cultures were overlaid with medium alone or medium containing lipopolysaccharide (LPS) (1 μg/ml) (Invivogen, San Diego, CA), respectively. All of these treatments were assayed in duplicate in two different wells. All cultures were incubated for an additional 24 h, and the supernatants were then removed and stored at −20°C until analysis with the Bio-Plex system.
Cytokine testing.
Bio-Plex 23-plex kits (Bio-Rad, Hercules, CA) were used to determine the concentrations of 23 cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, IFN-γ, CCL11 [eotaxin], G-CSF, GM-CSF, CXCL1 [KC {keratinocyte-derived chemokine}], CCL2 [MCP-1 {monocyte chemotactic peptide 1}]), CCL3 [MIP-1α {macrophage inflammatory protein 1α}], CCL4 [MIP-1β], CCL5 [RANTES {regulated upon activation, normal T-cell expressed and secreted}], and TNF) in brain tissue homogenates and in glial culture supernatant fluids. Invitrogen 10-plex and single-plex kits were used to analyze CXCL10 in brain tissues and in glial culture supernatants.
For the Bio-Plex assay, a wide range (1.95 to 32,000 pg/ml) of assay standards resuspended in culture medium was used to plot the standard curves for the 23 cytokines. All reagents needed for the assays were provided in the kits. Calibration of the instrument was performed for each use, along with the monthly recommended system validation, and all samples were assayed in duplicate. Data were obtained using the Bio-Plex Manager software program (Bio-Rad version 4.1.1) for standardization and standard curve acquisition followed by conversion to Excel (Microsoft Corporation, Seattle, WA) for further analysis.
For testing of brain tissue, 50 μl of a 1% homogenate was analyzed in duplicate by a multiplex assay. The concentrations of cytokines were determined by comparison to a set of standards. For glial cell cultures, 50 μl of a 200-μl total culture volume was analyzed, and the scrapie agent-induced cytokine concentration was calculated according to the following formula: scrapie agent-induced cytokine level = S − N, where S is the cytokine level after stimulation with 1% scrapie agent-infected brain homogenate minus the cytokine level detected in 1% scrapie agent-infected brain homogenate and N is the cytokine level after stimulation with 1% healthy uninfected brain homogenate minus the cytokine level detected in 1% healthy uninfected brain homogenate. Statistical analysis was done for each cytokine using the Wilcoxon matched-pair test on S and N values for pairs of individual glial cell cultures stimulated with scrapie agent-infected or with healthy uninfected brain tissues.
Preparation of RNA for real-time reverse transcriptase PCR analysis.
For the study of mRNA from cells, RNA was isolated from confluent flasks of cells using the Mini RNA Isolation Kit (Zymo Research, Orange, CA). The RNA was then treated with DNase (Ambion, Austin, TX) for 30 min to remove any contaminating DNA and purified using RNA Clean-up Kit (Zymo Research, Orange, CA). Each RNA was reversed transcribed into cDNA using Bio-Rad iScript cDNA synthesis kit (Bio-Rad, Hercules, CA).
Primers for gene detection.
F4/80 mRNA expression was detected using the forward primer F4/80-1321F (5′-ACA AGT GTC TCC CTC GTG CT-3′) and the reverse primer F4/80-1430R (5′-AAC ATG TGC TTT CCA CAG TC-3′). Gfap mRNA expression was detected using the forward primer GFAP-16F (5′-CGT TTC TCC TTG TCT CGA ATG AC-3′) and the reverse primer GFAP-112R (5′-TCG CCC GTG TCT CCT TGA-3′). Prnp mRNA expression was detected using the forward primer PRNP-590F (5′-GGA CCG CTA CTA CCG TGA AA-3′) and the reverse primer PRNP-768R (5′-TCA TCT TCA CAT CGG TCT CG-3′). Ccr1 mRNA expression was detected using the forward primer CCR1-535F (5′-TCA AAG CAT GAC CAG CAT CTA-3′) and the reverse primer CCR1-622R (5′-CTT GTA GTC AAT CCA GAA AGG TAA A-3′). Ccr2 mRNA expression was detected using the forward primer CCR2-276F (5′-CCA GTG TGA AGC AAA TTG GA-3′) and the reverse primer CCR2-351R (5′-TTG CCC ACA AAA CCA AAG AT-3′). Ccr5 mRNA expression was detected using the forward primer CCR5-1017F (5′-CGA AAA CAC ATG GTC AAA CG-3′) and the reverse primer CCR5-1195R (5′-CCA TTC CTA CTC CCA AGC TG-3′). Gapdh mRNA expression was detected using the forward primer GAPDH2-152F (5′-AAC GAC CCC TTC ATT GAC-3′) and the reverse primer GAPDH2-342RF (5′-TCC ACG ACA TAC TCA GCA-3′) (11).
Real-time PCR analysis of gene expression.
All primers used for real-time analysis were designed using Primer3 software (52). Primer sequences were checked against the National Center for Biotechnology Information (NCBI) database with BLAST to confirm that all primer pairs were specific for the gene of interest and that no homology to other genes was present. Reactions were run using SYBR green mix with Rox (Bio-Rad Laboratories) in a 10-μl volume with approximately 10 ng of cDNA and 1.8 μM forward and reverse primers. Samples were run in triplicate on an ABI PRISM 7900 sequence detection system (Applied Biosystems, Foster City, CA). Analysis of dissociation curves was used to confirm the amplification of a single product for each primer pair per sample. Confirmation of a lack of DNA contamination was achieved by analyzing samples that had not undergone reverse transcription. Untranscribed controls had at least a 1,000-fold-lower expression level than analyzed samples or were negative for all genes after 40 cycles. Gene expression was quantified by the cycle number at which each sample reached a fixed fluorescence threshold (CT). To control for variations in RNA amounts among samples, data were calculated as the difference in CT values (log2) between Gapdh and the gene of interest for each sample (ΔCT = CT of Gapdh − CT of the gene of interest). Data are presented as a percentage of Gapdh expression for each gene of interest.
RESULTS
Induction of cytokines in C57BL/10 mouse brain following infection with scrapie agent.
