Abstract
Background
Health-related quality of life (HRQOL) is a major clinical outcome for heart failure (HF) patients. We aimed to determine the frequency, durability, and prognostic significance of improved HRQOL after hospitalization for decompensated HF.
Methods and Results
We analyzed HRQOL, measured serially using the Minnesota Living with Heart Failure Questionnaire (MLHFQ), for 425 patients who survived to discharge in a multicenter randomized clinical trial of pulmonary artery catheter versus clinical assessment to guide therapy for patients with advanced HF. All patients enrolled had one or more prior HF hospitalizations or chronic high diuretic doses and one or more symptom and one sign of fluid overload at admission. Improvement, defined as a decrease of more than 5 points in MLHFQ total score, occurred in 68% of patients by 1 month and stabilized. The degree of 1 month improvement differed (P<0.0001 group × time interaction) between 6 month survivors and non-survivors. In a Cox regression model, after adjustment for traditional risk factors for HF morbidity and mortality, improvement in HRQOL by 1 month compared to worsening at one month or no change predicted time to subsequent event-free survival (P=0.013).
Conclusions
In patients hospitalized with severe HF decompensation, HRQOL is seriously impaired but improves substantially within 1 month for most patients and remains improved for 6 months. Patients for whom HRQOL does not improve by 1 month after hospital admission merit specific attention both to improve HRQOL and to address high risk for poor event-free survival.
Keywords: heart failure, quality of life
Health-related quality of life (HRQOL) is a subjective, multidimensional construct referring to how a given health condition affects a person's total well-being including functional capacity, psychological status, social functioning, and health perceptions. As longevity increases and more people must adjust to life with chronic conditions such as heart failure (HF), patient-centered outcomes such as HRQOL assume greater importance.1,2 Indeed, there is evidence that among symptomatic patients with HF, HRQOL is considered the most important outcome, surpassing quantity of life (survival) in value.3
Health-related quality of life in patients with HF is substantially impaired in several dimensions.4-6 Health-related quality of life is more severely impaired in HF than in several other common chronic conditions (i.e. hypertension, diabetes, arthritis, chronic lung disease, or angina).7,8 Some, but not all, investigators have found that poorer HRQOL also is associated with worse clinical outcomes, specifically higher mortality and rehospitalization rates.9,10 Inconsistencies in this literature may stem from the fact that most investigations have used one baseline value for HRQOL to predict outcomes. Health-related quality of life is dynamic, particularly around episodes of acute exacerbation when it improves in most patients during the month after discharge from a hospitalization.11 Using only one snapshot assessment may miss the true nature of HRQOL in a given individual. Thus, determination of the independent predictive value of HRQOL for morbidity and mortality outcomes may require consideration of whether HRQOL improves after a hospitalization for acute decompensated HF.
Improving outcomes requires appreciation of HRQOL at all stages of HF. Because of the difficulty of predicting death in HF, little is known about HRQOL in patients with the most advanced HF. The purpose of this study was to determine the frequency and durability of improvement in HRQOL after hospitalization for advanced HF, and whether improvements in HRQOL were associated with subsequent event-free survival.
Methods
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial12 offered a unique opportunity to examine questions about HRQOL in patients with advanced or end-stage HF. The ESCAPE trial was sponsored by a grant from the National Heart Lung and Blood Institute (NHLBI) and conducted at 26 academic heart failure and transplantation centers in the United States and Canada.
Sample and setting
A total of 433 patients hospitalized for NYHA class IV decompensated HF were enrolled in the ESCAPE trial. Patients were eligible for the ESCAPE trial if they had advanced HF with a left ventricular ejection fraction less than 30%, and were hospitalized for treatment of acute decompensated HF with at least 1 symptom and 1 sign of fluid overload, and a history of at least 1 prior HF hospitalization or chronic high maintenance diuretic doses. Patients were excluded from participation for significant co-existing conditions that could shorten life (e.g., cancers, liver failure) or if their plan of care included clinical need for a pulmonary artery catheter for management, mechanical circulatory or ventilatory support, intravenous milrinone within 48 hours, or dobutamine/dopamine within 24 hours, or listing for cardiac transplantation.12 Patients enrolled in this HRQOL substudy met the additional criterion of survival to discharge from the index hospitalization.
