Abstract
Alendronate inhibits osteoclastic activity. However, some studies suggest alendronate also has effects on osteoblast activity. We hypothesized alendronate would enhance osteoblastic differentiation without causing cytotoxicity of the osteoblasts. We evaluated the effect of alendronate on the osteogenic differentiation of mouse mesenchymal stem cells. D1 cells (multipotent mouse mesenchymal stem cells) were cultured in osteogenic differentiation medium for 7 days and then treated with alendronate for 2 days before being subjected to various tests using MTT assays, Alizarin Red, enzyme-linked immunosorbent assay, energy-dispersive xray spectrophotometry, reverse transcriptase–polymerase chain reaction, confocal microscopy, and flow cytometric analysis. D1 cells differentiated into osteoblasts in the presence of osteogenic differentiation medium as confirmed by positive Alizarin Red S staining, increased alkaline phosphatase activity and osteocalcin mRNA expression, a calcium peak by energy-dispersive xray spectrophotometry, and by positive immunofluorescence staining against CD44. Osteogenic differentiation was enhanced after treatment with alendronate as confirmed by Alizarin Red S staining, elevated alkaline phosphatase activity and osteocalcin mRNA expression, a greater calcium peak by energy-dispersive xray spectrophotometry, and by immunofluorescence staining against CD44 by flow cytometric analysis. These data suggest alendronate enhances osteogenic differentiation when treated with mouse mesenchymal stem cells in osteogenic differentiation medium.
Introduction
Bisphosphonates are well-known inhibitors of osteoclastic activity and are widely used to treat osteoporosis. Alendronate is one of the most potent antiosteoporotic agents known [36]. The pharmacologic action of alendronate relies on its interfering with the mevalonate pathway by inhibiting farnesyl pyrophosphate synthase [14] and thus reducing levels of geranylgeranyl diphosphate, which is required for prenylation of guanosine triphosphate-binding proteins (eg, Rab, Rac, Ras, Rho, and Cdc42) that are essential for osteoclast activity and survival [23, 40]. Consequently, alendronate interferes with the stability of the ruffled border and stimulates osteoclast apoptosis, which reduces bone resorption, lowers bone turnover, and promotes a positive bone balance [35].
Moreover, studies indicate bisphosphonates also influence osteoblasts [14, 19, 32, 33] and increase bone formation [15, 20], and others have reported bisphosphonates enhance osteoblast proliferation and maturation [15, 20, 34] and inhibit osteoblast apoptosis [33]. One study exploring the effects of bisphosphonates on immortalized human fetal osteoblasts showed decreased osteoblast cell proliferation and increased cytodifferentiation in a dose-dependent manner in cultures treated with pamidronate. Additionally, total cellular protein, alkaline phosphatase (ALP) activity, and Type I collagen secretion in osteoblasts also were increased. Consistent with these findings, the rate of bone formation also was increased in osteoblasts [34]. Similar findings were reported by von Knoch et al. [41] in a study of the effects of bisphosphonates (alendronate, risedronate, zoledronate) on proliferation and osteoblast differentiation of human bone marrow stromal cells (BMSCs). In their study using bone marrow stromal cells from patients undergoing primary THA for end-stage degenerative joint disease, they reported all bisphosphonates tested enhanced the proliferation of BMSC and initiated osteoblastic differentiation [41]. These observations support the suggestion that bisphosphonates have an anabolic effect on osteoblasts and subsequently promote bone formation, and therefore may be beneficial not only for treatment of osteoporosis, but also for treatment of fracture nonunion and even osteolysis.
We hypothesized alendronate would enhance osteoblastic differentiation compared with untreated osteoblasts as evidenced by the presence of calcification and osteoblast surface markers, increased ALP activity, and gene expression. We also hypothesized this effect can be achieved without causing cytotoxicity of the osteoblasts.