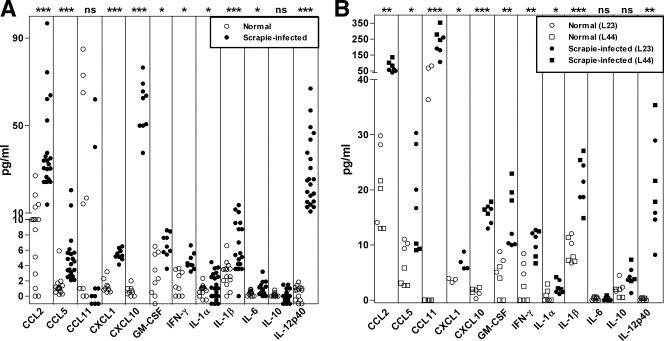
To measure the levels of cytokines in the brains of mice after infection with the scrapie agent, we analyzed brain homogenates from clinical, scrapie agent-infected mice (150 to 160 dpi) and healthy uninfected control C57BL/10 mice by multiplex bead array. Ten cytokines were found to be elevated in scrapie agent-infected brains compared to healthy control brains (Fig. 1A and Table 1). These 10 cytokines were CCL2, CCL5, CXCL1, CXCL10, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-6, and IL-12p40. In contrast, CCL3, CCL4, CCL11, G-CSF, IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, IL-12p70, IL-13, IL-17, and TNF were either not elevated or not detected in scrapie agent-infected brain. These results showed that there was a significant cytokine response to scrapie agent infection. However, the number of elevated cytokines and the levels observed were low compared to those seen in brain following a bacterial or viral infection where there is usually a prominent adaptive immune response (6, 27, 56). Interestingly, we found no increase in TNF, CCL3, or CCL4 protein, whereas several earlier reports noted an increase in these mRNAs. This difference suggested that TNF, CCL3, and CCL4 are regulated posttranscriptionally (18, 19).
FIG. 1.
Cytokine expression in scrapie agent-infected mouse brain. (A) Wild-type C57BL/10SnJ mice. Brain homogenates from 21 scrapie agent-infected mice and 14 uninfected age-matched mice were tested for cytokine levels by multiplex bead arrays. The cytokine levels of scrapie agent-infected mice are shown as solid circles, and the cytokine levels of healthy uninfected (normal) brains are open circles. Each symbol represents the value for one mouse. CCL2, CCL5, CXCL1, CXCL10, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-6, and IL-12p40 were expressed at significantly higher levels in the scrapie agent-infected brains than in the healthy brains. Statistical significance by the Mann-Whitney test is indicated as follows: *, P < 0.05; ***, P < 0.001; ns, not significant. Other cytokines tested, but not detected, in wild-type mouse brains were CCL3, CCL4, CCL11, G-CSF, IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, IL-12p70, IL-13, IL-17, and TNF. (B) Transgenic mice expressing anchorless PrP. Brain homogenates from homozygous transgenic scrapie agent-infected mice (lines 23 [L23] and 44 [L44]) were obtained at 350 to 400 dpi and were tested for cytokine levels by multiplex bead arrays. Uninfected age-matched transgenic mice were used as controls. The cytokine levels of scrapie agent-infected mice are shown as solid symbols, and the cytokine levels of healthy uninfected (normal) brains are shown as open symbols. Each symbol represents the value for one mouse. CCL2, CCL5, CCL11, CXCL1, CXCL10, GM-CSF, IFN-γ, IL-1α, IL-1β, and IL-12p40 were expressed at significantly higher levels in the scrapie agent-infected mice than in the uninfected mice. Statistical significance by the Mann-Whitney test is indicated as follows: *, P < 0.05; **, P < 0.005; ***, P < 0.001; ns, not significant. Other cytokines tested, but not detected, in brains were CCL3, CCL4, G-CSF, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17, and TNF.
TABLE 1.
Summary of scrapie agent-induced cytokines in vivo and in vitro in wild-type mice, PrP-null mice, and anchorless PrP transgenic mice
| Cytokinesa | Cytokine productionb |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild-type PrP |
PrP-null |
Anchorless PrP |
|||||||
| Brain | MG | AG | Brainc | MG | AG | Brain | MG | AG | |
| CCL2 (MCP-1) | + | − | + | − | − | − | + | + | + |
| CCL3 (MIP-1α) | − | − | + | − | − | − | − | − | − |
| CCL5 (RANTES) | + | − | + | − | − | − | + | − | + |
| CCL11 (eotaxin) | − | − | − | − | − | − | + | − | − |
| CXCL1 (KC) | + | − | + | − | − | − | + | ND | ND |
| CXCL10 (IP-10) | + | + | + | − | − | − | + | + | + |
| G-CSF | − | − | + | − | − | − | − | − | + |
| GM-CSF | + | − | − | − | − | − | + | ND | ND |
| IFN-γ | + | − | − | − | − | − | + | − | − |
| IL-1α | + | − | − | − | − | − | + | + | + |
| IL-1β | + | − | + | − | − | − | + | − | + |
| IL-6 | + | − | + | − | − | − | − | + | + |
| IL-12p40 | + | + | + | − | + | + | + | + | + |
| IL-12p70 | − | − | + | − | − | − | − | − | − |
| IL-13 | − | − | + | − | − | − | − | − | ND |
Only cytokines produced in scrapie agent-infected brain homogenates or in the supernatants of glial cells exposed to scrapie agent-infected brain homogenates are shown. The following cytokines were not detected in scrapie agent-infected brain homogenates and supernatant of glial cells exposed to scrapie agent-infected brain homogenates: CCL4 (MIP-1β), IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, IL-17, and TNF. Glial cultures stimulated with LPS were positive for all cytokines tested.
Significant cytokine production (+) or no significant cytokine production (−) from C57BL/10 mice and transgenic mice. The brains of C57BL/10 mice producing wild-type PrP, PrP-null C57BL/10 mice, and transgenic mice expressing anchorless PrP mice were tested for cytokines or used to derive glial cells (microglia [MG] and astroglia [AG]). ND, not determined.
Scrapie agent-inoculated PrP-null mice (n = 2) were euthanized at 500 dpi for analysis of brain homogenates.
Induction of cytokines by scrapie agent infection in transgenic mice expressing anchorless PrP.
