Abstract
To explore the role of a novel Obg-like ATPase 1 (OLA1) in cancer metastasis, small interference RNA (siRNA) was used to knockdown the protein, and the cells were subjected to in vitro cell migration and invasion assays. Knockdown of OLA1 significantly inhibited cell migration and invasion in breast cancer cell line MDA-MB-231. The knockdown caused no changes in cell growth but affected ROS production. In wound-healing assays, decreased ROS in OLA1-knockdown cells were in situ associated with the cells’ decreased motile morphology. Further, treatment of N-acetylcysteine, a general ROS scavenger, blunted the motility and invasiveness of MDA-MB-231 cells, similar to the effect of OLA1-knockdown. These results suggest that knockdown of OLA1 inhibits breast cancer cell migration and invasion through a mechanism that involves the modulation of intracellular ROS levels.
Keywords: Reactive oxygen species, Cell migration, Cancer metastasis, RNA interference
INTRODUCTION
The majority of cancer deaths are directly attributable to metastasis, the spread of cancer cells from the primary tumor to distant sites in the body. Disturbingly an estimated 40% of breast cancer patients relapse and develop metastatic disease (Weigelt et al., 2005); approximately 465 000 patients die of metastatic breast cancer annually worldwide (Hortobagyi et al., 2005). Cancer metastasis is a multi-step process involving tumor cell invasion at the primary site, access to the circulation, and colonization at distant sites (Steeg, 2006). Many of these steps depend on acquiring motility of cancer cells, which is driven by cycles of actin polymerization, protrusion at the front of the cell, and retraction at the rear (Olson and Sahai, 2009). Despite complex and redundant pathways that are established in the metastasis process (Wu, 2006), molecular changes underlying key metastatic phenotypes, such as uncontrolled migration, remain poorly understood (Ferraro et al., 2006; Steeg, 2006).
Recently, the role of reactive oxygen species (ROS) as central signaling molecules in metastasis and tumor progression has been demonstrated in experimental model systems (Radisky et al., 2005; Storz, 2005). ROS are continuously generated in cells as byproducts of normal energy metabolism, and are implicated in many physiological processes as well as disease progression including cancer metastasis (Nishikawa, 2008). High levels of ROS, which can be induced by several anti-cancer treatments, are found to suppress tumor metastasis by destroying cancer cells directly or through activation of cell death pathways. Conversely, a number of studies suggest that moderate levels of ROS stimulate cancer cell proliferation, migration, and invasion (Nishikawa, 2008; Wu, 2006). Apparently opposite effects of ROS have been observed. For example, in some cancer cells overproduction of endogenous ROS inhibits migratory activity, whereas depletion of ROS by introducing antioxidants or antioxidant enzymes to the cells stimulates migration (Fini et al., 2008; Shim et al., 2006). Clearly, additional studies are needed to resolve this contradiction and to further determine the mechanisms by which ROS affect cancer cell migration and invasion.
In our previous study, we demonstrated that OLA1, a novel Obg-like ATPase, functions as a negative regulator of the cellular antioxidant response through non-transcriptional/post-translational processes, which is distinct from most known antioxidant systems that depend on transcriptional pathways (Zhang et al., 2009). Knockdown of OLA1 in human cancer cells (HeLa) and non-cancerous cells (Beas2B) renders cells more resistant to oxidizing agents such as tert-butyl hydroperoxide (tBH) and diamide. When challenged with oxidants, OLA1-knockdown cells had decreased production of ROS and less depletion of cellular glutathione and total thiol content. Hence, we postulated that knockdown of OLA1 could have an effect on cancer cell migration and/or invasion. Further, establishing the function of OLA1 would reveal new insights into understanding of metastasis and defining new potential targets for anti-metastasis therapies. In the present study we show that knockdown of OLA1 inhibits migration and invasion of a breast cancer cell line (MDA-MB-231) and this effect is mediated through regulation of ROS level or redox status in the cancer cells.
MATERIALS AND METHODS
Chemicals and cell culture
All chemicals used in this study, except as otherwise indicated, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Human breast cancer cell line MDA-MB-231 (ATCC; Manassas, VA, USA) was grown at 37 °C in Dulbecco’s modification of Eagle’s medium (DMEM) supplemented with 10% (w/v) fetal bovine serum (FBS).
