Abstract
Type I diabetes mellitus inhibits fracture healing and leads to an increase in complications. As a pilot study, we used a closed fracture model in the diabetic rat to address the question of whether osteogenic protein-1 (OP-1) in a collagen carrier can overcome this inhibition by increasing the area of the newly mineralized callus and femoral torque to failure compared with diabetic animals with fractures treated without OP-1. Diabetes was created in 54 rats by injection of streptozotocin. After 2 weeks, a closed femur fracture was created using a drop-weight impaction device. Each fracture site was immediately opened and treated with or without 25 μg OP-1 in a collagen carrier. Animals were euthanized after 2 or 4 weeks. Fracture healing was assessed by callus area from high-resolution radiographs, callus strength from torsional failure testing, and undecalcified histologic analysis. The area of newly mineralized callus was greater in diabetic animals treated with 25 μg OP-1/carrier compared with diabetic animals with untreated fractures and with fractures treated with carrier alone. This increase in callus area did not translate into an equivalent increase in torque to failure. Osteogenic protein-1 showed some evidence of overcoming the inhibition of fracture healing in the diabetic rat.
Introduction
Type I diabetes mellitus is associated with an increased risk of complications with fractures, including delayed union, wound necrosis, and increased incidence of infection [6, 8, 23, 25, 28]. Many of these complications result from progressive small vessel arterial disease and peripheral neuropathy that develop with time and are largely untreatable [19, 29]. Type I diabetes mellitus alters the mechanical and biologic properties of bone [3, 22]. Impairment of histomorphometric, cellular, and biochemical indicators of bone formation, like osteocalcin have been linked to diabetes mellitus [15, 27, 32]. One study suggests delayed fracture healing in an animal model of diabetes is attributable in part to reduced cellular proliferation associated with decreased levels of platelet-derived growth factor (PDGF) [31]. Thus, it is possible biologic interventions capable of stimulating cellular proliferation and osteogenesis may promote fracture healing in patients with diabetes.
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-beta class of growth factors. Bone morphogenetic proteins upregulate the expression of vascular endothelial growth factor, a member of the PDGF superfamily [30]. In clinical and animal studies, BMPs promote fracture healing in a normal setting and in pathologic conditions, including infection [4, 5, 7, 14, 35].
A closed, transverse femoral fracture in streptozotocin-induced diabetic rats was examined to address the following question: Does treatment of a femoral fracture in diabetic animals with 25 μg OP-1 in a collagen carrier increase (1) the area of the newly mineralized reparative callus, and (2) femoral torque to failure compared with untreated fractures (no carrier and no OP-1) and with fractures treated with the collagen carrier alone (no OP-1)?
Materials and Methods
We injected 54 male Sprague-Dawley rats (200 g) with 50 mg/kg streptozotocin in 0.1 mol/L citrate buffer subcutaneously in the highly vascular region near the base of the tail to produce insulin-dependent diabetes mellitus (Table 1) [12, 17]. Eighteen additional animals were injected with citrate buffer only to create nondiabetic controls. After 2 weeks, a closed femur fracture was created in all animals using a drop-weight impaction device. Each fracture site was immediately opened and either left untreated (18 diabetic and the 18 nondiabetic rats), or treated with OP-1 in a collagen carrier (18 diabetic rats) or carrier alone (18 diabetic rats). Animals were euthanized after 2 or 4 weeks. Dependent outcome variables were fracture callus area from high-resolution radiographs and callus strength from torsional failure testing. The number of animals per treatment and time (n = 6) was determined by a power analysis based on data from a previous study using the same closed fracture model and collagen carrier with and without OP-1 in rats treated with and without prednisolone [14]. Based on an average variability in callus area and torque to failure of 20%, a difference in the means of these parameters in animals treated with and without OP-1 of 40%, a power level of 0.8, and a p value of 0.05, it was determined we would need six animals per treatment and a time to achieve significance. All procedures involving animals were approved by our Institutional Animal Care and Use Committee in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care guidelines.
Table 1.
Experimental design
| Time (weeks) | Assessment | Experimental groups | |||
|---|---|---|---|---|---|
| Nondiabetic | Diabetic | ||||
| Sham surgery* | Sham surgery* | 0 μg OP-1 + carrier | 25 μg OP-1 + carrier | ||
| 2 | Radiographic histologic | 5 | 5 | 6 | 4 |
| 4 | Radiographic histologic | 6 | 6 | 4 | 6 |
| Biomechanical | 6 | 4 | 6 | 5 | |
* Control animals in which closed fracture was created and fracture site was opened and then closed without treatment; OP-1 = osteogenic protein-1.