Measures
Health-related Quality of Life
Health-related quality of life was measured using the Minnesota Living with Heart Failure Questionnaire (MLHFQ), developed specifically to assess quality of life in patients with HF.13-15 The instrument measures patients' perception of how much their HF and its treatment affect their ability to live as they want. The MLHFQ is composed of 21 questions rated on a scale from 0 (no effect) to 5 (very much). Item ratings are summed for a total score that can range from 0 to 105. Higher scores reflect worse quality of life. Research nurses worked with the patients in order to reduce missing items in scales by clarifying questions that patients had about the items and as a result, there were very few missing items in the MLHFQ (i.e., fewer than 3% of patients very missing more than 2 items from the MLHFQ). In the few cases where items were missing, they were replaced with the mean of the items for that patient. This method was chosen in order to maintain the sample size. We conducted our analyses with MLHFQ scores using this method and with the more conservative approach of deleting any patient who did not complete 30% or more of items. Both methods yielded the same results, probably because of the small number of missing items.
Questions on the MLHFQ concern a variety of physical and psychological aspects of living with HF, and include activities of daily living, economic issues, ability to work, enjoyment of leisure time activities, relations with family and friends, sexual activity, side effects from medications, depression, and impact of HF symptoms. This instrument is widely used and particularly useful in the advanced HF population because it is short, easily understood by ill and elderly individuals, self-administered, and easy to score.
Event-free Survival
Event-free survival was defined as survival and the absence of rehospitalization for HF exacerbation during the follow-up period. Rehospitalization and mortality were determined by investigators at each of the enrolling sites. The primary end-point for the ESCAPE trial and for this analysis was days alive out of hospital during the 6 months of follow-up after patient entry into the study.
Covariates
Traditional clinical risk factors for rehospitalization and mortality were included as covariates based on modeling of mortality in this database and prior literature. These risk factors, collected at baseline as previously described,12 were left ventricular ejection fraction (LVEF), systolic blood pressure, serum sodium, blood urea nitrogen (BUN), 6-minute walk distance, whether the patient was too ill to walk for 6 minutes, and age. Patient group assignment in the ESCAPE trial was also included as a covariate.
Protocol
The protocol was approved by the Institutional Review Board at each enrolling site, and each patient gave written informed consent for participation in the study. Baseline data were collected during the index hospitalization. Medical care was designed to meet the following goals before patient discharge to insure clinical stability: no intravenous inotropic drugs for 48 hours; at least 24 hours on a stable oral drug regimen; stable fluid balance; completion of patient education; and arrangements for follow-up. Repeat assessment of HRQOL using the MLHFQ was done 1, 3, and 6 months after discharge. Patients were followed for occurrence of endpoints for 6 months.
Data analysis
Data are presented as means ± standard deviations or percents. The difference in the MLHFQ score from baseline to 1 month was used to predict 6 month hospitalization-free survival (5 months after the repeat assessment). Event-free time to survival was compared among the following 3 groups using Cox proportional hazards regression: (1) those with an improvement in HRQOL defined as a decrease in MLHFQ score of more than 5 points; 2) those with a decrement in HRQOL defined as an increase in MLHFQ score of more than 5 points; and (3) those with no change in HRQOL as defined by a change between these 2 values.15 The Cox regression analysis included adjustment for LVEF, systolic blood pressure, serum sodium, BUN, 6-minute walk distance, whether the patient was too ill to walk for 6 minutes, age, and patient group assignment. Alpha was set a priori at 0.05 for all analyses.
Results
Patient characteristics
Of the 433 patients enrolled in the ESCAPE trial, 425 survived to discharge. There were no differences in the characteristics of these 425 patients compared with the full cohort of 433 enrolled. A total of 274 (63%) of the 425 patients followed for this substudy died or were rehospitalized during the 6-month follow-up period; of these, 247 were rehospitalized, and 75 died. This number is not mutually exclusive as some patients died during or after a rehospitalization. A total of 13 patients died during the first month of follow-up after discharge, another 34 died within the 2- to 3-month follow-up period, and an additional 28 had died by 6-months follow-up. Of the 425, 313 provided 1 month data on the MLHFQ. Characteristics of those 313 are included in Table 1. Of the 112 patients who did not complete the MLHFQ at both time points, 13 died after discharge and before 1 month, 6 failed to complete the MLHFQ at baseline, and 94 failed to complete it at 1 month. One of these patients did not complete it at either time point. Reasons for failure to complete the MLHFQ varied but included patient refusal, conflicts in time schedule, and lack of patient and research assistant time. There were no differences in baseline characteristics between the 99 patients who failed to complete the MLHFQ at either the baseline or 1 month point, and those completed it at both time points.
Table 1.