Small interference RNA-mediated gene knockdown
Human OLA1 (NM_013341.3)-specific small interference RNA (siRNAs; on-target plus SMARTpools, No. L-015680-01) and the control siRNA (non-targeting siRNA, No. D-001810-10) were acquired from Dharmacon (Lafayette, CO, USA). Cells seeded in a 6-well plate were transfected with 50 nmol/L siRNA with the DharmaFECT1 siRNA transfection reagent (No. T-2001-03) according to the manufacturer’s instructions.
Intracellular ROS measurement
The measurement was obtained using the molecular probe 5 (and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA; Invitrogen, Carlsbad, CA, USA). Cells were suspended in phosphate buffered saline (PBS) and loaded with carboxy-H2DCFDA (1 μmol/L in phenol-free medium) at 37 °C for 30 min, and then treated with 400 μmol/L diamide for 1 h. Cells were washed with PBS and 2′,7′-dichlorofluorescein (DCF) fluorescence was evaluated by flow cytometry (FACscan, Becton-Dickinson, San Jose, CA, USA) using 492 nm for excitation and 520 nm for emission. A total of 10 000 events were collected for each histogram. For ROS measurement in situ, adherent cells in wound-healing assay were washed by pre-warmed PBS twice, and incubated with 20 μmol/L of carboxy-H2DCFDA in PBS at 37 °C for 15 min. Cells were again washed by PBS, and examined with a fluorescence microscope at fluorescein isothiocyanate (FITC)-channel (OlympusIX81, Olympus, Melville, NJ, USA).
Cytotoxicity/viability assay
Cells were seeded in a 96-well micro-plate at 1×104 cells/well and allowed to grow for a period as indicated in Results. The cells were then treated as indicated. Cell viability was evaluated by the [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (methyl tolyl sulfide, MTS)-based CellTiter 96 Aqueous One Solution Reagent (Promega, Madison, WI) following the manufacturer’s protocol.
Cell cycle detection
Cell cycle was determined by flow cytometry analysis using propidium iodide (PI) staining (BD Pharmingen, San Jose, CA, USA). Cell fluorescence was analyzed using a BD LSR II cytometer. Data were collected from at least 10 000 cells.
Western blot analysis
Immunoblot analysis was performed as previously described (Zhang et al., 2009). Antibody against OLA1 was purchased from Abcam Inc. (Cambridge, MA, USA), and antibody against β-actin from Sigma-Aldrich (St. Louis, MO, USA). Anti-rabbit IgG peroxidase-linked whole antibody and anti-mouse IgG peroxidase-linked whole antibody were purchased from GE Healthcare (Waukesha, WI, USA).
Wound-healing assay
Cells were transfected with OLA1 siRNA or non-targeting siRNA as a control and allowed to grow in 24-well plates until confluent. Cell monolayers were scratched with a sterile micropipette tip and the wound regions were allowed to heal by cell migration. Photographs were taken immediately (“0” time) and repeatedly at the indicated time points. By comparing the width of wound (the average distance between the two wound edges) at each time point with that at “0” time, the migration distance (in μm) was determined and used for assessing cell motility. The image analysis was done with ImageJ (National Institutes of Health, NIH).
Migration assay
The InnoCyte™ 96-well cell migration assay kit (CBA010) was obtained from Calbiochem (San Diego, CA, USA). It is provided in a 96-well format suitable with upper chamber inserts containing a membrane with an 8 μm pore-size at the bottom. The assay was performed following the manufacturer’s instructions. Briefly, cells were collected and washed by PBS twice and seeded in the upper chamber with serum-free medium, while the lower chamber was loaded with medium containing 10% (w/v) FBS or the serum-free medium (negative control). After incubation at 37 °C for 12 h, cells migrated through the membrane and attached to the underside of the upper chamber were collected with the labeling/cell detachment solution and labeled with calcein-acetoxymethyl (AM) dye. The fluorescence was measured at 485 nm/520 nm with a FLUOstar OPTIMA plate reader (BMG LABTECH, Nepean, Canada) and subsequently imaged with Xenogen IVIS200 optical imaging system (Caliper, Hopkinton, MA, USA).
