Abstract
Antioxidant vitamins may reduce cancer risk by limiting oxidative DNA damage. To summarize and quantify the current epidemiologic evidence of an association between antioxidant vitamin intake and endometrial cancer we conducted a systematic literature review and meta-analysis. One cohort and 12 case-control studies presenting relevant risk estimates were identified by conducting bibliographical searches through June 2008. Dose-response meta-analyses were conducted for beta-carotene, vitamin C, and vitamin E from food sources. Intake from supplements was not considered in the meta-analyses due to the few studies that reported relevant information. Based on case-control data, the random-effects summary odds ratios (OR) were, for beta-carotene: 0.88 (95% CI: 0.79–0.98) per 1,000 mcg/1,000 kcal (I2: 77.7%; p <0.01); for vitamin C: 0.85 (95% CI: 0.73–0.98) per 50 mg / 1,000 kcal (I2: 66.1%; p <0.01); and, for vitamin E: 0.91 (95% CI: 0.84–0.99) per 5 mg / 1,000 kcal (I2: 0.0%; p:0.45). In contrast, the only prospective study identified provided little indication of an association. Although the current case-control data suggest an inverse relationship of endometrial cancer risk with dietary intakes of beta-carotene, vitamin C, and vitamin E from food sources, additional studies are needed, particularly cohort studies, to confirm an association.
Keywords: endometrial carcinoma, diet, vitamins, antioxidants, vitamin E, vitamin C, carotenoids, meta-analysis, systematic literature review
INTRODUCTION
Endometrial cancer is the most common female gynecological cancer in the United States, ranking fourth in age-adjusted incidence and eighth in age-adjusted mortality among female cancers [1]. There is extensive experimental evidence showing the impact of dietary antioxidants in reducing oxidative stress and, therefore, influencing carcinogenesis [2]. However, only a few epidemiologic studies have evaluated the relationship between antioxidants and endometrial cancer.
In support of the Second World Cancer Research Fund International/American Institute for Cancer Research (WCRF/AICR) Report on Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective published in November 2007 [3], and commissioned by the WCRF, we conducted a systematic and comprehensive literature review of diet, nutrition, physical activity and endometrial cancer [4] to enhance and update the previous 1997 Report [5]. In this manuscript we focus on the evaluation of the role of carotenoids, vitamin C, and vitamin E on endometrial cancer risk, including dose-response meta-analyses to quantify the strength of the association. Building on the work conducted for the WCRF/AICR Report, we updated searches and meta-analyses for the purpose of this manuscript. To our knowledge, this is the first meta-analysis published on this topic.
MATERIALS AND METHODS
The general methods used followed the WCRF Specification Manual which can be found online at www.wcrf.org and have been described elsewhere [6–9]. The methods used in this manuscript diverge from the WCRF instructions in that we followed our own criteria for inclusion of studies, as well as our own methods for data tabulation and analysis and for interpretation of the evidence. In this manuscript additional sensitivity analyses were conducted, in which we repeated analyses excluding hospital-based studies.
Search strategy
Searches were conducted in July 2003, October 2004, and December 2005. Databases included Medline, ISI Web, Embase, Biosis, Ingenta, CINAHL, Science Direct, LILACS, Pascal, ExtraMed, and Allied CompMed. Results from the 2003 searches indicated that most citations were found in Medline and, therefore, some of the databases that did not produce any new results were not used in subsequent searches. These searches were complemented with manual searches of bibliographies in published papers. We also monitored the literature by using PubMed Alerts on “endometrial cancer” from January 2006 to June 2008.
Exposure terms for PubMed were provided by WCRF and can be found in the Specification Manual and in the Appendix of another manuscript [7]. General terms included diet[tiab] OR diets[tiab] OR dietetic[tiab] OR dietary[tiab] OR eating[tiab] OR intake[tiab] OR nutrient*[tiab] OR nutrition[tiab] OR vegetarian*[tiab] OR vegan*[tiab] OR "seventh day adventist"[tiab] OR macrobiotic[tiab] OR food and beverages[MeSH Terms]. Specifically for beta-carotene, vitamin C, or vitamin E, terms included supplements[tiab] OR supplement[tiab] OR vitamin*[tiab] OR carotenoid*[tiab] OR tocopherol[tiab].
Following WCRF instructions, our searches included endometrial hyperplasia, as this includes precancerous lesions. However, we found only a few papers evaluating the role of diet and nutrition on endometrial hyperplasia, and none that evaluated antioxidant vitamin intake. Outcomes terms included: (1): endometrial neoplasm [MeSH]; (2): malign* [tiab] OR cancer*[tiab] OR carcinoma*[tiab] OR tumor*[tiab] OR tumour*[tiab]; (3): endometr* [tiab] OR corpus uteri [tiab] OR uterine [tiab]; (4): #2 AND #3; (5): #3 AND hyperplasia [tiab].
Manuscript selection and data extraction
Overall search results and manuscript selection have been described elsewhere. In brief, citations identified from these searches were reviewed independently by two of us (LHK, EVB) for relevance. Papers identified through PubMed Alerts were similarly reviewed for relevance. Of the 325 papers identified through June 2008 evaluating some aspect of nutrition, diet, physical activity and endometrial cancer, 17 mentioned antioxidant vitamins [10–26]; all were written in English.
Data were extracted by trained research personnel on study characteristics, and results using an Access® program developed by Leeds University under WCRF sponsorship. Each entry was reviewed by at least one of us.
Statistical analysis
Meta-analyses were conducted separately by study design. Because there were only a few studies evaluating each antioxidant vitamin, we had limited power to assess publication bias through funnel plots, or to conduct sensitivity analyses and meta-regression. To be able to conduct dose-response meta-analyses, results were transformed into a common nutrient-density scale, as described elsewhere [4]. Study-specific odds ratios were estimated for the same energy-adjusted “dose”, which corresponded approximately to the difference between the median intakes of the highest and lowest categories of intake across the various studies. If confidence intervals were not reported, they were estimated based on the number of cases and controls in each category of exposure [27].
For studies reporting only categorical analyses, an estimate of mean intake for each category was computed following the methodology developed by Chêne and Thompson [28]. The iterative method described in Greenland and Longnecker was used to estimate a single logistic regression parameter per study [29]. This method imputes expected numbers of cases and controls (or cases for a prospective study) and computes the logistic regression slope parameter (which may be interpreted as the log relative risk) and standard error. Finally, we estimated fixed effects and random effects pooled logistic regression coefficients across studies. We used the random effects models in forest plots and for interpretation of the evidence, since it uses a combination of the "within study" variance and the "between study" variance for computing weights. The Chêne and Thompson and Greenland and Longnecker algorithms described above were implemented in the statistical language R (R Development Core Team, 2003).