Characteristics of patients enrolled in the ESCAPE trial who survived to discharge and who provided baseline and 1 month data on the MLHFQ (N=313).
| Characteristic | |
|---|---|
| Age, mean±SD, yrs | 56±13 |
| Female, % | 26.5 |
| Lives alone, % | 24.4 |
| LVEF, mean±SD, % | 19.6±6.7 |
| Baseline serum sodium, mean±SD, mmol/L | 137±4 |
| Baseline BUN, mean±SD, mg/dL | 34±21 |
| Baseline pulse pressure, mean±SD | 38±12 |
| Baseline systolic blood pressure, mean±SD, mmHg | 105±16 |
| Baseline hemoglobin, mean±SD, g/dL | 13±2 |
| Baseline 6-min walk, mean±SD, ft (n=279) | 456±415 |
| Too ill for 6-min walk, % | 23.1 |
| HF etiology, % | |
| Ischemic | 45.3 |
| Idiopathic | 25.1 |
| Other | 29.6 |
| Comorbidity, % | |
| History of stroke | 9.4 |
| History of MI/PCI/CABG | 48.7 |
| Diabetes mellitus | 35.2 |
| COPD | 15.8 |
| Discharge medications, % | |
| ACE inhibitors | 79.2 |
| Spironolactone | 53.0 |
| Beta-blocker | 66.0 |
| Diuretics | 89.7 |
| ARBs | 17.6 |
| Digoxin | 70.9 |
| Nitrates | 40.1 |
| Baseline MLHFQ total score, mean±SD | 74.2±17.4 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation.
Health-Related Quality of Life
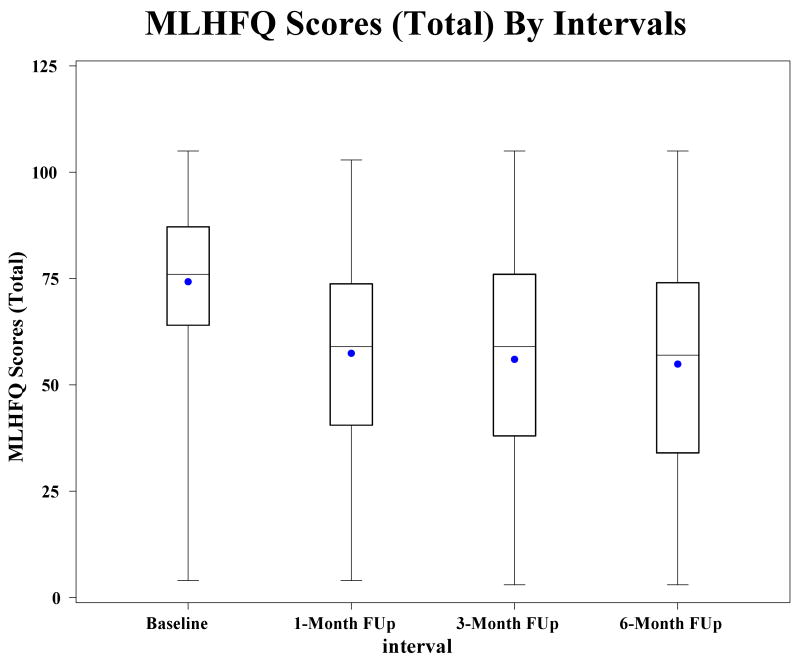
At baseline, the total MLHFQ score for the entire group of 74.2±17.4 reflected severe impairment in HRQOL (Table 1). At the 1 month follow-up point, the MLHFQ score for the entire group had decreased to 56.7 ± 22.7, meaning that HRQOL improved. Data on MLHFQ scores were available for patients at baseline, 1 month, 3 months, and 6 months in those who survived to the 6-month follow-up point, and demonstrate that the greatest improvement in HRQOL in those patients who survived 6 months occurs within the first month after discharge from hospitalization for an exacerbation (Figure 1). No gain or decrement was seen beyond that point. A total of 213 (68%) patients experienced an improvement in HRQOL from baseline to 1 month after discharge, while HRQOL worsened in 51 (16.3%) patients and remained the same in 49 (15.7%) (Table 2). The only baseline clinical or sociodemographic characteristic that distinguished among these groups was whether or not the patient was too ill to perform the 6-minute walk. There was a greater of proportion of patients in the group whose HRQOL worsened and who could not perform the 6-minute walk than in the group whose HRQOL remained the same or the group whose HRQOL improved. There was a trend (p=0.07) toward higher serum BUN in the group whose HRQOL worsened.
Figure 1.
Health-related quality of life (measured using the Minnesota Living with Heart Failure Questionnaire) at each of the time points in patients who survived to 6-month follow-up. P < 0.001 for improvement in score at 1 month; FUp, follow-up; MLHFQ, Minnesota Living with Heart Failure Questionnaire. Plots indicate the lowest and highest scores, the 25th and 75th percentiles, and the median. The mean is indicated by the dot. Sample sizes for baseline, 1 month, 3 months and 6 months are 425, 313, 287, and 227 respectively.
