Abstract
With an aging population the frequency of postmenopausal fractures is increasing. Methods to enhance the repair of osteoporotic bone repair therefore become more important to reduce the society burden of care. We asked if absorbable collagen sponges containing recombinant human bone morphogenetic protein-2 (rhBMP-2) have the potential to enhance bone repair. We randomly assigned 40 rats into the ovariectomy and sham operation groups. A segmental defect was created in the right tibia 12 weeks after ovariectomy. rhBMP-2-containing absorbable collagen sponges were implanted into the defect in half of the animals in each group. We analyzed radiographs and histological sections and performed three-point bending tests to assess repair. Radiological scores in the rhBMP-2 applied rats were higher than those in controls at the end of 8 weeks after tibial osteotomy. The specimens failed under higher loads in the rhBMP-2-applied groups and histology revealed a higher fracture healing score, including callus formation, bone union, marrow changes, and cortex remodeling. We observed no adverse tissue responses such as fibrous connective tissue formation and inflammatory cellular infiltration. rhBMP-2 in absorbable collagen sponges enhanced bone repair in segmental tibial defects of ovariectomized rats. The sponges with rhBMP-2 appeared to enhance bone repair.
Introduction
In most industrialized societies the absolute numbers and percentages of the aged population is increasing. The number of postmenopausal fractures is also increasing, creating a greater burden of care on societies. Some studies [6, 8, 12, 13, 18] suggest little or no change in the ability of osteoporotic bone to repair, while others [11, 21, 32] suggest impairment in the quality and quantity of fracture callus. Rapid mobilization is clinically important to decrease morbidity and mortality [28], as well as the societal social and economic burdens.
Growth factors offer the potential to shorten the healing time and improve the quality of repair. The potential role of growth factors in fracture repair is well-documented [23]. Recombinant human bone morphogenic protein-2 (rhBMP-2) accelerates spinal fusion [2] and fracture repair [28, 29]. One recent experimental study [14] suggested recombinant human platelet-derived growth factor combined with beta-tricalcium phosphate/collagen matrix accelerated fracture repair in an osteoporotic rat model. Another study [10] reported local delivery of a recombinant adenovirus carrying BMP-2 cDNA enhanced healing of fractures in an ovine model of osteoporosis. One other study [30] combined bone marrow stromal cells with autogenous cells transfected with human BMP-2 and reported more mature bone formation at 8 weeks in rat mandible. Two other studies [7, 17] have also reported enhanced repair with rhBMP-2 treatment in ovariectomized rats.
To confirm these studies we asked whether rhBMP-2-containing absorbable collagen sponge (ACS) implantation would radiographically, mechanically, and histologically enhance segmental fracture repair in long bones of ovariectomized rats.
Materials and Methods
In this preliminary study, we randomly assigned 40 6-month-old female Wistar albino rats into the ovariectomy (OVX, n = 20) and the sham operation (SO, n = 20) groups. Twelve weeks after OVX, a 4-mm-long central segmental tibial defect was created at the right tibia in all rats. The left tibia, which was not operated, served as the control. rhBMP-2 (BMP)-containing ACS were implanted into the segmental defect in half of the animals (n = 10) in each group and collagen sponges without the growth factor (control) were implanted into the other half. The rats were followed for 8 weeks after tibial osteotomy and implantation and assessed for radiology scores, load (N) to failure and histology scores.
The rats were anesthetized with an intramuscular injection of 40 mg/kg ketamine and 10 mg/kg xylazine for ovariectomy. A mid-dorsal abdominal incision was made under sterile conditions and the abdominal muscles were retracted. The ovaries were identified, clamped, ligated, and removed. The muscle layers were tied with a single interrupted 4-0 Vicryl suture (Ethicon, Somerville, NJ) and the skin incision was closed with wound clips. Sham surgery was completed as above with the visualization of the ovary but without clamping and removal of the organs. Weight gain and decrease of BMD indicated the ovariectomy had its intended effect. The OVX group was heavier than the SO group at the time of bone surgery (378 ± 16 g versus 329 ± 15 g; p = 0.002) and at termination of the experiment (392 ± 17 g versus 326 ± 15 g; p < 0.001). Bone mineral density of the left tibia (that was not operated) of the OVX-BMP and OVX-CONTROL groups decreased from 0.2704 ± 0.02 to 0.2633 ± 0.01 and 0.2776 ± 0.01 to 0.2673 ± 0.01, respectively, whereas the SO-BMP and SO-CONTROL groups increased from 0.2753 ± 0.01 to 0.2825 ± 0.01 and 0.2750 ± 0.01 to 0.2965 ± 0.02, respectively from the time of ovariectomy to bone surgery (F6,52 = 2.7, p < 0.05).
