Abstract
Gamma interferon (IFN-γ) is critical for the control of chronic infection with murine gammaherpesvirus 68 (γHV68). Current data indicate that IFN-γ has a lesser role in the control of acute replication of γHV68. Here, we show that IFN-γ-deficient mice on the BALB/c genetic background poorly control acute viral replication and succumb to early death by acute pneumonia. Notably, this acute, lethal pneumonia was dependent not only on the viral dose, but also on specific viral genes including the viral cyclin gene, previously identified to be important in promoting optimal chronic infection and reactivation from latency.
Murine gammaherpesvirus 68 (γHV68) is closely related to the human gammaherpesviruses Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus, making γHV68 infection of mice a good animal model for gammaherpesvirus pathogenesis (22).
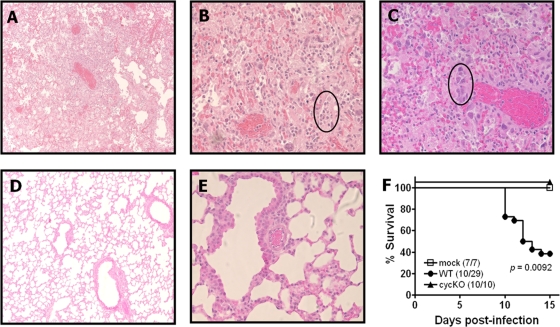
A number of studies have found gamma interferon (IFN-γ) to be crucial in controlling γHV68 reactivation from latency and persistent infection (9, 14, 18, 24) but not in controlling acute infection (10). Recently, we demonstrated that γHV68 infection of IFN-γ receptor-deficient mice results in pulmonary lymphoproliferation and B-cell lymphoma, similar to those observed in a number of gammaherpesvirus-associated human diseases (7). We found that this pathology was entirely independent of the viral cyclin. We then tested whether IFN-γ deficiency on the tumor-prone BALB/c background would result in more rapid or higher incidence of lymphomagenesis. Wild-type (WT) γHV68 clone WUMS and viral cyclin-deficient (cyclin knockout [cycKO]), viral bcl-2-deficient (M11 KO), and M1-deficient (M1 KO) γHV68 mutants were passaged and grown, and titers were determined as described previously (23). IFN-γ−/− mice on a BALB/c background [strain C.129S7(B6)-Ifngtm1Ts/J from Jackson Laboratory, Bar Harbor, ME] were intranasally infected at ≥8 weeks of age with 4 × 105 PFU of virus in 40 μl of Dulbecco's modified Eagle's medium, unless otherwise noted (2). Unexpectedly, we found that the majority of WT-infected mice died 9 to 14 days postinfection. Therefore, we harvested the lungs from the mice at the time of death and fixed the tissues, embedded them in paraffin, sectioned the samples, and stained them with hematoxylin and eosin (H&E) for histological examination. It was determined that the mice had succumbed to acute bronchopneumonia characterized by vascular congestion, interstitial and airway edema, inflammatory cell infiltrates, and viral inclusions (Fig. 1A to C). Upon sacrifice at 15 days postinfection, none of the cycKO virus-infected mice showed any signs of pneumonia (Fig. 1D and E), similar to WT-infected mice that had survived to day 15 (data not shown). We also compared virus titers in the lungs of these mice by mechanically disrupting the lower left lung lobe from each animal and performing plaque assays with the homogenates as described previously (15, 20). In accordance with the survival data and histological observations, we obtained detectable titers (mean ± standard error of the mean, 9.1 × 106 ± 4.7 × 106 PFU/ml) from only those WT-infected mice that had succumbed to infection (data not shown). No virus was detectable in samples from WT- and cycKO virus-infected mice that survived the length of the study (data not shown). Figure 1F depicts the relative survival rates of the mice from this study, which differed significantly (P= 0.0092 for WT- versus cycKO virus-infected mice). Finally, six cycKO virus-infected mice were monitored over 90 days postinfection, with no mortality (data not shown).
FIG. 1.
Infection of IFN-γ−/− mice with γHV68 results in acute lethal pneumonia. (A to E) Histological analyses of lung tissues from BALB/c IFN-γ−/− mice infected with WT (A to C) and cycKO (D and E) γHV68. Lungs were harvested at 10 days postinfection (for WT-infected mice) and 15 days postinfection (for cycKO virus-infected mice) and stained with H&E. Neutrophils (circled in panel B) and viral inclusions (circled in panel C) can be observed. Original magnifications, ×10 (A and D) and ×40 (B, C, and E). (F) Survival analysis of IFN-γ−/− mice infected with the indicated viruses. The fractions of surviving mice at 15 days postinfection are shown in parentheses. Lines representing 100% survival are separated for ease of visualization. Significance was determined using the Kaplan-Meier survival and log rank tests with GraphPad software. P = 0.0092 for results for WT versus cycKO infections.