Table 2.
Comparison of patient characteristics among patients whose MLHFQ scores improved, those whose worsened and those whose remained the same from baseline to 1 month follow-up (n = 313)
| Change in MLHFQ Score | ||||
|---|---|---|---|---|
| Characteristic | Worsened (n =51) |
No Change (n=49) |
Improved (n=213) |
p |
| Age, mean±SD, yrs | 58.5±14.2 | 55.3±14.2 | 55.6±12.7 | 0.34 |
| Female, % | 25.5 | 34.7 | 24.9 | 0.37 |
| Lives alone, % | 17.6 | 16.3 | 27.8 | 0.11 |
| LVEF, mean±SD, % | 20.9±7.1 | 20.2±6.8 | 19.1±6.7 | 0.20 |
| Baseline serum sodium, | 136.2±4.0 | 137.2±4.8 | 136.6±4.3 | 0.37 |
| mean±SD, mmol/L | ||||
| Baseline BUN, mean±SD, mg/dL | 42±27 | 31±21 | 32±18 | 0.07 |
| Baseline pulse pressure, mean±SD | 38.2±13.0 | 40.0±13.0 | 38±11.2 | 0.62 |
| Baseline systolic blood pressure, mean±SD, mmHg | 103±17 | 107±15 | 106±15 | 0.14 |
| Baseline hemoglobin, mean±SD, g/dL | 12±2 | 13±2 | 13±2 | 0.10 |
| Baseline 6-min walk, mean±SD, ft | 384.1±402.1 | 425.4±421.2 | 479.7±416.8 | 0.22 |
| Too ill for 6-min walk, % | 35.3 | 27.7 | 19.0 | 0.03 |
| HF etiology, % | 0.93 | |||
| Ischemic | 45.1 | 49.0 | 44.5 | |
| Idiopathic | 27.5 | 20.4 | 25.6 | |
| Other | 27.5 | 30.6 | 29.9 | |
| Comorbidity, % | ||||
| History of stroke | 10.0 | 4.1 | 10.5 | 0.44 |
| History of MI/PCI/CABG | 51.0 | 55.1 | 46.7 | 0.53 |
| Diabetes mellitus | 44.0 | 46.2 | 32.9 | 0.33 |
| COPD | 22.4 | 12.2 | 15.1 | 0.34 |
| Discharge medications, % | ||||
| ACE inhibitors | 70.6 | 79.6 | 81.1 | 0.25 |
| Spironolactone | 60.8 | 55.1 | 54,7 | 0.73 |
| Beta-blockers | 64.7 | 65.3 | 55.7 | 0.29 |
| Diuretics | 88.2 | 87.8 | 90.6 | 0.78 |
| Digoxin | 72.5 | 75.7 | 69.5 | 0.68 |
| Nitrates | 35.3 | 51.0 | 38.7 | 0.21 |
| ARBs | 17.6 | 18.4 | 17.4 | 0.90 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation.
There were no differences in baseline MLHFQ total scores between patients with (n = 218; 74±16) and without (n = 207; 74±18) an event during the follow-up period. Health-related quality of life improved in patients with and without an event by 1 month and remained at the 1 month level during follow-up to 6-months. There was, however, a group by time interaction (p<0.0001 group × time interaction), meaning that the pattern of MLHFQ total scores (i.e., degree of improvement) across time differed between patients who survived without an event and those who died or were rehospitalized by 6 months after discharge (Figure 2). Patients who died or were hospitalized between the 1 month and 6 month assessment did not experience improvements in HRQOL to the same degree as did those who had no events during follow-up.
Figure 2.
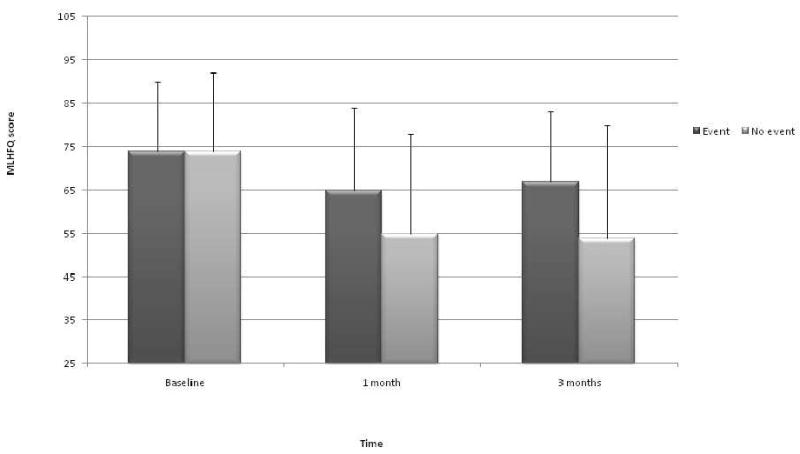
Health-related quality of life (measured using the Minnesota Living with Heart Failure Questionnaire and presented as means ± SD) compared between those who died or were rehospitalized (event) during 6-month follow-up and those with no event during follow-up. P<0.001 for group × time interaction; MLHFQ, Minnesota Living with Heart Failure Questionnaire.
