Abstract
Previous studies have demonstrated that the formation of spatial, contextual and trace conditioning memories are impaired in animal models of Alzheimer’s disease (AD), consistent with the observations that the first sign of cognitive decline in AD includes difficulties in the acquisition of new information or memory formation. Evidence is accumulating that memory retrieval is a dynamic process in which stored information becomes labile again and needs to be restabilized. However, it is poorly understood how this process referred to as memory reconsolidation is affected in animal models of AD. The present study was designed to use contextual fear conditioning to compare the changes in memory formation and subsequent reconsolidation processes in transgenic mice that overexpress human APP and PS1 harboring five familial AD mutations (5XFAD model). The results clearly demonstrate that cognitive dysfunction starts to occur primarily as reduced levels of contextual learning or memory formation in 5XFAD mice, but it is exacerbated by additional retrieval-dependent retrograde amnesia due to deficient reconsolidation as disease further develops.
Keywords: Alzheimer’s disease, amyloid, fear conditioning, reconsolidation, amnesia, APP transgenic, 5XFAD mice
Transgenic animal models of Alzheimer’s disease (AD) have made considerable contribution to our understanding of molecular and pathophysiological mechanisms of AD and related cognitive dysfunctions. Although to date no perfect model of AD has emerged, the mice that overexpress mutant forms of human amyloid precursor protein (APP), presenilin (PS) and/or tau genes successfully recapitulate many features of AD including β-amyloid (Aβ) deposits, neurofibrillary tangles, gliosis and synaptic degeneration, and exhibit memory deficits as evidenced by their poor performances on a broad battery of behavioral assays such as Morris water maze, Y-maze, fear conditioning and object or social recognition tasks (Ashe, 2001; Eriksen and Janus, 2007; Gotz and Ittner, 2008; Kobayashi and Chen, 2005; LaFerla and Oddo, 2005; McGowan et al., 2006; Ohno, 2006). However, these studies predominantly tested the acquisition of learning or memories shortly after training, and it remains unclear whether a subsequent prolonged period of memory reorganization is affected in APP transgenic mice. While new memories are stabilized after initial learning through a process called consolidation, it has become increasingly apparent that retrieval or reactivation of previously consolidated memories induces an additional labile phase during which the memories can be modified or strengthened (Eisenberg et al., 2003; Lee, 2008; Nader et al., 2000; Tronson et al., 2006). This process named reconsolidation requires molecular mechanisms or brain regions that are not necessarily the same as those of initial memory consolidation, representing an important distinctive component of long-term memory processing (Alberini, 2005; Dudai, 2006; Lee et al., 2004; Nader, 2003; Suzuki et al., 2004; Tronson and Taylor, 2007). To test the hypothesis that disrupted reconsolidation may be involved in AD-related memory dysfunction, I used a contextual fear conditioning paradigm and examined the effects of cerebral Aβ accumulation on memory reconsolidation in AD transgenic model mice, as compared to those on their initial hippocampal memory formation.
We previously reported that 5XFAD APP/PS1 transgenic mice (Tg6799 line) containing five familial AD (FAD) mutations represent one of the most early-onset and aggressive amyloid mouse models (Oakley et al., 2006; Ohno et al., 2006; Ohno et al., 2007). 5XFAD mice are engineered to co-overexpress and co-inherit mutant human APP (the Swedish mutation: K670N, M671L; the Florida mutation: I716V; the London mutation: V717I) and PS1 (M146L; L286V) transgenes under neuron-specific mouse Thy-1 promoter. In the 5XFAD mouse model, the Swedish mutation increases the production of total Aβ while the other four mutations specifically increase the production of Aβ42. Consequently, five FAD mutations act together to additively increase levels of cerebral Aβ peptides, especially neurotoxic Aβ42. 5XFAD lines (B6/SJL genetic background) were maintained by crossing hemizygous transgenic mice with B6/SJL F1 breeders (Taconic, Hudson, NY), and 5XFAD hemizygotes were used for the experiments with non-transgenic wild-type littermate mice served as controls. While the majority of AD transgenic mice take ~6–12 months, or longer, to form amyloid plaques (Eriksen and Janus, 2007), 5XFAD mice start to develop visible amyloid deposits as early as ~2 months of age consistent with their dramatically accelerated Aβ42 generation. Aβ deposition first emerges in the subiculum of hippocampal area and the layer 5 of the cortex and increases rapidly with age, spreading to fill much of the hippocampus and cortex in 5XFAD mice by 6 months of age. Behavioral testing was performed using 5XFAD mice at different stages of pathological development (3–4 months of age with moderate Aβ deposition, 6–7 months of age with massive Aβ deposition, and 10< months of age with severer Aβ deposition, marked synaptic degeneration and neuron loss) (Oakley et al., 2006; Ohno et al., 2007). All experiments were done blind with respect to the genotype of the mice and were conducted with the approval of the Nathan Kline Institute Animal Care and Use Committee.