Materials and Methods
We obtained MSCs and induced osteogenic differentiation by culturing the cells in osteogenic differentiation media (ODM). After 3 days, the cells were divided into four groups: Group 1 was the control group, which were cells treated with ODM alone but not with alendronate, and Groups 2, 3, and 4 constituted cells treated with ODM and with 0.1, 1, or 10 μg/mL alendronate, respectively. Cells then were tested 24 or 48 hours later to determine whether osteogenic differentiation had been enhanced in cells treated with alendronate compared with the control group. The experiments included Alizarin Red S staining, ALP activity assays, reverse transcriptase–polymerase chain reaction (RT-PCR) analysis, scanning electron microscope–energy-dispersive xray spectrometry (SEM-EDX) analysis, immunofluorescence staining, and flow cytometric analysis. Each experiment was repeated three times for each group, and the mean results were compared.
We cloned the primarily osteogenic D1 cells from MSCs as described previously [8]. D1 cells are a MSC line cloned from Balb/c mouse bone marrow cells. These cells differentiate into an osteogenic lineage when cultured in ODM, which contains ascorbic acid, dexamethasone, and β-glycerolphosphate. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (Gibco, BRL, Bethesda, MD) and antibiotics (Gibco). They were seeded at 1 × 104 cells/cm2 and maintained in culture for 3 days in a humidified 5% CO2 atmosphere at 37°C. Experiments were performed after cells had reached approximately 80% confluence. To induce osteogenic differentiation, 3 days after seeding, we changed culture media to ODM (DMEM supplemented with 50 μg/mL ascorbic acid [Sigma-Aldrich, St Louis, MO], 10−8 mol/L dexamethasone [Sigma-Aldrich], and 10 mmol/L β-glycerolphosphate [Sigma-Aldrich]). Three days after ODM changes, the cells were treated with 0.1, 1, or 10 μg/mL alendronate and were analyzed 24 or 48 hours later.
We used fluorescence microscopy, confocal microscopy, and flow cytometry to show changes in surface molecules, in this case the change from MSC to osteoblast, thus enabling identification of cells with CD44 or CD45 markers. For immunofluorescence staining, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 minutes, permeabilized with 0.1% Triton® X-100 (Sigma-Aldrich, Inc., Saint Louis, MO) for 15 minutes, and then blocked with 5% bovine serum albumen in PBS for 30 minutes. Coverslips then were incubated with primary antibodies against mouse CD44 (an antigen on osteoblasts, which acts as a receptor for osteopontin) [20, 28] at a dilution of 1:100 and against mouse CD45 (the leukocyte antigen) at 1:100, both at room temperature for 1 hour. CD45 (a hematopoietic cell marker) was used as a negative control for MSCs. We then washed cells with PBS and mounted them in 70% glycerol. Microscopic images were obtained using an Olympus BX50 fluorescence microscope (Olympus, Tokyo, Japan).
For flow cytometric analysis, 0.5 × 105 cells were incubated in staining buffer (PBS containing 2% fetal bovine serum and 0.1% sodium azide) with anti-CD44, anti-CD51, and anti-CD45 (BD Biosciences Pharmingen, San Diego, CA) for 30 minutes in ice. We used cells that stained with the appropriate isotype-matched immunoglobulin as negative controls. After staining, cells were fixed with 2% w/v paraformaldehyde and analyzed using a FACSCalibur™ equipped with CellQuest™ software (BD Biosciences, San Jose, CA).
We assayed specific ALP activities based on the release of p-nitrophenol from p-ntirophenyl phosphate. Optical densities of the p-nitrophenol produced were read at 405 nm using a Multiskan® EX ELISA reader (ThermoFisher Scientific, Inc, Waltham, MA). ALP activity was normalized versus total protein content, which was determined using a Qubit™ fluorometer and Quant-iT™ protein assay kits (Invitrogen, Kingston, Ontario, Canada).