RESULTS
We identified a total of 17 manuscripts from one randomized trial [10], 2 cohort studies [12, 17] and 12 case-control studies [13–26] presenting findings on antioxidant vitamins and endometrial cancer risk. One of the cohort studies [12] evaluated serum beta-carotene. Two manuscripts by Goodman et al. [13, 14] were from the same case-control study. Also, the study by Levi et al. [24] is an earlier report from the same study described in the paper by Negri et al. [23] The study characteristics in the cohort and case-control studies evaluating dietary intake of antioxidant vitamins are presented in Table 1. As shown in this table, included studies were conducted in Canada, Finland, the United States, China, Sweden, Greece, Switzerland, Italy and Mexico. Studies included also varied considerably in dietary assessment tools used. One cohort study [17] and one case-control study [25] focused on adenocarcinoma of the endometrium, while the outcome in all of the other studies was endometrial cancer. Adenocarcinoma accounts for approximately 90% of all endometrial cancers [30].
Table 1.
Characteristics of observational studies evaluating antioxidant vitamin intake and endometrial cancer risk
| Reference | Country | Cases/controls or cohort size | Age | Dietary assessment | Time frame1 | Hysterectomies excluded | Antioxidant vitamins evaluated |
|---|---|---|---|---|---|---|---|
| COHORT STUDIES | |||||||
| Jain et al., 2000 [17]2 | Canada | 221/3,697 | 40–59 | FFQ (86 items) | One month prior | Yes | Beta-carotene, lycopene, lutein, vitamin C, vitamin C with supplements, vitamin E |
| CASE-CONTROL STUDIES: Population-based | |||||||
| Shu et al., 1993 [15] | China | 268 / 268 | 18–74 | FFQ (63 items) | 10 years | Yes | Carotene, vitamin C |
| Potischman et al., 1993 [16] | United States | 399 / 296 | 20–74 | FFQ (Block, 60 items) | “past few years” | Yes | Vitamin C |
| Goodman et al., 1997 [13, 14] | United States | 332 / 511 | 18–84 | Dietary history (250 items) | 1 year | Yes | Beta-carotene from food, lycopene from food, lutein from food, vitamin C from food, alpha-tocopherol from food |
| Jain et al., 2000 [17] | Canada | 552 / 562 | 30–79 | Dietary history (n Items ?) | 1 year | Yes | Beta-carotene, beta-carotene with supplements, lycopene, lutein, vitamin C, vitamin E, vitamin E with supplements |
| McCann et al., 2000 [18] | United States | 232 / 639 | 40–85 | FFQ (172 items) | 2 years | Yes | Beta-carotene, alpha-carotene, lycopene, lutein, lutein + zeaxanthin, vitamin C, vitamin E |
| Terry et al., 2002 [19] | Sweden | 709 / 2887 | 50–74 | FFQ (n Items ?) | 1 year | Yes | Vitamin C supplement use, vitamin E supplement use |
| Xu et al., 2007 [21] | China | 1204 / 1212 | 30–69 | FFQ (71 foods) | 5 years | Yes | Beta-carotene, vitamin C, vitamin E, vitamin C supplement use, vitamin E supplement use |
| CASE-CONTROL STUDIES: Hospital-based | |||||||
| La Vecchia et al., 1986 [22] | Italy | 206 / 206 | <75 | Questionnaire (intake score-low medium, high- of 10 items) | current | Yes | Carotene |
| *Levi et al., 1993 [24] | Italy and Switzerland | 274 / 572 | 31–75 | Questionnaire (Weekly frequency of intake of 50 items) | “before symptoms” | Yes | Beta-carotene, vitamin C |
| Barbone et al., 1993 [25] | United States | 103 / 236 | FFQ (Willett, 116 items) | 1 year | Yes | Carotene, vitamin C, vitamin C supplements, vitamin E, vitamin E supplement use | |
| Tzonou et al, 1996 [20] | Greece | 145 / 298 | FFQ (115 items) | 1 year | ? | Beta-carotene, vitamin C | |
| *Negri et al.,1996 [23] | Italy and Switzerland | 368 / 713 | 31–75 | Questionnaire (Weekly frequency of intake of 50 items) | “2 years before symptoms” | Yes | Beta-carotene, vitamin C, vitamin E |
| Salazar-Martinez et al., 2005 [26] | Mexico | 85 / 629 | 18–81 | FFQ (116 items) | 1 year | Yes | Beta-carotene, vitamin C, vitamin E |
Abbreviations: FFQ, food frequency questionnaire; ?, unspecified
Time frame for dietary assessment
Case-cohort study
Same case-control study
Carotenoids
Most studies evaluating the relationship between dietary carotenoids and endometrial cancer focused on carotene or beta-carotene. We found one cohort study and eleven manuscripts from 10 case-control studies reporting on dietary beta-carotene/carotene, listed in Table 2. As previously mentioned, the manuscript by Levi et al. [24] is an earlier report from the same study described in the paper by Negri et al. [23]. Out of the ten case-control studies, seven suggested an inverse association with carotene [22, 25] or beta-carotene [13, 18, 21, 23, 26]. In contrast, two case-control studies suggested increased endometrial cancer risk with dietary beta-carotene [15, 20]. The remaining study [17] evaluated dietary beta-carotene and total beta-carotene and reported null associations for both. The cohort study [17] suggested an inverse association, but the confidence interval included one. In addition to these studies, the association between serum beta-carotene and subsequent risk of cancer after 8 years of follow up was evaluated using a nested case-control design within a longitudinal study in Finland [12]. There was no significant difference in serum beta-carotene levels between the 12 cases and 21 controls that comprised the endometrial cancer analysis. A relative risk estimate was not reported.
Table 2.
Studies evaluating carotenoid intake and endometrial cancer risk.