To create the bone defect we used ketamine and xylazine anesthesia, and 0.5 mL lidocaine was additionally injected into the cutaneous site of surgery. The tibia was exposed by a lateral longitudinal incision and then a 4-mm segmental defect was created in the midshaft with a saw; this defect size was previously judged as “critical” and would not heal without treatment [17]. After irrigation of the operating field with saline, an 8-mm-long ACS (Kurtsan, Istanbul) disc (ø10 mm) was squeezed and implanted into the defect area. In the two experimental groups, rhBMP-2 (Sigma, St. Louis, Mo.) was dissolved in 0.1 M sterile phosphate-buffered saline containing 0.2% human serum albumin and the ACS was soaked in this solution for 15 minutes before insertion. One μM rhBMP-2 was loaded to all collagen sponges. In the control groups, the ACS was soaked in the solution for the same duration that did not contain the rhBMP-2. Stability of the ACS and segmental defect was maintained by an intramedullary Kirschner wire (1 mm in diameter) (Fig. 1). The Kirschner wire was first inserted from the osteotomy site into the proximal segment of the tibia, crossed this segment retrograde and penetrated the knee joint. Later, the Kirschner wire crossed the defect and distal segment of the tibia in the antegrade direction by securing the ACS in the segmental defect. The wound was closed with silk sutures and alignment of the ACS and bones were controlled by radiograph.
Fig. 1.
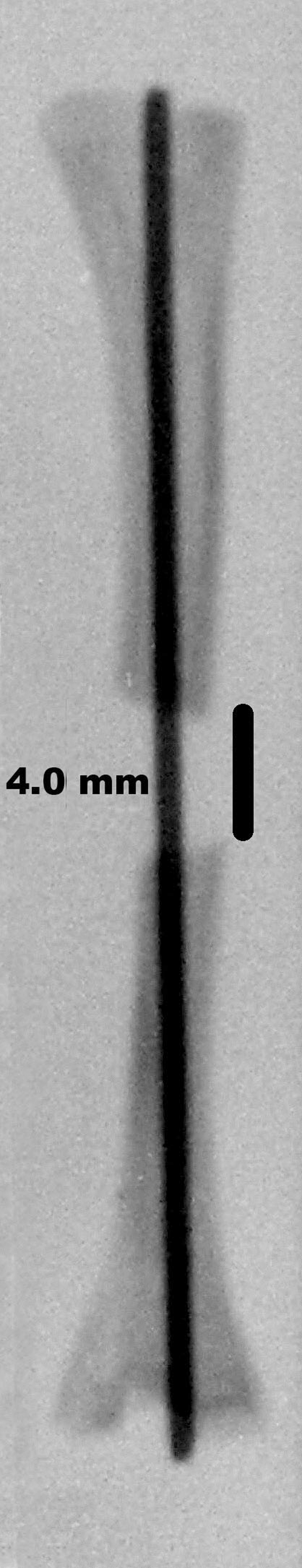
The 4-mm-long defect, shown in this radiograph of a rat tibia, was packed with absorbable collagen sponge that either contained or did not contain rhBMP-2.
Twenty mg/Kg tenoxicam (Tilcotil, Roche, Switzerland) and 50 mg/Kg cephalosporine-C (cefazolin, Antibioticos, Italy) were intramuscularly injected for postoperative analgesia and infection prophylaxis, retrospectively. Rats were allowed to move freely in their cages immediately after surgery without additional external fixation.
