Abstract
Background
Sleep Disordered Breathing (SDB) is present in over 50% of ambulatory patients with chronic heart failure. The prevalence and type of SDB in hospitalized patients with acutely decompensated heart failure (ADHF) are not known.
Methods
In-hospital sleep studies were performed on consecutive patients with ADHF who were not previously tested for SDB.
Results
395 consecutive patients with ADHF underwent successful sleep study recording during hospitalization. 298 patients (75%, 95% CI (71, 80%) had SDB; of these, 226 (57%, 95% CI (52, 62)) had predominantly obstructive SDB and 72 (18%, 95% CI (14, 22)) had predominantly central SDB. Only 25% (95% CI (20%, 29%)) of patients were free of SDB. Validation polysomnography between 6 and 8 weeks after discharge on a sub-group of unselected patients with obstructive SDB revealed a 100 % positive predictive value (95% CI. 94% to 100%) for Obstructive Sleep Apnea (OSA).
Conclusion
Similar to stable chronic heart failure, ADHF is associated with a high prevalence of SDB. The prevalence of predominantly obstructive SDB exceeded that of predominantly central SDB in ADHF patients. The presence of obstructive SDB during hospitalization predicted a diagnosis of OSA on polysomnography.
INTRODUCTION
Heart failure is the only cardiovascular disease without recent improvement in incidence or related mortality (1). Approximately 5 million Americans have heart failure, with an annual incidence of 10 per 1000 in individuals over 65 years, an already rising demographic segment. In the US, heart failure is the most frequent Medicare diagnosis, and most of the cost associated with it is related to hospitalizations with acutely decompensated heart failure (ADHF)(1–3). Patients with ADHF have 5–20% mortality (4, 5) and significant re-admission rate(6). Treatment options for ADHF remain limited and largely unchanged in the past two decades (7, 8). Identification and treatment of highly prevalent co-morbidities with known detrimental effects carries a high potential for positive impact(9).
Sleep Disordered Breathing (SDB), broadly categorized into central and obstructive sleep apnea, has far higher prevalence in patients with stable heart failure than in the general middle aged population (10–12). Recent prevalence studies suggest that the occurrence of central sleep apnea (CSA) may be declining due to current changes in the management of heart failure (13–15). Other studies have consistently demonstrated a very high prevalence of obstructive sleep apnea (OSA) in 38–53% of patients with stable heart failure (16–19). Treatment of (OSA) with Continuous Positive Airway Pressure (CPAP) in ambulatory patients with systolic heart failure improves cardiac function(20, 21). More recently, promising treatment options for CSA have also emerged(22). Therefore, the presence of a highly treatable and very prevalent disorder such as SDB in patients with heart failure may warrant a process for surveillance(23). A systematic approach to the diagnosis and treatment of SDB, particularly OSA, may be an important contribution to the management of heart failure. However, such an approach is not part of the current standard of practice (24, 25)
OSA worsens the control of hypertension, coronary artery disease, and atrial fibrillation, all are associated with ADHF (4, 26). It is likely, therefore, that OSA would be highly prevalent in patients presenting with ADHF. CSA, on the other hand, is more severe in heart failure patients with increased preload as is the case during decompensation of heart failure(27).
Therefore, we rationalized that the association between OSA and causes of ADHF (4, 26), along with the relationship between CSA and worsening cardiac filling pressures, will produce a high prevalence of SDB in patients with ADHF. The fact that SDB case-finding is not the standard of practice in ADHF patients, might be explained by a conception that either the prevalence is low, the screening is not feasible, or the condition is not treatable (23). To date, no study has reported on the feasibility of inpatient testing or evaluated the prevalence and type of SDB in patients with ADHF. We attempted to evaluate an inpatient approach to the identification of SDB, and to quantify and characterize the type of SDB in patients with AHDF. We also sought to evaluate any correlation between SDB in the inpatient setting and known SDB syndromes in the stable outpatient setting.
METHODS
Participants
Consecutive patients admitted to the heart failure services at the Ohio State University (OSU) Ross Heart Hospital with ADHF between December 30, 2006 and January 31, 2008 were eligible. The definition of ADHF was as follows: A primary admission diagnosis of congestive heart failure and elevated left ventricular pressure as indicated by at least one sign and one symptom of volume overload (pedal edema, crackles, consistent chest X-ray, increased left ventricular end-diastolic dimension (LVEDD), or elevated B-type natriutic peptide (BNP) level(7). This definition of ADHF included new onset acute heart failure or an exacerbation of already recognized heart failure. In particular, there was no cut-off criterion for left ventricular ejection fraction (LVEF). The study protocol was approved by the Ohio State University Institutional Review Board (#2007H0055).