| Reference | Country | Age | Cases / controls | Exposure | Contrast | RR/OR (95% CI) | p for trend | Covariates considered | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | E | S | H | R | ||||||||
| COHORT STUDIES | |||||||||||||
| BETA-CAROTENE | |||||||||||||
| Jain et al., 2000 [17] | Canada | 40–59 | 221 / 3,697 | Beta-carotene, mcg/day | 6771+ vs. < 3090 | 0.77 (0.52–1.14) | 1 | 1 | 1 | 1 | 1 | 2 | |
| CRYPTOXANTHIN | |||||||||||||
| Jain et al., 2000 [17] | Canada | 40–59 | 221 / 3,697 | Cryptoxanthin, mcg/day | 131.4+ vs. < 46.1 | 1.04 (0.71–1.52) | 1 | 1 | 1 | 1 | 1 | 2 | |
| LYCOPENE | |||||||||||||
| Jain et al., 2000 [17] | Canada | 40–59 | 221 / 3,697 | Lycopene, mcg/day | 13948+ vs. < 4438 | 0.63 (0.43 – 0.94) | 1 | 1 | 1 | 1 | 1 | 2 | |
| LUTEIN | |||||||||||||
| Jain et al., 2000 [17] | Canada | 40–59 | 221 / 3,697 | Lutein, mcg/day | 4102+ vs. 1740 | 0.92 (0.61 - 1.37) | 1 | 1 | 1 | 1 | 1 | 2 | |
| CASE-CONTROL STUDIES | |||||||||||||
| BETA-CAROTENE | |||||||||||||
| La Vecchia et al., 1986 [22] | Italy | <75 | 206 / 206 | Carotene index, 1000 IU | 200+ vs. < 100 | 0.27 (0.12-0.6) | 0.001 | 1 | 1 | 3 | |||
| Barbone et al., 1993 [25] | United States | 103 / 236 | Carotene, IU | 11169.4+ vs. < 7709.8 | 0.4 (0.2–0.8) | 0.007 | 1 | 1 | 1 | 1 | 1 | 3 | |
| *Levi et al., 1993 [24] | Italy and Switzerland | 30–75 | 274 / 572 | Beta-carotene, IU/month | 133500+ vs. < 86300 | 0.49 | <0.01 | 1 | 1 | ||||
| Shu et al., 1993 [15] | China | 18–74 | 268 / 268 | Carotene, mg/day | 3.83+ vs. < 1.37 | 1.3 | 0.72 | 1 | 1 | 1 | 1 | ||
| *Negri et al., 1996 [23] | Italy and Switzerland | 31–75 | 368 / 713 | Beta-carotene, mcg/day | 5545+ vs. < 2890 | 0.5 | <0.01 | 1 | 1 | 1 | |||
| Tzonou et al., 1996 [20] | Greece | Unknown | 145 / 298 | Beta-carotene, mg/day | Per 3 mg | 1.27 (0.98–1.64) | 1 | 1 | 1 | 1 | 1 | 5 | |
| Goodman et al., 1997 [13] | USA | 18–84 | 332 / 511 | Beta-carotene from food, mg/day | 5368+ vs. < 2006 | 0.6 | 0.09 | (1) | 1 | 1 | 1 | 1 | |
| McCann et al., 2000 [18] | United States | 40–85 | 232 / 639 | Beta-carotene, mcg/day | 8018+ vs. < 4024 | 0.4 (0.2–0.6) | 0.0001 | 1 | 1 | 1 | 1 | 1 | 3 |
| Jain et al., 2000 [17] | Canada | 30–79 | 552 / 562 | Beta carotene, mcg/day | 7342+ vs. < 3268 | 0.99 (0.69–1.4) | 0.81 | 1 | 1 | 1 | 1 | 1 | 2 |
| Beta carotene with supplements, mcg/day | 7393+ vs. < 3281 | 1.02 (0.72–1.45) | 0.62 | 1 | 1 | 1 | 1 | 1 | 2 | ||||
| Salazar-Martinez et al., 2005 [26] | Mexico | 18–81 | 85 / 629 | Beta-carotene, mg/day | 1547+ vs. <894 | 0.79 (0.41–1.5) | 0.77 | 1 | 1 | 1 | 1 | ||
| Xu et al., 2007 [21] | China | 30–69 | 1204 / 1212 | Beta-carotene | 2439+ vs. < 922 | 0.6 (0.4 – 0.8) | <0.01 | 1 | 1 | 1 | 1 | ||
| ALPHA-CAROTENE | |||||||||||||
| McCann et al., 2000 [18] | United States | 40–85 | 232 / 639 | Alpha-carotene, mcg/day | 1408+ vs. < 599 | 0.6 (0.4–1) | 0.03 | 1 | 1 | 1 | 1 | 1 | 3 |
| CRYPTOXANTHIN | |||||||||||||
| McCann et al., 2000 [18] | United States | 40–85 | 232 / 639 | Cryptoxanthin, mcg/day | 201+ vs. < 41 | 1.3 (0.8–2.1) | 0.82 | 1 | 1 | 1 | 1 | 1 | 3 |
| Jain et al., 2000 [17] | Canada | 30–79 | 552 / 562 | Cryptoxanthin, mcg/day | 118.3+ vs. < 30.6 | 1.23 (0.86–1.74) | 0.36 | 1 | 1 | 1 | 1 | 1 | 2 |
| LYCOPENE | |||||||||||||
| Goodman et al., 2000 [13] | USA | 18–84 | 332 / 511 | Lycopene from food, mg/day | Q4 vs. Q1 | 1.16 | 0.60 | (1) | 1 | 1 | 1 | 1 | |
| Jain et al., 2000 [17] | Canada | 30–79 | 552 / 562 | Lycopene, mcg/day | 9583+ vs. < 2634 | 0.85 (0.6 – 1.21) | 0.32 | 1 | 1 | 1 | 1 | 1 | 2 |
| McCann et al., 2000 [18] | United States | 40–85 | 232 / 639 | Lycopene, mcg/day | 7264+ vs. < 3505 | 0.6 (0.4 – 1.0) | 0.01 | 1 | 1 | 1 | 1 | 1 | 3 |
| LUTEIN/ZEAXANTHIN | |||||||||||||
| Goodman et al., 2000 [13] | USA | 30–79 | 332 / 511 | Lutein from food, mg/day | 3018+ vs. < 1470 | 0.5 | 0.3 | (1) | 1 | 1 | 1 | 1 | |
| Jain et al., 2000 [17] | Canada | 30–79 | 552 / 562 | Lutein, mcg/day | 3918+ vs. 1322 | 0.8 (0.56 – 1.15) | 0.13 | 1 | 1 | 1 | 1 | 1 | 2 |
| McCann et al., 2000 [18] | United States | 40–85 | 232 / 639 | Lutein + zeaxanthin | 7300+ vs. < 3501 | 0.3 (0.2 – 0.5) | 1 | 1 | 1 | 1 | 1 | 3 | |
Adjustment columns: A = Age; B = BMI/weight; E = Total Energy; S = Smoking; H = HRT/ERT use; R = Reproductive factors; (1): matched on age. Numbers in columns refer to the number of covariates adjusted for under that grouping. Abbreviations: OR: odds ratio; RR: Relative Risk; CI: Confidence Interval.
Same case control study
Lee et al. [10] evaluated the effect of beta-carotene supplementation in the Women’s Health Study. Among women assigned to receive beta-carotene (50 mg on alternate days, n=19,939) or placebo (n=19,937), there were no statistically significant differences for all cancers combined (RR: 1.03 (95% CI: 0.82–1.28) or site-specific cancers. For cancer of the uterus, there were 31 cases in the beta-carotene group and 27 in the placebo group (no relative risk or any other data presented).
