Summary
Microparticles (MP) are lipid vesicles from platelets, leukocytes and endothelial cells that are involved in early thrombogenesis. We evaluated a detailed time-course analysis of MPs on thrombogenesis and the associated tissue factor (TF) activity in wild-type, in gene-deleted for E- and P-selectins and with high levels of P-selectin expression after the initiation of venous thrombosis in mice. Inferior vena cava (IVC) ligation was performed on C57BL/6 mice (n =191, 59 = wild-type [WT], 55 = gene-deleted for E- and P – selectins [knock-outs, EPKO] and 77 = elevated levels of soluble P-selectin, named Delta Cytoplasmic Tail (ΔCT). Animals were euthanised at various time points to assess MP production, origin and thrombus weight. MPs were re-injected into separate mice at concentrations of 80,000 and 160,000 units, as well as from different ages. In addition, MPs from thrombosed animals were pooled and TF activity quantitated using a chromogenic assay. Thrombus weight correlated negatively with MPs derived from leukocytes, and positively with MPs derived from platelets for WT animals (p<0.05), while MPs from platelets presented a positive correlation to thrombus weight in the WT and EPKO groups (p<0.01). Total MPs correlated negatively with thrombus weight in the ΔCT group (p<0.05). MP re-injections led to greater thrombus weight, while older MP reinjections tended to form larger thrombus than younger. Finally, TF bearing MPs showed a significant correlation to MP concentrations (R=0.99). In conclusion, MPs appear to be an important element in venous thrombogenesis.
Keywords: Venous thrombosis, inflammation, microparticles, plasminogen, tissue factor, tissue factor activity, animal models
Introduction
For the past 30 years, through the pioneering theories of Gwen-dylen Stewart (1) a relationship between thrombosis and inflammation was suggested, adding new insights to the pathogenesis of deep venous thrombosis (DVT) based on Virchow's triad of stasis, changes in the vessel wall and thrombogenic changes in the blood (2). Stasis by itself, although an important factor, is usually not enough to produce thrombosis.
Inflammation and thrombosis are interrelated. For example, inflammation increases tissue factor (TF), platelet reactivity, fibrinogen, and leads to exposure of increased levels of phosphotidylserine, while decreasing thrombomodulin (TM) and inhibiting fibrinolysis (by increasing plasminogen activation inhibitor-1 [PAI-1]) (3). Cell adhesion molecules (CAMs) allow leukocyte transmigration, and selectins (P- and E-selectin) are integrally involved in thrombosis. Selectins are the first upregulated glycoproteins on activated endothelial cells and platelets. The cell adhesion molecule P-selectin has been found upregulated in the vein wall as early as six hours after thrombus induction, whereas E-selectin has been found upregulated at day 6 after thrombosis (4).
Microparticles (MPs) are small (less than 1 μm, about the size of a bacterium), phospholipid vesicles shed from platelets, leukocytes, and endothelial cells in a calcium dependent fashion (5-7). Recent studies suggest that procoagulant MP formation plays an integral part in the inflammatory component of venous thrombosis, leading to thrombus amplification. MPs are a normal constituent of blood and can be isolated from plasma by ultracentrifugation. MPs lack DNA, but recent evidence suggests they may carry RNA (8), and are protein-rich. Subpopulations of MPs rich in TF and phosphatidylserine have been identified (9, 10). Rafts and raft-derived MPs can concentrate TF in cavaolae where it is stored with tissue factor pathway inhibitor (TFPI) (10), an inhibitor of TF release. Fusion of MPs with activated platelets results in decryption of TF and the initiation of thrombosis (11).