Prediction of Event-Free Survival
To determine whether degree of improvement in HRQOL predicted subsequent event-free survival after controlling for traditional clinical risk factors, survival analysis with Cox proportional hazards regression was performed. In the Cox regression model, both before and after adjustment for LVEF, systolic blood pressure, serum sodium, BUN, 6-minute walk distance, whether the patient was too ill to walk for 6 minutes, age, and patient group assignment, improvement in HRQOL as reflected by a decrease in the MLHFQ score of more than 5 points predicted better event-free survival (p = 0.009; Table 3 and Figure 3). The odds ratio for poorer event-free survival was 3.3 in patients' whose MLHFQ scores worsened compared to improving. The only other variable predicting event-free survival in this model was serum sodium; lower serum sodium was predictive of worse event-free survival.
Table 3.
Hazard ratios for event-free survival from multivariable Cox regression
| Variable | Hazard ratio | 95th % Confidence Intervals | P value |
|---|---|---|---|
| MLHFQ score change* | |||
| Worse vs improve | 3.32 | 1.34 – 8.27 | 0.009 |
| Same vs improve | 2.26 | 0.82 – 6.29 | 0.118 |
| Age | 1.03 | 0.99 – 1.06 | 0.117 |
| Serum sodium | 0.93 | 0.86 – 0.99 | 0.04 |
| Serum BUN | 1.01 | 0.99 – 1.03 | 0.34 |
| Distance walked on the 6-minute walk | 0.99 | 0.99 – 1.00 | 0.07 |
| Too ill to perform the 6- minute walk | 0.42 | 0.14 – 1.33 | 0.14 |
| LVEF | 0.983 | 0.92 – 1.03 | 0.41 |
| Systolic blood pressure | 0.99 | 0.97 – 1.01 | 0.41 |
| Group assignment | 1.45 | 0.68 – 3.01 | 0.33 |
Legend: BUN = Blood urea nitrogen; LVEF = left ventricular ejection fraction; MLHFQ = Minnesota Living with Heart Failure Questionnaire; *MLHFQ score change = improve, improvement in HRQOL defined as a decrease in MLHFQ score of more than 5 points; worse, decrement in HRQOL defined as an increase in MLHFQ score of more than 5 points; and same, no change in HRQOL as defined by a change between these 2 values.
Figure 3.
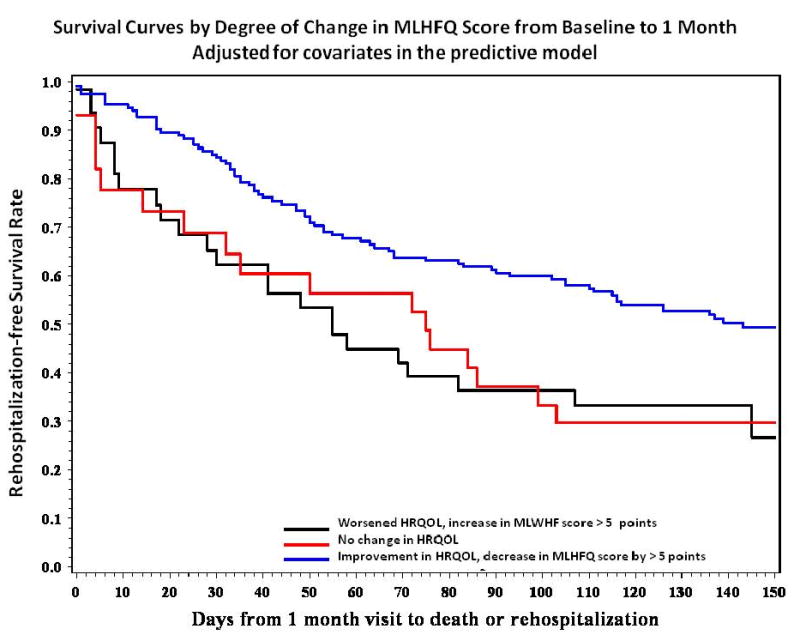
Survival curves (p = 0.009) derived from the multivariate Cox proportional hazards model based on degree of improvement in health-related quality of life (HRQOL) reflected by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) scores at baseline and 1 month, and adjusted for left ventricular ejection fraction, serum sodium, blood urea nitrogen, 6-minute walk distance, whether the patient was too ill to perform the 6-minute walk, age, systolic blood pressure, and patient group assignment. The adjusted curves were developed using the population-based adjustment method proposed by Makuck.30
Discussion
The findings from this study add new information to the existing body of literature about HRQOL in patients with HF in 3 important ways. First, this study was among the few to enroll and follow seriously ill patients with advanced HF prospectively after hospitalization, and to measure HRQOL serially in this cohort during 6 months. We demonstrated that among patients hospitalized with severe HF exacerbation, HRQOL is seriously impaired but improves substantially within 1 month of discharge and stabilizes. Second, among the group of patients with advanced HF who survived to 6 months, the improvement in HRQOL persisted throughout the 6-month follow-up. Third, patients for whom HRQOL does not improve merit special attention because of their high risk for early rehospitalization or mortality.