Other carotenoids have not been widely investigated. Only one case-control study evaluated alpha-carotene [18] and reported an OR of 0.6 (95% CI: 0.4 – 1.0). Two case-control studies of cryptoxanthin intake [17, 18] found slightly elevated risk, but the confidence intervals included 1.0. The only cohort study [17] evaluating the association did not find much evidence of a relationship. One cohort study [17] and three case-control [13, 17, 18] studies evaluated lutein. All four studies reported risk estimates below one. McCann et al. [18] evaluated lutein with zeaxanthin, and reported an odds ratio of 0.3 (95% CI: 0.2 – 0.5). The same four studies also evaluated lycopene. Two of the three case-control studies [17, 18] and the cohort study [17] reported a decreased risk. The relative risk reported in the cohort study was 0.63 (95% CI: 0.43 – 0.94).
Meta-analysis
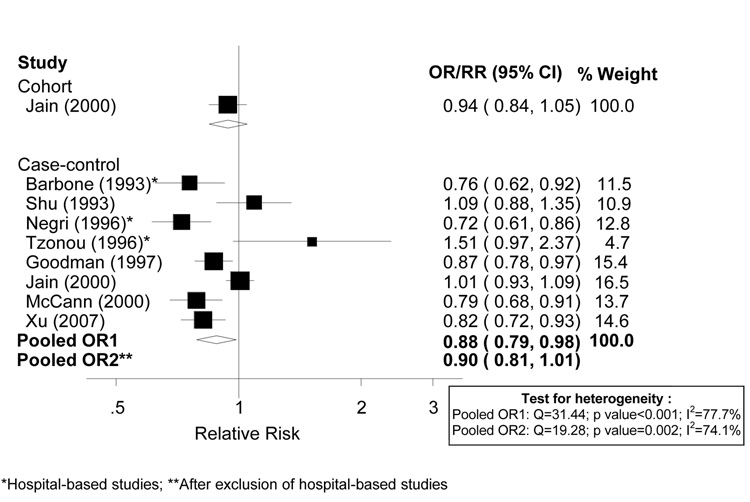
There were sufficient data only to conduct analyses on beta-carotene. The cohort study and ten case-control studies reporting on beta-carotene or carotene listed in Table 2 were considered for inclusion in meta-analyses. We excluded the studies by La Vecchia et al. (1986) and Salazar-Martinez et al. (2005) because they did not include an estimate of total energy intake, which was needed to convert the reported units into a common scale in mcg per 1000 kcal. La Vecchia et al. [22] reported a strong inverse association (OR=0.27, 95% CI 0.12-0.6) with carotene consumption, whereas Salazar-Martinez et al. [26] presented a weaker inverse association (OR: 0.79; 95% CI: 0.41–1.50). Dose-response analyses are shown in Figure 1. As shown in the figure, the derived ORs for the two studies reporting on carotene (Barbone et al., 1993; Shu et al., 1993) are similar to the other derived ORs from the other studies reporting on beta-carotene. There was significant heterogeneity among the eight case-control studies included in meta-analyses, with an I2 of 77.7% (p value for heterogeneity <0.001). The random-effects pooled OR was 0.88 (95% CI: 0.79–0.98) per 1,000 mcg /1,000 kcal. The fixed-effects summary estimate was practically identical (0.89; 95% CI: 0.85 – 0.94). The derived RR for the same increment for the cohort study was 0.94 (95% CI: 0.84–1.05). After excluding hospital-based studies, Barbone et al.[25], Negri et al.[23], and Tzonou et al.[20], the pooled estimate and heterogeneity remained essentially the same (random-effects summary OR: 0.90; 95% CI: 0.81 – 1.01; I2 : 74.1%).
Figure 1.
Random effects meta-analysis of studies evaluating dietary beta-carotene and endometrial cancer risk (per 1,000 mcg / 1,000 kcal of beta-carotene intake from food sources).
Vitamin C
As shown in Table 3, one cohort study [17] and 11 manuscripts [14–18, 20, 21, 23–26] from 10 case-control studies evaluated endometrial cancer risk and vitamin C. The cohort study [17] found no association with vitamin C from food sources and suggested an elevated risk for vitamin C supplements. Neither result was statistically significant. Out of the ten independent case-control studies examining vitamin C from food sources, five reported OR’s below one [14, 18, 21, 23, 25]. One study [16] suggested elevated risk, but the confidence interval included one. The remaining four studies reported no association [15, 17, 20, 26]. One of these case-control studies [17] also examined vitamin C from food and supplement sources and found no indication of a relationship.
Table 3.
Epidemiologic studies evaluating vitamin C intake and endometrial cancer risk.
| Reference | Country | Age | Cases/Controls | Exposure | Contrast | RR/OR (95% CI) | p for trend | Covariates considered | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | E | S | H | R | ||||||||
| COHORT STUDIES | |||||||||||||
| Jain et al., 2000 [17] | Canada | 40–59 | 221 / 3,697 | Vitamin C, mg/day | 196+ vs. < 109.9 | 1.07 (0.71–1.6) | 1 | 1 | 1 | 1 | 1 | 2 | |
| Vitamin C with supplements, mg/day | 205.7+ vs. < 112.6 | 1.32 (0.89–1.94) | 1 | 1 | 1 | 1 | 1 | 2 | |||||
| CASE-CONTROL STUDIES | |||||||||||||
| Barbone et al., 1993 [25] | United States | 103 / 236 | Vitamin C, mg/day | 150.1+ vs. < 100.3 | 0.7 (0.4–1.3) | 0.45 | 1 | 1 | 1 | 1 | 1 | 3 | |
| *Levi et al., 1993 [24] | Italy and Switzerland | 30–75 | 274 / 572 | Vitamin C, g/month | 3.7+ vs. < 2.7 | 0.46 | <0.01 | 1 | 1 | ||||
| Shu et al., 1993 [15] | China | 18–74 | 268 / 268 | Vitamin C, mg/day | 127.94+ vs. < 59.71 | 1.1 | 0.78 | 1 | 1 | 1 | 1 | ||
| Potischman et al., 1993 [16] | United States | 20–74 | 399 / 296 | Vitamin C, mg/day | 180+ vs. < 76 | 1.3 (0.7–2.2) | 1 | 1 | 1 | 1 | 1 | 1 | |