Low levels of MPs are found circulating in a normal physiological state and have been shown to increase with disease (10). Procoagulant MPs, derived more from activated leukocytes and less from activated platelets are recruited to the area of thrombosis, where they amplify coagulation via tissue factor and the extrinsic pathway. The co-localisation of fibrin, platelets, and leukocytes in the developing thrombus and the importance of P-selectin-mediated leukocyte-platelet interactions to the generation of tissue factor points out the central role of inflammation in venous thrombogenesis (4). The purpose of this study was: 1) to document a detailed time-course analysis of MPs after the experimental induction of venous thrombosis; 2) to determine the thrombogenicity of MPs by re-injecting MPs obtained from thrombosed animals into other animals undergoing inferior vena cava (IVC) occlusion; 3) to determine the effect of MP age on their thrombogenicity; and 4) to assess MP-associated TF expression. This was a continuation of our previous studies with MPs in which we evaluated wild-type (WT), E-selectin and P-selectin gene-deleted (EPKO) and animals with elevated levels of soluble (s) P-selectin (ΔCT) animals (4).
Material and methods
The experiment consisted of three phases:
Phase 1: Natural history of MPs. Mouse microparticle assay
C57BL/6 mice were anesthetised with isofluorane (1.5–2%), and IVC ligation was performed. This is our standard model for studying venous thrombosis in mice. Animal groups included wild types (WT, n = 59; Harlan, Indianapolis, IN, USA), and genetically modified either with deleted E- and P-selectins, the Eand P-selectin knock-outs (EPKO, n = 55; Daniel Bullard, University of Alabama, AL, USA) (12, 13) or with elevated levels of soluble P-selectin, the ΔCT mouse (ΔCT, n =77; Denisa Wagner, Cambridge, MA, USA) (14, 15). The ΔCT mouse demonstrates four-fold elevation in circulating soluble P-selectin. Animals were euthanised post ligation at 90 seconds (sec), 15 minutes (min), 30 min, 60 min, 90 min, 2 hours (h), 3 h, 1 day and 2 days. At euthanasia, animals had blood withdrawn using a syringe primed with 10% acid citrate dextrose (ACD) with a cardiac puncture, their IVC was removed, and thrombus weight was determined.
MPs were prepared from the blood samples. Platelet-poor plasma (PPP) was obtained by centrifuging blood at 1,500g and 23°C for 25 min and centrifuging once more for 2 min at 15,000g to remove contaminating cells from the plasma. PPP (200 μl) obtained from each mouse was diluted 1:3 with 600 μl of HEPES buffer [1 mM HEPES, 136 mM NaCl, 5 mM MgCl2, 50 mM KCl (pH 7.4)]. All samples were centrifuged for 2 h at 200,000g to separate the MPs. The supernatant was removed, and pelleted MP resuspended in HEPES buffer (pH 7.4). Antibodies that stain leukocytes and platelets were added to samples and included rat anti-murine PE CD11b (stains leukocytes) 48 μg/ml (Chemicon International, Temecula, CA, USA) and rat anti-murine FITC CD41 (stains platelets) 4 μg/ml (BD Pharmingen, San Diego, CA, USA). Unstained MPs were used for negative control and antibody-binding beads (Pharmingen, San Diego, CA, USA) used as positive controls for flourochromes. Test sample and antibodies were then mixed and incubated on ice for 20 min. Samples were centrifuged for 2 h at 200,000g, washed with phosphate-buffered saline (PBS), fixed with 0.5% paraformaldehyde, and stored at 4°C for fluorescence-activated cell scanning (FACS) analysis. To count the total MPs population, 25 μl of SPHERO Rainbow calibration 3.4 μm beads (approximately 250,000 beads) were added to tubes prior to FACS. Bead events (5,000) were counted and the total MPs determined by multiplying the MP events by 50. Samples were run on a Becton Dickinson Facscalibur System (Becton Dickinson, San Jose, CA) and analyzed using CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). Due to the variability in the absolute numbers of MP of one FACS run to another, control samples are included in every run, and the MP concentration is indexed from the experimental values to the control values.
Phase 2: MP re-injections
A. Thrombogenicity related to MP concentration and time of sacrifice
MPs were obtained from WT animals ligated and sacrificed 2 days later (n = 20). These MPs were pooled, their concentration determined by flow cytometry, and then re-injected into other animals undergoing the same IVC ligation protocol at either 80,000 MPs (n = 35) or 160,000 MPs (n = 32). These animals were euthanised at 90 sec, 120 min, 1 day or 2 days post-ligation and thrombus weight evaluated. These times represented early, mid and later times from the natural history of MPs (phase 1).