To assess the effects of alendronate on the transcriptions of genes encoding osteocalcin (5′-GAG GGC AAT AAG GTA GTG AAC AGA-3′; 5′-AAG CCA TAC TGG TCT GAT AGC TCG-3′), osteopontin (5′-CCA GGT TTC TGA TGA ACA GTA TCC-3′; 5′-ACT TGA CTC ATG GCT GCC CTT T-3′), and the housekeeping enzyme glyceraldehyde-3-phosphate dehydrogenase (5′-ATC ACT GCC ACC CAG AAG AC-3′; 5′-ATG AGG TCC ACC ACC CTG TT-3′), we homogenized D1 cells grown to 70% confluence on plates with or without alendronate using TRIzol® reagent (Molecular Research Center, Inc, Cincinnati, OH), and then isolated total RNA. RNA (0.5 μg) was reverse-transcribed in 20 μL buffer containing avian myeloblastosis virus reverse transcriptase (AMV RT) 5x, 2.5 μmol/L poly(dT), 1 mmol/L each of dATP, dCTP, dGTP, and dTTP, 20 U RNase inhibitor, and 20 U AMV RT. Reverse transcription was performed using the following conditions: initial incubation at room temperature for 10 minutes and then at 42°C for 15 minutes, 99°C for 5 minutes, and 5°C for 5 minutes in a GeneAmp® PCR System 2700 (Applied Biosystems, Foster City, CA). Aliquots of cDNA were amplified in 100 μL PCR buffer containing 15 mmol/L MgCl2, 0.1 nmol/L each of dATP, dCTP, dGTP, and dTTP, 0.35 IU Taq DNA polymerase, and 0.3 μmol/L 5′- and 3′-oligomers. PCR products were resolved by 1.5% agarose gel electrophoresis and visualized with ethidium bromide. We determined relative quantities of amplified products using an image analysis program (MultiGauge V3.0; Fujifilm, Tokyo, Japan).
We quantified calcification deposits in the matrices using an assay method described by Chen et al. [5]. Briefly, cell cultures were washed twice with distilled water, fixed for 1 hour in ice cold 70% (v/v) ethanol, and rinsed twice with deionized water. Cultures were stained for 10 minutes with Alizarin Red S, and then excess dye was removed gently using running water. We identified calcification deposits in the matrix, which appeared bright red, by light microscopy and photographed. Calcification was quantified by determining densities and areas of Alizarin Red S staining using an image analysis program (MultiGauge V3.0).
SEM-EDX analysis was performed to quantify calcium deposits. Briefly, we treated cells with different concentrations of alendronate for 48 hours, and then culture dishes were embedded in paraffin. SEM-EDX was performed using 12-mm sections. We fixed sections to the sample holder using a conductive carbon ribbon. A Hitachi S-4700 scanning electron microscope (Hitachi, Tokyo, Japan) equipped with EDX was used for this work. Maps of calcium distributions were acquired at 20 kV, and calcium quantities were determined by SEM-EDX analysis program.
We performed a methyl-tetra-zolium (MTT; 3[4,5-dimethyl-thiazoyl-2yl] 2,5-diphenyl-tetrazolium bromide; Sigma) assay to determine the number of living cells. Cells were treated with 0.1, 1, or 10 μg/mL alendronate and, after 48 hours, 10 μL of MTT solution (5 mg MTT/PBS) was added to each well and incubated for 4 hours. Finally the 100 μL dimethylsulfoxide was added per well to solubilize the MTT-formazan. After complete solubilization of the dye by vortexing the plate, we read absorbance on an ELISA reader (EL 340 Biokinetics Reader; Bio-Tek Instruments) at 570 nm wavelength. Cell viability (%) was expressed as a ratio of alendronate-treated cells to control cells ×100.
MTT and ALP results are reported as mean ± standard deviation relative to control. We used one-way ANOVA to assess differences between treatment groups. Similarly, gene expression is reported as mean ± standard deviation relative to control, and one-way ANOVA was used to assess differences. When a difference between groups was identified by ANOVA, we compared group means using a Student’s t test. We recorded all data in Microsoft Excel (Microsoft, Redmond, WA), and performed all statistical analyses using SPSS (SPSS Inc, Chicago, IL).