| *Negri et al., 1996 [23] | Italy and Switzerland | 31–75 | 368 / 713 | Vitamin C, mg/day | 183+ vs. < 92 | 0.6 | <0.05 | 1 | 1 | 1 | |||
| Tzonou et al., 1996 [20] | Greece | ? | 145 / 298 | Vitamin C, mg/day, continuous analysis | Per 70 mg | 1.13 (0.84–1.52) | 1 | 1 | 1 | 1 | 1 | 5 | |
| Goodman et al., 1997 [14] | United States, Multi-ethnic | 18–84 | 341 / 511 | Vitamin C, mg/day | 202+ vs. < 93.3 | 0.59 (0.34–1.03) | 0.10 | (1) | 1 | 1 | 1 | 1 | |
| Jain et al., 2000 [17] | Canada | 30–79 | 552 / 562 | Vitamin C, mg/day, categorical analysis | 251.4+ vs. < 130.4 | 0.87 (0.61–1.24) | 0.59 | 1 | 1 | 1 | 1 | 1 | 2 |
| Vitamin C, mg/day, continuous analysis | Per 120 mg | 0.95 (0.81–1.11) | 1 | 1 | 1 | 1 | 1 | 2 | |||||
| Vitamin C with supplements, mg/day | 414+ vs. < 163.9 | 1.01 (0.71–1.44) | 0.90 | 1 | 1 | 1 | 1 | 1 | 2 | ||||
| McCann et al., 2000 [18] | United States | 40–85 | 232 / 639 | Vitamin C, mg/day | 224+ vs. < 129 | 0.5 (0.3–0.8) | 0.02 | 1 | 1 | 1 | 1 | 1 | 3 |
| Salazar-Martinez et al., 2005 [26] | Mexico | 18–81 | 85 / 629 | Vitamin C, mg/day | 184+ vs. <78 | 0.95 (0.47–1.92) | 0.57 | 1 | 1 | 1 | 1 | ||
| Xu et al., 2007 [21] | China | 30–69 | 1204 / 1212 | Vitamin C | 72.7+ vs. < 29.8 | 0.5 (0.3 – 0.7) | <0.01 | 1 | 1 | 1 | 1 | ||
| VITAMIN SUPPLEMENTS | |||||||||||||
| Barbone et al., 1993 [25] | United States | 103 / 236 | Vitamin C supplements | Ever vs. never | 1.4 (0.9–2.4) | 1 | 1 | 1 | 1 | 1 | 3 | ||
| Terry et al., 2002 [19] | Sweden | 50–74 | 709 / 2877 | Vitamin C supplements | Daily vs. Never | 0.8 (0.6–1.2) | 0.30 | 1 | 1 | 1 | |||
Adjustment columns: A = Age; B = BMI/weight; E = Total Energy; S = Smoking; H = HRT/ERT use; R = Reproductive factors; (1): matched on age. Numbers in columns refer to the number of covariates adjusted for under that grouping. Abbreviations: OR: odds ratio; RR: Relative Risk; CI: Confidence Interval.
Same case-control study
Two additional case-control studies evaluated vitamin C supplements and presented conflicting results. Terry et al. [19] found slightly reduced risk for daily versus never use, while Barbone et al. [25] reported an increased risk for ever versus never use. Confidence limits for both risk estimates included the null.
Meta-analysis
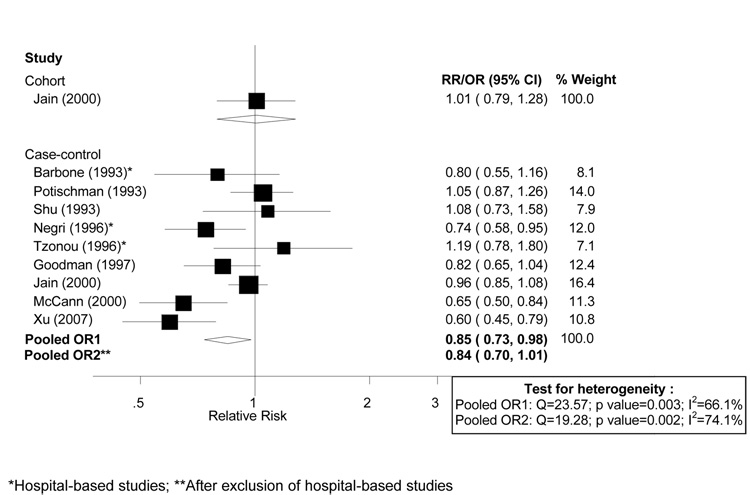
The cohort study and all but one [26] of the ten independent case-control studies (listed in Table 3) that reported vitamin C from food sources, were included in dose-response meta-analyses. Results by Levi et al. [24] were not included because they were based in part on the same study population as the study by Negri et al. [23]. All cutpoints were transformed into a common scale of mg/1,000 kcal of vitamin C intake. The study by Salazar-Martinez et al. [26] was not included in meta-analyses because it did not present an estimate of total energy intake and, therefore, transformation into a nutrient density measure was not possible. Confidence intervals were derived for the two studies that did not report them [15, 23]. Dose-response analyses are shown in Figure 2. There was moderate heterogeneity among case-control studies (I2: 66.1%, p for heterogeneity: 0.003). The random-effects pooled OR from the case-control data was 0.85 (95% CI: 0.73–0.98) per 50 mg /1,000 kcal of vitamin C. The fixed-effects pooled estimate for the same increment was 0.88 (95% CI: 0.82 – 0.95). The derived RR for the cohort study corresponding to the same increment was 1.01 (95% CI: 0.79–1.28). Sensitivity analyses were conducted by excluding hospital-based studies: Barbone et al.[25], Negri et al.[23] and Tzonou et al.[20]. Removing these studies essentially did not influence the results (Figure 2).
Figure 2.
Random effects meta-analysis of studies evaluating dietary vitamin C and endometrial cancer risk (per 50 mg / 1,000 kcal of vitamin C from food sources).
Vitamin E
One cohort study [17] and seven case-control studies [13, 17, 18, 21, 23, 25, 26] evaluated vitamin E intake from food and endometrial cancer risk (Table 4). The cohort study [17] and one case-control study [26] found no association. The remaining six case-control studies reported odds ratios less than 1, although all of the confidence intervals included the null value. One of these case-control studies also evaluated vitamin E from food and supplements [17] and reported an odds ratio of 0.91 (95% CI: 0.64 – 1.29). Two case-control studies, Terry et al. [19]. and Barbone et al. [25], reported on vitamin E supplement use, suggesting elevated risks of 1.3 (95% CI: 0.9 – 1.9) and 1.4 (95% CI: 0.8 – 2.3), respectively.
Table 4.