B. Thrombogenicity related to MP age
The same IVC ligation model was performed on C57BL/6 mice (n = 13). Re-injection of MPs (80,000 MPs) of different ages (2 h [n = 4] and 2 days [n = 9] ) were obtained from a pool of other animals, and then comparative thrombus weight evaluation was performed 2 days after ligation.
Phase 3: MP TF activity
TF activity was evaluated ex vivo from MPs obtained from WT (n = 38) animals euthanised 2 days after ligation. MPs were obtained from both thrombosed mice (n = 31) and non-thrombosed but ligated mice (n = 7). The MPs were pooled and TF activity determined using a standard kit (American Diagnostica, Stamford, CT, USA). The assay involved the combination of soluble factor VIIa, human factor X, TF/ TFPI free plasma and the MP sample (spun down as above and pellet resuspended in TF/TFPI free plasma). Activated Factor X hydrolyzed Spectrozyme Xa (American Diagnostica Inc.) in a linear fashion at 37°C and the result read on a spectrophotometer. Enzyme activity was determined by measuring the increase in absorbance of the free chromophore (pNA) generated per unit time at λ405 nm. In the development of this assay, wells without factor VIIa or factor X did not demonstrate significant TF activity (unpublished). We selected doses of 160,000, 80,000, 50,000, 25,000 and 10,000 MPs per reaction well, each sample run in triplicate. The actual assay was performed in its entirety twice and the positive control was a known amount of TF (standard curve), while the negative control was MP free buffer.
Thrombus weight
This technique was used as an indirect measure of thrombus content. At sacrifice, the IVC and its associated thrombus was removed and weighed (wet weight in grams) (4). We have found that the major component of weight is thrombus as opposed to the vein wall tissue itself and in comparing one animal to another or one group to another, the vein wall contribution to weights tends to cancel out.
Statistical analysis
Statistical analysis included Student t-test and correlation analysis (Sigmastat 2.03 Software, Systat Software Inc., San Jose, CA, USA) with an alpha of 5% and confidence interval (CI) of 95%. Bonferroni's test was used to determine the significant differences between group means in the natural history of MPs analysis, and Kruskal-Wallis one-way analysis of variance test was used for MP fold change analysis.
All mice were housed and cared for by the University of Michigan Unit for Laboratory Animal Medicine and were free of pathogens. The University of Michigan Committee on Use and Care of Animals approved this research protocol.
Results
Phase 1
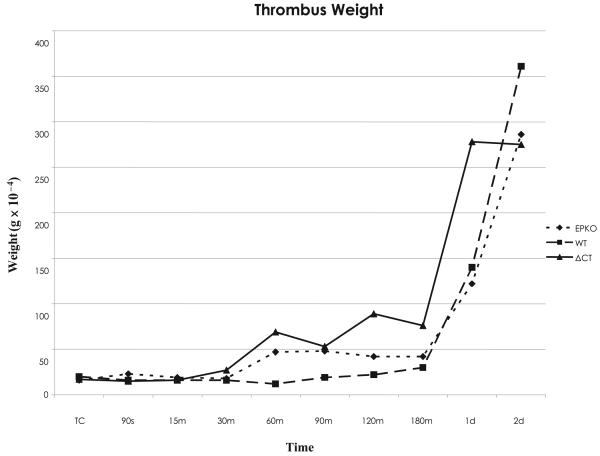
Thrombus formation appeared to accelerate at 180 min in our mouse model, and continued to increase up through day 2. Statistical differences from true controls (TC) were observed on ΔCT animals beginning at 60 min, while for WT differences were not significant until day 1 and for EPKO animals not until day 2 (Fig. 1).
Figure 1. Thrombus weight versus different times in the WT, EPKO and ΔCT groups.
Using Bonferroni's multiple group comparison (p<0.005), for WT animals significant differences from TW began at day 1 after thrombosis, while for EPKO animals significant differences were detected at 90 seconds and two days after thrombosis. For ΔCT mice, significant differences began as early as 60 minutes after thrombosis and continued through day 2.