A clinical process was established to perform in-hospital sleep studies on all newly admitted patients with ADHF. While the clinical process is still ongoing, this report includes patients who were hospitalized between December 30, 2006 and January 31, 2008.
Study Protocol
An order for the sleep study was incorporated in the computerized order entry set (28) for all patients hospitalized with ADHF at the Ohio State University Ross Heart Hospital. Patients were subsequently excluded if they had a preexistent diagnosis of SDB. Training of nursing and support staff on administering the study was concluded prior to implementation of the computerized order entry process. As such, the study was ordered by default and subsequently performed on the second night of admission on all newly admitted patients with ADHF. No previous knowledge of the patient’s history or weight was available or included in the ordering or interpreting processes. While no screening for SDB occurred, a clinical study coordinator ensured every day that the orders generated by the system included patients with ADHF who met the inclusion criteria and did not have an existing diagnosis of SDB.
The administering nurses had the discretion to withhold the study if a patient was hemodynamically unstable or developed clinical deterioration during the study requiring administration of supplemental oxygen. Patients with hemodynamic instability, defined as mean arterial pressure (MAP) less than 55 mmHg off vasopressors or being on vasopressor treatment, and patients requiring mechanical ventilatory support were included after stabilization and on the night prior to discharge.
In the first 6 months of the study (12/2006-6-2007), patients who had abnormal studies in and resided in the hospital’s draw area were asked to return to the OSU Sleep laboratory. The remainder of patients was referred to either the OSU Sleep Laboratory or a local AASM accredited sleep laboratories. After 06/01/2007 we delegated the follow up arrangement to the case managers and regional sleep centers. Figure 1 describes the disposition of patients in the study.
Figure 1.
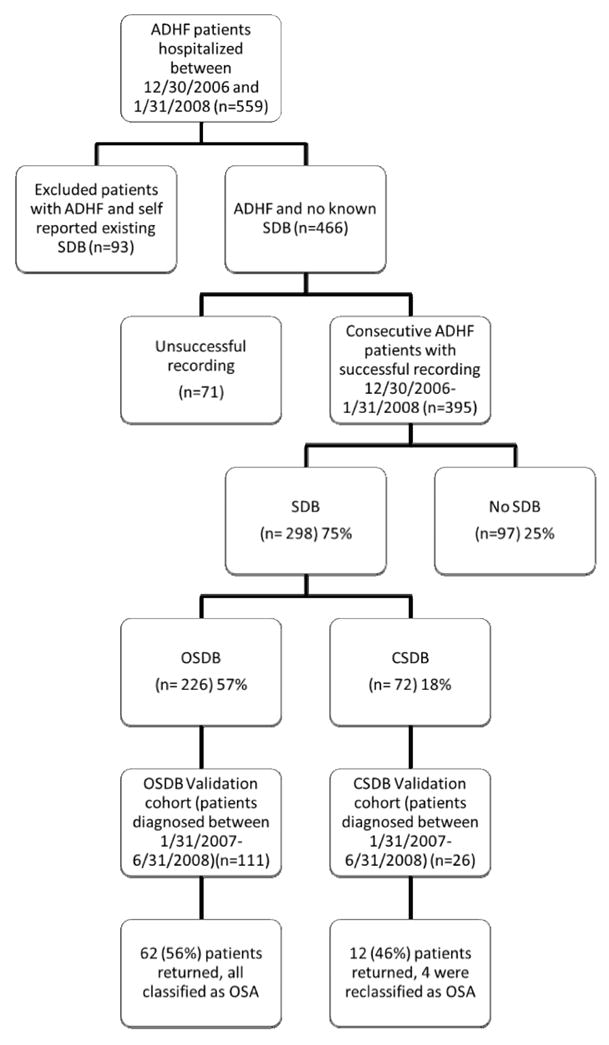
Disposition of the 395 consecutive patients with acutely decompensated heart failure (ADHF) who underwent successful recording during hospitalization between 12/30/2007 and 1/31/2008. SDB: Sleep Disordered Breathing; OSDB: Obstructive Sleep Disordered Breathing; CSDB: Central Sleep Disordered Breathing; OSA: Obstructive Sleep Apnea; CSA: Central Sleep Apnea; AHI Apnea hypopnea index. The figure includes all patients with ADHF who were screened during the study period (559). Note that there was no difference in any of the characteristics listed in table 1 between the 71 patients with failed sleep studies and the 395 with successful sleep studies.
Procedures
1- In-hospital sleep study
This was an attended cardiorespiratory study that measures nasal pressure, respiratory effort, oxygen saturation, heart rate, and body position (Stardust II, Respironics, Inc Murrysville, PA). The sleep study is classified as a type III sleep study according to the American Academy of Sleep Medicine (AASM) (29). The study was attended by previously trained night shift nurses who explained the purpose of the study to the patient and noted light out and light on times, along with any interruption to sleep. On the following morning, the study recorders were downloaded and transmitted via the hospital network for interpretation by a Sleep specialist who was blinded to patients’ diagnosis or risk factors for SDB.