Two weeks after streptozotocin (or buffer only) injection, we anesthetized all diabetic and nondiabetic animals with an intraperitoneal injection of ketamine (80–120 mg/kg) and xylazine (2–3 mg/kg) and prepared them for aseptic surgery. A medial incision was made to open the left knee of each animal. We made a femoral intercondylar entrance hole through the open knee. The medullary canal was hand-reamed with an 18-gauge hypodermic needle so each medullary cavity would be uniformly prepared to receive a stainless steel intramedullary pin (33 mm in length, 1.4 mm in diameter). Each pin had a tapered end that provided for an intimate fit of the pin in the medullary canal proximally. We inserted the pin in a retrograde manner into the femoral intramedullary canal of each animal, and a closed transverse middiaphyseal femoral fracture was created with a drop-weight impaction device [2]. To fracture the femur in a controlled, reproducible way, the animal’s lower limb was supported on the stage of the fracture device so the middiaphysis of the femur was situated under the impactor. The stage was vertically adjustable so the femur could be positioned against the impactor creating a 44-N preload as measured by a load cell placed in series with the impactor. A 960-g weight then was dropped 8 cm against the impactor to create a transverse fracture in the middiaphysis. The intent of preloading the impactor against the femur was to minimize soft tissue damage, fracture comminution, and variability in the fracture pattern. We obtained a radiograph immediately after surgery to confirm the pattern and location of the fracture.
At the same surgical setting, we aseptically opened each fracture site laterally with a 5-mm longitudinal incision, maintaining periosteal integrity. Eighteen diabetic animals received 25 μg OP-1 in 0.1 mL sterile water mixed with 50 mg lyophilized bovine Type I collagen matrix (Stryker Biotech, Hopkinton, MA) that was packed around the fracture site (Table 1). This 25-μg dose of OP-1 was reportedly sufficient to induce new bone formation with a closed femoral fracture in rats treated with prednisolone and with an intramuscular osteoinduction model in the rat challenged with bacteria [5, 14]. The fracture sites in 18 other diabetic animals received the collagen carrier mixed with 0.1 mL sterile water without OP-1. Finally, the fracture sites in the remaining 18 diabetic animals and in the 18 nondiabetic animals were surgically opened and closed without any OP-1 or carrier placed at the site, providing sham surgery controls. The deep and superficial layers of soft tissue at the pin insertion and fracture sites were closed with suture, and the animals were allowed to recover in their cages. An analgesic (0.013–0.026 mg/kg buprenorphine) was administered intramuscularly immediately after surgery. Animals typically exhibited normal weightbearing by the second postoperative day. All surgical procedures were performed by one orthopaedic surgeon (XC).
Six rats from each of the four treatment groups were euthanized 2 and 4 weeks after creation of the closed fracture using inhalant anesthesia (glass-covered tank with a sponge soaked with 1 mL of halothane) and an overdose of euthanasia solution (intraperitoneal administration of 100 mg/kg Beuthanasia-D [Schering-Plough Animal Health Corp, Kenilworth, NJ]). The 2- and 4-week times were chosen because extracellular matrix proteins are maximally expressed in the hard callus by 15 days and maximum torque to failure peaks at 4 weeks and remained at the same level to 8 weeks [18, 21]. The fractured femurs from these animals were harvested and fixed in 10% formalin (Fischer Scientific, Pittsburgh, PA), a formaldehyde/methanol solution buffered with phosphate-buffered saline. We first measured the area of newly mineralized callus from these specimens using quantitative high-resolution radiography. These same specimens subsequently were prepared for decalcified histologic analysis. Fractured and intact contralateral femurs from the remaining six animals in each treatment group at 4 weeks were harvested, wrapped in saline-soaked gauze, stored in plastic bags, and frozen for eventual torsional failure testing.
We measured serum glucose levels using a plasma glucometer just before streptozotocin (or buffer only) injection, at the time of fracture creation 2 weeks after injection, and just before euthanasia 2 or 4 weeks later [12]. Animals were considered diabetic if serum glucose concentrations were maintained at a level greater than 300 mg/dL throughout the study and nondiabetic if less than 300 mg/dL.