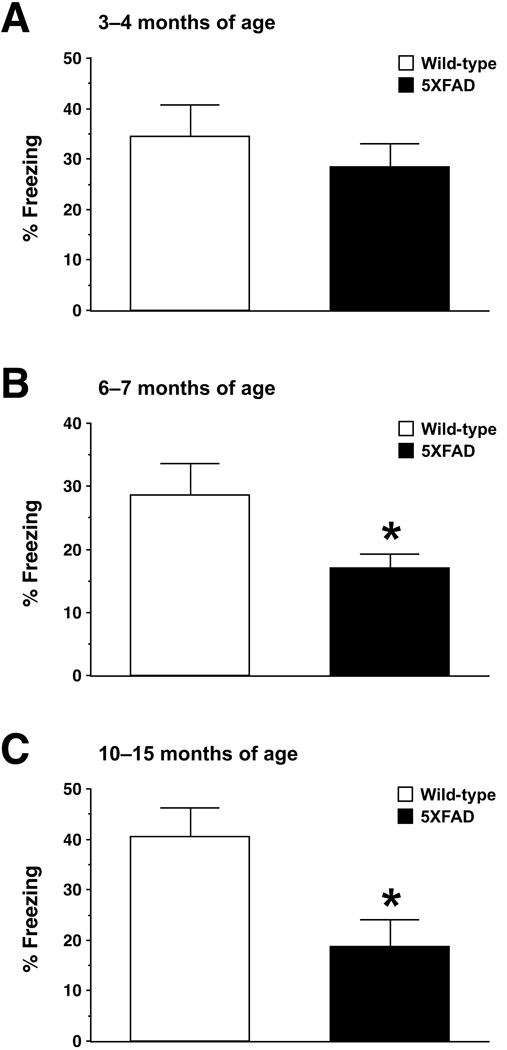
In experimental animals, memory consolidation and reconsolidation processes have been most successfully addressed using contextual fear conditioning (Lee et al., 2004; Suzuki et al., 2004), in which mice learn to associate a distinctive context (CS: conditioned stimulus) with aversive footshocks (US: unconditioned stimulus) (Fanselow, 2000). I first tested how contextual fear conditioning was affected in 5XFAD mice at different ages (Fig. 1), as described previously (Kimura et al., 2008; Ohno et al., 2001). During training, mice were placed in the conditioning chamber for 3 min and then received two unsignaled footshocks (1.0 mA, 2 s) at 1 min intervals through the grid floor. After the last shock delivery, mice were left in the chamber for another 30 s. Contextual fear memory was measured by scoring freezing behavior (the absence of all movement except for that needed for breathing) for 3 min when the mice were placed back into the same conditioning chamber 24 h after training. The automated FreezeFrame system (Coulbourn Instruments, Allentown, PA), which digitizes the video signal at 4 Hz and compares movement frame by frame, was used to score the amount of freezing. While 5XFAD mice at 3–4 months of age showed freezing that was indistinguishable from that of wild-type controls (F(1,30) = 0.63, p > 0.05) (Fig. 1A), 5XFAD mice at 6–7 months of age (F(1,38) = 4.73, p < 0.05) (Fig. 1B) and at 10–15months of age (F(1,33) = 7.49, p < 0.05) (Fig. 1C) exhibited significantly lower levels of freezing as compared to wild-type littermates when re-exposed to the chamber 24 h after training with 2 CS/US pairings. Therefore, 5XFAD mice show age-dependent deficits in hippocampus-dependent formation of contextual fear memory. This is consistent with our recent findings that hippocampal synaptic dysfunctions, as assessed by reductions in both basal transmission and long-term potentiation (LTP: a measure of NMDA receptor-dependent synaptic plasticity representing a cellular basis of learning and memory) at Schaffer collateral-CA1 synapses, occur in 5XFAD mice at 6 months of age but are not found at <4 months of age (Kimura and Ohno, 2009). It is reasonable to speculate that 24-h memory failures of 5XFAD mice may be due to deficient initial acquisition and memory consolidation given that a broad range of signaling pathways essential for both mnemonic processes (e.g., NMDA receptors, various kinases, transcription factors, and immediate-early genes) are impaired in a series of APP transgenic mouse models (Dewachter et al., 2009; Dickey et al., 2004; Dickey et al., 2003; Gong et al., 2004).