Patients in this study had HRQOL scores reflecting their severity of HF. The mean score at baseline was worse than that seen in most previous studies of hospitalized patients with HF4,9,16 demonstrating the truly advanced nature of HF among the patients enrolled in this study. Even among this group of patients, in whom the 6-month rehospitalization or mortality rate was 63%, HRQOL improved substantially from hospitalization to 1 month post-discharge by an average of 16 points. Although no further improvement was seen after the 1-month period, HRQOL scores overall remained stable to the 6-month follow-up point. These data add to the small body of existing literature demonstrating that HRQOL improves in the early months after a hospitalization for decompensated HF11,17,18 and expand existing data by extending this finding to serial assessment in advanced HF.
The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) was the first large scale study to examine HRQOL in a group of patients with severe advanced HF close to the time of death.19 In this study, HRQOL was reported to be good even in the weeks to days prior to death. However, overall quality of life was measured by patients or surrogates using a single item estimation to which the possible responses were “poor”, “fair”, “good”, or “very good or excellent”. Single-item estimates of HRQOL underestimate the full impact of a condition, assessment by surrogates often is not accurate, and the use of cross-sectional instead of repeated within patient assessments likely produced some inaccuracies in HRQOL measurement. The data from the current study and from others9,10,20–23 using reliable and valid disease-specific instruments demonstrate that HRQOL is substantially impaired and worsens in HF patients close to death. These data provide clinicians with a much clearer view of the realities of advanced HF.
Patients commonly ask questions that are difficult to answer about their expected course after an acute exacerbation of chronic HF. Many clinicians struggle with providing such information owing to the uncertain course that is typical of HF.21 Determination of HRQOL during an admission for severe decompensated HF and within the month after discharge provides clinicians with useful information for discussions with patients and families about the expected course of HRQOL and survival. The finding that advanced HF patients whose HRQOL failed to improve after hospital discharge were at increased risk for rehospitalization or mortality adds to the practical prognostic information available to clinicians.
Since the first demonstration that HRQOL was predictive of rehospitalization in patients with HF independent of traditional risk factors for this outcome,10 a small number of investigators have examined the relation of HRQOL with rehospitalization and mortality.9,20,22,23 In the earliest work in this area with patients with HF, Konstam and colleagues examined the relationship of HRQOL with rehospitalization and mortality during mild-moderate HF over a mean follow-up of 36.5 months in 5025 of the 6796 patients enrolled in the treatment and prevention arms of the Studies of Left Ventricular Dysfunction (SOLVD) trials.10 Baseline HRQOL was assessed using a number of items from different instruments including the Profile of Mood States, the Medical Outcomes Study Short Form general health survey, and the Functional Status Questionnaire. The items assessed HRQOL with regard to physical functioning, emotional distress, social health, intimacy, life satisfaction, and perceived health. The investigators demonstrated that the HRQOL aspects of activities of daily living, general health, and HF symptoms were predictive of rehospitalization and mortality independent of ejection fraction, age, treatment with angiotensin-converting enzyme inhibitor or placebo, and NYHA classification. This study was important for establishing the role of subjective patient perspectives of health status in determining clinical outcomes in patients with HF. Equally important was the demonstration of the relation between HRQOL and these clinical outcomes in patients both with and without overt HF.