Nine of the initial 72 diabetic and nondiabetic animals could not be used for the radiographic, histologic, and biomechanical assessments in this study because of infection (n = 2), unintended fracture through the distal femoral condyles (n = 1), a comminuted fracture pattern (n = 1), unintended fracture while applying preload before fracture creation (n = 1), fracture location too distal to the intended middiaphyseal fracture site (n = 2), and nonunion (n = 2) (Table 1). All animals receiving streptozotocin had serum glucose levels greater than 300 mg/dL during the entire study period; no diabetic animals were excluded because glucose levels decreased below 300 mg/dL.
The formalin-fixed specimens slated for radiographic and subsequent histologic evaluation were first imaged using high-resolution radiography (Faxitron X-ray System; Hewlett-Packard, McMinnville, OR) with slow-speed film. We attempted to position specimens so an anteroposterior view could be obtained. The radiograph of each sample was digitized and imported into an image analysis system (BioQuant workstation; R&M Biometrics, Nashville, TN). The areas of callus were outlined manually on the workstation by each of three investigators (LSK, XC, WDL) in a blinded manner, were expressed in squared millimeters based on a scale factor from the known dimensions of the intramedullary pin appearing in the films, and were averaged. The bone outlined on the image analysis workstation was the newly mineralized callus that occurred beyond the original periosteal surface of the cortex as defined by the length of the pin.
After completing the radiographic assessment, femurs were processed for decalcified histologic analysis. Specimens were demineralized in 1.35 N hydrochloric acid and 0.003 mol/L ethylenediaminetetraacetic acid. After demineralization, we removed the intramedullary pins and the specimens were dehydrated in ethanol, cleared in xylene, and embedded in paraffin. Longitudinal 5-μm-thick sections were cut in the coronal plane with a rotary microtome and were stained by Masson’s trichrome to clearly delineate newly synthesized types of tissue. Mature mineralized tissues were stained green, cell nuclei blue, and unmineralized and other soft tissues red. We qualitatively examined the histologic sections.
Each fractured femur and respective intact contralateral femur to be used for mechanical testing was thawed in their plastic storage bag in a saline bath at room temperature. We removed the fixation pin from the intramedullary canal of each fractured femur so as to not disturb the fracture callus that had formed. Each femur then was placed in an alignment fixture, and the distal and proximal ends of the femur were potted in low-melting-temperature metal alloy. Femurs were loaded in torsion to failure in an axial-torsion test machine (EnduraTEC Systems Corp, Eden Prairie, MN) at a rate of 0.5° per second. We recorded torque versus angular displacement data and used them to compute the torque to failure, energy absorbed to failure, and torsional stiffness of the fracture site or intact contralateral femur. The pattern of failure also was recorded.
The radiographic callus area was assessed by two-way analysis of variance (ANOVA) with factors of treatment group (nondiabetic and diabetic sham surgery, diabetic with carrier only, and diabetic with carrier and 25 μg OP-1) and time from fracture (2 and 4 weeks). The mechanical test data at 4 weeks (torque and energy to failure and linear stiffness) were analyzed using repeated-measures two-way ANOVA with factors of fractured femur/intact contralateral femur and treatment group. Post hoc pair-wise comparisons were made using the Student-Newman-Keuls method. These analyses were used to determine whether a closed femoral fracture in diabetic rats treated with 25 μg OP-1 in collagen carrier has greater area of the newly mineralized reparative callus and greater femoral torque to failure compared with untreated fractures (no carrier and no OP-1) and with fractures treated with the carrier alone (no OP-1) in diabetic rats.
Results
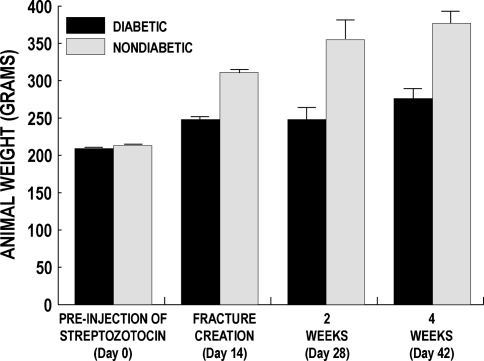
The mean serum glucose concentrations of animals injected with streptozotocin increased (p < 0.001) to 300 mg/dL or more by the time of fracture surgery 2 weeks later and remained above 300 mg/dL during the study because this was a model of uncontrolled diabetes (Table 2). All animals continued to gain weight during the study, although the weights of those injected with streptozotocin increased at a lower rate than nondiabetic control animals (Fig. 1). The uncontrolled streptozotocin-induced diabetic disease process weakened the intact contralateral femurs during the 6-week study, similar to other diabetic models [10–12, 24]. The mean torque to failure of the intact femurs in the nondiabetic group at 4 weeks was 1.6 to 2.1 times greater (p < 0.009 for all comparisons) than the failure torques of the intact femurs in the three diabetic treatment groups (Table 3).