We also studied scrapie agent induction of cytokines in a type of scrapie agent-induced disease where the pathology was induced by the presence of amyloid and not by typical spongiform degeneration seen in most prion diseases. For these experiments, we used two lines of transgenic mice expressing anchorless PrP (lines 23 and 44), which show no differences in their response to scrapie agent infection (15). These mice lack the usual GPI anchor for PrP. Thus, the PrP is not attached to the cell plasma membrane but rather is secreted into the extracellular fluid. These mice are susceptible to scrapie agent infection, but they do not develop typical prion disease. Instead they develop slowly progressive amyloid brain pathology. In scrapie agent-infected transgenic mice, no elevation of cytokines was seen at 150 or 250 dpi (data not shown). However, at approximately 350 dpi, elevation in the levels of nine cytokines was observed (Fig. 1B and Table 1), indicating that the timing of cytokine production was correlated with development of brain pathology. The two transgenic lines tested had similar cytokine responses to scrapie agent (Fig. 1B). These cytokines were the same as those detected after scrapie agent infection of C57BL/10 mice, with the exception of IL-6 and CCL11 (Fig. 1B and Table 1). The similarity of the cytokines induced by scrapie agent infection in the C57BL/10 and transgenic mouse models with differing pathogenic processes suggested that the cytokines themselves were not the primary mediators of the pathogenesis but were more likely to be part of a host response to the brain damage induced in both these models.
Scrapie agent induction of cytokines in primary astroglia and microglia.
During scrapie agent infection, there is a strong activation of both microglia and astroglia in the brain, and both of these cell types are known to produce cytokines in various disease states. To determine which brain cytokines might be produced by scrapie agent-stimulated astroglia and microglia, we studied primary astroglial or microglial cultures stimulated with scrapie agent-infected brain homogenates. Cytokines were tested in supernatant fluid of paired cultures exposed to either scrapie agent-infected or healthy uninfected brain homogenate for 24 h, and these values were subtracted to obtain the cytokine level attributable to stimulation by scrapie agent (Fig. 2).
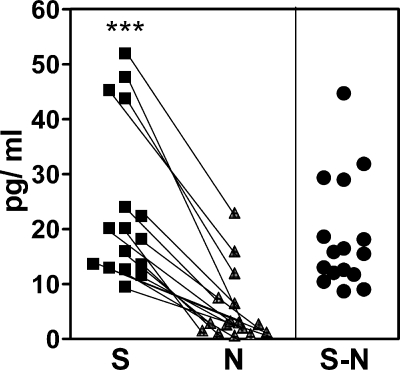
FIG. 2.
In vitro stimulation of IL-12p40 in microglial cells by scrapie agent-infected and healthy uninfected brain homogenates. S (▪) is the cytokine level in glial culture supernatant 24 h after stimulation with scrapie agent-infected brain homogenate minus the background cytokine level detected in scrapie agent-infected brain homogenate prior to use as a stimulant. N (▴) is the cytokine level in glial culture supernatant 24 h after stimulation with healthy brain homogenate minus the background cytokine level detected in healthy brain homogenate prior to use as a stimulant. Values for paired cultures in each experiment are linked by lines. S − N subtracted values for each pair are shown in the right-hand side of the figure (•). The S and N values were significantly different (P < 0.001) by the Mann-Whitney matched-pair test (***). Data shown in Fig. 3A, 4A, and 5A are S − N values.
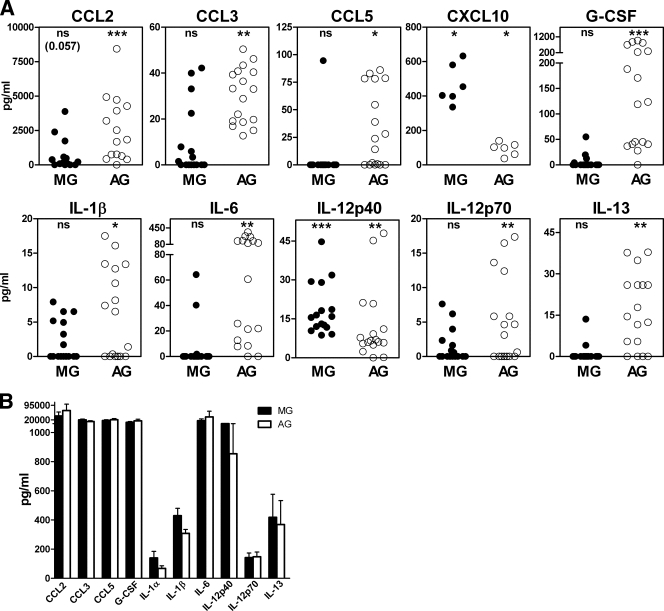
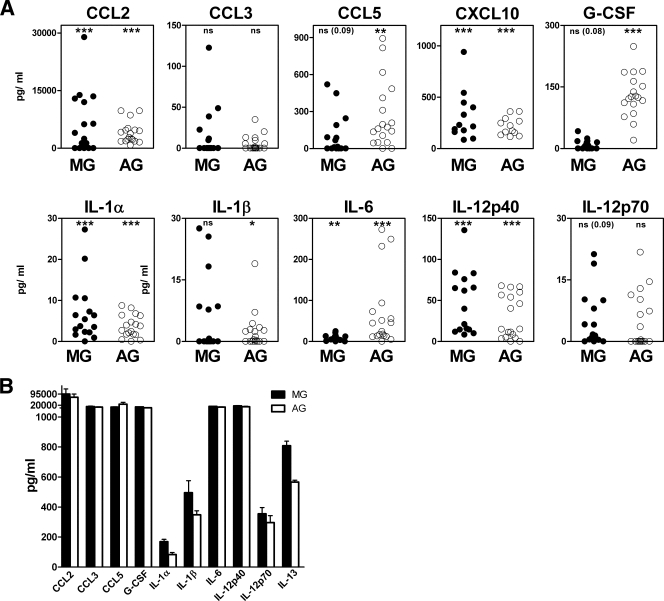
Astroglia stimulated with scrapie agent-infected brain homogenate produced CCL2, CCL3, CCL5, G-CSF, IL-1β, IL-6, IL-12p70, IL-13 (Fig. 3A), and CXCL1 (Table 1), which were not elevated in microglia. However, scrapie agent-stimulated astroglia and microglia both made IL12p40 and CXCL10 in vitro (Fig. 3A). In control experiments, stimulation by LPS, a strong stimulator of cytokines in many cell types, elevated all cytokines to levels higher than those induced by scrapie agent-infected brain homogenates (10 cytokines shown in Fig. 3B).
FIG. 3.