Epidemiologic studies evaluating vitamin E intake and endometrial cancer risk.
| Reference | Country | Age | Cases / Controls | Exposure | Contrast | RR/OR (95% CI) | p for trend | Covariates considered | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | E | S | H | R | ||||||||
| COHORT STUDIES | |||||||||||||
| Jain, et al., 2000 [17] | Canada | 40–59 | 221 / 3,697 | Vitamin E, mg/day | 23.5+ vs. < 15.8 | 1.09 (0.74–1.61) | 1 | 1 | 1 | 1 | 1 | 2 | |
| CASE-CONTROL STUDIES | |||||||||||||
| Barbone et al., 1993 [25] | United States | 103 / 236 | Vitamin E, mg | 9.5+ vs. < 7.9 | 0.7 (0.4–1.2) | 0.35 | 1 | 1 | 1 | 1 | 1 | 3 | |
| Negri et al., 1996 [23] | Italy and Switzerland | 31–75 | 368 / 713 | Vitamin E, mg/day | 13.9+ vs. < 8.7 | 0.9 | NS | 1 | 1 | 1 | |||
| Goodman et al., 1997 [13] | United States | 18–84 | 341 / 511 | Vitamin E, alpha tocopherol, mg/day | 13.1+ vs. < 6.83 | 0.86 (0.43–1.71) | 0.75 | (1) | 1 | 1 | 1 | 1 | |
| Jain et al., 2000 [17] | Canada | 30–79 | 552 / 562 | Vitamin E, mg/day, continuous analysis | Per 12 mg/day | 0.93 (0.82–1.05) | 1 | 1 | 1 | 1 | 1 | 2 | |
| Vitamin E, mg/day, categorical analysis | 17.5+ vs. < 5.5 | 0.87 (0.62–1.23) | 0.22 | 1 | 1 | 1 | 1 | 1 | 2 | ||||
| Vitamin E with supplements, mg/day | 37.8+ vs. < 8.2 | 0.91 (0.64–1.29) | 0.70 | 1 | 1 | 1 | 1 | 1 | 2 | ||||
| McCann et al., 2000 [18] | United States | 40–85 | 232 / 639 | Vitamin E, mg/day | 10.2+ vs. < 6.4 | 0.6 (0.3–1.2) | 0.18 | 1 | 1 | 1 | 1 | 1 | 3 |
| Salazar-Martinez et al., 2005 [26] | Mexico | 18–81 | 85 / 629 | Vitamin E, mg/day | 9.4+ vs. <6.3 | 1.09 (0.51–2.36) | 0.11 | 1 | 1 | 1 | 1 | ||
| Xu et al., 2007 [21] | China | 30–69 | 1204 / 1212 | Vitamin E | 10.4+ vs. < 6.0 | 0.8 (0.6 – 1.1) | <0.01 | 1 | 1 | 1 | 1 | ||
| VITAMIN E SUPPLEMENTS | |||||||||||||
| Barbone et al., 1993 [25] | United States | 103 / 236 | Vitamin E supplements | Ever vs. never | 1.4 (0.8–2.3) | 1 | 1 | 1 | 1 | 1 | 3 | ||
| Terry et al., 2002 [19] | Sweden | 50–74 | 709 / 2877 | Vitamin E supplement use. | Daily vs. Never | 1.3 (0.8–1.9) | 0.53 | 1 | 1 | 1 | |||
Adjustment columns: A = Age; B = BMI/weight; E = Total Energy; S = Smoking; H = HRT/ERT use; R = Reproductive factors; (1): matched on age. Numbers in columns refer to the number of covariates adjusted for under that grouping. Abbreviations: OR: odds ratio; RR: Relative Risk; CI: Confidence Interval.
Meta-analysis
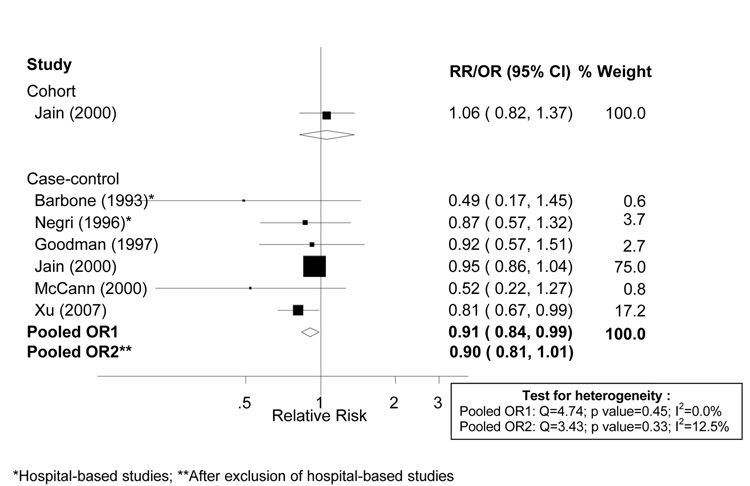
The cohort study [17] and all but one [26] of the seven case-control studies which reported on vitamin E from food sources were included in the meta-analysis. The remaining study [26] was not included as it did not provide a value for total energy intake which was needed to transform the exposure units to a common scale. Confidence intervals were estimated for one study which did not report them [23], and all cutpoints were transformed to a common scale. Dose-response analyses are shown in Figure 3. There was no evidence of heterogeneity among studies, with an I2 value of 0.0% (p = 0.448). The random effects pooled odds ratio was 0.91 (95% CI: 0.84 – 0.98) and the fixed effects pooled odds ratio was 0.91 (95% CI: 0.84 – 0.99) per 5 mg/1000 kcal of vitamin E intake. Additional sensitivity analyses conducted by excluding the two hospital-based studies [23, 25] essentially did not influence results.
Figure 3.
Random effects meta-analysis of studies evaluating dietary vitamin E and endometrial cancer risk (per 5 mg per 1,000 kcal of vitamin E from food sources).
DISCUSSION
Our systematic literature review and meta-analyses suggest a modest reduction in endometrial cancer risk with higher consumption of beta-carotene, vitamin C, and vitamin E from food sources, based on data from case-control studies. We estimated a 12% reduction of endometrial cancer risk per 1,000 mcg/1,000 kcal for beta-carotene, a 15% decreased risk per 50 mg/1,000 kcal vitamin C, and a 9% reduction in risk in per 5 mg/1,000 kcal vitamin E. However, there was little indication of an association in the only prospective study evaluating these associations. There was also little indication of a benefit from using beta-carotene, vitamin C, or vitamin E supplements.
To our knowledge this is the first published systematic literature review and meta-analysis of the relationship between these dietary anti-oxidant vitamins and endometrial cancer. In the 1997 WCRF/AICR Report [5], based on a narrative (and not comprehensive) review of the literature, while the evidence was suggestive of a decreased risk associated with carotenoids, the evidence was deemed “insufficient”, as only four case-control studies were identified [15, 22, 24, 25]. In the 2007 WCRF/AICR Report [3], the evidence was deemed “limited - no conclusion” for beta-carotene, based on our meta-analysis [4] of one cohort study [17] and seven case control studies [13, 15, 17, 18, 20, 23, 25]. The classification of “limited- no conclusion” was given to exposures for which available data were so limited or inconsistent that no firm conclusion could be made. Since publication of the 2007 report we identified and included in the meta-analysis one additional case-control study [21], which strengthened the evidence of an inverse association from case-control study data. Beta-carotene has been considered for decades an anticarcinogenic agent based on its potent antioxidant [31], antimutagenic [32], and immuno-regulatory [33] actions. However, the role of beta-carotene in carcinogenesis has been controversial following the results from the CARET [34] and ATBC [35] trials, in which it was shown that beta carotene supplementation unexpectedly increased lung cancer risk in smokers. Whether beta-carotene from food sources as opposed to supplements may still play an important role in cancer prevention is unclear. The doses of beta-carotene in these trials were substantially higher than that typically found in the diet. In addition, food sources of beta-carotene typically contain other compounds that may decrease risk of cancers, including other antioxidant carotenoids.