Table 2.
Serum glucose levels during the study period
| Time (days) | Experimental groups | |||
|---|---|---|---|---|
| Nondiabetic | Diabetic | |||
| Sham surgery* | Sham surgery* | 0 μg OP-1 + carrier | 25 μg OP-1 + carrier | |
| Prestreptozotocin injection, Day 0 | 130 ± 3 | 133 ± 3 | 132 ± 6 | 133 ± 5 |
| Fracture creation, treatment, Day 14 | 209 ± 29 | 384 ± 15† | 402 ± 18† | 414 ± 12† |
| 2 weeks postoperatively, Day 28 | 213 ± 49 | 334 ± 13† | 372 ± 27† | 385 ± 21† |
| 4 weeks postoperatively, Day 42 | 208 ± 24 | 388 ± 24† | 440 ± 16† | 400 ± 19† |
Serum glucose levels in mg/dL are given as mean and standard error of the mean; *control animals in which closed fracture was created and fracture site was opened and then closed without treatment; †greater than prestreptozotocin injection levels (p < 0.001); OP-1 = osteogenic protein-1.
Fig. 1.
Weights of diabetic and nondiabetic animals were measured just before streptozotocin injection (Day 0), 2 weeks later when fracture was created (Day 14), and 2 or 4 additional weeks later at study end point (Day 28 or 42). The bars represent the mean ± standard error of the mean. All animals continued to gain weight during the study, although the weights of those injected with streptozotocin increased at a lower rate than that of nondiabetic control animals.
Table 3.
Torsional biomechanical testing at 4 weeks
| Mechanical parameter | Experimental groups | |||
|---|---|---|---|---|
| Nondiabetic | Diabetic | |||
| Sham surgery* | Sham surgery* | 0 μg OP-1 + carrier | 25 μg OP-1 + carrier | |
| Failure torque (Nm) | ||||
| Fractured femur | 0.191 ± 0.033 | 0.147 ± 0.016 | 0.161 ± 0.024 | 0.210 ± 0.073 |
| Intact contralateral femur | 0.471 ± 0.027† | 0.227 ± 0.019†,‡ | 0.296 ± 0.021†,‡ | 0.293 ± 0.041‡ |
| Energy to failure (Nm/0) | ||||
| Fractured femur | 1.106 ± 0.211 | 1.169 ± 0.364 | 0.617 ± 0.101 | 0.940 ± 0.462 |
| Intact contralateral femur | 2.728 ± 0.255 | 1.328 ± 0.095 | 1.911 ± 0.282 | 1.874 ± 0.263 |
| Torsional stiffness (Nm/0) | ||||
| Fractured femur | 0.024 ± 0.008 | 0.021 ± 0.005 | 0.027 ± 0.004 | 0.045 ± 0.013 |
| Intact contralateral femur | 0.046 ± 0.005 | 0.026 ± 0.007 | 0.032 ± 0.005 | 0.033 ± 0.006 |
Values are given as mean and standard error of the mean; *control animals in which closed fracture was created and fracture site was opened then closed without treatment; †intact femurs greater than fractured femurs for same experimental group (p < 0.012); ‡less than intact nondiabetic sham surgery controls (p < 0.009); OP-1 = osteogenic protein-1.