Cytokine expression in C57BL/10SnJ glial cells exposed to scrapie agent-infected brain homogenate. (A) Exposure to scrapie agent-infected or healthy uninfected brain homogenates. Purified microglia (MG) or astroglia (AG) were stimulated by overlay with scrapie agent-infected or healthy brain homogenates. Supernatants were analyzed by multiplex assay 24 h after stimulation. The data show the scrapie agent-induced cytokine level as explained in Materials and Methods. Statistical analysis was done using the Wilcoxon matched-pair test comparing the S and N values for cultures from 16 independent experiments for each cytokine (6 experiments for CXCL10). Subtracted values for scrapie agent-infected brain stimulation minus healthy brain stimulation (S − N) are shown (see the legend to Fig. 2 and Materials and Methods for details). The cytokine levels from microglia are shown as solid circles, and the cytokine levels from astroglia are shown as open circles. MG produced IL-12p40 and CXCL10, and AG produced these two cytokines plus CCL2, CCL3, CCL5, G-CSF, IL-1β, IL-6, IL-12p70, IL-13, and CXCL1 (not shown). Statistical significance by the Mann-Whitney matched-pair test is indicated as follows: *, P < 0.05; **, P < 0.005; ***, P < 0.001; ns, not significant. (B) Stimulation of glia by LPS. Supernatants of microglia and astroglia cultures stimulated for 24 h with LPS at a concentration of 1 μg/ml were analyzed for cytokines using Bio-Plex multiple-cytokine assay kits. The values represent the means plus standard errors of the means (error bars) for seven different experiments.
Scrapie agent induction of cytokines is altered in PrP-null cells.
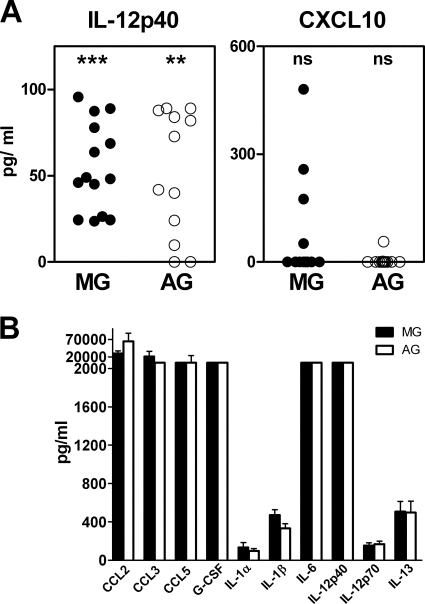
To test the role of PrP expression in cytokine induction in primary glial cell cultures, we used glia from PrP-null mice. In both PrP-null astroglia and microglia, IL-12p40 was elevated by exposure to scrapie agent compared to healthy uninfected brain homogenates (Fig. 4A and Table 1). All the other cytokines previously found to be specifically elevated in wild-type astroglia and microglia by scrapie agent-infected brain were not elevated in PrP-null glia, indicating that PrP expression was required for the stimulation process. In contrast to scrapie agent-infected brain homogenates, LPS gave a broad stimulation of all cytokines tested in PrP-null glia, showing that PrP-null glia were capable of making a wide variety of cytokines (Fig. 4B).
FIG. 4.
Scrapie agent-induced cytokine profiles in astroglia and microglia from PrP-null mice. Supernatants of PrP-null glial cell cultures were analyzed for cytokines by using Bio-Plex multiple-cytokine assay kits 24 h after overlay with scrapie agent-infected or healthy uninfected brain homogenates. Scrapie agent-induced cytokine level is shown as explained in Materials and Methods. Statistical analysis was done using the Wilcoxon matched-pair test comparing the S and N values for cultures from 14 independent experiments for each cytokine. Subtracted values for scrapie agent-infected brain stimulation minus healthy brain stimulation (S − N) are shown (see the legend to Fig. 2 and Materials and Methods for details). (A) Only IL-12p40 was significantly elevated in astoglia and microglia. Twenty-three other cytokines were found to be negative; CXCL10/IP-10 is shown as an example. Statistical significance by the Mann-Whitney matched-pair test is indicated as follows: ns, not significant; **, P < 0.005; ***, P < 0.001). (B) LPS stimulation. The levels of cytokines produced by microglia (MG) and astroglia (AG) are shown. The values represent the means plus standard errors of the means (error bars) for seven different experiments after stimulation for 24 h with LPS at a concentration of 1 μg/ml. Ten cytokines are shown; however, all 24 cytokines tested were produced at elevated levels.
Scrapie agent induction of cytokines in glial cells expressing anchorless PrP.
On the basis of the preceding results, PrP expression by glia was required for scrapie agent stimulation of most cytokines. Furthermore, mice expressing either wild-type anchored PrP or transgenic anchorless PrP both made significant cytokine responses to scrapie agent infection in vivo (Fig. 1A and B). Therefore, we next tested whether primary astroglial or microglial cells expressing only anchorless PrP could produce a cytokine response after exposure to scrapie agent-infected brain homogenate. Astroglia stimulated with scrapie agent-infected brain produced elevated levels of eight cytokines: CCL2, CCL5, CXCL10, G-CSF, IL-1α, IL-1β, IL-6, and IL-12p40 (Fig. 5A and Table 1). Microglia stimulated with scrapie agent-infected brain had elevated levels of five cytokines: CCL2, CXCL10, IL-1α, IL-6, and IL-12p40 (Fig. 5A and Table 1). Therefore, GPI anchoring of PrP was not required for scrapie agent stimulation of production of these cytokines by glial cells. As before, stimulation by LPS gave very high levels of all cytokines tested (Fig. 5B).
FIG. 5.
(A) Scrapie agent-induced cytokine profiles in astroglia and microglia from transgenic mice expressing anchorless PrP. Supernatants of glial cell cultures from transgenic mice were analyzed for amounts of cytokines by using the Bio-Plex multiple-cytokine assay kits 24 h after overlay with brain homogenates. Stimulated culture supernatants of microglia (MG) and astroglia (AG) were analyzed for scrapie agent-induced cytokine level as described in Materials and Methods. Statistical analysis was done using the Wilcoxon matched-pair test comparing the S and N values for cultures from 18 independent experiments for each cytokine. Subtracted values for scrapie agent-infected brain stimulation minus healthy uninfected brain stimulation (S − N) are shown (see the legend to Fig. 2 and Materials and Methods for details). After scrapie agent stimulation, astroglia significantly produced CCL2, CCL5, CXCL10, G-CSF, IL-1α, IL-1β, IL-6, and IL-12p40. Statistical significance by the Mann-Whitney matched-pair test is indicated as follows: ns, not significant; *, P < 0.05; **, P < 0.005; ***, P < 0.001). Other cytokines were not significantly elevated. Microglia significantly produced CCL2, CXCL10, IL-1α, IL-6, and IL-12p40 after scrapie agent stimulation. Other cytokines were not significantly elevated. (B) LPS stimulation. Supernatants of MG and AG cultures stimulated for 24 h with LPS at a concentration of 1 μg/ml were analyzed for cytokines using Bio-Plex multiple-cytokine assay kits. The values represent the means plus standard errors of the means (error bars) for eight different experiments.