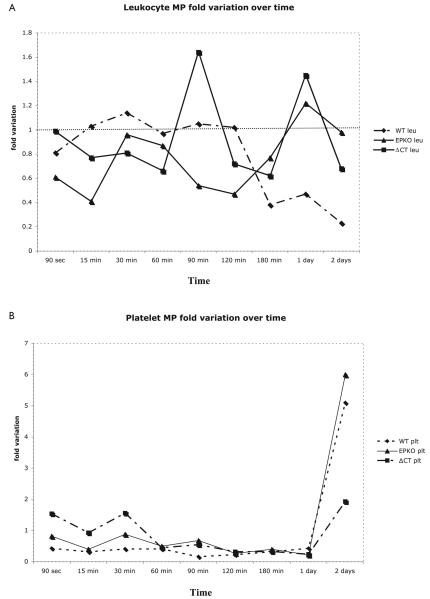
The number of animals used in this phase is listed in Table 1. MPs fold variation is presented in Figure 2A and B. Evaluating all time points, thrombus weight correlated negatively with MPs derived from leukocytes (MPs leu), and positively with MPs derived from platelets (MPs plt) for WT animals (−0.751 [p < 0.05] and 0.942 [p < 0.01]), while MPs plt correlated in a positive fashion to thrombus weight in the EPKO animals (0.897 [p < 0.01]). For the ΔCT mice, MPs not related to leukocytes or platelets and total MPs correlated negatively with thrombus weight (−0.864 [p < 0.01] and − 0.698 [p < 0.05]) (Table 2).
Table1. Total numbers of animals evaluated in phase 1.
| Times | Groups | ||
|---|---|---|---|
| WT | EPKO | ΔCT | |
| TC | 9 | 5 | 11 |
| 90 sec | 5 | 5 | 6 |
| 15 min | 5 | 5 | 6 |
| 30 min | 5 | 5 | 6 |
| 60 min | 5 | 5 | 5 |
| 90 min | 5 | 5 | 5 |
| 120 min | 5 | 5 | 5 |
| 180 min | 5 | 5 | 8 |
| 1 day | 6 | 5 | 10 |
| 2 days | 9 | 10 | 15 |
| Total | 59 | 55 | 77 |
Figure 2. MP variation over time.
A) Fold variation on leukocyte derived MP versus different times on the WT, EPKO and ΔCT group. Using Kruskall-Wallis one-way analysis of variance, on 90 seconds WT versus EPKO versus ΔCT (p<0.031), WT vs. EPKO (NS). B) Fold variation on platelet-derived MP versus different times on the WT, EPKO and ΔCT group. Using Kruskall-Wallis one-way analysis of variance, differences were observed on day 2: WT versus EPKO versus ΔCT (p=0.014), WT versus EPKO (NS), WT versus ΔCT (NS), and EPKO versus ΔCT (p<0.003).
Table 2. Correlations between MPs fold change and thrombus weight on the WT, EPKO and ΔCT groups.
| MPs | Correlation coefficient |
P-value |
|---|---|---|
| WT | ||
| Leukocyte MP | −0.751 | p < 0.05 |
| Platelet MP | 0.942 | p < 0.01 |
| Both + | −0.655 | p = 0.056 |
| Both − | −0.412 | NS |
| Total | 0.110 | NS |
| EPKO | ||
| Leukocyte MP | 0.513 | NS |
| Platelet MP | 0.897 | p< 0.01 |
| Both + | 0.403 | NS |
| Both − | −0.07 | NS |
| Total | −0.486 | NS |
| ΔCT | ||
| Leukocyte MP | 0.165 | NS |
| Platelet MP | 0.151 | NS |
| Both + | 0.160 | NS |
| Both − | −0.864 | p < 0.01 |
| Total | −0.698 | p < 0.05 |
Phase 2A
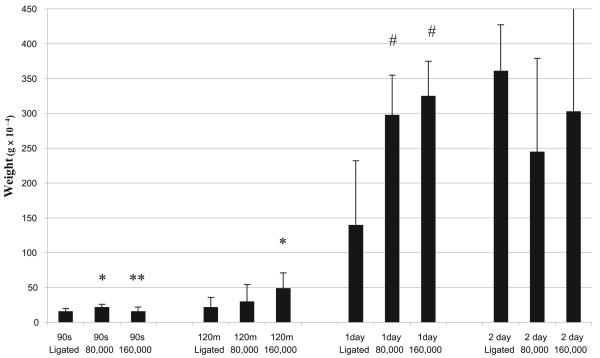
At the 90-sec time point, ligation plus re-injection of 80,000 MPs produced greater thrombus weight, compared to ligation without re-injection and compared to ligation and re-injection of 160,000 MPs (p < 0.05) (Fig. 3). Re-injection of 160,000 MPs produced higher thrombus weight at 120 min (p < 0.05), while both amounts of MP re-injections (80,000 and 160,000) presented highly significant greater thrombus weights on day 1 compared to the non-re-injection animals (p < 0.01). This relationship was not noted at day 2.