Cell invasion assay
InnoCyte™ laminin-based 96-well cell invasion assay kit (CBA043, Calbiochem, San Diego, CA, USA) was used to evaluate cell invasion activity. This system is similar to the above migration assay; however, a laminin protein layer is coated on the bottom membrane. Invasive cells that can degrade the laminin layer will migrate through and attach to the underside of the membrane. The assay was performed with procedures essentially identical to the migration assay.
Cytoskeleton staining
Cells were transfected with OLA1-specifc siRNA or non-targeting siRNA and subjected to the wound-healing assay as described above. At 12 h post-scratching, cells were fixed with 4% (w/v) paraformaldehyde, permeabilized with 0.1% (w/v) Triton, and then incubated with Phalloidin-F554 (Invitrogen) and 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) for 1 h in dark. Images were captured and analyzed with a fluorescence microscope (Olympus IX81, Center Valley, PA, USA).
RESULTS
Knockdown of OLA1 inhibits MDA-MB-231 cell motility and invasion activity
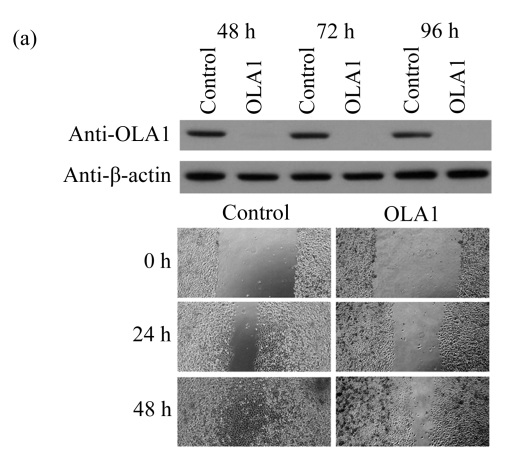
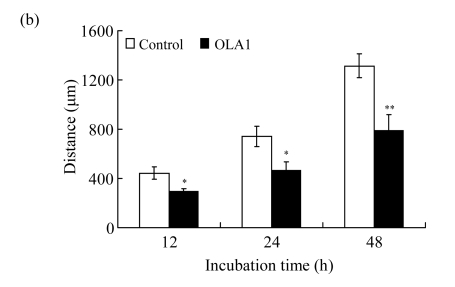
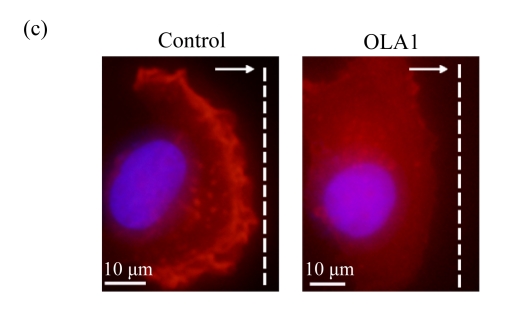
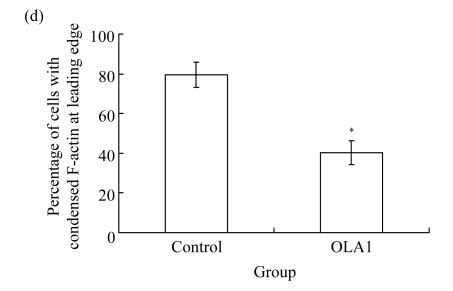
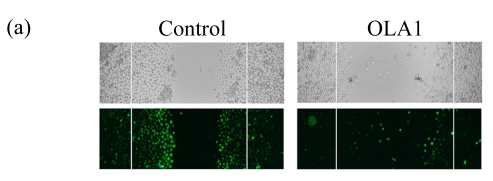
To explore the role of OLA1 in cancer cell migration, MDA-MB-231 cells, a highly metastatic breast cancer adenocarcinoma cell line, were transfected with OLA1-specific Smartpool siRNAs, and analyzed for cell motility and invasion in vitro. The siRNA-mediated knockdown of OLA1 was confirmed by immunoblotting analysis. As shown in Fig.1a, at 48 to 96 h after the siRNA transfection, OLA1 protein was suppressed to a negligible level as compared with the cells transfected with non-targeting siRNA. These transfected cells were first subjected to the wound-healing assay. At 48 h post transfection (“0” time), the monolayer cells were scratched and cell migration into the wound area was followed at various time points. OLA1-knockdown cells exhibited significantly decreased migration rate as compared with the control cells (Figs.1a and 1b). At 48 h after the scratch was made the control cells had fully “healed” the wound by migrating into the space; however, OLA1-knockdown cells migrated only ~60% of the distance, leaving the wound unclosed (Fig.1b). In addition, cells in the wound-healing assay were stained with phalloidin for actin distribution (Fig.1c). When examined at 12 h, 78% of the control cells bordering the wound spot exhibited motile morphologies, with a leading edge that contained condensed F-actin. In contrast, only 42% of the OLA1-knockdown cells bordering the wound site had the F-actin-rich leading edge (Fig.1d). These data suggest that knockdown of OLA1 affects MDA-MB-231 cells on horizontal migratory activity induced by wound.