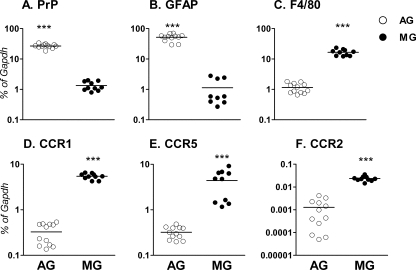
Microglia express less PrP mRNA than astroglia do.
Since PrP expression was required for scrapie agent stimulation of most cytokines, we hypothesized that the production of 11 cytokines by astroglia compared to only two cytokines by microglia in wild-type mice (Fig. 3A and Table 1) might be due in part to a difference in levels of PrP expression on these glial cell types. Therefore, we tested PrP expression by reverse transcriptase PCR and found that astroglia expressed 20-fold-more PrP mRNA than did microglia (Fig. 6A). These levels were not altered by stimulation with scrapie agent or healthy uninfected brain homogenates. We found similar results at the protein level using immunofluorescence on glial cell cultures (data not shown). Higher PrP expression in astroglia versus microglia might be related to the larger number of cytokines produced by astroglia after stimulation by scrapie agent-infected brain homogenates.
FIG. 6.
Expression of PrP mRNA in microglia and astroglia. Expression of mRNA encoding PrP and five other genes in microglia (MG) and astroglia (AG) was determined using quantitative real-time reverse transcriptase PCR. Data are shown as the percent difference relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression for the following genes: PrP (A), GFAP (B), F4/80 (C), CCR1 (D), CCR5 (E), and CCR2 (F). Two different litters of mice were analyzed in separate experiments with six wells of PCR per experiment. Statistical analysis was performed by a one-tailed Mann-Whitney test (***, P < 0.001). Microglia expressed approximately 20-fold-less PrP mRNA than astroglia did. Microglia expressed significantly higher levels of chemokine receptors CCR1, CCR2, and CCR5 than astroglia did. Each symbol shows the amount of mRNA from one mouse, and the black bar shows the mean value.
In control experiments, the mRNA levels of the astroglia-specific marker, GFAP was 45-fold higher in astrocyte culture than in microglial culture, and the microglia-specific marker, F4/80, was 15-fold higher in microglial culture than in astrocyte culture, indicating that the cultures were indeed highly enriched for either astroglia or microglia (Fig. 6B and C).
The lack of high PrP expression on microglia suggested that other receptors might be contributing to the release of IL-12p40 and CXCL10 by microglia after scrapie agent stimulation. We tested three chemokine receptors, chemokine (C-C motif) receptor 1 (CCR1), CCR2, and CCR5, capable of binding chemokine CCL2, CCL5, and CCL11, respectively, found in scrapie agent-infected brain (Fig. 1A). We found that microglia expressed 10- to 16-fold-higher levels of CCR1, CCR2, and CCR5 mRNAs than did astroglia (Fig. 6D, F, and E, respectively). Since some of the ligands for these receptors were found to be elevated in scrapie agent-infected brain, these ligands might act to directly stimulate microglia.
DISCUSSION
In the present experiments, the levels of 10 out of 24 cytokines tested were found to be significantly increased in the brains of scrapie agent-infected mice. This is the first report of the examination of protein levels of many cytokines in a prion agent-infected brain. We found upregulation of CCL2, CCL5, CXCL10, IL-1α, IL-1β, and IL-6, which were noted earlier in quantitative experiments measuring mRNA (12, 28, 32, 33, 47) as well as in some qualitative experiments detecting cytokine protein by immunohistochemistry (21, 66, 67). However, we also noted an increase in CXCL1, GM-CSF, IFN-γ, and IL-12p40, which were not previously reported. CCL3, CCL4, CCL11, G-CSF, IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, IL-12p70, IL-13, IL-17, and TNF were not elevated or not detected in scrapie agent-infected brain. Compared to bacterial or viral infections of the CNS (6, 27, 49), the brain cytokine response after scrapie agent infection in mice was restricted to fewer cytokines and lower levels. This was particularly surprising considering the extensive microgliosis and astrogliosis present at sites of prion disease pathology (45, 49, 65). One reason for the lower cytokine levels might be the fact that PrPres, the major abnormal molecule produced in scrapie, is derived by misfolding of a self-protein, PrPsen. Therefore, PrPres might not be recognized by pattern recognition receptors which induce a strong innate immune response following detection of foreign microbes (38). The lack of a lymphocytic response to scrapie agent in the CNS might also contribute to the low cytokine levels observed.
The mechanisms of cytokine induction in scrapie and other prion diseases are not well understood at this time. Several lines of evidence suggest that the disease-associated protease-resistant PrPres is likely to be involved in this process. First, PrPres is often colocalized with brain damage (9, 30, 61, 64); second, in PrP-null mice, which do not generate PrPres or develop brain pathology, there is no cytokine response to scrapie agent inoculation (Table 1). In our present experiments, PrPsen expression was found to be required for scrapie agent-induced stimulation of 10 of the 11 cytokines produced by astroglia. This finding correlated with our data showing that astroglia expressed higher levels of PrPsen mRNA than did microglia (Fig. 6). PrPsen is known to bind PrPres in cell-free experiments (14, 29) as well as in experiments involving live cells (58). Such binding might trigger cytokine release from astroglia, and if so, PrPres might be an important molecule in initiating the cytokine response to scrapie. However, it was surprising that PrP anchoring to the plasma membrane was not required for this stimulatory process, as brain and primary glia from transgenic mice expressing anchorless PrP were stimulated by scrapie agent to produce most of these same cytokines (Table 1). This result suggested that secreted or cytoplasmic anchorless PrPsen might form complexes with PrPres and bind glial scavenger receptors which might contribute to signaling for microglial and astroglial activation (2, 16, 68).
Interestingly, IL-12p40 production by both astroglia and microglia did not require PrPsen expression. Significant induction by scrapie agent-infected brain homogenates was observed in PrP-null glia (Fig. 4), and PrPres interactions with endogenous PrPsen could not have initiated this process. However, microglia were found to express high levels of the CCR1, CCR2, and CCR5 cytokine receptors (Fig. 3), and other cytokine receptors, including chemokine (C-X-C motif) receptor 3 (CXCR3), have also been found on both astroglia and microglia. Several ligands for these receptors were elevated significantly in scrapie agent-infected brain homogenates and were also produced by astroglia and microglia in vitro. Thus, these receptor-ligand interactions might have been involved in a PrPsen-independent stimulation of microglia and possibly also astroglia.