There was also mention in the 1997 WCRF/AICRF Report [5] of inconsistent findings and insufficient evidence for vitamin C, based on four case-control studies [15, 16, 24, 25], and the 2007 WCRF/AICR Report [3] found the evidence “limited-no conclusion”, based on our meta-analysis [4] of one cohort [17] and eight case-control studies [13, 15–18, 20, 23, 25] examining the association. Since publication of the 2007 Report, one more case-control study evaluating this association has been published [21] and was included in the meta-analysis presented in this manuscript. This study reported a strong inverse association with vitamin C. Vitamin C is a water-soluble antioxidant essential to maintain the extracellular matrix through its role in hydroxylation of proline, an amino acid integral to the synthesis and structural integrity of collagen. The role of vitamin C on cancer chemoprevention has been attributed to its ability to stimulate immune function, inhibit nitrosamine formation, and block the metabolic activation of carcinogens, as well as its potential for preventing oxidative stress [36]. It has been demonstrated in a number of studies that vitamin C helps to minimize DNA damage[37].Vitamin C may also influence cellular differentiation, possibly through modulation of gene expression [37]. While the evidence relating supplemental vitamin C to cancer prevention may be equivocal [38], the meta-analysis conducted here relates to vitamin C from foods. As such, any observed estimated effect may not be attributable solely to vitamin C, but may reflect a combination of the multiple beneficial vitamins and other factors found in foods rich in vitamin C, or in dietary patterns that emphasize these foods.
Vitamin E was not mentioned in the 1997 WCRF/AICR Report and the 2007 Report also found the evidence to be “limited -no conclusion”, based on our meta-analysis [4] of one cohort study [17] and five case-control studies [13, 17, 18, 23, 25]. Since the publication of the report, we identified one more case-control study [21] evaluating the association, which provided additional evidence for an inverse association. Vitamin E is a lipid-soluble antioxidant that is known to help prevent lipid peroxidation. As oxidative stress is thought to be one central mechanism in carcinogenesis, high vitamin E levels may generally help prevent carcinogenesis due to oxidative damage. For example, there is some suggestion that vitamin E may enhance DNA repair [39]. Vitamin E may also play an important role in immune function [40]. As the meta-analysis conducted here refers to vitamin E from food sources rather than supplemental vitamin E, a possible inverse association of vitamin E with endometrial cancer may be acting as a marker for other foods or dietary patterns associated with higher levels of vitamin E intake.
We found high heterogeneity among studies for vitamin C and beta-carotene. Because of the small number of studies evaluating these associations we were not able to conduct meta-regression or detailed sensitivity analyses to explore sources of heterogeneity. However, type of study (population-based vs. hospital-based) did not seem to explain heterogeneity. As shown in our tables and figures, most studies included in our meta-analyses adjusted for the most important confounding variables, BMI and total energy intake. In contrast, another important confounder, cigarette smoking, was not taken into account by all studies. However, this did not seem to be an important source of heterogeneity, as, for example, an inverse association between beta-carotene and endometrial cancer was suggested in studies adjusting [18, 25] and not adjusting [21, 23] for smoking. Other potential sources of heterogeneity are the different dietary assessment tools used (as shown in Table 1, the number of items in the questionnaires vary considerably among studies) and possible various degrees of residual confounding in the different studies.
In conclusion, our meta-analyses suggest a possible inverse association of dietary antioxidants vitamin C, vitamin E, and beta-carotene from food sources with endometrial cancer risk and point to the need to evaluate their potential protective effect in well-designed large cohort studies. As supplemental sources of these antioxidants are widely used in the US and some other countries, more studies reporting on the effects of supplemental intake would also be helpful to determine whether findings from food and supplemental sources are congruent. Furthermore, little is known regarding the interaction between antioxidants and other lifestyle factors. Based on the current literature, the 2007 WCRF/AICR grading of the evidence for the associations of vitamin C, vitamin E, and beta-carotene intakes with endometrial cancer risk of “limited-no conclusion” remains appropriate.
ACKNOWLEDGEMENTS
We would like to thank James Thomas for his valuable help with the data extraction access program. This work was funded in part by the WCRF and by the National Cancer Institute (NIH-K07 CA095666 to Dr. Bandera). However, interpretation of the evidence does not represent the views of WCRF or the NCI.