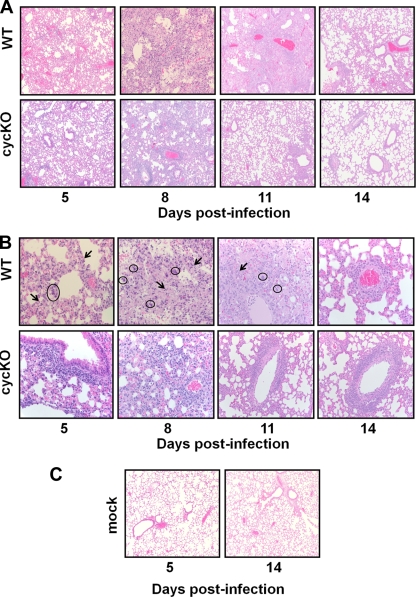
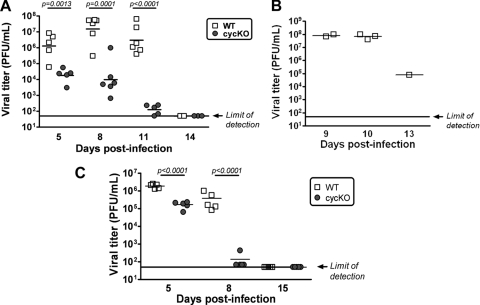
These observations prompted us to perform a thorough kinetic analysis of infection. We infected BALB/c IFN-γ−/− mice with WT or cycKO virus, sacrificed them at various times postinfection, and harvested lung tissues for histological examination by H&E staining and determination of viral titers by plaque assay. On day 5, the lungs of both WT and cycKO virus-infected mice showed signs of mild, acute pneumonia (Fig. 2A and B). Lungs from mock-infected mice were completely normal (Fig. 2C). There appeared to be greater cellularity and infiltration, as well as higher viral titers, in the lungs of WT-infected mice than in those of cycKO virus-infected mice (72-fold difference; P = 0.0013) (Fig. 3A). However, by day 8, we already noted differences in the severity of disease and the viral load between WT- and cycKO virus-infected mice. WT-infected mice had progressed to more severe disease, with most of the lung affected, while cycKO virus-infected lungs demonstrated no change from day 5 (Fig. 2A and B). The viral titers in the lungs of WT-infected mice were also higher than those in the lungs of cycKO virus-infected mice on day 8 (1,535-fold difference; P = 0.0001) (Fig. 3A). By day 11, some of the WT-infected mice had succumbed to disease. The lungs of WT-infected mice 11 days postinfection, even those of mice that had not succumbed to disease, looked as severe as they did on day 8 (Fig. 2A and B). In comparison, cycKO virus-infected mice had already cleared the pneumonia by day 11 and showed only signs of mild perivascular inflammation (Fig. 2A and B). The viral loads in WT-infected mice on day 11 were significantly higher than those in cycKO virus-infected mice (23,823-fold difference; P < 0.0001) (Fig. 3A), consistent with the histological findings. We found that WT- and cycKO virus-infected mice that survived to day 14 postinfection showed no signs of pneumonia and only mild perivascular inflammation (Fig. 2A and B). None of the cycKO virus-infected mice succumbed over the 15-day course of the study. By day 14 postinfection, both WT and cycKO virus-infected mice had no detectable viral titers in the lungs (Fig. 3A). Viral titers in BALB/c IFN-γ−/− mice that succumbed prior to sacrifice are shown in Fig. 3B. To determine whether the observed replication defect in cycKO virus-infected BALB/c IFN-γ−/− mice was due specifically to the lack of IFN-γ, we also infected normal BALB/c mice with WT or cycKO γHV68. In normal BALB/c mice, cycKO virus replication was also decreased relative to WT virus replication, but to a lesser extent than that in BALB/c IFN-γ−/− mice. In contrast to BALB/c IFN-γ−/− mice, both WT- and cycKO virus-infected BALB/c mice efficiently cleared virus infection and showed no mortality at up to 15 days postinfection (Fig. 3C), although they did demonstrate signs of mild inflammation that resolved (data not shown). The contribution of genetic background to pathogenesis and replication is unknown at this point; however, a previous report also describes mortality associated with intranasal infection of IFN-γ−/− mice on a C57BL/6 background (6).