Anteroposterior and lateral radiographs were obtained on Agfa Mamoray MR3-II (Belgium) films with a Fisher Imaging HF-X (Denver, Col.) mammography machine 8 weeks after tibial osteotomy and implantation. The distance of the xray source to the bones was 75 cm. The setting of the machine was 25 kV, 100 mA, and 160 mAs. Films were developed using the Ecomat 21 (ELK Medical Products, Tokyo, Japan) automatic developing machine. Radiographs were evaluated by two independent observers (ORC, CA) who were blinded to the groups. They scored the radiographs for callus formation, quality of union, and bone remodeling according to Yang et al. [33] and Johnson et al. [15] (Table 1). Interobserver reliability (r) was 0.89.
Table 1.
Radiographic scoring system for fracture healing (maximum expected score is 8 for bone fracture repair)
| Categories | Scores | |||
|---|---|---|---|---|
| 3 | 2 | 1 | 0 | |
| Periosteal reaction | Full-across the defect | Moderate (> 50%) | Mild (< 50%) | None |
| Bone union | Full bone bridge union | Moderate bridge (> 50%) | Mild bridge (< 50%) | No new bone in the fracture line—nonunion |
| Remodeling | Full remodeling cortex | Mild remodeling (< 50%) | No remodeling | |
Total BMD (g/cm2) of rats was measured using the Lunar-DPX-IQ (Madison, WI) dual energy xray absorptiometry (DXA) device equipped with the small-animal software before and after ovariectomy at Week 12, and a third time at Week 8 after tibial osteotomy when the experiment was terminated. Animals were euthanized by cervical dislocation under 100 mg/Kg ketamine HCL (Ketalar, Eczacibasi, Istanbul) anesthesia. The tibia were dissected free from muscles and soft tissues and isolated by knee and ankle disarticulation. The whole tibia and the BMP-containing ACS implanted defect sites were evaluated with radiology, biomechanical tests, and histology.
Biomechanical tests were carried out on six tibia from each of the four groups (a total of 24 tibia). Load-to-failure strength was determined using the three-point bending test on a Lloyd universal mechanical testing device (Model LS 500, Southampton, UK). The tibiae were thawed to room temperature from −20ºC an hour before the tests. An axial load was applied at the midspan in the anterior-posterior direction with a span length of 32 mm [1]. Cross-head speed was 5 mm/min and the 1000 N load cell was used [31].
Four specimens from each group (a total of 16 specimens) were fixed in 10% phosphate-buffered formalin (pH 7.0) at room temperature, rinsed in buffer, and decalcified in deCastro solution. Specimens were dehydrated in a graded series of ethanol before embedding in paraffin. Four- to six-μm-thick longitudinal sections (n = 10) from the 4-mm defect where the rhBMP-2-containing ACS were implanted were prepared with a rotary microtome (Microm, HM 360, Germany). Hematoxylin and eosin and Mallory trichrome stained sections were evaluated for overall morphology, new bone formation, and tissue response. Stained sections (a minimum of 10 sections obtained from different levels of each tissue) were examined with a Leica DMR microscope (Germany); images were captured using the Leica DC500 digital camera (Germany). Histological findings were evaluated by two investigators (PK, DZ) for tissue response and fracture healing. Tissue response to the BMP containing ACS was scored according to Royals et al. [24] and fracture healing was scored according to An and Friedman [3] (Table 2). Interobserver reliability (r) for fracture repair and tissue response was 0.94 and 1.00, respectively.
Table 2.