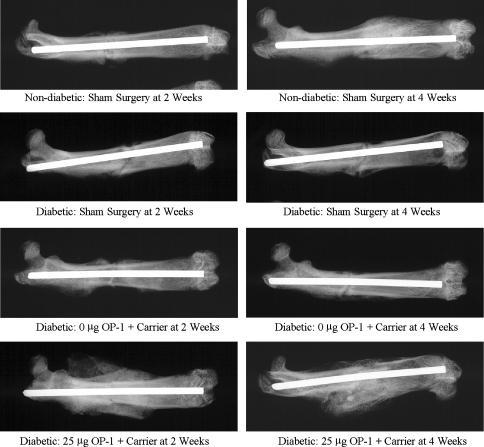
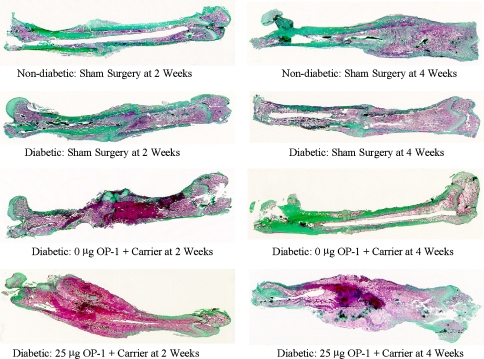
The mean area of newly mineralized callus in the periosteal envelope at 2 and 4 weeks was three to 30 times greater (p < 0.001 for all comparisons) when the closed fractures in diabetic animals were treated with 25 μg OP-1 in the collagen carrier compared with untreated fractures (no carrier or OP-1) and with fractures treated with carrier alone (carrier with no OP-1) in diabetic animals (Table 4; Fig. 2). Two weeks after fracture surgery, streptozotocin-induced diabetes resulted in diminished tissue maturation and organization compared with that seen in the nondiabetic controls (Fig. 3). The core of the fracture callus was filled with a dense fibrous meshwork lined with immature, rapidly forming woven bone, and there was little soft callus-associated fibrocartilage. Fibrocartilage persisted in the fracture site at 4 weeks compared with that of nondiabetic controls, which were well mineralized and appeared to be undergoing active remodeling. Treatment with OP-1 promoted the formation of a florid proliferation of woven, disorganized bone surrounding islands of mineralized fibrocartilage. Collagen granules (carrier) were surrounded by proliferating fibroblastic and inflammatory cell populations. Some consolidation and organization of the fracture callus was apparent by 4 weeks with a decrease in the collagen granules and associated host inflammatory cells. The callus was composed of mineralizing trabecular-like structures with little mineralized cartilage and woven bone.
Table 4.
Radiographic fracture callus area at 2 and 4 weeks
| Time (weeks) | Experimental groups | |||
|---|---|---|---|---|
| Nondiabetic | Diabetic | |||
| Sham surgery* | Sham surgery* | 0 μg OP-1 + carrier | 25 μg OP-1 + carrier | |
| 2 weeks | 4.5 ± 1.3† | 1.5 ± 0.5† | 2.6 ± 0.8† | 44.2 ± 5.9 |
| 4 weeks | 13.8 ± 2.9† | 8.4 ± 2.4† | 6.9 ± 3.1† | 35.9 ± 5.7 |
Areas in mm2 are given as mean and standard error of the mean; *control animals in which closed fracture was created and fracture site was opened and then closed without treatment; †less than treatment with 25 μg OP-1/carrier at same time (p < 0.001); OP-1 = osteogenic protein-1.
Fig. 2.
Representative high-resolution radiographs of femurs in the four treatment groups at 2 and 4 weeks after closed fracture are shown. The area of newly mineralized callus at 2 and 4 weeks in fractures of diabetic animals after treatment with osteogenic protein-1 (OP-1) was greater than with carrier-only and sham surgery groups.
Fig. 3.
Histologic sections cut from the same specimens in Fig. 2 are shown (Stain, Masson’s trichrome; original magnification, ×2.5). Mineralized tissues are stained green and soft tissues are red. OP-1 = osteogenic protein-1.
The mean torque to failure of fractured femurs in the diabetic animals treated with 25 μg OP-1 in the collagen carrier was similar to that of diabetic animals with untreated fractures (p = 0.381) and diabetic animals with fractures treated with carrier alone (p = 0.508) (Table 3). The mean torque to failure of the fractured femurs in the diabetic animals treated with 25 μg of OP-1 was similar (p = 0.351) to that of the respective intact contralateral femurs. The mean failure torques of the fractured femurs were 1.5 to 2.5 times less than the mean failure torques of their respective intact contralateral femurs in the nondiabetic (p < 0.001) and diabetic sham surgery (p = 0.012) groups and collagen carrier-only group (p = 0.002).
Discussion
Type I diabetes mellitus negatively alters the mechanical and biologic properties of bone [3, 22] and has been associated with an increased risk of complications with fracture healing, including delayed union, wound necrosis, and increased incidence of infection [6, 8, 23, 25, 28]. An intervention that would overcome this inhibition of fracture healing would be useful clinically. This pilot study addressed the question of whether treatment of a closed femoral fracture in streptozotocin-induced diabetic rats with 25 μg OP-1 in a collagen carrier can increase the area of the newly mineralized reparative callus and femoral torque to failure compared with that of diabetic animals with untreated fractures (no carrier and no OP-1) and with fractures treated with the collagen carrier alone (no OP-1).