2- Polysomnography
This was a standard clinical sleep study performed in the Ohio State University Sleep Laboratory within 8 weeks of discharge from the hospital. The polysomnography montage (MedCare Diagnostics, Buffalo, NY) was a standard overnight clinical montage with flow measured by nasal pressure and oro-nasal thermistor, thoraco-abdominal wall movement measured by inductance plethysmography; cardiac frequency by ECG; Arterial oxygen saturation measured by pulse oximetry. A bilateral electro-oculogram, four channels of electroencephalogram, chin and anterior tibial electromyograms, and body position sensor were all available.
Interpretation of Sleep Studies
For the in-hospital sleep study, SDB was defined as an Apnea Hypopnea Index (AHI) ≥15 events/hour. Apnea was scored when complete or near complete cessation of flow occurred. Hypopnea was scored when a 50% reduction in the flow signal occurred in association with 3% desaturation. A duration of 10 seconds was required for all events, and a minimum 3% desaturation was required for scoring hypopneas (29). An AHI cutoff of 15 events/hour was selected for the in-hospital study to mitigate against expected increase in SDB events during severe heart failure. Mostly apneas were used to classify SDB as central or obstructive. If more than 50% of the apneas were central the SDB was considered as Central SDB (CSDB). If more than 50% of the apneas were obstructive the disorder was considered as Obstructive (OSDB). If hypopneas occurred in the setting of periodic breathing (crescendo-decrescendo breathing with a cycle length >50 seconds), they were classified as central. The scoring criteria for the polysmnography were according to event definition by the AASM(30). The polysomnography was scored by technicians blinded to the findings of the in-hospital studies. The sleep physicians interpreting the polysomnography were aware that the patient underwent an inhospital sleep studies, but were not aware of the results.
Statistical Analysis
Descriptive statistics, such as frequencies and percentages and their confidence interval or standard error, were used to characterize the subjects and to report the prevalence and type of SDB in the study population. Comparisons on a profile of patient characteristics were used to understand the differences between normal obstructive SDB, and central SDB groups. Pearson’s correlations were used first in all patients then in the SDB group to evaluate potential relationships between the severity of SDB according to the AHI and selected clinical variables: Body mass index (BMI), LVEF, LVEDD, BNP, and age. The BMI and age were selected because they correlate with OSA in the general population(31). LVEF, LVEDD, and BNP were chosen as markers of heart failure disease severity. For correlations, 95% confidence intervals were provided. Polychotomous logistic regression (SAS version 9.1, SAS Institute Inc., Cary, NC) was used to model the relation between the three groups as referents (obstructive SDB, central SDB, and negatives) and the selected variables.
RESULTS
Patient Characteristics
During the study period (December 30, 2006 – January 31, 2008) we identified 559 patients who met the described criteria of ADHF. Of these, 93 patients have had a test for SDB and were excluded. The remaining 466 patients received orders for the in-hospital sleep study. Successful recording on the second day of hospitalization was obtained in 395 patients. Based on the 395 consecutive ADHF patients, Table 1 provides a profile of baseline characteristics. For example the mean LVEF was 33 (95% CI (32, 35)). The 71 patients who did not have successful recording had similar characteristics as the 395 patients with successful recording. In particular there was no difference in age, BMI, type of cardiomyopathy, LVEFF, or LVEDD. The most common reason for recording failure was device malfunction, inadequate number of recorders on a particular night, or multiple channel failure on the recorded study.
Table 1.
Characteristics of all 395 patients with ADHF
| Patient characteristic (number of Patients with data) | Mean or % (SE) | 95% Confidence Interval |
|---|---|---|
| Age (395) | 59 (0.7) | (57, 60) |
| Sex (male) (395) | 62 % (2%) | (58 %, 67 %) |
| Ischemic cardiomyopathy (395) | 57 (0.8) | (56, 59) |
| Left ventricular ejection fraction (370) | 33 (0.9) | (32, 35) |
| BMI kg/cm2 (393) | 32 (0.4) | (31, 33) |
| Admission BNP pg/mL (294) | 888 (59) | (773, 1003) |
| Atrial fibrillation (394) | 35 % (2%) | (30 %, 39 %) |
BNP: B-type natriuretic peptide on admission; BMI: body mass index; SE: Standard error
Prevalence and Type of Sleep Disordered Breathing
Using a cutoff AHI of ≥15 events/hour, 298 patients (75%, 95% CI (71, 80%)) had SDB; 226 (57%, 95% CI (52, 62) had predominantly obstructive SDB (OSDB) and 72 (18%, 95% CI (14, 22)) had predominantly central SDB (CSDB). Only 97 patients (25% (95% CI 20%, 29%)) were free of SDB.