Two-category histological scoring system for fracture healing and tissue response
| Categories | Parameters | Scores | ||||
|---|---|---|---|---|---|---|
| 3 | 2 | 1 | 0 | |||
| Fracture healing | Callus formation | Full-across the defect | Moderate (> 50%) | Mild (< 50%) | None | |
| Bone union | Full bone bridge union | Moderate bridge (> 50%) | Mild bridge (< 50%) | No new bone in the fracture line-nonunion | ||
| Marrow changes | Adult type fatty marrow | > 50% replaced by new tissue | < 50% replaced by new tissue | Fibrous tissue or red | ||
| Cortex remodeling | Full remodeling cortex | Moderate remodeling (> 50%) | Mild remodeling (< 50%) | No remodeling | ||
| Parameters | 4 | 3 | 2 | 1 | 0 | |
| Tissue response | Fibrous connective tissue formation | Severe deposition of dense collagen connective tissue around implant | Disruption of normal tissue architecture and presence of moderately dense fibrous connective tissue | Presence of moderate connective tissue | Presence of delicate spindle shaped cells or mild fibroplasia | No difference from normal control tissue, no presence of connective tissue at or around implant site |
| Inflammatory cellular infiltration | Severe cellular infiltrate response to implant or tissue necrosis at or around the site | Presence of large numbers of lymphocytes, macrophages and foreign body giant cells, also notable presence of eosinophils and neutrophils | Presence of several lymphocytes, macrophages with a few foreign body giant cells and a small focus of neutrophils | Presence of a few lymphocytes or macrophages, no presence of foreign body giant cells, eosinophils, or neutrophils | No difference from normal control tissue, no presence of macrophages, foreign body cells, lymphocytes, eosinophils, or neutrophils at or around implant site | |
Maximum expected score is 12 for bone fracture repair and 0 for tissue response.
Pearson product correlation coefficient was calculated for interobserver reliability. A series of one-way analysis of variance were conducted to evaluate differences in radiology scores, histology scores, and load-to-failure between the four groups. The data met the assumptions of a parametric test. Pair-wise comparison was conducted by the Tukey test when statistically significant (p < 0.05) values were obtained between groups.
Results
Bone mineral density of the operated tibia of the OVX-BMP group increased (p = 0.001) from the time of implantation to the time of termination, whereas the BMD values of the OVX-CONTROL group between these two time points remained almost constant (Table 3). The BMD of the SO-BMP group at the time of bone surgery was not different than the BMD of the OVX-CONTROL and the OVX-BMP groups. At termination, however, the BMD of the SO-BMP and the SO-CONTROL groups were higher (p = 0.002) than those of the OVX-BMP and the OVX-CONTROL groups. When the operated right and unoperated left tibia were compared at termination, the BMD value of the operated side was higher (p ≤ 0.01) in all groups.
Table 3.
Comparison of the BMD values of the right tibia that had a segmental defect and the implant
| Timepoint | Group | Average | SD |
|---|---|---|---|
| Before ovariectomy | SO-CONTROL | 0.2763a | 0.02 |
| SO-BMP | 0.2711 | 0.01 | |
| OVX-CONTROL | 0.2721 | 0.02 | |
| OVX-BMP | 0.2795 | 0.02 | |
| At the time of bone surgery (12 weeks after ovariectomy) | SO-CONTROL | 0.2975b | 0.02 |
| SO-BMP | 0.2700c | 0.01 | |
| OVX-CONTROL | 0.2675d | 0.08 | |
| OVX-BMP | 0.2638e | 0.01 | |
| At termination (8 weeks after bone surgery) | SO-CONTROL | 0.3039f | 0.01 |
| SO-BMP | 0.3064g | 0.01 | |
| OVX-CONTROL | 0.2650h | 0.01 | |
| OVX-BMP | 0.2796i | 0.01 |
Statistical significant differences: a–j p = 0.006; b–c,d,e p ≤ 0.02; f,g–h,i p ≤ 0.001.
SD = standard deviation.
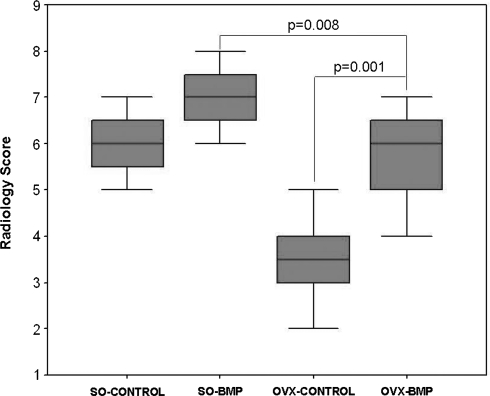
Radiological mean score of the OVX-BMP group was higher (p = 0.001) than that of the OVX-CONTROL group at termination. Radiological mean score of the OVX-BMP was lower (p = 0.008) than that of the SO-BMP group but not the SO-CONTROL group at termination (Fig. 2).