Figure 3. Re-injections (80,000 and 160,000 MPs) plus IVC ligation versus ligation alone (ligated) and thrombus weight on 90 sec, 120 min, 1 and 2 day evaluation.
Phase 2B
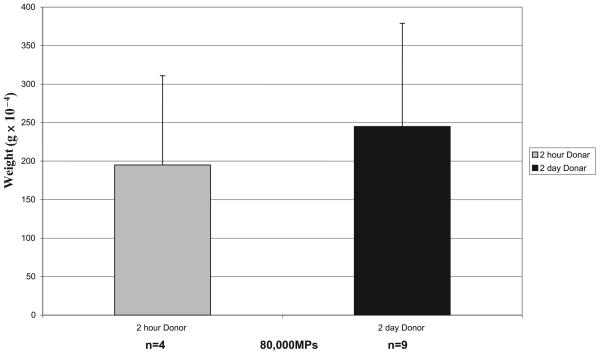
Animals re-injected with older MPs (2 days vs. 2 h) produced greater thrombus weight (245 × 10−4 vs.194 × 10−4 g), an increase of 26%, but not statistically significant (Fig. 4).
Figure 4. Re-injections (80,000 MPs) 2-hour- versus 2-day-old MPs and thrombus weight evaluation.
Differences not significant, although the difference between 2-day donor and 2-hour donor was 26%.
Phase 3
TF activity on MPs derived from thrombosed animals (n = 31) showed a highly significant direct correlation to MP concentrations (R=0.99). MPs from animals with IVC occlusion, but no thrombus (n=7), averaged 30 to 48% less TF activity than MPs from animals with thrombus (Table 3).
Table 3. Tissue factor (TF) activity (expressed in pM) of MPs measured ex vivo (n = 38, clot = 31, non clot = 7).
A) Correlation between MPs and thrombus weight (R = 0.99). B) Animals without thrombus demonstrated a 48% decrease in TF (80,000) and a 30% decrease in TF (160,000).
| Number of MPs | TF (pM) average |
|---|---|
| A | |
| 10,000 | 13.7 |
| 25,000 | 40.5 |
| 50,000 | 75.9 |
| 80,000 | 124.3 |
| 160,000 | 205.2 |
| B | |
| No clot 80,000 | 64.6 |
| No clot 160,000 | 144 |
Discussion
Venous stasis results in the generation of prothrombotic MPs that promote and amplify venous thrombosis (4, 17-19). MPs co-expressing TF and leukocyte markers have been shown to accumulate in growing thrombi (20, 21), and these prothrombotic MPs express TF and possess a phosphatidylserine-rich anionic surface. This surface is capable of assembling coagulation cascade complexes (21). MPs have been found to normalise tail bleeding times in haemophilic mice (22) and human pericardial-derived MPs expressing TF have been demonstrated to increase thrombosis in a rat venous stasis model (23, 24). Additionally, MPs are not only prothrombotic, but also appear to inhibit fibrinolysis (25). In patients with DVT, MPs have been found elevated as well as platelet-leukocyte conjugates (26, 27).