FIG. 2.
Temporal analysis of γHV68-infected IFN-γ−/− mice reveals more prolonged and severe pneumonia in WT-infected mice than in cycKO virus-infected mice. BALB/c IFN-γ−/− mice were infected with WT or cycKO γHV68 and sacrificed on day 5, 8, 11, or 14 postinfection. Mock-infected mice were sacrificed on days 5 and 14. Lungs were harvested at the specified time points for histological analysis. (A and B) H&E staining of lung tissues from WT- and cycKO virus-infected mice. Viral inclusions (circled) and neutrophils (indicated by arrows) can be observed. Original magnifications, ×10 (A) and ×40 (B). (C) H&E staining of lung tissues from mock-infected BALB/c IFN-γ−/− mice. Original magnification, ×10.
FIG. 3.
WT-infected IFN-γ−/− mice fail to control acute infection. Plaque assays were performed with lung homogenates from BALB/c and BALB/c IFN-γ−/− mice infected for temporal analysis (as described in the legend to Fig. 2). Viral titers in BALB/c IFN-γ−/− mice that were sacrificed on day 5, 8, 11, or 14 postinfection (A) or succumbed to WT infection on the indicated days (B) and those in BALB/c mice sacrificed on days 5, 8, and 15 postinfection (C) are shown. The limit of detection of the assay (50 PFU) is indicated. P values for statistically significant differences between WT and cycKO virus infections, listed in panels A and C, were determined by an unpaired t test.
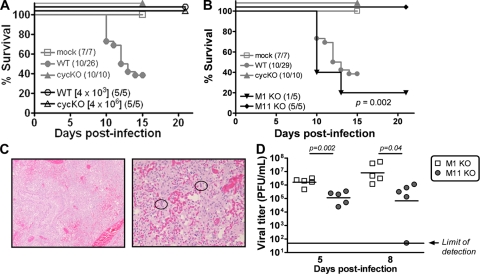
Overall, we found that 16 of 26 WT-infected mice (61.5%) died between 10 and 14 days postinfection (Fig. 1F and 4A and B). Since not all of the WT-infected mice succumbed to infection, we tested whether there was dose dependence associated with disease and mortality. In contrast to the results of initial experiments, mice infected with 4 × 103 PFU of WT virus (a dose 100-fold lower than that initially used) uniformly survived for 21 days postinfection (Fig. 4A), consistent with data in previous reports (10). H&E analysis of the lungs harvested at 21 days indicated no signs of pneumonia and only mild perivascular inflammation (data not shown). Notably, mice infected with 4 × 106 PFU of cycKO virus (a dose 10-fold higher than that initially used) all survived infection (Fig. 4A).
FIG. 4.
The regulation of acute infection and pneumonia is dependent on virus dose and specific gene expression. (A and B) Survival analyses of BALB/c IFN-γ−/− mice infected with different doses of WT or cycKO virus as indicated (A) and M1 or M11 KO virus at 4 × 105 PFU (B). The fractions of surviving mice at 21 days postinfection are shown in parentheses. Survival results for mock, WT, and cycKO virus infections (reported in Fig. 1F) are included in gray for reference. The significance of survival results was determined using the Kaplan-Meier survival curves and the log rank test. Results for M1 KO virus versus mock infections, P = 0.002. (C) H&E staining of lung tissues from M1 KO virus-infected mice that succumbed at day 10 postinfection. Examples of viral inclusions are circled. Original magnifications, ×10 (left) and ×40 (right). (D) Viral titers in M1 KO or M11 KO virus-infected mice sacrificed at the indicated times postinfection. The limit of detection of the assay (50 PFU) is indicated. P values for statistically significant differences between M1 KO and M11 KO virus infections were determined by an unpaired t test.
The observation that none of the cycKO virus-infected mice died was surprising, since the cycKO virus previously showed no defect in acute replication or pathogenesis (4, 5, 21), with a single exception for a low-dose inoculation following intranasal infection (19). In contrast, the cycKO virus has profound defects in reactivation from latency and decreased persistence (4, 5, 15, 20, 21). To investigate whether other mutant viruses with alterations in reactivation from latency may also lead to altered pathogenesis, we tested the abilities of two viral mutants to induce lethal, acute pneumonia in BALB/c IFN-γ−/− mice: (i) the M11 (viral bcl-2) KO virus, which exhibits reduced levels of reactivation from latency (3, 4, 8) and has properties similar to those of the cycKO virus, and (ii) the M1 KO virus, which has increased levels of reactivation from latency (1, 11). As suspected, mice infected with the M1 KO virus exhibited disease kinetics and mortality similar to those of WT-infected mice, whereas M11 KO virus-infected mice remained healthy and viable (Fig. 4B). WT- and M1 KO virus-infected mice that survived infection showed no signs of viral replication by or before day 21 (data not shown). The M1 KO virus-infected mice that succumbed to infection (4 of 5 [80%]; P = 0.002) showed signs of severe pneumonia (Fig. 4C) and viral titers similar to those in WT-infected mice (Fig. 4D). In contrast, viral titers in M11 KO virus-infected mice were more similar to those in cycKO virus-infected mice.