Results
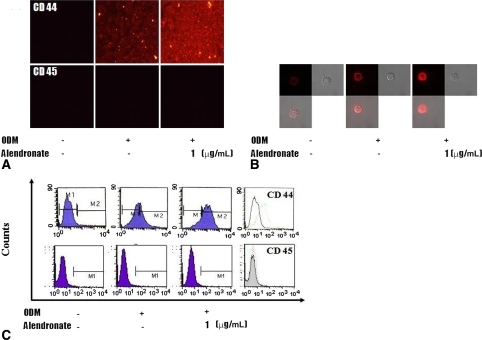
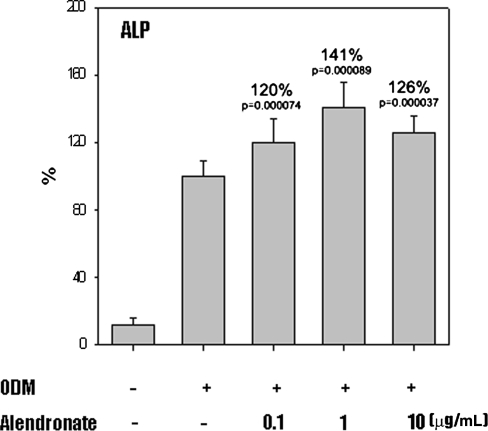
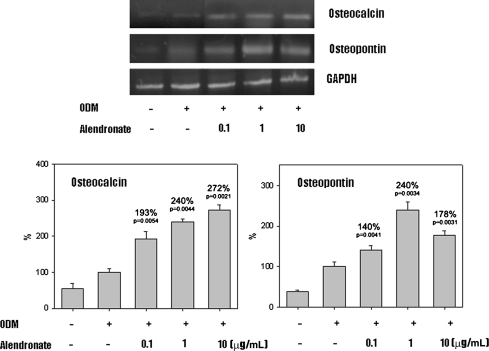
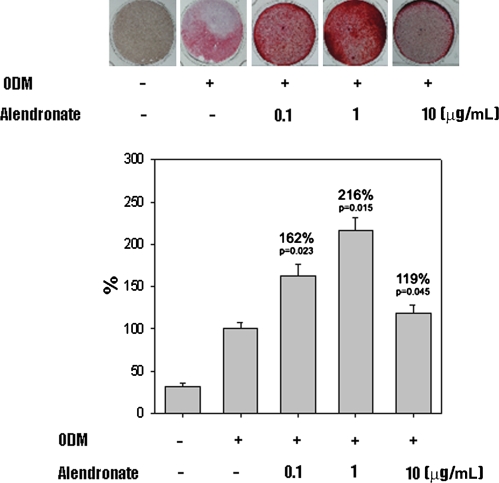
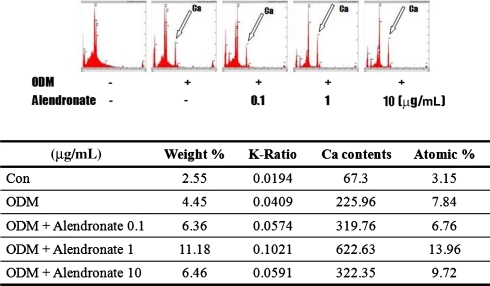
In the presence of ODM, all D1 cells differentiated into osteoblasts. Cells cultured in ODM with or without alendronate expressed CD44 (Fig. 1). However, CD45 was not detected in any cells. Flow cytometry showed CD44 expression was increased on cells treated with alendronate, which suggests alendronate enhanced osteoblastic differentiation. Activities of ALP, a marker of early osteoblast differentiation, were increased by 120%, 141%, and 126% by alendronate at 0.1 (p = 0.000074), 1 (p = 0.000089), and 10 μg/mL (p = 0.000037), respectively, compared with cells cultured in ODM alone (Fig. 2). The mRNA expressions of osteocalcin and osteopontin of alendronate-treated cells also were compared with cells cultured in ODM only (Fig. 3). Osteocalcin mRNA expression was increased to 193%, 240%, and 272% by alendronate at 0.1 (p = 0.0054), 1 (p = 0.0044), and 10 μg/mL (p = 0.0021), respectively, versus the controls. Osteopontin mRNA expression also was increased to 140%, 240%, and 178% by alendronate at 0.1 (p = 0.0041), 1 (p = 0.0034), and 10 μg/mL (p = 0.0031), respectively, versus the controls. Microscopic analysis and Alizarin Red S staining of the cultured cells showed many nodules composed of aggregated cells entrapped in a mineralized extracellular matrix (Fig. 4). Control cells cultured in ODM alone were stained red by Alizarin Red S, and this stain was at much higher intensity when cells were cultured in ODM containing 0.1, 1, or 10 μg/mL alendronate. Density measurements obtained using an image analysis program revealed cells cultured in ODM and alendronate at these concentrations had higher intensities of 162% (p = 0.023), 216% (p = 0.015), and 119% (p = 0.045) versus the untreated control. SEM-EDX provided evidence of mineralization through the appearance of a calcium peak, and the degree of mineralization was increased by alendronate (Fig. 5). SEM-EDX showed no calcium peak for undifferentiated D1 cells but revealed calcium peaks in cells cultured in ODM with or without alendronate.