Fig.1.
Effect of OLA1-knockdown on cell motility and invasion activity
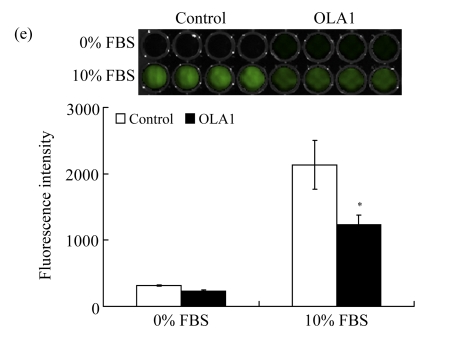
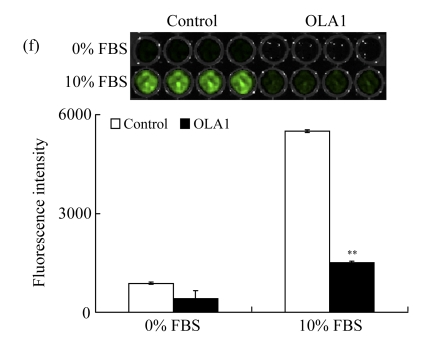
MDA-MB-231 cells were transfected with OLA1-specific siRNAs or the control siRNA for 48 h and then subjected to cell migration and invasion assays. (a) Representative images of cell migration in wound-healing assays. ‘0 h’ indicates the time when the scratch was made at 48 h post-transfection (upper insert). The effectiveness of the knockdown was evaluated by Western blot analysis, and β-actin was used as loading control; (b) Quantitative analysis of the migratory distances in wound-healing assays. The results are displayed as mean±SD of triplicate samples; (c) F-actin distribution in the cells bordering the wound. F-actin was stained with F554-phalloidin (red), while the nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). The white dash line and arrow indicate the wound margin and the direction of cell migration, respectively. Representative images of the control cells (left) and the OLA1-knockdown cells (right) are shown; (d) Quantitative analysis of the cells that present motile morphology with condensed F-actin at the leading edge. Only cells in the first row bordering the wound edge were counted with a minimum of 200 cells per measurement. The results are displayed as mean±SD of triplicate samples; (e) InnoCyte™ cell migration assay (12 h). The green fluorescence intensity (at 485 nm/520 nm) indicates the number of migratory cells. Images of the green fluorescence were taken using an IVIS200 optical imaging system. The results are displayed as mean±SD of triplicate samples; (f) InnoCyte™ laminin-coated cell invasion assay (18 h). The green fluorescence intensity (at 485 nm/520 nm) indicates the number of invasive cells. The green fluorescence signals was imaged using the IVIS200 optical imaging system. The results are displayed as mean±SD of triplicate samples. * P<0.05; ** P<0.01, as compared with the control cells
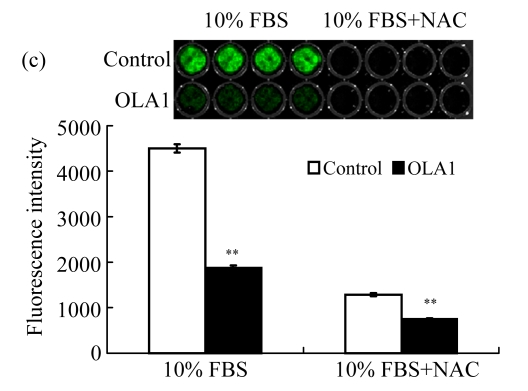
Next, we examined the motility of OLA1-knockdown cells with InnoCyte™ cell migration assay, a measurement of serum-induced transwell migration. In the assay migrated cells were collected and stained with calcein-AM solution to produce green fluorescence. As shown in Fig.1e, with the presence of serum in the lower chamber, considerable fluorescent signals were produced in both the OLA1-knockdown and the control cells. However, the total signals of OLA1-knockdown cells were significantly lower than those of the control cells, only 57% of the latter, which indicates a decreased rate of serum-induced migration in the OLA1-knockdown cells.