Characteristics and predictors of the type of SDB
The characteristics of patients in the three groups (negative, obstructive, and central) are compared in Table 2. Note that the significance values were not corrected for multiplicity here, because this analysis is considered exploratory. The same applies to the confidence intervals. Compared to patients without SDB, patients with OSDB were more likely to be older males, have higher body mass index (BMI), and have ischemic cardiomyopathy.
Table 2.
Comparison of characteristics among the three groups of patients with ADHF
| Characteristic | OSDB Mean (SE) | CSDB Mean (SE) | Negative Mean (SE) | P Value negatives vs. OSDB | P Value negative vs. CSDB | P Value CSDB vs. OSDB |
|---|---|---|---|---|---|---|
| Age | 60 (0.9) | 58 (1.8) | 56 (1.6) | 0.03 | 0.37 | 0.40 |
| Male sex | 69% (3%) | 75% (5%) | 38% (5%) | 0.0001 | 0.0001 | 0.30 |
| Cardiomyopathy | ||||||
| Ischemic | 62% (3%) | 64% (6%) | 44% (5%) | 0.003 | 0.01 | 0.82 |
| Dilated | 23% (3%) | 14% (4%) | 35% (5%) | 0.02 | 0.001 | 0.11 |
| Others | 15% (2%) | 22% (5%) | 21% (4%) | 0.22 | 0.80 | 0.16 |
| LVEF | 34 (1.2) | 27 (1.7) | 38 (1.8) | 0.06 | 0.0001 | 0.0008 |
| BMI kg/cm2 | 33 (0.6) | 29 (0.9) | 31 (0.8) | 0.03 | 0.12 | 0.0001 |
| LVEDD | 57 (1.1) | 63 (1.6) | 54 (1.2) | 0.14 | 0.0001 | 0.0037 |
| BNP pg/dl | 746 (66) | 1341 (161) | 873 (130) | 0.35 | 0.02 | 0.001 |
| Atrial fibrillation | 39% (3%) | 32% (6%) | 28% (5%) | 0.06 | 0.57 | 0.31 |
SDB: Sleep Disordered Breathing; OSDB: Obstructive Sleep Disordered Breathing; CSDB; BNP: B-type natriuretic peptide on admission; SE: Standard error; LVEF: left ventricular ejection fraction; LVEDD: Left ventricular end diastolic diameter; BMI: body mass index; SE: Standard error
When compared to negatives, patients with CSDB were also more likely to be males. Additionally, central SDB patients had lower LVEF, and higher LVEDD, and BNP than both negatives and patients with OSDB. There was no difference in BMI between the negatives and central SDB patients.
Correlation between SDB and selected predictors
In order to further evaluate the relationships described above and to confirm potential predictors of SDB, we calculated Pearson correlation coefficients between AHI and five variables (LVEF, LVEDD, BMI, Age, BNP). We also evaluated the association with atrial fibrillation using logistic regression. For the overall group of ADHF patients, there was a correlation between AHI and BMI (r2 0.17, (CI: 0.07, 0.26) and AHI and LVEDD (r2 0.19, (CI: 0.07, 0.30). There was no significant association between the four other variables (LVEFF, age, BNP, and atrial fibrillation) and AHI. For the SDB group (n=298), similar correlations between LVEDD and BMI with AHI were found. The correlation between BMI and AHI was r2 0.18(CI (0.07, 0.29); and between LVEDD and AHI was r2= 0.15 (CI: 0.01, 0.28). This further analysis identified only elevated BMI and LVEDD as predictors of having SDB in patients with ADHF. For the group of patients with OSDB the correlation between BMI and AHI was largest (Pearson r2= 0.3, (CI: 0.17, 0.41)). For the group with CSDB (n=72), no significant correlations were found between any of six clinical variables and severity of SDB (AHI). Multi-regression modeling did not change these relationships.
Follow-up Testing and Validation of inpatient testing
Patients who underwent an abnormal in-hospital study in the first 6 months of the study period (January – June 2007) were invited back to undergo a standard outpatient in-lab polysomnography in our sleep laboratory (Figure 1). Sixty two patients out of the 111 validation cohort of OSDB patients returned for their validation polysomnography. The most common reason for not returning for the validation polysomnography was living away from the hospital’s draw area. Twelve patients (out of 26 invited) with CSDB underwent their validation polysomnography. Table 3 presents the comparison in sleep and cardiac characteristics between the group of OSDB patients with validated studies and those without a validation study. Overall, while there was a slight difference in BMI, no other differences existed in cardiac parameters, symptoms, or AHI on the in-hospital study between the two groups. Additionally, the severity of SDB (average AHI) remained unchanged on the validation Polysomnography (mean± standard error37.4 ± 2.5 for the in-hospital study vs. 41.7±3.9 on the outpatient polysomnography) (Table 3.3). All patients with OSDB were diagnosed with OSA on the validation polysomnography in the OSDB group with a validated study. This gives the inpatient test 100% positive predictive value (95% CI 94,100%) for a diagnosis of OSA on a subsequent outpatient polysomnography. In the validated group of patients with l CSDB the average AHI did not change on the validation polysomnography (table 3.3). Among the 12 patients with CSDB who returned for validation polysomnography, 4 patients were diagnosed as having OSA, and 8 were diagnosed as CSA.