In subsequent work, investigators confirmed that the symptom aspect of HRQOL was an independent predictor of long-term mortality and rehospitalization risk in HF.24 In another investigation of the SOLVD cohort, HRQOL predicted outcomes, but only in patients younger than 55 years of age.25 Others, using a disease-specific HRQOL instrument (i.e., the Kansas City Cardiomyopathy Questionnaire),20,22 a generic HRQOL instrument (i.e., the Nottingham Health Profiles),21 or both (i.e., Minnesota Living with Heart Failure Questionnaire and Medical Outcomes Study Short Form 36 or Duke Health Profile)9,23 to measure HRQOL at 1 time point have demonstrated that HRQOL predicts future hospitalization or mortality independent of clinical risk factors. The relation between HRQOL and outcomes is not uniformly stable, however, and inconsistencies may be the result of using HRQOL measured at only 1 time point to predict outcomes. These experiences focused on patients with lower symptom burden and longer anticipated survival than those in the ESCAPE population.
Why might HRQOL be associated with morbidity and mortality outcomes in patients with HF? Is it simply that HRQOL is associated with some other, unmeasured, predictor of outcomes that reflects severity of HF? This study was not designed to answer these questions, but the latter possibility seems unlikely given that HRQOL remains strongly predictive even after controlling for a number of known, objective, clinical predictors of outcomes that do reflect severity of HF. Health-related quality of life is strongly influenced by depression levels and by patient perception of symptom burden.26-28 These factors both have components that are conceptually and practically distinct from objective measures of HF severity. Depression is thought to be related independently to poor outcomes by physiologic alterations that include increased neurohormonal activity, increased pro-inflammatory activity, and changes in platelet activation and by behavioral mechanisms including decreased adherence.28 Health-related quality of life may be related to poor outcomes through these same mechanisms.
Data from the current study add to the accumulating body of evidence demonstrating the predictive power of patient-reported HRQOL. Given the inadequacies of current prognostic models,29 and the relative difficulties in obtaining all of the clinical data used in predictive models, use of a subjective measure like HRQOL to assist in making decisions about use of resources based on risk is an attractive alternative.
Limitations
Findings from this study should be interpreted in light of the limitation inherent in the assessment of self-reported constructs. Patients who are too ill or do not survive to report their HRQOL are likely to have poor HRQOL and thus our findings are limited by the potential for a survivor bias. Given that we demonstrated poorer outcomes in patients whose HRQOL worsened, it is likely that our findings would only be strengthened by addition of HRQOL data from non-survivors and non-responders. The study is also limited by the relatively short follow-up period of 6 months. It would be enlightening in future studies to see the relationship of HRQOL to outcomes over a longer course.
Conclusion
We demonstrated that even among patients hospitalized for severe decompensated HF who initially report very poor HRQOL, many experience improvement in HRQOL at 1-month follow-up. Clinicians can use these findings to provide patients and their families with information about the typical course of HRQOL after a hospitalization. Patients whose HRQOL fails to improve at 1-month follow-up are at heightened risk for rehospitalization or mortality, and this risk is independent of traditional clinical risk factors for these outcomes. Measurement of HRQOL should assume greater importance in the management and risk stratification of patients with advanced HF.
Acknowledgments
Funded by NIH, ESCAPE Contract No. NHLBI-HV-98-24.
Footnotes
Disclosures: There are no conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sullivan M. The new subjective medicine: taking the patient's point of view on health care and health. Soc Sci Med. 2003;56:1595–1604. doi: 10.1016/s0277-9536(02)00159-4. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan RM. The significance of quality of life in health care. Qual Life Res. 2003;12:3–16. doi: 10.1023/a:1023547632545. [DOI] [PubMed] [Google Scholar]
- 3.Stanek EJ, Oates MB, McGhan WF, Denofrio D, Loh E. Preferences for treatment outcomes in patients with heart failure: symptoms versus survival. J Card Fail. 2000;6:225–232. doi: 10.1054/jcaf.2000.9503. [DOI] [PubMed] [Google Scholar]
- 4.Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984. doi: 10.1016/j.ahj.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Evangelista LS, Dracup K, Moser DK, Westlake C, Erickson V, Hamilton MA, Fonarow GC. Two-year follow-up of quality of life in patients referred for heart transplant. Heart Lung. 2005;34:187–193. doi: 10.1016/j.hrtlng.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Gott M, Barnes S, Parker C, Payne S, Seamark D, Gariballa S, Small N. Predictors of the quality of life of older people with heart failure recruited from primary care. Age Ageing. 2006;35:172–177. doi: 10.1093/ageing/afj040. [DOI] [PubMed] [Google Scholar]