Fig. 1A–C.
The photographs show increased surface molecular expression (expression of CD44) for MSCs cultured in ODM and treated with alendronate by (A) fluorescence microscopy (×100), (B) confocal microscopy (×400), and (C) flow cytometry.
Fig. 2.
A graph shows increased ALP activity normalized protein concentration of MSCs cultured in ODM with alendronate treatment. Data are presented as a percentage of control (n = 3).
Fig. 3.
Osteocalcin and osteopontin gene expression of MSCs cultured in ODM and treated with alendronate were elevated compared with the control group. Data are presented as a percentage of control (n = 3). GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
Fig. 4.
Light microscopy photographs (original magnification, ×100) and graphs show higher intensity of Alizarin Red S staining for MSCs treated with alendronate. Data are presented as a percentage of control (n = 3). ODM = osteogenic differentiation media.
Fig. 5.
The diagrams show energy-dispersive xray spectrometry analysis performed 48 hours after MSCs cultured in ODM were treated with alendronate. The calcium peak is indicated by arrows. The chart shows the ratio of calcium contents and percentage of atomic weight represented by calcium of MSCs cultured in ODM and treated with alendronate. The chart and diagrams show increases in calcium content were in a dose-dependent manner.
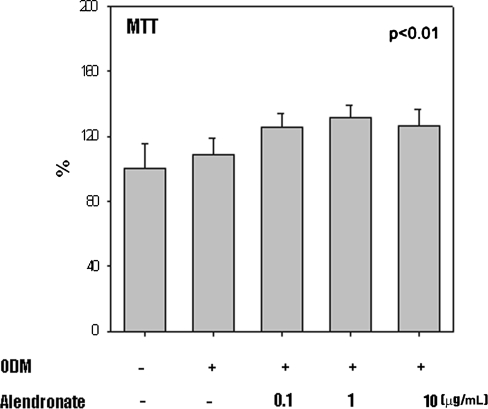
MTT assays at the doses of alendronate administered showed no cytotoxicity (Fig. 6.).
Fig. 6.
The graph shows methyl-tetra-zolium (MTT) activity of MSCs cultured in ODM and treated with alendronate and reaffirms that all cells were viable posttreatment with alendronate. Data are presented as a percentage of control (n = 3).
Discussion
Bisphosphonates are used to treat postmenopausal osteoporosis and are administered as prophylactics for secondary osteoporosis. In fact, bisphosphonates are the most effective known inhibitors of bone resorption. Although they were first used to treat Paget’s disease [26, 28], bisphosphonates now are the preferred drugs to treat hypercalcemia of malignancy and postmenopausal osteoporosis [17, 30]. They also were evaluated for treating inflammation-related bone loss [1, 10], fibrous dysplasia [7, 36], and other disorders of the musculoskeletal system, such as osteogenesis imperfecta [2, 3]. Moreover, one study reported bisphosphonates improve the durability of total joint arthroplasties [38]. We therefore hypothesized alendronate would enhance osteoblastic differentiation compared with ODM-treated D1 cells and that this effect would be achieved without cytotoxicity.