The present data raise the question of whether glial activation including the cytokine response to prion infection is part of the pathogenic process or part of the host response to brain damage induced by other factors. This conundrum has been faced in other neurodegenerative and neuroinflammatory diseases, such as Alzheimer's disease (20), amyotrophic lateral sclerosis (4), Parkinson's disease (51), and multiple sclerosis (13). In these diseases, the general opinion seems to be that glia contribute to both disease control and disease progression. Data from studies of prion diseases has also given mixed conclusions. For example, mice lacking expression of cytokine receptor IL-1 receptor type I (54) or chemokine receptor CXCR3 (48) showed increased survival following scrapie agent infection, indicating that signaling via these receptors might contribute to disease pathogenesis. In contrast, other studies using mice lacking expression of CCR1 (31) or having a mutation affecting TLR4 (Toll-like receptor 4) signaling (55) showed decreased survival after scrapie, indicating that signaling via these receptors might contribute to damage control during prion infection. Last, mice with deficient expression of cytokine TNF or IL-6 had no alteration in survival after intracerebral scrapie agent infection (34). Together these data indicate that glial activation and the accompanying cytokine responses to prion infection might have negative, positive, or no effect on the disease outcome. The similarity of the scrapie agent-induced cytokine responses in wild-type mice and transgenic mice expressing anchorless PrP, which differ in neuropathology (Fig. 1 and Table 1), suggested that the cytokine levels found in the present experiments were mostly responses to brain damage rather than initiators of a particular pathogenic mechanism. However, the presence of cytokines might also exacerbate the prion disease pathology as appears to be the case in Alzheimer's disease (17, 39).
Acknowledgments
We thank Brent Race and Kimberly Meade-White for technical assistance with the mouse experiments and Suzette A. Priola, Byron Caughey, and Kim J. Hasenkrug for critical reading of the manuscript.
This work was supported by the NIAID Division of Intramural Research at NIH.
Footnotes
Published ahead of print on 26 August 2009.
REFERENCES
- 1.Baker, C. A., and L. Manuelidis. 2003. Unique inflammatory RNA profiles of microglia in Creutzfeldt-Jakob disease. Proc. Natl. Acad. Sci. USA 100:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell, M. D., R. Lopez-Gonzalez, L. Lawson, D. Hughes, I. Fraser, S. Gordon, and V. H. Perry. 1994. Upregulation of the macrophage scavenger receptor in response to different forms of injury in the CNS. J. Neurocytol. 23:605-613. [DOI] [PubMed] [Google Scholar]
- 3.Beringue, V., M. Demoy, C. I. Lasmezas, B. Gouritin, C. Weingarten, J. P. Deslys, J. P. Andreux, P. Couvreur, and D. Dormont. 2000. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J. Pathol. 190:495-502. [DOI] [PubMed] [Google Scholar]
- 4.Boillee, S., C. Vande Velde, and D. W. Cleveland. 2006. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52:39-59. [DOI] [PubMed] [Google Scholar]
- 5.Brandner, S. 2003. CNS pathogenesis of prion diseases. Br. Med. Bull. 66:131-139. [DOI] [PubMed] [Google Scholar]
- 6.Brown, A. R., J. Webb, S. Rebus, R. Walker, A. Williams, and J. K. Fazakerley. 2003. Inducible cytokine gene expression in the brain in the ME7/CV mouse model of scrapie is highly restricted, is at a strikingly low level relative to the degree of gliosis and occurs only late in disease. J. Gen. Virol. 84:2605-2611. [DOI] [PubMed] [Google Scholar]
- 7.Brown, D. R. 2001. Microglia and prion disease. Microsc. Res. Tech. 54:71-80. [DOI] [PubMed] [Google Scholar]
- 8.Brown, D. R., B. Schmidt, and H. A. Kretzschmar. 1996. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380:345-347. [DOI] [PubMed] [Google Scholar]
- 9.Budka, H., A. Aguzzi, P. Brown, J. M. Brucher, O. Bugiani, F. Gullotta, M. Haltia, J. J. Hauw, J. W. Ironside, K. Jellinger, et al. 1995. Neuropathological diagnostic criteria for Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol. 5:459-466. [DOI] [PubMed] [Google Scholar]
- 10.Burwinkel, M., C. Riemer, A. Schwarz, J. Schultz, S. Neidhold, T. Bamme, and M. Baier. 2004. Role of cytokines and chemokines in prion infections of the central nervous system. Int. J. Dev. Neurosci. 22:497-505. [DOI] [PubMed] [Google Scholar]
- 11.Butchi, N. B., S. Pourciau, M. Du, T. W. Morgan, and K. E. Peterson. 2008. Analysis of the neuroinflammatory response to TLR7 stimulation in the brain: comparison of multiple TLR7 and/or TLR8 agonists. J. Immunol. 180:7604-7612. [DOI] [PubMed] [Google Scholar]
- 12.Campbell, I. L., M. Eddleston, P. Kemper, M. B. Oldstone, and M. V. Hobbs. 1994. Activation of cerebral cytokine gene expression and its correlation with onset of reactive astrocyte and acute-phase response gene expression in scrapie. J. Virol. 68:2383-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carson, M. J. 2002. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia 40:218-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caughey, B. 2000. Formation of protease-resistant prion protein in cell-free systems. Curr. Issues Mol. Biol. 2:95-101. [PubMed] [Google Scholar]
- 15.Chesebro, B., M. Trifilo, R. Race, K. Meade-White, C. Teng, R. LaCasse, L. Raymond, C. Favara, G. Baron, S. Priola, B. Caughey, E. Masliah, and M. Oldstone. 2005. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308:1435-1439. [DOI] [PubMed] [Google Scholar]
- 16.Coraci, I. S., J. Husemann, J. W. Berman, C. Hulette, J. H. Dufour, G. K. Campanella, A. D. Luster, S. C. Silverstein, and J. B. El-Khoury. 2002. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am. J. Pathol. 160:101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dheen, S. T., C. Kaur, and E. A. Ling. 2007. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 14:1189-1197. [DOI] [PubMed] [Google Scholar]