Our study has several limitations. Streptozotocin-induced diabetes mellitus is not the same disease process as Type I diabetes mellitus in humans. However, streptozotocin-induced diabetes in a closed fracture rat model provides a useful and relatively controlled approach for challenging fracture healing and using a pharmacologic or biologic intervention to correct it with potential for an eventual treatment of human patients with diabetes who have fractures. The streptozotocin-induced diabetes was uncontrolled because no insulin therapy was initiated; this is unlikely the case in humans. The single injection of streptozotocin was sufficient to elevate the serum glucose concentration to greater than 300 mg/dL 2 weeks after injection and maintain it above that threshold at a relatively constant level for the remainder of the study with only a 5% variation with time. However, it is possible this variation in serum glucose level resulting from the uncontrolled nature of the diabetes, although small, may have caused some of the variation in the area of newly mineralized callus or the failure strength of the healed fractures, which was approximately 15%. Some limitations were associated with the area measurements from the high-resolution radiographs. Interobserver variability and intraobserver variability with these measurements were not formally assessed. In addition, these area measurements were influenced by variation in placement of the femurs from the true anteroposterior projection. Finally, the 200-gram rats used in this study were immature and still growing. Introduction of streptozotocin likely disrupted their normal growth pattern. It would have been more appropriate to use adult rats before making them diabetic.
We found the area of newly mineralized callus in the periosteal envelope at 2 and 4 weeks in fractures in diabetic animals after treatment with OP-1 was greater than in treatment groups without OP-1. We also found that the torque to failure of fractures in diabetic animals at 4 weeks after treatment with OP-1 was similar to that in the treatment groups without OP-1. Treatment with OP-1 restored the mechanical strength of the fractured femurs (i.e., similar to their respective intact contralateral femurs), albeit these diabetic intact femurs were relatively weakened compared with intact femurs of the nondiabetic animals. Although a power analysis justified the six animals per treatment and time, some of these animals were lost to experimental complications. Nonetheless, these numbers were sufficient to determine a major effect of OP-1 on the area of newly formed callus. This was not the case with the torque to failure, however. The percentage increase in area of reparative callus with OP-1, compared with treatment groups without OP-1, did not necessarily translate into an equivalent percentage increase in torque to failure. Although our choices of the 25-μg dose of OP-1 and 4-week point initially seemed justified by the literature [4, 15, 18, 21], our torsion failure testing results indicated the diabetic challenge in this model may have required a higher dose of OP-1 and longer followup to observe a difference in failure parameters. A time greater than 4 weeks may have allowed the callus to further remodel and become stronger. In addition, the mechanical failure parameters of the treated femurs were not normalized with respect to their intact contralateral femurs because the failure strengths of the intact diabetic femurs were less than the intact nondiabetic femurs. Rather, a comparison of the mean failure data for the fractured and intact femurs indicated how well a treatment restored a fractured femur to its intact counterpart and how that intact counterpart varied with and without diabetes.
Although animal models in which diabetes mellitus was genetically bred into the animal or chemically induced do not precisely mimic human Type I diabetes mellitus, they have been used to document reduced osseointegration with metallic implants, delayed healing of fractures and bone defects, reduction in bone strength, and diminished bone mineral density and longitudinal bone growth [1, 10–12, 24, 26, 34]. Although diabetes mellitus inhibits fracture healing in rat models, treatment of the diabetes or fracture site can compensate for this impairment [1, 9, 12, 23]. Treatment of diabetes in these models with systemic insulin ameliorated impairment of fracture healing [1, 9, 23]. Application of insulin locally to a fracture site directly mediated fracture healing [13]. Local application of recombinant human fibroblastic growth factor in a closed fracture model in a streptozotocin-induced diabetic rat restored the impaired ability of the fracture to heal [20].
A supraphysiologic amount of OP-1 may maintain osteoinductive activity in the diabetic setting by numerous possible mechanisms. Responding progenitor elements (stromal, periosteal, and perivascular cells) may retain their inductive response to BMPs, thereby overcoming an inhibitory environment [4]. Because BMP receptor II is downregulated in diabetes mellitus [33], it is possible treatment of a diabetic fracture with exogenous BMP may stimulate expression of BMP receptors [35]. It also is possible administration of exogenous BMP may compensate for the delay in Type X collagen expression and associated chondrocyte maturation observed in diabetes [16]. However, the precise mechanism by which OP-1 may enhance fracture healing in the diabetic environment is unknown and needs to be addressed in future work.