In sum, our data indicate that IFN-γ plays a critical role in controlling acute γHV68 replication and pathogenesis on the BALB/c genetic background. This role may involve pulmonary cytokine responses to γHV68, as described previously for BALB/c and C57BL/6 mice (25). The observed early death was dependent on the dose of virus and was critically dependent on two viral genes, the viral cyclin and viral bcl-2 genes. These two genes have previously been observed to play little role in acute replication (10) but prominent roles in promoting reactivation from latency and persistent replication in immunocompromised mice (3-5, 8, 15, 20, 21; A. L. Suarez and L. F. van Dyk, submitted for publication). Based on these observations, we hypothesize two explanations for the viral cyclin- and viral bcl-2-dependent lethality in BALB/c IFN-γ−/− mice. First, these mice may succumb to acute pneumonia due to uncontrolled, early reactivation from latency, a process in which both the viral cyclin and viral bcl-2 are known to be important. One caveat to this explanation, however, is that it is difficult to assess reactivation from latency in the context of active viral replication. A second, not mutually exclusive explanation is that viruses in BALB/c IFN-γ−/− mice undergo replication in additional cell types in which viral replication in healthy mice is not typically observed and that the viral cyclin and viral bcl-2 are important within these cell types (e.g., in the case of viral persistence in endothelial cells). We find the latter explanation particularly intriguing given our recent data that γHV68 persistence in endothelial cells is impaired in viruses lacking either the viral cyclin or the viral bcl-2 (15; Suarez and van Dyk, submitted). A cell type-specific effect of IFN-γ on γHV68 reactivation has also been reported previously (13).
In conclusion, these findings provide strong support for the role of an antiviral cytokine, IFN-γ, in controlling early γHV68 replication and pathogenesis. Furthermore, they provide evidence of expanded roles for the viral cyclin and viral bcl-2 in acute mortality in immunocompromised mice. This is significant since previous analyses of cycKO and M11 KO viruses have shown that they do not generally have acute replication defects and are equivalently capable of causing mortality in mice lacking T and B cells (4, 12, 21) and in young mice after intracranial inoculation (4). Based on these data, we hypothesize that the unanticipated role of the viral cyclin and bcl-2 genes in acute pathogenesis is uniquely revealed in IFN-γ−/− hosts. We further hypothesize that altered cell tropism in IFN-γ−/− mice may be central to the requirement for these viral genes in acute pathogenesis.
Acknowledgments
We thank Jill Slanksy for BALB/c IFN-γ−/− mice (National Jewish Health; originally from Jackson Laboratory) and Sam Speck (Emory University, Atlanta, GA) for M1 KO virus. We also thank members of the van Dyk lab and Eric Clambey for discussions.
This research was supported by National Institutes of Health grants T32 AI07537-09 and T32 AI007405-18 to K.S.L. and National Institutes of Health grant R01 CA103632 and a Burroughs Wellcome Foundation career award to L.F.V.D. We declare no competing financial interests.
Footnotes
Published ahead of print on 26 August 2009.