Fig. 2.
Radiological scores were higher in the OVX-BMP group compared to the OVX-CONTROL group at Week 8 of implantation. Radiological score of the SO-BMP group but not the SO-CONTROL group was higher than that of the OVX-BMP group.
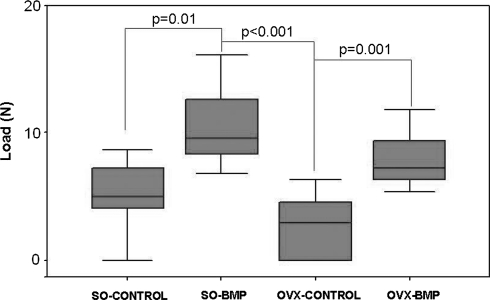
The mean load to failure value of the OVX-BMP group was higher (p = 0.001) than that of the OVX-CONTROL group at termination (Fig. 3). The highest mean load to failure value was obtained in the SO-BMP group. This value was higher (p = 0.01 and p < 0.001, respectively) than those of the SO-CONTROL and the OVX-CONTROL values. The mean load values of the nonoperated left tibiae however remained higher (p < 0.001) than the operated right tibiae.
Fig. 3.
The mean load-to-failure values of the SO-BMP and the OVX-BMP groups were higher than that of the SO-CONTROL and the OVX-CONTROL groups values at Week 8 of implantation, respectively.
Callus formation, cortex remodeling, and bridging at the fracture sites occurred in all groups. The cortical bone adjacent to fracture site was circumferentially filled with thick and regular cancellous bone in the two BMP groups. The periosteum was mitotically active and thickened. New trabecular bone islands at the periphery of the segmental fracture were observed in these groups. The spongy bone with its bone trabeculae was surrounded by active osteoblasts and resorptive osteoclasts. Together with the bone marrow islands in between the spongy bone and bone trabeculae presented a picture of a well-organized bone tissue in the SO-BMP group (Table 4). Callus formation was inferior; thus mild and/or no remodeling of the cortex was observed in the OVX groups when compared to that of the SO groups (Fig. 4A–D). However, the OVX-BMP group qualitatively appeared to provide better union, cortex remodeling, and callus formation when compared to that of the OVX-CONTROL group (Fig. 4C–D). We observed no fibrous connective tissue formation or inflammatory cellular infiltration in any of the groups.
Table 4.
Raw histological data of groups*
| Variable | Score at termination (week 8 of implantation) | ||||
|---|---|---|---|---|---|
| Parameters/groups | SO-CONTROL | SO-BMP | OVX-CONTROL | OVX- BMP | |
| Fracture repair | Callus formation | 3 | 3 | 2 | 3 |
| Bone union | 2 | 3 | 1 | 2 | |
| Marrow changes | 2 | 3 | 1 | 2 | |
| Cortex remodeling | 2 | 3 | 1 | 2 | |
| Total score | 9 | 12 | 5 | 9 | |
| Tissue response | Fibrous connective tissue formation | 0 | 0 | 0 | 0 |
| Inflammatory cellular infiltration | 0 | 0 | 0 | 0 | |
| Total score | 0 | 0 | 0 | 0 | |
* Due to low sample size, raw data is presented.
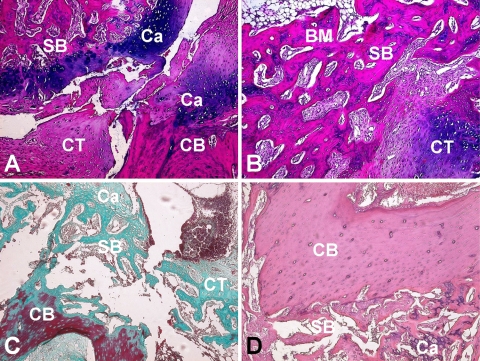
Fig. 4A–D.