We found preliminary evidence in this pilot study that OP-1 has the potential to overcome the osteogenic inhibition of streptozotocin-induced diabetes in this closed femoral fracture model in the rat with the treatments and times studied. However, definitive conclusions can be made only after more extensive work including larger numbers of animals, longer followup, and a range of doses of OP-1. Since their commercial introduction, there has been increased use of BMPs clinically in circumstances when fracture healing may be impaired such as in open fractures and fractures in individuals who use tobacco. Presuming further preclinical work confirms our initial findings, we believe clinical assessment of BMP administration in patients with diabetes should be considered.
Acknowledgments
We thank Craig Bourgeault for assistance with mechanical testing.
Footnotes
One or more of the authors (LSK, WDL) have received funding from Stryker Biotech (Hopkinton, MA) and the Midwest Orthopaedic Research Foundation (Minneapolis, MN). The osteogenic protein-1 and collagen carrier were donated by Stryker Biotech.
Each author certifies that his institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Beam HA, Parsons JR, Lin SS. The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res. 2002;20:1210–1216. [DOI] [PubMed]
- 2.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. [DOI] [PubMed]
- 3.Carnevale V, Romagnoli E, D’Erasmo E. Skeletal involvement in patients with diabetes mellitus. Diabetes Metab Res Rev. 2004;20:196–204. [DOI] [PubMed]
- 4.Chen X, Kidder LS, Lew WD. Osteogenic protein-1 induced bone formation in an infected segmental defect in the rat femur. J Orthop Res. 2002;20:142–150. [DOI] [PubMed]
- 5.Chen X, Kidder LS, Schmidt AH, Lew WD. Osteogenic protein-1 induces bone formation in the presence of bacterial infection in a rat intramuscular osteoinduction model. J Orthop Trauma. 2004;18:436–442. [DOI] [PubMed]
- 6.Costigan W, Thordarson DB, Debnath UK. Operative management of ankle fractures in patients with diabetes mellitus. Foot Ankle Int. 2007;28:32–37. [DOI] [PubMed]
- 7.Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van der Bent C, Papapoulos SE, Lowik CW. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. [DOI] [PubMed]
- 8.Folk JW, Starr AJ, Early JS. Early wound complications of operative treatment of calcaneus fractures: analysis of 190 fractures. J Orthop Trauma. 1999;13:369–372. [DOI] [PubMed]
- 9.Follak N, Kloting I, Ganzer D, Merk H. Scanning electron microscopic examinations on retarded bone defect healing in spontaneously diabetic BB/O(ttawa)K(arlsburg) rats. Histol Histopathol. 2003;18:111–120. [DOI] [PubMed]
- 10.Follak N, Kloting I, Merk H. Influence of diabetic metabolic state on fracture healing in spontaneously diabetic rats. Diabetes Metab Res Rev. 2005;21:288–296. [DOI] [PubMed]
- 11.Follak N, Kloting I, Wolf E, Merk H. Improving metabolic control reverses the histomorphometric and biomechanical abnormalities of an experimentally induced bone defect in spontaneously diabetic rats. Calcif Tissue Int. 2004;74:551–560. [DOI] [PubMed]
- 12.Funk JR, Hale JE, Carmines D, Gooch HL, Hurwitz SR. Biomechanical evaluation of early fracture healing in normal and diabetic rats. J Orthop Res. 2000;18:126–132. [DOI] [PubMed]
- 13.Gandhi A, Beam HA, O’Connor JP, Parsons JR, Lin SS. The effects of local insulin delivery on diabetic fracture healing. Bone. 2005;37:482–490. [DOI] [PubMed]
- 14.Gilley RS, Wallace LJ, Bourgeault CA, Kidder LS, Chen X, Bechtold JE. Influence of bone morphogenetic protein on glucocorticoid-inhibited fracture healing in a closed femoral fracture rat model. Trans Orthop Res Soc. 2003;28:502.