Fig. 1.
Age-dependent impairments of contextual fear conditioning in 5XFAD mice. (A–C) 5XFAD mice at 3–4 months (A), 6–7 months (B) and 10–15 months (C) of ages, and their respective wild-type littermate mice were trained with 2 CS/US pairings for contextual fear conditioning. 5XFAD mice at 6–7 and 10–15 months of age but not at 3–4 months of age show significantly lower levels of contextual freezing than wild-type controls when tested 24 h after training (n = 15–21 mice per group). * p < 0.05 versus wild-type controls. All data are presented as mean ± SEM.
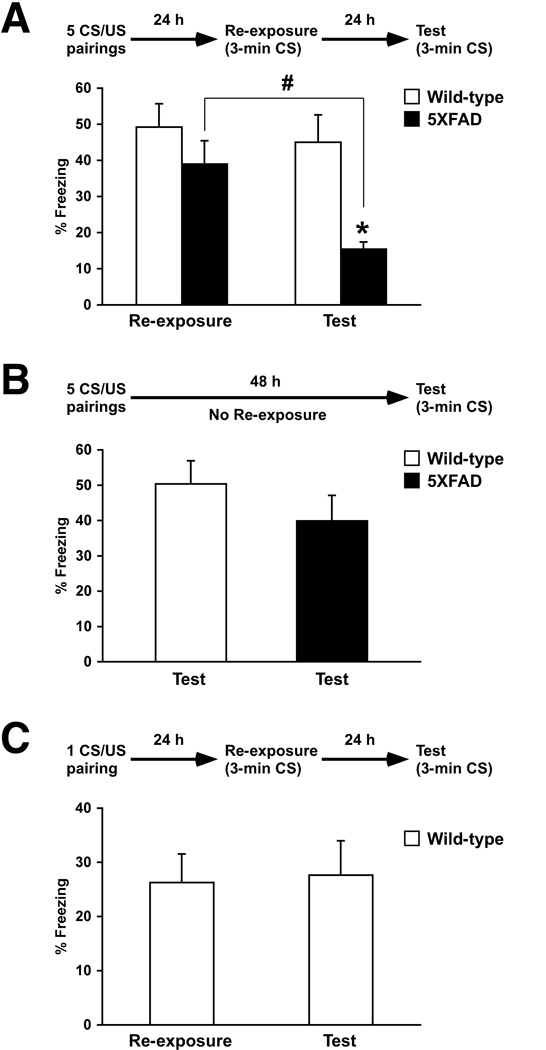
Retrieval of memories is the key event that initiates reconsolidation processing and is experimentally triggered by re-exposing subjects to the CS or training context without the US. Memory reconsolidation consists of two phases, a reactivation-dependent destabilization process and the subsequent protein synthesis-dependent restabilization process (Nader, 2003). A disruption of reconsolidation results in retrograde amnesia for previously consolidated memories, and recent evidence supports the idea that the mechanisms underlying initial consolidation and reconsolidation is at least partially dissociable (Alberini, 2005; Lee et al., 2004; Tronson and Taylor, 2007). Nevertheless, it has not been studied whether deficient reconsolidation may contribute to the mechanisms involved in AD-associated cognitive impairments. To address this issue, I tested the abilities of 5XFAD mice to reconsolidate contextual fear memory at different ages by re-exposing the mice to the conditioning chamber for 3 min in the absence of footshocks 24 h after training. Although 5XFAD mice at 10–12 months of age showed significantly reduced levels of contextual memory 24 h after mild training (2 CS/US pairings) (Fig. 1C), they showed robust freezing that was almost equivalent to wild-type controls during the re-exposure 24 h after training with 5 CS/US pairings at 1 min intervals (Fig. 2A). Therefore, more intensive training ensured initial hippocampus-dependent memory formation and consolidation in 5XFAD mice at this age, allowing the specific testing of their subsequent reconsolidation processes. Importantly, when mice were once again placed back into the training chamber for 3 min to test contextual memory 24 h later, one-way ANOVA and post-hoc Fisher’s PLSD test revealed that 5XFAD mice showed significantly lower levels of freezing as compared to those of wild-type controls and those during re-exposure (F(3,48) = 5.73, p < 0.05) (Fig. 2A). Furthermore, when different groups of mice were tested for contextual memory 48 h after 5 CS/US pairings without the intervening re-exposure session, contextual freezing was indistinguishable between 5XFAD and wild-type mice (F(1,23) = 1.13, p > 0.05) (Fig. 2B), suggesting that a process