- 18.Dumitru, C. D., J. D. Ceci, C. Tsatsanis, D. Kontoyiannis, K. Stamatakis, J. H. Lin, C. Patriotis, N. A. Jenkins, N. G. Copeland, G. Kollias, and P. N. Tsichlis. 2000. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103:1071-1083. [DOI] [PubMed] [Google Scholar]
- 19.Fan, J., N. M. Heller, M. Gorospe, U. Atasoy, and C. Stellato. 2005. The role of post-transcriptional regulation in chemokine gene expression in inflammation and allergy. Eur. Respir. J. 26:933-947. [DOI] [PubMed] [Google Scholar]
- 20.Farfara, D., V. Lifshitz, and D. Frenkel. 2008. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer's disease. J. Cell. Mol. Med. 12:762-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felton, L. M., C. Cunningham, E. L. Rankine, S. Waters, D. Boche, and V. H. Perry. 2005. MCP-1 and murine prion disease: separation of early behavioural dysfunction from overt clinical disease. Neurobiol. Dis. 20:283-295. [DOI] [PubMed] [Google Scholar]
- 22.Giese, A., D. R. Brown, M. H. Groschup, C. Feldmann, I. Haist, and H. A. Kretzschmar. 1998. Role of microglia in neuronal cell death in prion disease. Brain Pathol. 8:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickman, S. E., E. K. Allison, and J. El Khoury. 2008. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J. Neurosci. 28:8354-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulse, R. E., P. E. Kunkler, J. P. Fedynyshyn, and R. P. Kraig. 2004. Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J. Neurosci. Methods 136:87-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karpus, W. J., and R. M. Ransohoff. 1998. Chemokine regulation of experimental autoimmune encephalomyelitis: temporal and spatial expression patterns govern disease pathogenesis. J. Immunol. 161:2667-2671. [PubMed] [Google Scholar]
- 26.Khaleduzzaman, M., J. Francis, M. E. Corbin, E. McIlwain, M. Boudreaux, M. Du, T. W. Morgan, and K. E. Peterson. 2007. Infection of cardiomyocytes and induction of left ventricle dysfunction by neurovirulent polytropic murine retrovirus. J. Virol. 81:12307-12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kielian, T. 2004. Microglia and chemokines in infectious diseases of the nervous system: views and reviews. Front. Biosci. 9:732-750. [DOI] [PubMed] [Google Scholar]
- 28.Kim, J. I., W. K. Ju, J. H. Choi, E. Choi, R. I. Carp, H. M. Wisniewski, and Y. S. Kim. 1999. Expression of cytokine genes and increased nuclear factor-kappa B activity in the brains of scrapie-infected mice. Brain Res. Mol. Brain Res. 73:17-27. [DOI] [PubMed] [Google Scholar]
- 29.Kocisko, D. A., J. H. Come, S. A. Priola, B. Chesebro, G. J. Raymond, P. T. Lansbury, and B. Caughey. 1994. Cell-free formation of protease-resistant prion protein. Nature 370:471-474. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs, G. G., M. Preusser, M. Strohschneider, and H. Budka. 2005. Subcellular localization of disease-associated prion protein in the human brain. Am. J. Pathol. 166:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaCasse, R. A., J. F. Striebel, C. Favara, L. Kercher, and B. Chesebro. 2008. Role of Erk1/2 activation in prion disease pathogenesis: absence of CCR1 leads to increased Erk1/2 activation and accelerated disease progression. J. Neuroimmunol. 196:16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, H. P., Y. C. Jun, J. K. Choi, J. I. Kim, R. I. Carp, and Y. S. Kim. 2005. The expression of RANTES and chemokine receptors in the brains of scrapie-infected mice. J. Neuroimmunol. 158:26-33. [DOI] [PubMed] [Google Scholar]
- 33.Lu, Z. Y., C. A. Baker, and L. Manuelidis. 2004. New molecular markers of early and progressive CJD brain infection. J. Cell. Biochem. 93:644-652. [DOI] [PubMed] [Google Scholar]
- 34.Mabbott, N. A., A. Williams, C. F. Farquhar, M. Pasparakis, G. Kollias, and M. E. Bruce. 2000. Tumor necrosis factor alpha-deficient, but not interleukin-6-deficient, mice resist peripheral infection with scrapie. J. Virol. 74:3338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manson, J. C., A. R. Clarke, M. L. Hooper, L. Aitchison, I. McConnell, and J. Hope. 1994. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol. Neurobiol. 8:121-127. [DOI] [PubMed] [Google Scholar]
- 36.McHattie, S. J., D. R. Brown, and M. M. Bird. 1999. Cellular uptake of the prion protein fragment PrP106-126 in vitro. J. Neurocytol. 28:149-159. [DOI] [PubMed] [Google Scholar]
- 37.Minagar, A., P. Shapshak, R. Fujimura, R. Ownby, M. Heyes, and C. Eisdorfer. 2002. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 202:13-23. [DOI] [PubMed] [Google Scholar]
- 38.Palm, N. W., and R. Medzhitov. 2009. Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 227:221-233. [DOI] [PubMed] [Google Scholar]
- 39.Patel, N. S., D. Paris, V. Mathura, A. N. Quadros, F. C. Crawford, and M. J. Mullan. 2005. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer's disease. J. Neuroinflammation 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pauli, U. 1994. Control of tumor necrosis factor gene expression. Crit. Rev. Eukaryot. Gene Expr. 4:323-344. [DOI] [PubMed] [Google Scholar]
- 41.Peyrin, J. M., C. I. Lasmezas, S. Haik, F. Tagliavini, M. Salmona, A. Williams, D. Richie, J. P. Deslys, and D. Dormont. 1999. Microglial cells respond to amyloidogenic PrP peptide by the production of inflammatory cytokines. Neuroreport 10:723-729. [DOI] [PubMed] [Google Scholar]
- 42.Powell, M. J., S. A. Thompson, Y. Tone, H. Waldmann, and M. Tone. 2000. Posttranscriptional regulation of IL-10 gene expression through sequences in the 3′-untranslated region. J. Immunol. 165:292-296. [DOI] [PubMed] [Google Scholar]
- 43.Prusiner, S. B. 1991. Molecular biology of prion diseases. Science 252:1515-1522. [DOI] [PubMed] [Google Scholar]
- 44.Race, B., K. Meade-White, M. B. Oldstone, R. Race, and B. Chesebro. 2008. Detection of prion infectivity in fat tissues of scrapie-infected mice. PLoS Pathog. 4:e1000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rezaie, P., and P. L. Lantos. 2001. Microglia and the pathogenesis of spongiform encephalopathies. Brain Res. Brain Res. Rev. 35:55-72. [DOI] [PubMed] [Google Scholar]