In the current study, in phase 1, WT animals demonstrated that MPs derived from leukocytes correlated negatively with thrombus weight, while MPs derived from platelets correlated positively. MPs derived from platelets also correlated positively with thrombus weight in the EPKO animals, while MPs derived from leukocytes did not correlate to thrombus weight in these animals. Although the observations are indirect, they lend support to the idea that MPs derived from platelets may be a marker for ongoing thrombosis in WT and EPKO animals, while MPs derived from leukocytes are involved in thrombogenesis in the WT animals, suggested by their consumption. In those animals in which both E- and P-selectin were gene deleted (EPKO), it has been previously shown that MPs derived from platelets and not leukocytes are critical in the venous thrombotic thrombotic process (4). This previous work is supported by the present study. In the animals with elevated levels of soluble P-selectin, the total MP population was involved in thrombogenesis as evidenced by its negative correlations to thrombus weight.
What is known about this topic?
– Inflammation and thrombosis are interrelated.
– Microparticles (MPs) are small phospholipid vesicles shed from platelets, leukocytes, and endothelial cells in a calcium-dependent fashion.
– Procoagulant MP formation plays an integral part in the inflammatory component of venous thrombosis, leading to thrombus amplification.
What does this paper add?
– MPs are involved in the thrombogenic process with differences between MPs derived from leukocytes and platelets and differences between wild type animals and animals with either selectin depletion or selectin over-expression.
– Re-injections of MPs plus inferior vena cava (IVC) ligation leads to higher thrombus weight than IVC ligation alone, while older MPs tend to produce higher thrombus weight than younger MPs. This supports the prothrombotic nature of these MPs.
– Tissue factor on MPs correlates to numbers of MPs.
To determine the prothrombotic effect of the MPs, we re-injected these into ligated mice. We found a significant increase in thrombus weight compared to IVC ligation alone, especially prominent at the one day time point after thrombus initiation. This observation supports the prothrombotic role in thrombus amplification by MPs. By day 2, this relationship was no longer noted, likely due to other mechanisms associated with the thrombotic process as the thrombus ages. Additionally, re-injection of older (2 days) MPs tended to induce greater thrombosis than younger (2 hours) MPs, but our data does not support any conclusions due to lack of statistical significance.
Finally, it was found that MP concentration and TF activity directly correlated at a highly significant level (R = 0.99). Importantly, in animals with IVC ligation but no clot, the amount of TF was decreased. These data support the importance of TF associated with MPs in the thrombogenic process (19, 28, 29). Further studies are planned to follow-up the current experiment. In these studies, MPs will be labeled from different origins and observed directly if they are incorporated or consumed into the venous thrombi, in order to confirm what we have inferred indirectly from the correlations noted in the current study.
Conclusions
MPs are involved in the thrombogenic process with differences between MPs derived from leukocytes and platelets and differences between WT animals and animals with either selectin depletion or selectin over-expression.
Re-injections of MPs plus IVC ligation leads to higher thrombus weight than IVC ligation alone, while older MPs tend to produce higher thrombus weight than younger MPs.
TF on MPs correlates to numbers of MPs.
Acknowledgments
Financial support: This study was supported by NIH HL070766, NIH NCRR 5P41RR018627 and State of Michigan, MEDC GR239.