that is initiated by retrieval and not a long-lasting process in consolidation is affected in 5XFAD mice. Together, these results support the notion that retrieval-dependent reconsolidation of contextual fear memory is impaired in 5XFAD mice at 10–12 months of age. However, although the levels of freezing were not significantly different between 5XFAD and wild-type mice 24 h after 5 CS/US pairings (Fig. 2A), the initial underlying memory trace might be potentially weaker in 10- to 12-months-old 5XFAD mice and thus more susceptible to disruption by reactivation. To address this possibility, contextual memory in wild-type mice after weaker training (one CS/US pairing) was tested for reconsolidation (Fig. 2C). Wild-type control mice with the weaker conditioning exhibited much lower levels of freezing during re-exposure (26.3 ± 5.3 %, mean ± SEM) than 5XFAD mice that were trained with stronger 5 CS/US pairings (39.0 ± 6.4 %), but they retained similar levels of freezing during memory testing 24 h later (F(1,22) = 0.03, p > 0.05) in contrast to 5XFAD mice. Therefore, lower levels of initial freezing do not necessarily result in unstable reconsolidation. Taken collectively, these data demonstrate that retrieval-dependent amnesia due to impaired memory reconsolidation is specifically observed in 5XFAD mice at 10–12 months of age.
Fig. 2.
Deficient reconsolidation of contextual fear memory in older 5XFAD mice. Experimental design used is presented at the top of each panel. (A) 5XFAD mice at 10–12 months of age and their wild-type littermate mice were trained with 5 CS/US pairings for contextual fear conditioning. Mice received a single 3-min re-exposure to the conditioning context 24 h after training, and were then tested for contextual memory 24 h later. Levels of freezing during re-exposure were not different between 5XFAD and wild-type mice. In contrast, levels of freezing during subsequent memory testing in 5XFAD mice were significantly lower than those of wild-type controls (* p < 0.05) and as compared to those during re-exposure (#p < 0.05) (n = 12–14 mice per group). (B) When mice received no re-exposure intervening between training with 5 CS/US pairings and memory testing 48 h later, no difference in freezing levels was found between 5XFAD mice and wild-type controls (n = 11–14 mice per group). (C) Weaker training with 1 CS/US pairing dramatically reduced freezing during re-exposure in wild-type mice, but similar levels of freezing were retained during memory testing 24 h later (n = 12 mice per group). All data are presented as mean ± SEM.
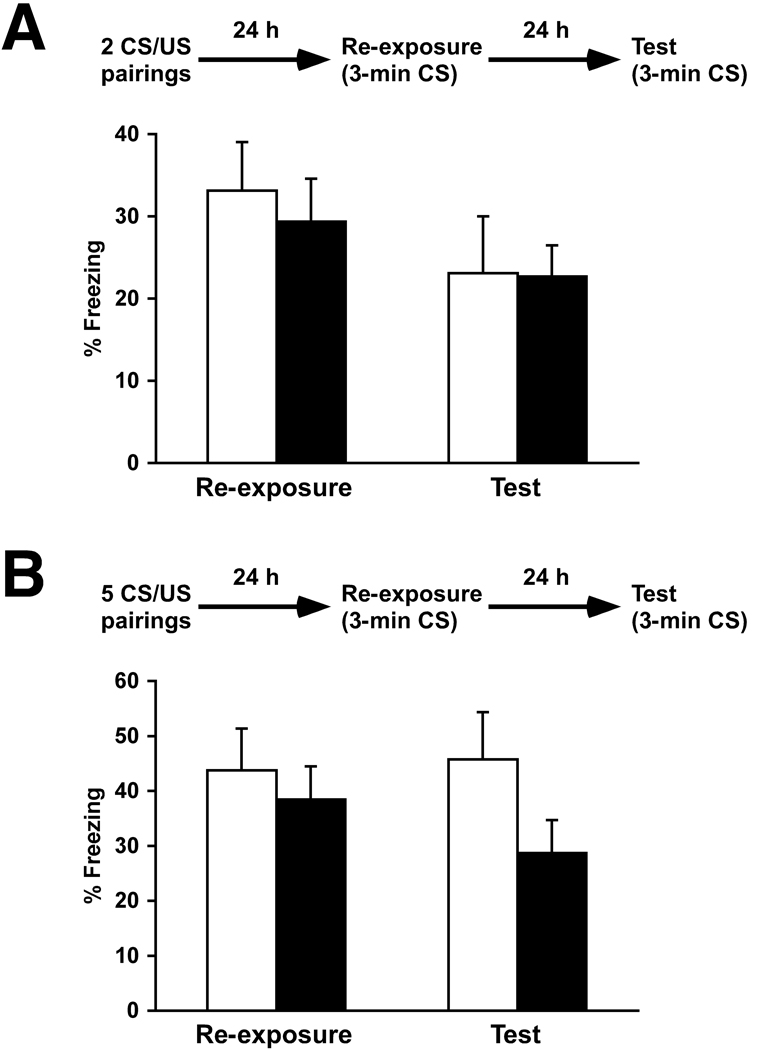