As this study was only a preliminary in vitro study, we are unsure if the findings of this experiment would be reflected in vivo. The effects were relatively small and we did not compare the effects of alendronate with other known osteoblastic differentiation factors such as insulin-like growth factor, BMP-2, and prostaglandins. Further study is required to determine if the effects of alendronate as seen in this study are comparable to those of these other factors and whether they can be replicated in vitro and in vivo.
The mode of action of bisphosphonates on osteoclasts has been described [4, 11–14, 18, 32, 36, 37, 39]. Bisphosphonates inactivate osteoclasts, which then undergo apoptosis and thus reduce bone resorption, reduce bone turnover, and promote a positive bone balance [35]. Bisphosphonates have well known RANKL inhibition on fracture healing and bone strength in animal models [9]. A study by Mackie et al. [24] on expression of RANKL and other factors in osteoclast development using pamidronate and clodronate found a dose-related inhibition of cellular proliferation and down-regulation of RANKL in those cells; however, a study by Naidu et al. [27] showed no effect on RANKL in response to zoledronate or alendronate treatment. In addition to inhibiting bone resorption by osteoclasts, bisphosphonates also have an anabolic effect on osteoblasts [38]. Mathov et al. [25] reported numerous bisphosphonates (ie, olpadronate, pamidronate, etidronate) induced rat calvaria-derived osteoblast proliferation, and Giuliani et al. [16] reported bisphosphonates stimulate the formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures. Im et al. [20] investigated the effects of alendronate and risedronate on primary human trabecular bone cell cultures on MG-63 osteoblast-like cell lines and found bisphosphonates promoted osteoblast proliferation and maturation, as evidenced by increased cell numbers and ALP activity and by the enhanced expressions of BMP-2, Type I collagen, and osteocalcin. Reinholz et al. [34] reported similar findings in their study on pamidronate and zoledronate.
Alkaline phosphatase activity and osteocalcin and osteopontin expressions are considered markers of osteoblast differentiation [6, 22], as early progenitor cells do not express osteoblast markers such as ALP, osteocalcin, or osteopontin [19] and only cells that have differentiated to a mature osteoblast phenotype express these markers [19]. We found ALP activity and expressions of osteocalcin and osteopontin were enhanced by alendronate in a dose-dependent fashion. We consider it likely if alendronate stimulates cellular differentiation, it also might stimulate proliferation of osteocalcin and ALP-expressing cells. The adhesion molecule CD44 is a cell surface transmembrane glycoprotein and is encoded by a single gene. Moreover, by acting as a receptor for hyaluronic acid, CD44 is involved in lymphocyte activation, recirculation and homing, adhesion to the extracellular matrix, angiogenesis and cellular proliferation, differentiation, and migration [39]. CD44 modulation also may play an important role in MSC differentiation. Some studies suggest CD44 can be used as a marker for osteoblasts [21, 29]. In our study, fluorescence microscopy, confocal microscopy, and FACS analysis showed increased presence of CD44, which mirrored the increase in osteoblastic differentiation when cells were treated with alendronate. Our findings suggest CD44 is a reasonable marker of osteoblast differentiation, and D1 cells treated with ODM and alendronate differentiate to osteoblasts, thus confirming alendronate can affect osteoblast differentiation. However, our study only provides clues as to how this is achieved at the molecular level. Therefore, additional investigations at the molecular level, involving receptors, channels, enzymes, and signal transduction mediators, are required to determine the mechanistic basis of the actions of alendronate and other bisphosphonates. We confirmed the presence of calcium in deposits by Alizarin Red S staining and EDX. Intensities of Alizarin Red S staining and calcium peak heights were greater in the presence of alendronate and peaked at an alendronate concentration of 1 μg/mL. These observations indicate the main mineral components of calcified bone matrix, or the precursors of calcified bone matrix, were formed [31]. Our findings concur with those of a previous study, in which it was suggested bisphosphonates enhanced the development of osteoblasts from the matrix maturation stage to the mineralization stage [34]. Additionally, our data suggest osteoblast cells differentiate with alendronate treatment.