Table 3.
| Table 3-1. Comparison between OSDB patients with validation outpatient polysomnography and eligible patients who did not have validation polysomnography OSDB | ||||
|---|---|---|---|---|
| OSDB patients with validation PSGs (62) Mean or % (SE)(N) | OSDB Non-validated patients (49) Mean or % (SE)(N) | Difference between validated OSDB patients and non-validated patients | 95% CI for the Difference | |
| Age | 55 (1.8) (62) | 61 (2.1) (49) | −6 | −11.4, 0.4 |
| Male | 69% (6%) (62) | 67% (7%) (49) | 2% | −16%, 20% |
| BMI | 35.5 (1.1) (62) | 30.8 (1.3) (49) | 4.7 | 1.4, 8.0 |
| LVEF | 35.3 (2.2) (60) | 31.2 (2.8) (48) | 4.1 | −2.8, 11.0 |
| LVEDD | 56.4 (2.1) (51) | 58.6 (2.2) (38) | −2.2 | −8.2, 3.9 |
| BNP | 475 (79) (46) | 1113 (180) (44) | −637 | −1023, −252 |
| Ischemic cardiomyopathy | 56% (6%) (62) | 69% (7%) (49) | −13% | −31%, 5% |
| Snoring | 54% (6%) (61) | 80% (6%) (40) | −26% | −45%, −7% |
| ESS | 10.4 (0.7) (43) | 9.7 (0.9) (43) | 0.7 | −1.5, 2.9 |
| Atrial fibrillation | 41% (6%) (61) | 39% (7%) (49) | 2% | −17%, 21% |
| Inpatient AHI | 37.4 (2.5) (62) | 32.5 (1.9) (49) | 5.0 | −1.7, 11.6 |
| Table 3-2. Comparison of AHI between the in-hospital study and the polysomnography in the validated OSDB and CSDB patients | ||||
| PSG AHI Mean (SE) (N) | Inpatient AHI Mean (SE) (N) | Difference between PSG AHI and Stardust AHI | 95% CI for the Difference | |
| OSDB | 41.7 (3.9) (62) | 37.4 (2.5) (62) | 4.3 | −1.1, 9.6 |
| CSDB | 36.4 (7.2) (12) | 49.1 (5.9) (12) | −12.7 | −29.9, 4.5 |
OSDB: Obstructive Sleep Disordered Breathing; CSDB; BNP: B-type natriuretic peptide on admission; SE: Standard error; LVEF: left ventricular ejection fraction; LVEDD: Left ventricular end diastolic diameter; ESS: Epworth Sleepiness Scale; BMI: body mass index; SE: Standard error; N: number of patients with available data; Inpatient AHI: Apnea hypopnea index on the inpatient sleep study
OSDB: Obstructive Sleep Disordered Breathing; CSDB: Central Sleep Disordered Breathing; LVEDD: Left ventricular end diastolic diameter; LVEF: Left ventricular ejection fraction; BNP: B-type natriutic peptide on admission; SE: Standard error; ESS: Epworth Sleepiness Scale; AHI: Apnea hypopnea index, Afib: Atrial Fibrillation, PSG: Polysomnography
DISCUSSION
In this study, we describe a systematic surveillance program for SDB in patients hospitalized with ADHF. This is the first study to evaluate the prevalence of SDB in this population and demonstrate feasibility and reliability of inpatient testing. Using an attended inpatient sleep study, with an AHI cut-off of 15 events/hour, we studied 395 consecutive unscreened patients hospitalized with ADHF. We found that 75% of these ADHF patients demonstrated SDB. Of these, the majority (57%) had a predominantly obstructive type and a minority (18%) demonstrated central type of SDB. Although only 56% of invited patients returned for the validation polysmnography, we found that those who returned were quite similar to those who did not. The validity of inpatient diagnosis of central SDB for an outpatient diagnosis of CSA was not tested in this study. However, the presence of obstructive SDB during the hospitalization was 100% predictive of having OSA on follow up polysomnography after resolution of the decompensation. Increased LVEDD and BMI were the only risk factors for having SDB. Patients with OSDB tended to be older males with higher BMI. Patients with CSDB in this series of ADHF patients had worse cardiac function parameters (lower LVEF, higher LVEDD, and higher BNP).