- 15.Glajchen N, Epstein S, Ismail F, Thomas S, Fallon M, Chakrabarti S. Bone mineral metabolism in experimental diabetes mellitus: osteocalcin as a measure of bone remodeling. Endocrinology. 1988;123:290–295. [DOI] [PubMed]
- 16.Gooch HL, Hale JE, Fujioka H, Balian G, Hurwitz SR. Alterations of cartilage and collagen expression during fracture healing in experimental diabetes. Connect Tissue Res. 2000;41:81–91. [DOI] [PubMed]
- 17.Horcajada-Molteni MN, Chanteranne B, Lebecque P, Davicco MJ, Coxam V, Young A, Barlet JP. Amylin and bone metabolism in streptozotocin-induced diabetic rats. J Bone Miner Res. 2001;16:958–965. [DOI] [PubMed]
- 18.Jingushi S, Joyce ME, Bolander ME. Genetic expression of extracellular matrix proteins correlates with histologic changes during fracture repair. J Bone Miner Res. 1992;7:1045–1055. [DOI] [PubMed]
- 19.Powers AC. Diabetes mellitus, chapter 323. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 16th Ed. New York, NY: McGraw-Hill; 2005:2165–2169.
- 20.Kawaguchi H, Kurokawa T, Hanada K, Hiyama Y, Tamura M, Ogata E, Matsumoto T. Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology. 1994;135:774–781. [DOI] [PubMed]
- 21.Kokubu T, Hak DJ, Hazelwood SJ, Reddi AH. Development of an atrophic nonunion model and comparison to a closed healing fracture in rat femur. J Orthop Res. 2003;21:503–510. [DOI] [PubMed]
- 22.Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and turnover in diabetes. Diabetes. 1995;44:775–782. [DOI] [PubMed]
- 23.Loder RT. The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop Relat Res. 1998;232:210–216. [PubMed]
- 24.Macey LR, Kana SM, Jingushi S, Terek RM, Borretos J, Bolander ME. Defects of early fracture-healing in experimental diabetes. J Bone Joint Surg Am. 1989;71:722–733. [PubMed]
- 25.McCormack RG, Leith JM. Ankle fractures in diabetics: complications of surgical management. J Bone Joint Surg Br. 1998;80:689–692. [DOI] [PubMed]
- 26.McCracken M, Lemons JE, Rahemtulla F, Prince CW, Feldman D. Bone response to titanium alloy implants placed in diabetic rats. Int J Oral Maxillofac Implants. 2000;15:345–354. [PubMed]
- 27.Sasaki T, Kaneko H, Ramamurthy NS, Golub LM. Tetracycline administration restores osteoblast structure and function during experimental diabetes. Anat Rec. 1991;231:25–34. [DOI] [PubMed]
- 28.Segalman KA, Clark GL. Un-united fractures of the distal radius: a report of 12 cases. J Hand Surg Am. 1998;23:914–919. [DOI] [PubMed]
- 29.Tierney LM, McPhee SJ, Papadakis MA. Current Medical Diagnosis & Treatment. 42nd Ed. New York, NY: McGraw-Hill; 2003:1180–1184.
- 30.Tischer E, Gospodarowicz D, Mitchell R, Silva M, Schilling J, Lau K, Crisp T, Fiddes JC, Abraham JA. Vascular endothelial growth factor: a new member of the platelet-derived growth factor family. Biochem Biophys Res Commun. 1989;165:1198–1206. [DOI] [PubMed]
- 31.Tyndall WA, Beam HA, Zarro C, O’Connor JP, Lin SS. Decreased platelet derived growth factor expression during fracture healing in diabetic animals. Clin Orthop Relat Res. 2003;408:319–330. [DOI] [PubMed]
- 32.Verhaeghe J, Suiker AM, Visser WJ, Van Herck E, Van Bree R, Bouillon R. The effects of systemic insulin, insulin-like growth factor-I and growth hormone on bone growth and turnover in spontaneously diabetic BB rats. J Endocrinol. 1992;134:485–492. [DOI] [PubMed]
- 33.Wang SN, Lapage J, Hirschberg R. Loss of tubular bone morphogenetic protein-7 in diabetic nephropathy. J Am Soc Nephrol. 2001;12:2392–2399. [DOI] [PubMed]
- 34.Waud CE, Marks SC Jr, Lew R, Baran DT. Bone mineral density in the femur and lumbar vertebrae decreases after twelve weeks of diabetes in spontaneously diabetic-prone BB/Worcester rats. Calcif Tissue Int. 1994;54:237–240. [DOI] [PubMed]
- 35.Yeh LC, Zavala MC, Lee JC. Osteogenic protein-1 and interleukin-6 with its soluble receptor synergistically stimulate rat osteoblastic cell differentiation. J Cell Physiol. 2002;190:322–331. [DOI] [PubMed]