(A) In the SO-CONTROL group about 50% of the fibrous tissue of the marrow was replaced by cartilage. Remodeling was mild to moderate and mild bridging was observed. Endochondral bone formation was observed (Stain, hematoxylin and eosin; original magnification ×100). (B) Callus formation across the defect and bone bridge union was almost full in the SO-BMP group. Adult type fatty marrow started to appear in this group (Stain, hematoxylin and eosin; original magnification ×100). (C) In the OVX-CONTROL group, callus formation was very mild. Bridging in the fracture line was sparse and fibrous tissue was dominating the marrow (Stain, Goldners’ Masson Trichrome; original magnification ×100). (D) In the OVX-BMP group well-organized young bone trabeculae were observed. Note that cortex remodeling was moderate to full and bone marrow was mostly replaced by new tissue. (Stain, hematoxylin and eosin; original magnification ×100). HE: Hematoxylin & Eosin; MT: Goldners’ Masson Trichrome; CB: Cortical Bone; SB: Spongy Bone; CT: Soft Callus/Connective Tissue; BM: Bone Marrow; Ca: Cartilage.
Discussion
Osteoporosis is a metabolic bone disease characterized by decrease in bone mass, deterioration of microstructure, and increased susceptibility to fracture [5]. Current research on osteoporosis focuses on the prevention and treatment of bone fractures, however, the exact etiology and reason of fracture is not well-known [4]. Knowledge about osteoporotic fractures is improving and various growth factors have recently been used to enhance bone repair [11, 14]. We therefore asked whether rhBMP-2-containing absorbable collagen sponge (ACS) implantation would radiographically, mechanically, and histologically enhance segmental fracture repair in long bones of ovariectomized rats.
Several limitations should be mentioned. First, while we used only one dose of rhBMP-2 (1 μM, a dose reported in the literature [16, 19]) our observations suggested the BMP was active. The optimal clinical dose of BMP-2 is reportedly 1.5 mg/ml when applied with a bovine collagen I matrix [26]; this dose is similar to the dose we used in our studies. We therefore did not perform a dose-response experiment in our study. Second, we evaluated the defects at one time point only due to Ethical Committee regulations for preliminary studies. Rat tibial diaphyseal fractures heal between 3 and 9 weeks with a median of 6 to 8 weeks [3]. We therefore evaluated our samples at Week 8 only in this study. Third, we did not establish a control group that did not contain the ACS due to technical reasons. Fixation with a Kirschner wire allowed only partial load bearing [1] at the ACS implantation site and the relatively large defect would not likely have been able to remain fixed without the ACS.
The application of rhBMP-2 with ACS into the segmental defects of tibia improved radiographically observed repair, while ovariectomy diminished it. Radiological findings of this study were consistent with other studies [9, 17, 27] (Table 5). In one recent study [9], bridging, thickening, and remodeling of the callus after BMP administration using polypropylene fumarate and tricalcium phosphate as the carrier were better than those in the control group after 6, 12, and 15 weeks. Although that study [9] used a more simple radiological scoring system than this study at Week 8 after tibial osteotomy periosteal reaction, bone bridge union and remodeling were full in this study. Defect mineralization by radiology as assessed in another previous study [17] was not assessed in this study. Indeed, BMD using DXA was assessed in this study. Ovariectomy caused a decrease and rhBMP-2 administration increased BMD at the site of the tibial defect.
Table 5.