Prior studies of SDB prevalence in heart failure mainly included stable outpatients with chronic systolic heart failure. In one landmark study, Javaheri et al (11) evaluated ambulatory elderly males with systolic heart failure and found that 51% of these patients had SDB. Forty percent of all patients with heart failure had CSA and 11% had OSA. Sin et al (12) also studied predominantly male patients with systolic heart failure who were previously referred to the sleep laboratory, and reported similar prevalence of SDB with a higher occurrence of OSA. Patients with ADHF are a distinct population from ambulatory patients with stable systolic heart failure. And the available data on prevalence and distribution of SDB in stable patients with systolic dysfunction may have limited application to hospitalized patients with ADHF. Our study enrolled consecutive hospitalized patients with decompnesated heart failure regardless of sex, type of cardiac dysfunction, or the duration of prior cardiac dysfunction. As a result, 38% of our cohort was women (compared to 15% in Sin et al, and 0% in Javaheri et al) (12). In addition, our sample did not exclude patients with predominantly diastolic etiology of their decompensated heart failure. These patients represent a large portion of heart failure patients(32) (3). In more recent studies of ambulatory heart failure patients, the prevalence of SDB was still very high and similar to that reported by Javaheri and Sin. However, the distribution of central and obstructive sleep apnea appeared to change; with higher prevalence of OSA was in these ambulatory patients with systolic heart failure(16–19). This was particularly the case in one study that included hospitalized as well as ambulatory patients with heart failure(19). There is evidence that recent changes in the treatment of chronic heart failure and the use of b- blockers may affect a decline in the occurrence of CSA(14). These changes in the management of chronic heart failure, along with the increasing obesity in the general adult population (33, 34) may explain the increasing prevalence of OSA in these studies and in ours.
Increased cardiac filling pressure in patients with severe systolic heart failure correlates with respiratory control instability and subsequently CSA (27, 35). Therefore one might expect higher prevalence of CSA than OSA in patients with worse cardiac function. However, the role of increased cardiac filling pressure in the pathogenesis of SDB in heart failure patients remains incompletely understood. Recently it was demonstrated that increased venous return worsens upper airway collapsibility and gives rise to obstructive events (36, 37). One can speculate that this effect may be more pronounced in patients with ADHF who have more cervical venous congestion than patients with stable heart failure. A complimentary explanation may be the strong relation between respiratory control instability itself and upper airway collapsibility. It is known that upper airway obstruction may follow central apnea (38) and that central sleep apnea may result from upper airway instability(39). Therefore, it is conceivable that patients who are predisposed to increased upper airway resistance due to obesity or cervical venous congestion may manifest predominantly obstructive, but also some central events during states of further increased filling pressure. The high prevalence of OSA, a disorder of obesity and aging, may be simply a corollary of the rising weight of the middle –aged population, and the older age of heart failure patients(1). Elevated BMI correlated with having OSDB in our patients with ADHF, and OSDB patients tended to be older males, all of which are the same risk factors for OSA in the general population(40) and in patients with stable heart failure(12). This supports that OSDB is independent from the heart failure and its decopmensation. The persistence of SDB on the validation portion of our study after resolution of the decompensation further supports that SDB in these patients, while possibly worsened by the decompnesation, has an independent etiology from the underlying decompensation.
We used a cardiorespiratory sleep recording device to evaluate for SDB. Similar devices have been validated in the unattended setting (41–43), and their sensitivity is likely enhanced with use in the in-hospital attended setting. Additionally, an identical device was shown to have excellent ability in the unattended setting to discriminate between OSA and CSA in patients with heart failure compared to polysomnography (44). These devices are now approved by the American Academy of Sleep Medicine (AASM) for the diagnosis of OSA (45) in populations with high pretest probability; albeit, not yet in the heart failure population. If the inhospital technique had a high rate of false positives due to a possible role for ADHF in worsening SDB (27, 36, 46), the polysomography after stabilization of the decompensation would have produced at least some false positives. The confidence interval on the positive predictive value was quite narrow. The persistence of OSDB into the outpatient setting is well supported by these findings. Due to the limited number of patients, the persistence of CSDB is less clear. The reason for the persistence of OSDB is not evaluated in this study. Increased cervical venous congestion during decompensated may have contributed to the higher than expected prevalence of OSDB in the inpatient setting. Increased cardiac filling pressure is not expected to be a significant problem 8 weeks after discharge. However, a recent study suggest that AHI in patients with OSA is related to fluid shift in the supine position(47). Such fluid shift may be even more significant in patients with heart failure and subsequently may account for the persistence of OSDB into the outpatient setting. Improved sleep efficiency after discharge and the ability to measure sleep on the polysomnography may also result in higher AHI since sleep time is the denominator for AHI in polysomnographic studies.