I also tested memory reconsolidation in 5XFAD mice at younger ages, and compared the onset of their impairments in initial memory formation and the subsequent reconsolidation. First, 5XFAD mice at 3–4 months of age and wild-type littermate controls showed comparable levels of freezing during the 3-min re-exposure that was given 24 h after mild training with 2 CS/US pairings (Fig. 3A), confirming normal memory formation and consolidation in 5XFAD at this age. When mice were once again placed back into the conditioning chamber 24 h after re-exposure, no significant difference in freezing levels was found between 5XFAD and wild-type mice or between the re-exposure session and subsequent memory testing (F(3,38) = 0.84, p > 0.05) (Fig. 3A). Therefore, memory reconsolidation is not affected in 3- to 4- months-old 5XFAD mice. Next, 5XFAD mice at 6–7 months of age were trained with stronger protocols (5 CS/US pairings), since reduced levels of memory formation following mild training with 2 CS/US pairings start to be observed at this age (Fig. 1B) and would preclude the analyses of specific changes in their subsequent reconsolidation processes. In contrast to the findings in older 5XFAD mice (10–12 months of age) (Fig. 2A), one-way ANOVA revealed no significant differences either in freezing of 5XFAD mice compared to wild-type controls or in freezing levels during the re-exposure and subsequent memory test sessions (F(3,34) = 1.09, p > 0.05) (Fig. 3B). However, post-hoc Fisher’s PLSD test indicated a trend toward a decrease in freezing of 5XFAD relative to wild-type mice during memory testing 24 h after re-exposure (p = 0.11). Together, it is conceivable that slight reconsolidation deficiencies may start to emerge in 5XFAD mice at 6–7 months of age, while they become more pronounced to reach statistical significance at a later time point after the onset of impairments in initial memory formation or consolidation in 5XFAD mice (Fig. 2A).
Fig. 3.
Normal reconsolidation of contextual fear memory in younger 5XFAD mice. Experimental design used is presented at the top of each panel. (A) 5XFAD mice at 3–4 months of age and their wild-type littermate mice were trained with 2 CS/US pairings for contextual fear conditioning. Mice received a single 3-min re-exposure to the conditioning context 24 h after training, and were then tested for contextual memory 24 h later (n = 9–12 mice per group). (B) 5XFAD mice at 6–7 months of age and their wild-type littermate mice were similarly tested for reconsolidation except that they were trained with 5 CS/US pairings (n = 9–10 mice per group). In either case, levels of freezing were not significantly different between 5XFAD (closed columns) and wild-type mice (open columns) or between the re-exposure and subsequent memory test sessions. However, a trend toward a decrease in reconsolidation was observed in 5XFAD mice at 6–7 months of age as compared to wild-type littermate controls during memory testing 24 h after re-exposure (p = 0.11). All data are presented as mean ± SEM.