- 46.Riemer, C., S. Neidhold, M. Burwinkel, A. Schwarz, J. Schultz, J. Kratzschmar, U. Monning, and M. Baier. 2004. Gene expression profiling of scrapie-infected brain tissue. Biochem. Biophys. Res. Commun. 323:556-564. [DOI] [PubMed] [Google Scholar]
- 47.Riemer, C., I. Queck, D. Simon, R. Kurth, and M. Baier. 2000. Identification of upregulated genes in scrapie-infected brain tissue. J. Virol. 74:10245-10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riemer, C., J. Schultz, M. Burwinkel, A. Schwarz, S. W. Mok, S. Gultner, T. Bamme, S. Norley, F. van Landeghem, B. Lu, C. Gerard, and M. Baier. 2008. Accelerated prion replication in, but prolonged survival times of, prion-infected CXCR3−/− mice. J. Virol. 82:12464-12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rock, R. B., G. Gekker, S. Hu, W. S. Sheng, M. Cheeran, J. R. Lokensgard, and P. K. Peterson. 2004. Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 17:942-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolls, A., R. Shechter, and M. Schwartz. 2009. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 10:235-241. [DOI] [PubMed] [Google Scholar]
- 51.Roodveldt, C., J. Christodoulou, and C. M. Dobson. 2008. Immunological features of alpha-synuclein in Parkinson's disease. J. Cell. Mol. Med. 12:1820-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365-386. [DOI] [PubMed] [Google Scholar]
- 53.Savarin-Vuaillat, C., and R. M. Ransohoff. 2007. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics 4:590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schultz, J., A. Schwarz, S. Neidhold, M. Burwinkel, C. Riemer, D. Simon, M. Kopf, M. Otto, and M. Baier. 2004. Role of interleukin-1 in prion disease-associated astrocyte activation. Am. J. Pathol. 165:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spinner, D. S., I. S. Cho, S. Y. Park, J. I. Kim, H. C. Meeker, X. Ye, G. Lafauci, D. J. Kerr, M. J. Flory, B. S. Kim, R. B. Kascsak, T. Wisniewski, W. R. Levis, G. B. Schuller-Levis, R. I. Carp, E. Park, and R. J. Kascsak. 2008. Accelerated prion disease pathogenesis in Toll-like receptor 4 signaling-mutant mice. J. Virol. 82:10701-10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanton, J. B., D. P. Knowles, D. R. Call, B. A. Mathison, and T. V. Baszler. 2009. Limited transcriptional response of ovine microglia to prion accumulation. Biochem. Biophys. Res. Commun. 386:345-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Streit, W. J. 2002. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40:133-139. [DOI] [PubMed] [Google Scholar]
- 58.Taguchi, Y., Z. D. Shi, B. Ruddy, D. W. Dorward, L. Greene, and G. S. Baron. 2009. Specific biarsenical labeling of cell surface proteins allows fluorescent- and biotin-tagging of amyloid precursor protein and prion proteins. Mol. Biol. Cell 20:233-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thellung, S., A. Corsaro, V. Villa, V. Venezia, M. Nizzari, M. Bisaglia, C. Russo, G. Schettini, A. Aceto, and T. Florio. 2007. Amino-terminally truncated prion protein PrP90-231 induces microglial activation in vitro. Ann. N. Y. Acad. Sci. 1096:258-270. [DOI] [PubMed] [Google Scholar]
- 60.Trifilo, M. J., M. Sanchez-Alavez, L. Solforosi, J. Bernard-Trifilo, S. Kunz, D. McGavern, and M. B. Oldstone. 2008. Scrapie-induced defects in learning and memory of transgenic mice expressing anchorless prion protein are associated with alterations in the gamma aminobutyric acid-ergic pathway. J. Virol. 82:9890-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Everbroeck, B., I. Dobbeleir, M. De Waele, E. De Leenheir, U. Lubke, J. J. Martin, and P. Cras. 2004. Extracellular protein deposition correlates with glial activation and oxidative stress in Creutzfeldt-Jakob and Alzheimer's disease. Acta Neuropathol. 108:194-200. [DOI] [PubMed] [Google Scholar]
- 62.Veerhuis, R., J. J. Hoozemans, I. Janssen, R. S. Boshuizen, J. P. Langeveld, and P. Eikelenboom. 2002. Adult human microglia secrete cytokines when exposed to neurotoxic prion protein peptide: no intermediary role for prostaglandin E2. Brain Res. 925:195-203. [DOI] [PubMed] [Google Scholar]
- 63.Walsh, D. T., S. Betmouni, and V. H. Perry. 2001. Absence of detectable IL-1beta production in murine prion disease: a model of chronic neurodegeneration. J. Neuropathol. Exp. Neurol. 60:173-182. [DOI] [PubMed] [Google Scholar]
- 64.Wells, G. A. 1993. Pathology of nonhuman spongiform encephalopathies: variations and their implications for pathogenesis. Dev. Biol. Stand. 80:61-69. [PubMed] [Google Scholar]
- 65.Williams, A., P. J. Lucassen, D. Ritchie, and M. Bruce. 1997. PrP deposition, microglial activation, and neuronal apoptosis in murine scrapie. Exp. Neurol. 144:433-438. [DOI] [PubMed] [Google Scholar]
- 66.Williams, A., A. M. Van Dam, D. Ritchie, P. Eikelenboom, and H. Fraser. 1997. Immunocytochemical appearance of cytokines, prostaglandin E2 and lipocortin-1 in the CNS during the incubation period of murine scrapie correlates with progressive PrP accumulations. Brain Res. 754:171-180. [DOI] [PubMed] [Google Scholar]
- 67.Williams, A. E., A. M. van Dam, W. K. Man-a-Hing, F. Berkenbosch, P. Eikelenboom, and H. Fraser. 1994. Cytokines, prostaglandins and lipocortin-1 are present in the brains of scrapie-infected mice. Brain Res. 654:200-206. [DOI] [PubMed] [Google Scholar]
- 68.Wyss-Coray, T., J. D. Loike, T. C. Brionne, E. Lu, R. Anankov, F. Yan, S. C. Silverstein, and J. Husemann. 2003. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 9:453-457. [DOI] [PubMed] [Google Scholar]
- 69.Xiang, W., O. Windl, G. Wünsch, M. Dugas, A. Kohlmann, N. Dierkes, I. M. Westner, and H. A. Kretzschmar. 2004. Identification of differentially expressed genes in scrapie-infected mouse brains by using global gene expression technology. J. Virol. 78:11051-11060. [DOI] [PMC free article] [PubMed] [Google Scholar]