According to WHO criteria for case-finding, highly prevalent conditions with known negative impact, for which treatment is available and diagnosis is feasible, warrant screening(23). This study demonstrated the feasibility of systematic inpatient testing for SDB and the high prevalence of this independent comorbidity. The benefit of such practice will require further studies to evaluate the effect of inpatient treatment of OSA on ADHF outcomes. Once these studies are concluded and the benefit of inpatient treatment of OSA is demonstrated, such systematic approaches could be considered in patients with ADHF.
Footnotes
Disclosure: RNK and WTA have received research grants from Respironics, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell JB. The economic burden of heart failure. Clin Cardiol. 2000 Mar;23(3 Suppl):III6–10. doi: 10.1002/clc.4960231503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 Jan 29;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 4.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006 Nov;27(22):2725–36. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 5.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. Jama. 2005 Feb 2;293(5):572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 6.Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997 Jan 13;157(1):99–104. [PubMed] [Google Scholar]
- 7.Elkayam U, Tasissa G, Binanay C, Stevenson LW, Gheorghiade M, Warnica JW, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007 Jan;153(1):98–104. doi: 10.1016/j.ahj.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005 Feb;26(4):384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 9.Naughton MT. The link between obstructive sleep apnea and heart failure: underappreciated opportunity for treatment. Curr Heart Fail Rep. 2006 Dec;3(4):183–8. doi: 10.1007/s11897-006-0020-z. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993 Apr 29;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 11.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998 Jun 2;97(21):2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 12.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999 Oct;160(4):1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 13.Bradley TD, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005 Nov 10;353(19):2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 14.Tamura A, Kawano Y, Naono S, Kotoku M, Kadota J. Relationship between beta-blocker treatment and the severity of central sleep apnea in chronic heart failure. Chest. 2007 Jan;131(1):130–5. doi: 10.1378/chest.06-0919. [DOI] [PubMed] [Google Scholar]
- 15.Tamura A, Kawano Y, Kadota J. Carvedilol reduces the severity of central sleep apnea in chronic heart failure. Circ J. 2009 Feb;73(2):295–8. doi: 10.1253/circj.cj-08-0678. [DOI] [PubMed] [Google Scholar]
- 16.Oldenburg O, Lamp B, Topfer V, Faber L, Teschler H, Horstkotte D. [Prevalence of sleep-related breathing disorders in ischemic and non-ischemic heart failure] Dtsch Med Wochenschr. 2007 Mar 30;132(13):661–6. doi: 10.1055/s-2007-973599. [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007 Mar;9(3):251–7. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Ferrier K, Campbell A, Yee B, Richards M, O’Meeghan T, Weatherall M, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005 Oct;128(4):2116–22. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 19.Schulz R, Blau A, Borgel J, Duchna HW, Fietze I, Koper I, et al. Sleep apnoea in heart failure. Eur Respir J. 2007 Jun;29(6):1201–5. doi: 10.1183/09031936.00037106. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003 Mar 27;348(13):1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 21.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004 Feb 1;169(3):361–6. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 22.Teschler H, Dohring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001 Aug 15;164(4):614–9. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 23.Jungner JMGWaG. Principles and Practice of Screening of Disease. Journal. 1968;(34) [serial on the Internet] [Google Scholar]
- 24.Pack AI G, LR Routine Polysomnography is Not Indicated in Congestive Heart Failure. Journal of Clinical Sleep Medicine. 2006;01( 01):19–22. [PubMed] [Google Scholar]
- 25.Franklin KA. Sleep apnoea screening in heart failure? Not until benefit is proven! Eur Respir J. 2007 Jun;29(6):1073–4. doi: 10.1183/09031936.00030507. [DOI] [PubMed] [Google Scholar]
- 26.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005 Feb;149(2):209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999 Mar 30;99(12):1574–9. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 28.Ahmad A, Teater P, Bentley TD, Kuehn L, Kumar RR, Thomas A, et al. Key attributes of a successful physician order entry system implementation in a multi-hospital environment. J Am Med Inform Assoc. 2002 Jan–Feb;9(1):16–24. doi: 10.1136/jamia.2002.0090016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Executive summary on the systematic review and practice parameters for portable monitoring in the investigation of suspected sleep apnea in adults. Am J Respir Crit Care Med. 2004 May 15;169(10):1160–3. doi: 10.1164/rccm.169.1160. [DOI] [PubMed] [Google Scholar]