Comparison of similar studies to the current study
| Variable | Bostrom et al. [7] | Egermann et al. [10] | Tang et al. [30] | Sarban et al. [current study] |
|---|---|---|---|---|
| Experimental model | Ulnar defect in rabbit (not an osteoporosis model) | Tibial osteotomy in overiectomized (OVX) sheep | Mandibular defect in OVX rat | Tibial defect in OVX rat |
| Source of BMP | rhBMP-2 | Adenoviral transduction of mesenchymal stem cells and osteoblasts | Transfection of bone marrow stromal cells with plasmid | rhBMP-2 |
| Carrier scaffold | Polylactic glycolic acid/blood clot | — | Macroporous coral hydroxyapatite | Absorbable collagen sponge |
| Radiology | Dose dependent response in the healing of defect | QCT analyses showed larger cross-sectional callus area. | Bone formation on the transfected bone marrow stromal cell seeded scaffold | Mean radiological score was significantly higher in the BMP-2/OVX group than the only OVX group |
| Biomechanics | Torsion test revealed BMP-2 group more stiffer than the control but it was not dose dependent | Bending and torsion tests showed stiffer callus tissue in the adenoviral BMP-2 group | — | Three-point bending test revealed higher load value in the BMP-2/OVX group than the its peer without BMP-2 application |
| Histology | Qualitative analyses revealed a dose dependent bone healing rate | Quantitative analyses showed no difference between the groups in terms of the number of inflammatory cells | Qualitative analyses showed the critic size defect completely filled by the trabecular bone at the end of 8-week in experimental group | Quantitative analyses revealed superior callus formation (better bone union, cortex remodeling and callus formation) in the BMP-2 groups |
| Bone mineral density measurement | — | QCT analyses showed higher mineral density in the adenoviral BMP-2 | — | DXA measurements revealed higher BMD in the BMP-2 groups (data not presented) |
Bone strength was higher in the rhBMP-2-applied tibia when compared to the control tibia in both groups. When human platelet derived growth factor (PDGF) was combined with a tricalcium phosphate and collagen matrix in another study [14], in contrast to rhBMP-2 application, increase in mechanical strength was not obtained. We therefore presume rhBMP-2 instead of PDGF has a positive effect on bone strength. In previous studies [17, 20] where rat femora were tested under torsion and bending, respectively, BMP-silk composites in the former and vitamin D administration in the later did not improve the mechanical properties. We studied bending rather than torsion. One other experimental study [21] revealed an increase in failure load, stiffness, stress, and energy after OVX. In that study in which skeletally mature mongrel dogs with 2.5-cm-long bilateral radial osteotomies were assessed, a dose-dependent increase in mechanical properties with rhBMP-2-soaked collagen sponge application was observed. Our findings were similar to those in that study, however, we did not assess the dose effect. Ovariectomy, on the other hand, did not alter mechanical properties of the nonoperated tibia. Our observations on the nonoperated tibia after OVX were consistent with those from other studies [6, 7, 22].
Histological findings revealed superior callus formation and calcification in the BMP groups compared to the control groups. Ovariectomy deteriorated callus calcification and rhBMP-2 application enhanced not only calcification but also callus organization. Ossification was also much better in the OVX-BMP group compared to that of the OVX-CONTROL group. These findings were consistent with other studies [25, 30] reporting mature bone at the end of 8 weeks in defects created in the ramus of the mandible of osteoporotic rats.
Our observations suggest rhBMP-2-containing ACS is a promising approach for bone repair in OVX rats. Bone strength increased in the rhBMP-2 applied tibia. Callus formation and calcification were better in the BMP applied groups compared to the control groups. Findings were in line with previous experimental studies [6, 13, 14, 16, 18, 21] which indicated that OVX deteriorated fracture healing and rhBMP-2 enhances bone repair.
Acknowledgments
We thank Dr. Hasan Bilgili of Ankara University for conducting the ovariectomies; Dr. Sedat Ulger for evaluating the radiological images; Dr. Tevfik Sabuncu, Dr. Müge Harma, and Dr. Mehmet Harma of Harran University for their help in creating the osteoporosis rat model; Arzu Tasci of Middle East Technical University for assisting with the biomechanical tests and Dr. Sedat Isikli of Hacettepe University for statistical analysis. Orhan Resit Cubukcu MD and Celal Aydin MD of Middle East Technical University Medical Center Radiodiagnostic Unit independently evaluated and scored the xray pictures. Dilara Zeybek MD of Hacettepe University Faculty of Medicine, Department of Histology and Embryology, assisted blinded evaluation and scoring of the histological sections.
Footnotes
This project was partly supported by Harran University Research Fund (HUBAK 436). Kurtsan Inc. (Istanbul, Turkey) supplied the absorbable collagen sponges for this study.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Middle East Technical University.
References
- 1.Akkus O, Korkusuz F, Akin S, Akkas N. Relation between mechanical stiffness and vibration transmission of fracture callus: an experimental study on rabbit tibia. Proc Instn Mech Eng. 1998;212:327–336. [DOI] [PubMed]
- 2.Alanay A, Chen C, Lee S, Murray SS, Brochmann EJ, Miyazaki M, Napoli A, Wang JC. The adjunctive effect of a binding peptide on bone morphogenetic protein enhanced bone healing in a rodent model of spinal fusion. Spine. 2008;33:1709–1713. [DOI] [PubMed]
- 3.An YH, Friedman RJ. Animal models of bone defect repair. In: An YH, Friedman RJ, eds. Animal Models in Orthopedic Research. Boca Raton, FL: CRC Press LLC; 1999:241–260.