REFERENCES
- 1.Clambey, E. T., H. W. Virgin, and S. H. Speck. 2000. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J. Virol. 74:1973-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 3.de Lima, B. D., J. S. May, S. Marques, J. P. Simas, and P. G. Stevenson. 2005. Murine gammaherpesvirus 68 bcl-2 homologue contributes to latency establishment in vivo. J. Gen. Virol. 86:31-40. [DOI] [PubMed] [Google Scholar]
- 4.Gangappa, S., L. F. van Dyk, T. J. Jewett, S. H. Speck, and H. W. Virgin IV. 2002. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 195:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoge, A. T., S. B. Hendrickson, and W. H. Burns. 2000. Murine gammaherpesvirus 68 cyclin D homologue is required for efficient reactivation from latency. J. Virol. 74:7016-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni, A. B., K. L. Holmes, T. N. Fredrickson, J. W. Hartley, and H. C. Morse III. 1997. Characteristics of a murine gammaherpesvirus infection immunocompromised mice. In Vivo 11:281-291. [PubMed] [Google Scholar]
- 7.Lee, K. S., S. D. Groshong, C. D. Cool, B. K. Kleinschmidt-Demasters, and L. F. van Dyk. 2009. Murine gammaherpesvirus 68 infection of IFNγ unresponsive mice: a small animal model for gammaherpesvirus-associated B-cell lymphoproliferative disease. Cancer Res. 69:5481-5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loh, J., Q. Huang, A. M. Petros, D. Nettesheim, L. F. van Dyk, L. Labrada, S. H. Speck, B. Levine, E. T. Olejniczak, and H. W. Virgin. 2005. A surface groove essential for viral Bcl-2 function during chronic infection in vivo. PLoS Pathog. 1:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora, A. L., E. Torres-Gonzalez, M. Rojas, J. Xu, J. Ritzenthaler, S. H. Speck, J. Roman, K. Brigham, and A. Stecenko. 2007. Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-γ receptor-deficient mice. Am. J. Respir. Crit. Care Med. 175:1139-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarawar, S. R., R. D. Cardin, J. W. Brooks, M. Mehrpooya, A. M. Hamilton-Easton, X. Y. Mo, and P. C. Doherty. 1997. Gamma interferon is not essential for recovery from acute infection with murine gammaherpesvirus 68. J. Virol. 71:3916-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simas, J. P., R. J. Bowden, V. Paige, and S. Efstathiou. 1998. Four tRNA-like sequences and a serpin homologue encoded by murine gammaherpesvirus 68 are dispensable for lytic replication in vitro and latency in vivo. J. Gen. Virol. 79(Pt. 1):149-153. [DOI] [PubMed] [Google Scholar]
- 12.Sparks-Thissen, R. L., D. C. Braaten, K. Hildner, T. L. Murphy, K. M. Murphy, and H. W. Virgin. 2005. CD4 T cell control of acute and latent murine gammaherpesvirus infection requires IFNγ. Virology 338:201-208. [DOI] [PubMed] [Google Scholar]
- 13.Steed, A., T. Buch, A. Waisman, and H. W. Virgin IV. 2007. Gamma interferon blocks gammaherpesvirus reactivation from latency in a cell type-specific manner. J. Virol. 81:6134-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steed, A. L., E. S. Barton, S. A. Tibbetts, D. L. Popkin, M. L. Lutzke, R. Rochford, and H. W. Virgin. 2006. Gamma interferon blocks gammaherpesvirus reactivation from latency. J. Virol. 80:192-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suarez, A. L., and L. F. van Dyk. 2008. Endothelial cells support persistent gammaherpesvirus 68 infection. PLoS Pathog. 4:e1000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145:818-826. [PMC free article] [PubMed] [Google Scholar]
- 17.Tarakanova, V. L., F. Suarez, S. A. Tibbetts, M. A. Jacoby, K. E. Weck, J. L. Hess, S. H. Speck, and H. W. Virgin IV. 2005. Murine gammaherpesvirus 68 infection is associated with lymphoproliferative disease and lymphoma in BALB β2 microglobulin-deficient mice. J. Virol. 79:14668-14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibbetts, S. A., L. F. van Dyk, S. H. Speck, and H. W. Virgin IV. 2002. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J. Virol. 76:7125-7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upton, J. W., and S. H. Speck. 2006. Evidence for CDK-dependent and CDK-independent functions of the murine gammaherpesvirus 68 v-cyclin. J. Virol. 80:11946-11959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dyk, L. F., H. W. Virgin IV, and S. H. Speck. 2003. Maintenance of gammaherpesvirus latency requires viral cyclin in the absence of B lymphocytes. J. Virol. 77:5118-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dyk, L. F., H. W. Virgin IV, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virgin, H. W., IV, R. M. Presti, X. Y. Li, C. Liu, and S. H. Speck. 1999. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J. Virol. 73:2321-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weck, K. E., A. J. Dal Canto, J. D. Gould, A. K. O'Guin, K. A. Roth, J. E. Saffitz, S. H. Speck, and H. W. Virgin. 1997. Murine gamma-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus-induced vascular disease. Nat. Med. 3:1346-1353. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg, J. B., M. L. Lutzke, R. Alfinito, and R. Rochford. 2004. Mouse strain differences in the chemokine response to acute lung infection with a murine gammaherpesvirus. Viral Immunol. 17:69-77. [DOI] [PubMed] [Google Scholar]