5XFAD mice showed a decline in various types of hippocampus-dependent memory including spatial memory in a Morris water maze and temporal memory in auditory trace fear conditioning in our previous studies (Ohno et al., 2006) as well as contextual fear memory in this study. Importantly, these memory impairments start to occur at ~6 months of age in accordance with the onset of hippocampal synaptic dysfunctions such as reduced levels of baseline transmission and deficient LTP (Kimura and Ohno, 2009). These are also consistent with the observation that Aβ levels and amyloid burden dramatically increase in 5XFAD mouse brains between 4 and 6 months of age (Oakley et al., 2006), although Aβ continues to further accumulate afterward. Recent studies have provided compelling evidence that memories that are initially consolidated by the hippocampus undergo subsequent time-dependent memory reorganization or stabilization processes (Dudai, 2004; McClelland et al., 1995). For example, as memories mature, they become increasingly independent of the hippocampus and are gradually stabilized and eventually transformed into remote memories in cortical networks (Frankland and Bontempi, 2005; Squire and Bayley, 2007). We recently reported that remote memory stabilization is impaired in 5XFAD mice at <4 months of age when tested 30 days after contextual fear conditioning, which is probably due to their cortical Aβ-dependent dysfunction and precedes the onset of hippocampal synaptic and memory encoding failures (Kimura and Ohno, 2009). On the other hand, memory reconsolidation represents a restabilization process that occurs following the reactivation of memory through retrieval (Nader, 2003). Interestingly, the present study demonstrate that reconsolidation is also impaired in 5XFAD mice, whereas it starts to occur some time after initial contextual memory formation is reduced in contrast to the earlier onset of remote memory failures. Therefore, 5XFAD mice show disruptions in memory reorganization or stabilization processes following initial hippocampal coding at different time points relative to the onset of hippocampal mnemonic dysfunction, presumably reflecting their different sensitivities to increases in cerebral Aβ levels. To my knowledge, this is a first demonstration of deficient memory reconsolidation in animal models of AD. The present results suggest that while memory impairments in early AD result from reduced levels of initial memory acquisition and/or consolidation, retrieval-dependent retrograde amnesia due to disrupted reconsolidation may additionally weaken memory traces and thus worsen cognitive decline as the disease further progresses.
What is the mechanism by which memory reconsolidation is impaired in 5XFAD mice? Although post-retrieval restabilization of memory is thought to be a process that is distinct from initial consolidation, there is a considerable overlap in underlying molecular mechanisms (Alberini, 2005; Tronson and Taylor, 2007). For example, both consolidation and reconsolidation of fear memories require NMDA receptor-dependent activation of signaling pathways (e.g., extracellular signal-regulated kinase (ERK)/MAP kinase and PKA) leading to the activation of transcription factors (e.g., CREB) and de novo protein synthesis (Duvarci et al., 2005; Kida et al., 2002; Mamiya et al., 2009; Nader et al., 2000; Suzuki et al., 2004; Tronson et al., 2006). In contrast, a double dissociation has been found between the transcription factor zif268, which is selectively required for reconsolidation, and brain-derived neurotrophic factor (BDNF), which is selectively required for consolidation of contextual fear conditioning (Lee et al., 2004). Importantly, these signaling molecules or activities are reported to consistently decrease in brains of APP transgenic mice at basal expression levels or at their capabilities to respond to a new environment and memory tasks (Blanchard et al., 2008; Dewachter et al., 2009; Dickey et al., 2004; Dickey et al., 2003; Gong et al., 2006; Gong et al., 2004; Ma et al., 2007; Snyder et al., 2005; Wu et al., 2006), providing some mechanistic basis that may link Aβ accumulation and impairments of memory consolidation or reconsolidation. Our future analyses of these signaling cascades with relevance to age-dependent behavioral changes in 5XFAD mice will more rigorously determine specific molecular mechanisms that account for the occurrence of impairments in consolidation and reconsolidation associated with AD development.
In summary, the present study demonstrates that age-dependent and specific disruption of memory reconsolidation adds to the well documented decline in hippocampus-dependent learning or memory consolidation in mouse models of AD, suggesting that memory retrieval-dependent amnesia may contribute to aggravating cognitive dysfunction associated with disease progression. It will be important to pay more attention to the failures in memory reorganization or stabilization processes such as reconsolidation and cortical remote memory consolidation that follow initial hippocampal coding in future studies with AD animal models, especially in evaluating possible therapeutic strategies for the treatment of AD.