Notably, we found at the highest concentration examined (10 μg/mL), alendronate had a lower effect on D1 proliferation than at 0.1 and 1 μg/mL. A similar observation was reported by Reinholz et al. [34] who reported an inhibitory effect on immortalized human fetal osteoblast cell proliferation with treatment of 10 μg/mL pamidronate with evidence of cell death at concentrations greater than 10 μg/mL pamidronate. Im et al. [20] similarly observed, at the highest concentration of alendronate used (10−4 mol/L), cellular proliferation was inhibited. In both of these studies, it was suggested this effect was probably the result of a toxic effect of bisphosphonates.
We found the ODM/alendronate system enhanced the differentiation of mouse MSCs into osteoblasts in a relatively dose-dependant manner. However, the real benefits of alendronate can be established only by histologic and clinical investigations. Moreover, our findings suggest alendronate has a beneficial effect at the cellular level and suggest it has clinical potential.
Footnotes
One of the authors (TRY) received funding from a Chonnam National University (Korea) research fund.
References
- 1.Adamia S, Maxwell CA, Pilarski LM. Hyaluronan and hyaluronan synthases: potential therapeutic targets in cancer. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:3–14. [DOI] [PubMed]
- 2.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. [DOI] [PubMed]
- 3.Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. [DOI] [PubMed]
- 4.Boonekamp PM, van der Wee-Pals LJ, van Wijk-van Lennep MM, Thesing CW, Bijvoet OL. Two modes of action of bisphosphonates on osteoclastic resorption of mineralized matrix. Bone Miner. 1986;1:27–39. [PubMed]
- 5.Chen CH, Ho ML, Chang JK, Hung SH, Wang GJ. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int. 2005;16:2039–2045. [DOI] [PubMed]
- 6.Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation. J Clin Periodontol. 1996;23:492–496. [DOI] [PubMed]
- 7.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–2082. [DOI] [PubMed]
- 8.Dahir GA, Cui Q, Anderson P, Simon C, Joyner C, Triffitt JT, Balian G. Pluripotential mesenchymal cells repopulate bone marrow and retain osteogenic properties. Clin Orthop Relat Res. 2000;379(suppl):S134–S145. [DOI] [PubMed]
- 9.Delos D, Yang X, Ricciardi BF, Myers ER, Bostrom MP, Camacho NP. The effects of RANKL inhibition on fracture healing and bone strength in a mouse model of osteogenesis imperfecta. J Orthop Res. 2008;26:153–164. [DOI] [PMC free article] [PubMed]
- 10.Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, Riccardi D. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci USA. 2004;101:5140–5145. [DOI] [PMC free article] [PubMed]
- 11.Evans CE, Braidman IP. Effects of two novel bisphosphonates on bone cells in vitro. Bone Miner. 1994;26:95–107. [DOI] [PubMed]
- 12.Fast DK, Felix R, Dowse C, Neuman WF, Fleisch H. The effects of diphosphonates on the growth and glycolysis of connective tissue cells in culture. Biochem J. 1978;172:97–107. [DOI] [PMC free article] [PubMed]
- 13.Felix R, Guenther HL, Fleisch H. The subcellular distribution of [14C]dichloromethylene bisphosphonate and [14C]1-hydroxyethylidene-1,1-bisphosphonate in cultured calvaria cells. Calcif Tissue Int. 1984;36:108–113. [DOI] [PubMed]
- 14.Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA. Alendronate mechanism of action: geranylgeraniol, an intermediate in the melavonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci USA. 1999;96:133–138. [DOI] [PMC free article] [PubMed]
- 15.Fromigue O, Body JJ. Bisphosphonates influence the proliferation and the maturation of normal human osteoblasts. J Endocrinol Invest. 2002;25:539–546. [DOI] [PubMed]
- 16.Giuliani N, Pedrazzoni M, Negri G, Passeri G, Impicciatore M, Girasole G. Biphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone. 1998;22:455–461. [DOI] [PubMed]