Furthermore, the invasion ability of OLA1-knockdown cells was evaluated with InnoCyte™ laminin-coated cell invasion assay. The mechanism is similar to the cell migration assay, but one layer of laminin is coated on the bottom of the membrane of the upper chamber, resembling the Matrigel-based invasion assay. The results are summarized in Fig.1f. The OLA1-knockdown cells showed a substantial reduction in invasion ability; the number of cells that penetrated the laminin layer and passed through the bottom membrane was only 27% of the controls. These experiments indicate that knockdown of OLA1 in MDA-MB-231 cells inhibits cell motility and invasion activity in vitro.
Knockdown of OLA1 has no effect on cell growth but inhibits ROS production
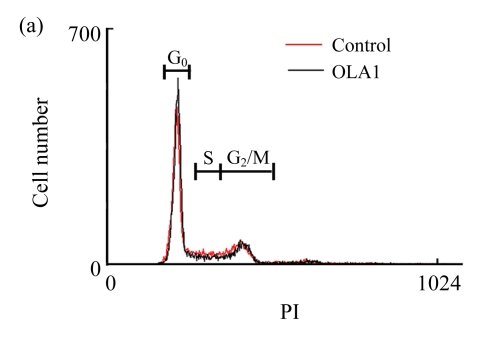
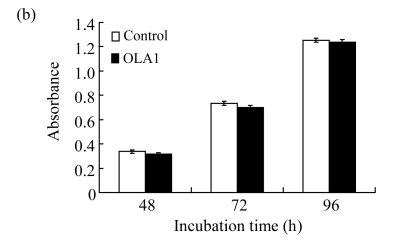
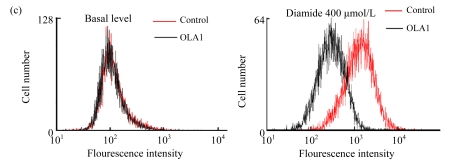
In order to define a mechanistic explanation for these findings, we first tested if OLA1-knockdown would affect cell growth and viability. The siRNA transfected cells were grown for 48 h and then stained with PI for cell cycle analysis by flow cytometry. No significant differences in cell cycle were found between the OLA1-knockdown and the control cells (Fig.2a). Cell proliferation was determined with an MTS-based assay. As shown in Fig.2b, OLA1-knockdown cells exhibited proliferative activities comparable to the control cells between 48 to 96 h post-transfection, the time frame within which the above migration and invasion assays were carried out (“0” h to 48 h). Therefore, the inhibition of cell migration and invasion in OLA1-knockdown cells cannot be explained by either reduction of cell proliferation or loss of cell viability. These results are consistent with our recent analysis using different cell lines, e.g., HeLa, in which knockdown of OLA1 caused no changes in cell growth and baseline cell death (Zhang et al., 2009). However, OLA1-knockdown cells were found to have reduced intracellular ROS production and to be more resistant to thiol-depleting agents such as diamide (Zhang et al., 2009). Following these observations, we investigated the role of OLA1 in ROS production in MDA-MB-231 cells. As shown in Fig.2c, ROS production in OLA1-knockdown cells and the control cells was measured by DCFDA staining and flow cytometry analysis. No differences of basal ROS levels were detected between these cell groups. However, when treated with diamide, OLA1-knockdown cells generated significantly less ROS when compared with the control cells, as indicated by an attenuated right-shift in DCF signals (Fig.2c).
Fig.2.