Abbreviations
- WCRF
World Cancer Research Fund International
- AICR
American Institute for Cancer Research
- SLR
Systematic Literature Review
- OR
Odds Ratio
- RR
Relative Risk
- CI
Confidence Interval
- FFQ
food frequency questionnaire
- BMI
body mass index
- HRT
hormone replacement therapy
REFERENCES
- 1.Cancer Facts and Figures: American Cancer Society. 2008 [Google Scholar]
- 2.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington, DC: American Cancer Institute for Cancer Research; 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Second Report on Food, Nutrition, Physical Activity and the Prevention of Cancer. World Cancer Research Fund International/American Institute for Cancer Research; 2007. The association between food, nutrition, and physical activity and the risk of endometrial cancer and underlying mechanisms. [Google Scholar]
- 5.World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington, DC: American Cancer Institute for Cancer Research; 1997. [DOI] [PubMed] [Google Scholar]
- 6.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Fruits and vegetables and endometrial cancer risk: a systematic literature review and meta-analysis. Nutr Cancer. 2007;58:6–21. doi: 10.1080/01635580701307929. [DOI] [PubMed] [Google Scholar]
- 7.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Consumption of animal foods and endometrial cancer risk: a systematic literature review and meta-analysis. Cancer Causes Control. 2007;18:967–988. doi: 10.1007/s10552-007-9038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Dietary lipids and endometrial cancer: the current epidemiologic evidence. Cancer Causes Control. 2007;18:687–703. doi: 10.1007/s10552-007-9021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Association between dietary fiber and endometrial cancer: a dose-response meta-analysis. Am J Clin Nutr. 2007;86:1730–1737. doi: 10.1093/ajcn/86.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women's Health Study. J Natl Cancer Inst. 1999;91:2102–2106. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 11.Jain MG, Rohan TE, Howe GR, Miller AB. A cohort study of nutritional factors and endometrial cancer. Eur J Epidemiol. 2000;16:899–905. doi: 10.1023/a:1011012621990. [DOI] [PubMed] [Google Scholar]
- 12.Knekt P, Aromaa A, Maatela J, et al. Serum vitamin A and subsequent risk of cancer: cancer incidence follow-up of the Finnish Mobile Clinic Health Examination Survey. Am J Epidemiol. 1990;132:857–870. doi: 10.1093/oxfordjournals.aje.a115728. [DOI] [PubMed] [Google Scholar]
- 13.Goodman MT, Hankin JH, Wilkens LR, et al. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Res. 1997;57:5077–5085. [PubMed] [Google Scholar]
- 14.Goodman MT, Wilkens LR, Hankin JH, Lyu LC, Wu AH, Kolonel LN. Association of soy and fiber consumption with the risk of endometrial cancer. Am J Epidemiol. 1997;146:294–306. doi: 10.1093/oxfordjournals.aje.a009270. [DOI] [PubMed] [Google Scholar]
- 15.Shu XO, Zheng W, Potischman N, et al. A population-based case-control study of dietary factors and endometrial cancer in Shanghai, People's Republic of China. Am J Epidemiol. 1993;137:155–165. doi: 10.1093/oxfordjournals.aje.a116655. [DOI] [PubMed] [Google Scholar]
- 16.Potischman N, Swanson CA, Brinton LA, et al. Dietary associations in a case-control study of endometrial cancer. Cancer Causes Control. 1993;4:239–250. doi: 10.1007/BF00051319. [DOI] [PubMed] [Google Scholar]
- 17.Jain MG, Howe GR, Rohan TE. Nutritional factors and endometrial cancer in Ontario, Canada. Cancer Control. 2000;7:288–296. doi: 10.1177/107327480000700312. [DOI] [PubMed] [Google Scholar]
- 18.McCann SE, Freudenheim JL, Marshall JR, Brasure JR, Swanson MK, Graham S. Diet in the epidemiology of endometrial cancer in western New York (United States) Cancer Causes Control. 2000;11:965–974. doi: 10.1023/a:1026551309873. [DOI] [PubMed] [Google Scholar]
- 19.Terry P, Vainio H, Wolk A, Weiderpass E. Dietary factors in relation to endometrial cancer: a nationwide case-control study in Sweden. Nutr Cancer. 2002;42:25–32. doi: 10.1207/S15327914NC421_4. [DOI] [PubMed] [Google Scholar]
- 20.Tzonou A, Lipworth L, Kalandidi A, et al. Dietary factors and the risk of endometrial cancer: a case--control study in Greece. Br J Cancer. 1996;73:1284–1290. doi: 10.1038/bjc.1996.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu WH, Dai Q, Xiang YB, et al. Nutritional factors in relation to endometrial cancer: a report from a population-based case-control study in Shanghai, China. Int J Cancer. 2007;120:1776–1781. doi: 10.1002/ijc.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Vecchia C, Decarli A, Fasoli M, Gentile A. Nutrition and diet in the etiology of endometrial cancer. Cancer. 1986;57:1248–1253. doi: 10.1002/1097-0142(19860315)57:6<1248::aid-cncr2820570631>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Negri E, La Vecchia C, Franceschi S, Levi F, Parazzini F. Intake of selected micronutrients and the risk of endometrial carcinoma. Cancer. 1996;77:917–923. doi: 10.1002/(sici)1097-0142(19960301)77:5<917::aid-cncr17>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Levi F, Franceschi S, Negri E, La Vecchia C. Dietary factors and the risk of endometrial cancer. Cancer. 1993;71:3575–3581. doi: 10.1002/1097-0142(19930601)71:11<3575::aid-cncr2820711119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Barbone F, Austin H, Partridge EE. Diet and endometrial cancer: a case-control study. Am J Epidemiol. 1993;137:393–403. doi: 10.1093/oxfordjournals.aje.a116687. [DOI] [PubMed] [Google Scholar]
- 26.Salazar-Martinez E, Lazcano-Ponce E, Sanchez-Zamorano LM, Gonzalez-Lira G, Escudero-De los Rios P, Hernandez-Avila M. Dietary factors and endometrial cancer risk. Results of a case-control study in Mexico. Int J Gynecol Cancer. 2005;15:938–945. doi: 10.1111/j.1525-1438.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 27.Rothman KJ. Modern epidemiology. 1st ed. Boston: Little, Brown; 1986. pp. 174–175. [Google Scholar]
- 28.Chene G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996;144:610–621. doi: 10.1093/oxfordjournals.aje.a008971. [DOI] [PubMed] [Google Scholar]
- 29.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 30.Burke TW, Eifel PJ, Muggia FM. Cancer of the uterine body. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer : principles & practice of oncology. 6th ed. Philadelphia: Lippincott; 2001. [Google Scholar]
- 31.Bendich A. From 1989 to 2001: What Have We Learned About the "Biological Actions of Beta-Carotene"? J Nutr. 2004;134:225S–230S. doi: 10.1093/jn/134.1.225S. [DOI] [PubMed] [Google Scholar]
- 32.Krinsky NI. Effects of carotenoids in cellular and animal systems. Am J Clin Nutr. 1991;53:238S–246S. doi: 10.1093/ajcn/53.1.238S. [DOI] [PubMed] [Google Scholar]
- 33.Chew BP, Park JS. Carotenoid Action on the Immune Response. J Nutr. 2004;134:257S–261S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 34.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 35.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 36.Lee KW, Lee HJ, Surh YJ, Lee CY. Vitamin C and cancer chemoprevention: reappraisal. Am J Clin Nutr. 2003;78:1074–1078. doi: 10.1093/ajcn/78.6.1074. [DOI] [PubMed] [Google Scholar]
- 37.Duarte TL, Lunec J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005;39:671–686. doi: 10.1080/10715760500104025. [DOI] [PubMed] [Google Scholar]
- 38.Seifried HE, McDonald SS, Anderson DE, Greenwald P, Milner JA. The antioxidant conundrum in cancer. Cancer Res. 2003;63:4295–4298. [PubMed] [Google Scholar]
- 39.Lunec J, Halligan E, Mistry N, Karakoula K. Effect of vitamin E on gene expression changes in diet-related carcinogenesis. Ann N Y Acad Sci. 2004;1031:169–183. doi: 10.1196/annals.1331.016. [DOI] [PubMed] [Google Scholar]
- 40.Beharka A, Redican S, Leka L, Meydani SN. Vitamin E status and immune function. Methods Enzymol. 1997;282:247–263. doi: 10.1016/s0076-6879(97)82112-x. [DOI] [PubMed] [Google Scholar]