- 17.Gundberg CM, Hauschka PV, Lian JB, Gallop PM. Osteocalcin: isolation, characterization, and detection. Methods Enzymol. 1984;107:516–544. [DOI] [PubMed]
- 18.Hughes DE, MacDonald BR, Russell RG, Growen M. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest. 1989;83:1930–1935. [DOI] [PMC free article] [PubMed]
- 19.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487. [DOI] [PubMed]
- 20.Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials. 2004;25:4105–4115. [DOI] [PubMed]
- 21.Jamal HH, Aubin JE. CD44 Expression in fetal rat bone: in vivo and in vitro analysis. Exp Cell Res. 1996;223:467–477. [DOI] [PubMed]
- 22.Karu T. High-tech helps to estimate cellular mechanisms of low power laser therapy. Lasers Surg Med. 2004;34:298–299. [DOI] [PubMed]
- 23.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. [DOI] [PubMed]
- 24.Mackie PS, Fisher JL, Zhou H, Choong PF. Bisphosphonates regulate cell growth and gene expression in the UMR 106-01 clonal rat osteosarcoma cell line. Br J Cancer. 2001;84:951–958. [DOI] [PMC free article] [PubMed]
- 25.Mathov I, Plotkin LI, Sgarlata CL, Leoni J, Bellido T. Extracellular signal-regulated kinases and calcium channels are involved in the proliferative effect of bisphosphonates on osteoblastic cells in vitro. J Bone Miner Res. 2001;16:2050–2056. [DOI] [PubMed]
- 26.Mester E, Jaszsagi-Nagy E. The effects of laser radiation on wound healing and collagen synthesis. Studia Biophys. 1973;35:227–230.
- 27.Naidu A, Dechow PC, Spears R, Wright JM, Kessler HP, Opperman LA. The effects of bisphosphonates on osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:829–837. [DOI] [PubMed]
- 28.Oron U, Yaakobi T, Oron A, Hayam G, Gepstein L, Rubin O, Wolf T, Ben Haim S. Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation. Laser Surg Med. 2001;28:204–211. [DOI] [PubMed]
- 29.Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–234. [DOI] [PubMed]
- 30.Owen TA, Holthuis J, Markose E, van Wijnen AJ, Wolfe SA, Grimes SR, Lian JB, Stein GS. Modifications of protein-DNA interactions in the proximal promoter of a cell-growth-regulated histone gene during onset and progression of osteoblast differentiation. Proc Natl Acad Sci USA. 1990;87:5129–5133. [DOI] [PMC free article] [PubMed]
- 31.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. [DOI] [PubMed]
- 32.Plasmans CM, Jap PH, Kuijpers W, Slooff TJ. Influence of a diphosphonate on the cellular aspect of young bone tissue. Calcif Tissue Int. 1980;32:247–266. [DOI] [PubMed]
- 33.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. [DOI] [PMC free article] [PubMed]
- 34.Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000;60:6001–6007. [PubMed]
- 35.Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–2696. [DOI] [PMC free article] [PubMed]
- 36.Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA. Bisphosphonate action: alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095–2105. [DOI] [PMC free article] [PubMed]
- 37.Schmidt A, Rutledge SJ, Endo N, Opas EE, Tanaka H, Wesolowski G, Leu CT, Huang Z, Ramachandaran C, Rodan SB, Rodan GA. Protein-tyrosine phosphatase activity regulates osteoclast formation and function: inhibition by alendronate. Proc Nat Acad Sci USA. 1996;93:3068–3073. [DOI] [PMC free article] [PubMed]
- 38.Shanbhag AS. Use of bisphosphonates to improve the durability of total joint replacements. J Am Acad Orthop Surg. 2006;14:215–225. [DOI] [PubMed]
- 39.Sikavitsas VI, Temenoff JS, Mikos AG. Biomaterial and bone mechanotransduction. Biomaterials. 2001;22:2581–2593. [DOI] [PubMed]
- 40.van Beek E, Lowik C, van der Pluijm G, Papapoulos S. The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone explants in vitro: a clue to the mechanism of action of nitrogen-containing bisphosphonates. J Bone Miner Res. 1999;14:722–729. [DOI] [PubMed]
- 41.von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, Rubash HE, Shanbhag AS. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26:6941–6949. [DOI] [PubMed]