Knockdown of OLA1 has no effect on cell growth but inhibits ROS production
(a) Cell cycle analysis: 48 h after transfection with the control siRNA or the OLA1 siRNA, the cells were stained with PI and analyzed by flow cytometry. Histograms shown are representative from 3 independent experiments. Phases of the cell cycle are indicated above the histograms; (b) Cell growth rate as monitored by MTS assay following the siRNA transfection. At the indicted time periods post-transfection, cells were incubated with MTS for 2 h, and measured for absorbance at 540 nm. Data are represented as mean±SD from 3 independent experiments; (c) Knockdown of OLA1 attenuated the production of ROS. Unchallenged cells (baseline) and cells treated with 400 μmol/L of diamide for 1 h were subjected to carboxy-H2DCFDA labeling and flow cytometry analysis. Histograms shown are representative from 3 independent experiments
ROS suppression mediates the effect of OLA1-knockdown on cell motility
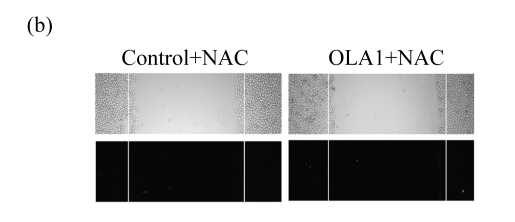
We then hypothesized that the decreased cell motility in OLA1-knockdown cells is mediated by the reduction of intracellular ROS. In order to confirm the direct involvement of ROS in cell migration, we performed wound-healing assays in which cellular ROS were visualized in situ during cell migration. Confluent monolayers of siRNA-transfected MDA-MD-231 cells were scratched, and the cultures were followed by DCFDA staining to monitor the production of ROS. As expected (Ikeda et al., 2005; Moldovan et al., 2000), in the control cultures strong DCF fluorescence signals were observed in the cells flanking the wound margins, with the highest signals in the cells with the most motile activity. The signals decreased gradually in the direction away from the wound sites (Fig.3a). However, in the OLA1-knockdown cultures, only a few DCF-positive cells were found scattered in the wound margins, and no gradient of DCF fluorescence signals could be identified. We confirmed that the DCF fluorescence signals represented the levels of intracellular ROS, since co-treatment of the cultures with N-acetylcysteine (NAC) abolished the signal (Fig.3b).
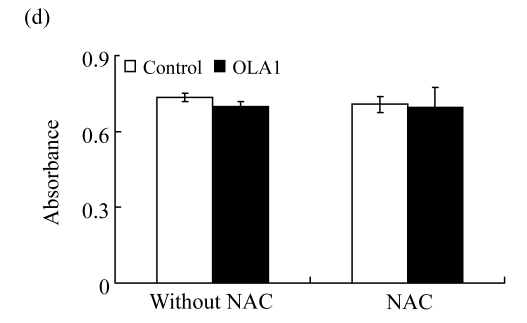
Fig.3.
ROS production during cell migration and its inhibition by NAC
(a) ROS production during wound-induced cell migration. Twelve hours after the wounding, cells were labeled with carboxy-H2DCFDA and examined under a fluorescence microscope. The green fluorescence signal indicates the accumulation of intracellular ROS. Both bright field images (top panel) and fluorescence images (bottom panel) are shown; (b) ROS production in “wound-healing” cultures treated with NAC. Cultures were pre-treated with 5 mmol/L NAC for 1 h, and then subjected to wound-healing assay in the presence of NAC, followed with ROS visualization. Images shown are representative of 3 independent experiments; (c) Effect of NAC on cell invasion. InnoCyte™ laminin-coated cell invasion assay was conducted with or without the presence of NAC (5 mmol/L) for 18 h. The green fluorescence intensity indicates the number of invasive cells. Green fluorescence signals were imaged using an IVIS200 optical imaging system. The results are displayed as mean±SD of triplicate samples; (d) The effect of NAC on cell growth as determined by MTS assay. OLA1-knockdown and the control cells were incubated with 5 mmol/L NAC for 24 h, and then subjected to MTS assay. ** P<0.01, as compared with the control cells
We sought to determine whether the decrease in ROS production was a cause or a result of cell migration. NAC has been widely used as a general antioxidant to neutralize ROS (Pendyala et al., 2008). The NAC-induced decreases in ROS levels have been attributed to the decreased motility in human endothelial cells (Ikeda et al., 2005; Moldovan et al., 2000) and rat fibroblast cells expressing Ras (Alexandrova et al., 2006). As summarized in Figs.3a and 3b, when the MDA-MD-231 cells were incubated with NAC (5 mmol/L), the treatment not only abolished the wound-induced ROS formation, but also significantly inhibited the cell migration in both the control and OLA1-knockdown cells. Moreover, when NAC (5 mmol/L) was included in the cell invasion assays, the MDA-MB-231 cells exhibited dramatic decreases in invasiveness (Fig.3c). The control-transfected cells showed a 72% reduction in invasion, to a level that was intermediate between those in the OLA1-knockdown cells without NAC treatment and in the cells treated with NAC (Fig.3c). The effect of NAC on cell migration and invasion was unlikely due to its toxicity to the cells, since no loss of cell viability was detected in MDA-MB-231 cells treated with NAC at the same dose for 24 h (Fig.3d). These studies suggest that suppression of ROS production can be a causal effect that leads to the inhibition of cell motility and invasion activity.