- 30.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999 Aug 1;22(5):667–89. [PubMed] [Google Scholar]
- 31.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005 Oct;99(4):1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 32.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003 Jan 8;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 33.Center for Disease Control and Prevention NCfhS. Health. In: Services UDoHaH., editor. US Census National Health Interview Survey. 2006. [Google Scholar]
- 34.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006 Apr 5;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 35.Chenuel BJ, Smith CA, Skatrud JB, Henderson KS, Dempsey JA. Increased propensity for apnea in response to acute elevations in left atrial pressure during sleep in the dog. J Appl Physiol. 2006 Jul;101(1):76–83. doi: 10.1152/japplphysiol.01617.2005. [DOI] [PubMed] [Google Scholar]
- 36.Chiu KL, Ryan CM, Shiota S, Ruttanaumpawan P, Arzt M, Haight JS, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006 Dec 15;174(12):1378–83. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 37.Shiota S, Ryan CM, Chiu KL, Ruttanaumpawan P, Haight J, Arzt M, et al. Alterations in Upper Airway Cross-sectional Area in Response to Lower Body Positive Pressure in Healthy Subjects. Thorax. 2007 Apr 18; doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995 May;78(5):1806–15. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 39.Harms CA, Zeng YJ, Smith CA, Vidruk EH, Dempsey JA. Negative pressure-induced deformation of the upper airway causes central apnea in awake and sleeping dogs. J Appl Physiol. 1996 May;80(5):1528–39. doi: 10.1152/jappl.1996.80.5.1528. [DOI] [PubMed] [Google Scholar]
- 40.Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002 Apr 22;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 41.Whittle AT, Finch SP, Mortimore IL, MacKay TW, Douglas NJ. Use of home sleep studies for diagnosis of the sleep apnoea/hypopnoea syndrome. Thorax. 1997 Dec;52(12):1068–73. doi: 10.1136/thx.52.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zucconi M, Ferini-Strambi L, Castronovo V, Oldani A, Smirne S. An unattended device for sleep-related breathing disorders: validation study in suspected obstructive sleep apnoea syndrome. Eur Respir J. 1996 Jun;9(6):1251–6. doi: 10.1183/09031936.96.09061251. [DOI] [PubMed] [Google Scholar]
- 43.Man GC, Kang BV. Validation of a portable sleep apnea monitoring device. Chest. 1995 Aug;108(2):388–93. doi: 10.1378/chest.108.2.388. [DOI] [PubMed] [Google Scholar]
- 44.Quintana-Gallego E, Villa-Gil M, Carmona-Bernal C, Botebol-Benhamou G, Martinez-Martinez A, Sanchez-Armengol A, et al. Home respiratory polygraphy for diagnosis of sleep-disordered breathing in heart failure. Eur Respir J. 2004 Sep;24(3):443–8. doi: 10.1183/09031936.04.00140603. [DOI] [PubMed] [Google Scholar]
- 45.Nancy A, Collop WMA, Brian Boehlecke, David Claman, et al. Portable Monitoring Task Force for the American Academy of Sleep Medicine. Clinical Guidelines for the Use of Unattended Portable Monitors in the Diagnosis of Obstructive Sleep Apnea in Adult Patients. Journal of Clinical Sleep Medicine. 2007;3(7) [PMC free article] [PubMed] [Google Scholar]
- 46.Tkacova R, Niroumand M, Lorenzi-Filho G, Bradley TD. Overnight shift from obstructive to central apneas in patients with heart failure: role of PCO2 and circulatory delay. Circulation. 2001 Jan 16;103(2):238–43. doi: 10.1161/01.cir.103.2.238. [DOI] [PubMed] [Google Scholar]
- 47.Redolfi S, Yumino D, Ruttanaumpawan P, Yau B, Su MC, Lam J, et al. Relationship between overnight rostral fluid shift and Obstructive Sleep Apnea in nonobese men. Am J Respir Crit Care Med. 2009 Feb 1;179(3):241–6. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 48.Bendjelid K, Schutz N, Suter PM, Fournier G, Jacques D, Fareh S, et al. Does continuous positive airway pressure by face mask improve patients with acute cardiogenic pulmonary edema due to left ventricular diastolic dysfunction? Chest. 2005 Mar;127(3):1053–8. doi: 10.1378/chest.127.3.1053. [DOI] [PubMed] [Google Scholar]
- 49.Chadda K, Annane D, Hart N, Gajdos P, Raphael JC, Lofaso F. Cardiac and respiratory effects of continuous positive airway pressure and noninvasive ventilation in acute cardiac pulmonary edema. Crit Care Med. 2002 Nov;30(11):2457–61. doi: 10.1097/00003246-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Bellone A, Barbieri A, Ricci C, Iori E, Donateo M, Massobrio M, et al. Acute effects of non-invasive ventilatory support on functional mitral regurgitation in patients with exacerbation of congestive heart failure. Intensive Care Med. 2002 Sep;28(9):1348–50. doi: 10.1007/s00134-002-1424-1. [DOI] [PubMed] [Google Scholar]