- 7.Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, McGlynn EA, Ware JE., Jr Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA. 1989;262:907–913. [PubMed] [Google Scholar]
- 8.Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J. 2002;23:1867–1876. doi: 10.1053/euhj.2002.3255. [DOI] [PubMed] [Google Scholar]
- 9.Alla F, Briancon S, Guillemin F, Juilliere Y, Mertes PM, Villemot JP, Zannad F. Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail. 2002;4:337–343. doi: 10.1016/s1388-9842(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 10.Konstam V, Salem D, Pouleur H, Kostis J, Gorkin L, Shumaker S, Mottard I, Woods P, Konstam MA, Yusuf S. Baseline quality of life as a predictor of mortality and hospitalization in 5,025 patients with congestive heart failure. SOLVD Investigations. Studies of Left Ventricular Dysfunction Investigators. Am J Cardiol. 1996;78:890–895. doi: 10.1016/s0002-9149(96)00463-8. [DOI] [PubMed] [Google Scholar]
- 11.Riegel B, Moser DK, Glaser D, Carlson B, Deaton C, Armola R, Sethares K, Shively M, Evangelista L, Albert N. The Minnesota Living With Heart Failure Questionnaire: sensitivity to differences and responsiveness to intervention intensity in a clinical population. Nurs Res. 2002;51:209–218. doi: 10.1097/00006199-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 12.ESCAPE Investigators and ESCAPE Study Coordinators Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 13.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Am Heart J. 1992;124:1017–1025. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 14.Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71:1106–1107. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 15.Rector TS, Tschumperlin LK, Kubo SH, Bank AJ, Francis GS, McDonald KM, Keeler CA, Silver MA. Use of the Living with Heart Failure Questionnaire to ascertain patients' perspectives on improvement in quality of life versus risk of drug-induced death. J Card Fail. 1995;1:201–206. doi: 10.1016/1071-9164(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 16.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 17.Riegel B, Carlson B, Glaser D, Romero T. Changes over 6-months in health-related quality of life in a matched sample of Hispanics and non-Hispanics with heart failure. Qual Life Res. 2003;12:689–698. doi: 10.1023/a:1025132623647. [DOI] [PubMed] [Google Scholar]
- 18.Chin MH, Goldman L. Gender differences in 1-year survival and quality of life among patients admitted with congestive heart failure. Medical Care. 1998;36:1033–1046. doi: 10.1097/00005650-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Levenson JW, McCarthy EP, Lynn J, Davis RB, Phillips RS. The last six months of life for patients with congestive heart failure. J Am Geriatr Soc. 2000;48:S101–109. doi: 10.1111/j.1532-5415.2000.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 20.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110:546–551. doi: 10.1161/01.CIR.0000136991.85540.A9. [DOI] [PubMed] [Google Scholar]
- 21.Mejhert M, Kahan T, Persson H, Edner M. Predicting readmissions and cardiovascular events in heart failure patients. Int J Cardiol. 2006;109:108–113. doi: 10.1016/j.ijcard.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, Conard MW, Williams RE. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47:752–756. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Artalejo F, Guallar-Castillon P, Pascual CR, Otero CM, Montes AO, Garcia AN, Conthe P, Chiva MO, Banegas JR, Herrera MC. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165:1274–1279. doi: 10.1001/archinte.165.11.1274. [DOI] [PubMed] [Google Scholar]
- 24.Ekman I, Cleland JG, Swedberg K, Charlesworth A, Metra M, Poole-Wilson PA. Symptoms in patients with heart failure are prognostic predictors: insights from COMET. J Card Fail. 2005;11:288–292. doi: 10.1016/j.cardfail.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Stull DE, Clough LA, Van Dussen D. Self-report quality of life as a predictor of hospitalization for patients with LV dysfunction: a life course approach. Res Nurs Health. 2001;24:460–469. doi: 10.1002/nur.10006. [DOI] [PubMed] [Google Scholar]
- 26.Heo S, Doering LV, Widener J, Moser DK. Predictors and effect of physical symptom status on health-related quality of life in patients with heart failure. Am J Crit Care. 2008;17:124–132. [PubMed] [Google Scholar]
- 27.Heo S, Moser DK, Riegel B, Hall LA, Christman N. Testing a published model of health-related quality of life in heart failure. J Card Fail. 2005;11:372–379. doi: 10.1016/j.cardfail.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 28.York KM, Hassan M, Sheps DS. Psychobiology of depression/distress in congestive heart failure Heart Fail Rev. 2008 doi: 10.1007/s10741-008-9091-0. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frankel DS, Piette JD, Jessup M, Craig K, Pickering F, Goldberg LR. Validation of prognostic models among patients with advanced heart failure. J Card Fail. 2006;12:430–438. doi: 10.1016/j.cardfail.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Makuch RW. Adjusted survival curve estimation using covariates. J Chronic Dis. 1982;35(6):437–43. doi: 10.1016/0021-9681(82)90058-3. [DOI] [PubMed] [Google Scholar]