DISCUSSION
We report here that knockdown of OLA1, a newly discovered regulatory protein of oxidative stress response, inhibits cell migration and invasion ability in breast cancer MDA-MB-231 cells as verified by 3 complimentary but distinct in vitro assays: wound-healing (Fig.1a), transwell migration (Fig.1e), and invasion assay (Fig.1f). We also found that the inhibition is not due to loss of cell viability or proliferation, but is associated with decreased production of ROS (Figs.2 and 3). Although excessive ROS are toxic, moderate levels of ROS can serve as signaling intermediates, regulating many normal and pathological cellular processes including growth, immune defense, and migratory response (Valko et al., 2007). Despite apparently conflicting reports on whether ROS enhances or decreases cell migration, our study supports a positive role of ROS in cell motility. We demonstrated that the decreased levels of ROS in OLA1-knockdown cells, as measured by flow cytometry (Fig.2c) and visualized in situ in a wound-healing assay (Fig.3a), are well correlated with the decreased motility and invasion activity in these cells. Based on the observation that treatment with NAC, a scavenger of ROS, largely impairs the cell motility and invasiveness (Fig.3), as well as prior reports in the literature that presented similar NAC effects (Huo et al., 2009; Pendyala et al., 2008), we reason that decreased ROS in OLA1-knockdown cells can be a causative mechanism underlying the migration/invasion impairment. On the other hand, the mechanism by which knockdown of OLA1 alters intracellular ROS has been previously proposed (Zhang et al., 2009). OLA1 may function through post-translational pathways to down-regulate antioxidant enzymes, likely the enzymes responsible for thiol redox balance. Alternatively, it may interact with these enzymes as a suppressive co-factor or inhibitor. Consequently, the knockdown of OLA1 results in an enhancement of ROS scavenging and other antioxidant activities.
In wound-healing assays (Fig.1), the OLA1-knockdown cultures decreased the number of motile cells at the wound margins. Cells with “motile morphologies” are characterized with a leading edge containing condensed F-actin (Fig.1c), in which active actin polymerization and nucleation occur. Recent studies suggested that actin cytoskeleton reorganization, which is the “driving force” for cell migration, can be positively regulated by intracellular ROS (Moldovan et al., 2000). ROS may interact with actin directly by facilitating the exposure of new barbed ends, thus promoting the actin polymerization. ROS may also regulate the actin dynamics indirectly by interacting with a number of actin-binding proteins including gelsolin, cofilin, IQGAP, and FAK (Moldovan et al., 2006). In our present study, it is likely that in OLA1-knockdown cells the decreased ROS levels affect the actin polymerization, leading to retarded migration and invasion (Figs.2 and 3).
Our present study suggests that inhibition of cancer migration and invasion could be achieved by suppression of OLA1. This finding sheds light on a new therapeutic opportunity to battle metastatic diseases. As reported previously (Gradia et al., 2009; Koller-Eichhorn et al., 2007), OLA1 contains a guanine nucleotide-binding domain (G domain) and belongs to the p-loop ATPase family. Members of this family have been identified as drug targets (Chène, 2002). However, animal models of metastasis are required to clearly demonstrate the effect of OLA1-knockdown on cancer cells in vivo. Moreover, protein assays are needed to validate the druggability of OLA1. We believe that studies on OLA1 function, especially on its interactions with other signaling molecules or effector proteins, will benefit the development of therapeutic strategies based on targeting OLA1 or its downstream proteins.