Acknowledgments
This work was supported by grants from National Institute of Mental Health (R01 MH067251) and Alzheimer’s Association (IIRG-08-91231) to M.O.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Ashe KH. Learning and memory in transgenic mice modeling Alzheimer's disease. Learn Mem. 2001;8:301–308. doi: 10.1101/lm.43701. [DOI] [PubMed] [Google Scholar]
- Blanchard J, Martel G, Guillou JL, Nogues X, Micheau J. Impairment of spatial memory consolidation in APP751SL mice results in cue-guided response. Neurobiol Aging. 2008;29:1011–1021. doi: 10.1016/j.neurobiolaging.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Dewachter I, Filipkowski RK, Priller C, Ris L, Neyton J, Croes S, Terwel D, Gysemans M, Devijver H, Borghgraef P, et al. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol Aging. 2009;30:241–256. doi: 10.1016/j.neurobiolaging.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Gordon MN, Mason JE, Wilson NJ, Diamond DM, Guzowski JF, Morgan D. Amyloid suppresses induction of genes critical for memory consolidation in APP + PS1 transgenic mice. J Neurochem. 2004;88:434–442. doi: 10.1111/j.1471-4159.2004.02185.x. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Loring JF, Montgomery J, Gordon MN, Eastman PS, Morgan D. Selectively reduced expression of synaptic plasticity-related genes in amyloid precursor protein + presenilin-1 transgenic mice. J Neurosci. 2003;23:5219–5226. doi: 10.1523/JNEUROSCI.23-12-05219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinas-emitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Janus CG. Plaques, tangles, and memory loss in mouse models of neurodegeneration. Behav Genet. 2007;37:79–100. doi: 10.1007/s10519-006-9118-z. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues β-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Ittner LM. Animal models of Alzheimer's disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Kimura R, Ohno M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol Dis. 2009;33:229–235. doi: 10.1016/j.nbd.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R, Silva AJ, Ohno M. Autophosphorylation of αCaMKII is differentially involved in new learning and unlearning mechanisms of memory extinction. Learn Mem. 2008;15:837–843. doi: 10.1101/lm.1049608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi DT, Chen KS. Behavioral phenotypes of amyloid-based genetically modified mouse models of Alzheimer's disease. Genes Brain Behav. 2005;4:173–196. doi: 10.1111/j.1601-183X.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Oddo S. Alzheimer'ls disease: Aβ, tau and synaptic dysfunction. Trends Mol Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Ma QL, Harris-White ME, Ubeda OJ, Simmons M, Beech W, Lim GP, Teter B, Frautschy SA, Cole GM. Evidence of Aβ- and transgene-dependent defects in ERK-CREB signaling in Alzheimer's models. J Neurochem. 2007;103:1594–1607. doi: 10.1111/j.1471-4159.2007.04869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McGowan E, Eriksen J, Hutton M. A decade of modeling Alzheimer's disease in transgenic mice. Trends Genet. 2006;22:281–289. doi: 10.1016/j.tig.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M. Genetic and pharmacological basis for therapeutic inhibition of β- and γ-secretases in mouse models of Alzheimer's memory deficits. Rev Neurosci. 2006;17:429–454. doi: 10.1515/revneuro.2006.17.4.429. [DOI] [PubMed] [Google Scholar]
- Ohno M, Chang L, Tseng W, Oakley H, Citron M, Klein WL, Vassar R, Disterhoft JF. Temporal memory deficits in Alzheimer's mouse models: rescue by genetic deletion of BACE1. Eur J Neurosci. 2006;23:251–260. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- Ohno M, Cole SL, Yasvoina M, Zhao J, Citron M, Berry R, Disterhoft JF, Vassar R. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol Dis. 2007;26:134–145. doi: 10.1016/j.nbd.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Frankland PW, Chen AP, Costa RM, Silva AJ. Inducible, pharmacogenetic approaches to the study of learning and memory. Nat Neurosci. 2001;4:1238–1243. doi: 10.1038/nn771. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Squire LR, Bayley PJ. The neuroscience of remote memory. Curr Opin Neurobiol. 2007;17:185–196. doi: 10.1016/j.conb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Ciallella JR, Flood DG, O'Kane TM, Bozyczko-Coyne D, Savage MJ. Comparative analysis of cortical gene expression in mouse models of Alzheimer's disease. Neurobiol Aging. 2006;27:377–386. doi: 10.1016/j.neurobiolaging.2005.02.010. [DOI] [PubMed] [Google Scholar]