References
- 1.Stewart GJ, Ritchie WGM, Lynch PR. Venous endothelial damage produced by massive sticking and emigration of leukocytes. Am J Pathol. 1974;74:507–532. [PMC free article] [PubMed] [Google Scholar]
- 2.Virchow R. Gesammelte Abhandlungen zur Wissenschaftlichen Medizin. A M Von Meidinger Sohn; Frankfurt: 1856. pp. 525–530. [Google Scholar]
- 3.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1:1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 4.Myers DD, Hawley AE, Farris DM, et al. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38:1075–1089. doi: 10.1016/s0741-5214(03)01033-4. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert GE, Sims PJ, Wiedmer T, et al. Platelet-derived MPs express high affinity receptors for factor VIII. J Biol Chem. 1991;266:17261–17268. [PubMed] [Google Scholar]
- 6.Mesri M, Altieri DC. Endothelial cell activation by leukocyte MPs. J Immunol. 1998;161:4382–4387. [PubMed] [Google Scholar]
- 7.Sabatier F, Roux V, Anfosso F, et al. Interaction of endothelial MPs with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;99:3962–3970. doi: 10.1182/blood.v99.11.3962. [DOI] [PubMed] [Google Scholar]
- 8.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 9.Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez MC, Tesse A, Zobairi F, et al. Shed membrane MPs from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol. 2005;288:H1004–H1009. doi: 10.1152/ajpheart.00842.2004. [DOI] [PubMed] [Google Scholar]
- 11.Sevinsky JR, Rao LV, Ruf W. Ligand-induced pro-tease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J Cell Biol. 1996;133:293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bullard DC, Qin L, Lorenzo I, et al. P-Selectin/ICAM-1 double mutant mice: acute emigration of neutrophils into the peritoneum is completely absent but is normal into pulmonary alveoli. J Clin Invest. 1995;95:1782–1788. doi: 10.1172/JCI117856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullard DC, Kunkel EJ, Kubo H, et al. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med. 1996;183:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andre P, Hartwell D, Hrachovinova I, et al. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci USA. 2000;97:13835–13840. doi: 10.1073/pnas.250475997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartwell DW, Mayadas TN, Berger G, et al. Role of P-selectin cytoplasmic domain in granular targeting in vivo and in early inflammatory responses. J Cell Biol. 1998;143:1129–1141. doi: 10.1083/jcb.143.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osterud B. The role of platelets in decrypting monocyte tissue factor. Semin Hematol. 2001;38(4 Suppl 12):2–5. doi: 10.1016/s0037-1963(01)90139-8. [DOI] [PubMed] [Google Scholar]
- 17.Myers DD, Jr, Rectenwald JE, et al. Decreased venous thrombosis with an oral inhibitor of P- selectin. J Vasc Surg. 2005;42:329–336. doi: 10.1016/j.jvs.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 18.Myers DD, Wakefield TW. Inflammation-dependent thrombosis. Front Biosci. 2005 Sep 1;10:2750–2757. doi: 10.2741/1732. [DOI] [PubMed] [Google Scholar]
- 19.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3:1590–1596. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 20.Giesen PL, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci USA. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jy W, Horstman LL, Wang F, et al. Platelet factor 3 in plasma fractions: its relation to microparticle size and thromboses. Thromb Res. 1995;80:471–482. doi: 10.1016/0049-3848(95)00202-2. [DOI] [PubMed] [Google Scholar]
- 22.Hrachovinová I, Cambien B, Hafezi-Moghadam A, et al. Interaction of P-selectin and PSGL-1 generates MPs that correct hemostasis in a mouse model of hemophilia A. Nat Med. 2003;9:1020–1025. doi: 10.1038/nm899. [DOI] [PubMed] [Google Scholar]
- 23.Biró E, Sturk-Maquelin KN, Vogel GM, et al. Human cell-derived MPs promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–2568. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 24.Podor TJ, Singh D, Chindemi P, et al. Vimentin exposed on activated platelets and platelet MPs localizes vitronectin and plasminogen activator inhibitor complexes on their surface. J Biol Chem. 2002;277:7529–7539. doi: 10.1074/jbc.M109675200. [DOI] [PubMed] [Google Scholar]
- 25.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28:387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- 26.Rectenwald JE, Myers DD, Jr, Hawley AE, et al. D-dimer, P-selectin, and MPs: novel markers to predict deep venous thrombosis. A pilot study. Thromb Hae-most. 2005;94:1312–1317. doi: 10.1160/TH05-06-0426. [DOI] [PubMed] [Google Scholar]
- 27.Chirinos JA, Heresi GA, Velasquez H, et al. Elevation of endothelial MPs, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45:1467–1471. doi: 10.1016/j.jacc.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 28.Rauch U, Nemerson Y. Circulating tissue factor and thrombosis. Current Opin Hematol. 2000;7:273–277. doi: 10.1097/00062752-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]