Acknowledgments
We thank Drs. Daniel LEE and Brandi MATTSON, the Methodist Hospital Institute, USA, for help in the preparation of the manuscript.
Footnotes
Project supported by the National Basic Research Program (973) of China (No. 2004CB518707) and the Methodist Hospital Research Institute, USA
References
- 1.Alexandrova AY, Kopnin PB, Vasiliev JM, Kopnin BP. ROS up-regulation mediates Ras-induced changes of cell morphology and motility. Exp Cell Res. 2006;312(11):2066–2073. doi: 10.1016/j.yexcr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Chène P. ATPases as drug targets: learning from their structure. Nat Rev Drug Discov. 2002;1(9):665–673. doi: 10.1038/nrd894. [DOI] [PubMed] [Google Scholar]
- 3.Ferraro D, Corso S, Fasano E, Panieri E, Santangelo R, Borrello S, Giordano S, Pani G, Galeotti T. Pro-metastatic signaling by c-Met through RAC-1 and reactive oxygen species (ROS) Oncogene. 2006;25(26):3689–3698. doi: 10.1038/sj.onc.1209409. [DOI] [PubMed] [Google Scholar]
- 4.Fini MA, Orchard-Webb D, Kosmider B, Amon JD, Kelland R, Shibao G, Wright RM. Migratory activity of human breast cancer cells is modulated by differential expression of xanthine oxidoreductase. J Cell Biochem. 2008;105(4):1008–1026. doi: 10.1002/jcb.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gradia DF, Rau K, Umaki AC, de Souza FS, Probst CM, Correa A, Holetz FB, Avila AR, Krieger MA, Goldenberg S, et al. Characterization of a novel Obg-like ATPase in the protozoan Trypanosoma cruzi . Int J Parasitol. 2009;39(1):49–58. doi: 10.1016/j.ijpara.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, Hudis CA, Khaled H, Liu MC, Martin M, Namer M, et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6(5):391–401. doi: 10.3816/CBC.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 7.Huo Y, Qiu WY, Pan Q, Yao YF, Xing K, Lou MF. Reactive oxygen species (ROS) are essential mediators in epidermal growth factor (EGF)-stimulated corneal epithelial cell proliferation, adhesion, migration, and wound healing. Exp Eye Res. 2009 doi: 10.1016/j.exer.2009.07.012. in press [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol. 2005;25(11):2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 9.Koller-Eichhorn R, Marquardt T, Gail R, Wittinghofer A, Kostrewa D, Kutay U, Kambach C. Human OLA1 defines an ATPase subfamily in the Obg family of GTP-binding proteins. J Biol Chem. 2007;282(27):19928–19937. doi: 10.1074/jbc.M700541200. [DOI] [PubMed] [Google Scholar]
- 10.Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86(5):549–557. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- 11.Moldovan L, Mythreye K, Goldschmidt-Clermont PJ, Satterwhite LL. Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc Res. 2006;71(2):236–246. doi: 10.1016/j.cardiores.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008;266(1):53–59. doi: 10.1016/j.canlet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009;26(4):273–287. doi: 10.1007/s10585-008-9174-2. [DOI] [PubMed] [Google Scholar]
- 14.Pendyala S, Gorshkova IA, Usatyuk P, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. 2008;11(4):747–764. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436(7047):123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shim H, Shim E, Lee H, Hahn J, Kang D, Lee YS, Jeoung D. CAGE, a novel cancer/testis antigen gene, promotes cell motility by activation ERK and p38 MAPK and downregulating ROS. Mol Cells. 2006;21(3):367–375. [PubMed] [Google Scholar]
- 17.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 18.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10(1-3):1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 19.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Weigelt B, Peterse JL, van′t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 21.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25(4):695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Rubio V, Lieberman MW, et al. OLA1, an Obg-like ATPase, suppresses antioxidant response via nontranscriptional mechanisms. Proc Natl Acad Sci USA. 2009;106(36):15356–15361. doi: 10.1073/pnas.0907213106. [DOI] [PMC free article] [PubMed] [Google Scholar]