- 4.Atik OS. FRAX™ and Turkey: osteoporotic fracture risk assessment. Joint Dis Relat Surg. 2008;19:100.
- 5.Atik OS, Gunal I, Korkusuz F. Burden of osteoporosis. Clin Orthop Relat Res. 2006;443:19–24. [DOI] [PubMed]
- 6.Blythe JG, Buchsbaum HJ. Fracture healing in estrogen-treated and castrated rats. Obstet Gynecol. 1976;48:351–352. [PubMed]
- 7.Bostrom M, Lane J, Tomin E, Browne M, Berberian W, Turek T, Smith J, Wozney J, Schildhauer T. Use of bone morphogenetic protein-2 in the rabbit ulna nonunion model. Clin Orthop Rel Res. 1996;327:272–282. [DOI] [PubMed]
- 8.Chao EY, Inoue N, Koo TK, Kim YH. Biomechanical considerations of fracture treatment and bone quality maintenance in elderly patients and patients with osteoporosis. Clin Orthop Relat Res. 2004;425:12–25. [DOI] [PubMed]
- 9.Chu TM, Warden SJ, Turner CH, Stewart RL. Segmental bone repair using a load-bearing biodegradable carrier of bone morphogenetic protein-2. Biomaterials. 2007;28:459–467. [DOI] [PMC free article] [PubMed]
- 10.Egermann M, Baltzer AW, Adamaszek S, Evans C, Robbins P, Schneider E, Lill CA. Direct adenoviral transfer of bone morphogenetic protein-2 cDNA enhances fracture healing in osteoporotic sheep. Hum Gene Ther. 2006;17:507–517. [DOI] [PubMed]
- 11.Egermann M, Schneider E, Evans CH, Baltzer AW. The potential of gene therapy for fracture healing in osteoporosis. Osteoporos Int. 2005;16(Suppl 2):S120–S128. [DOI] [PubMed]
- 12.Giannoudis P, Tzioupis C, Almalki T, Buckley R. Fracture healing in osteoporotic fractures: is it really different? A basic science perspective. Injury. 2007;38(Suppl 1):S90–S99. [DOI] [PubMed]
- 13.Gürkan L, Ekeland A, Gautvik KM, Langeland N, Rønningen H, Solheim LF. Bone changes after castration in rats. A model for osteoporosis. Acta Orthop Scand. 1986;57:67–70. [DOI] [PubMed]
- 14.Hollinger JO, Onikepe AO, MacKrell J, Einhorn T, Bradica G, Lynch S, Hart CE. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-BB and an injectable beta-tricalcium phosphate/collagen matrix. J Orthop Res. 2008;26:83–90. [DOI] [PubMed]
- 15.Johnson KD, Frierson KE, Keller TS, Cook C, Scheinberg R, Zerwekh J, Meyers L, Sciadini MF. Porous ceramics as bone graft substitutes in long bone defects: a biomechanical, histological, and radiographic analysis. J Orthop Res. 1996;14:351–369. [DOI] [PubMed]
- 16.Keskin DS, Tezcaner A, Korkusuz P, Korkusuz F, Hasirci V. Collagen-chondroitin sulfate-based PLLA-SAIB-coated rhBMP-2 delivery system for bone repair. Biomaterials. 2005;26:4023–4034. [DOI] [PubMed]
- 17.Kirker-Head C, Karageorgiou V, Hofmann S, Fajardo R, Betz O, Merkle HP, Hilbe M, von Rechenberg B, McCool J, Abrahamsen L, Nazarian A, Cory E, Curtis M, Kaplan D, Meinel L. BMP-silk composite matrices heal critically sized femoral defects. Bone. 2007;41:247–255. [DOI] [PMC free article] [PubMed]
- 18.Lindholm TS. Studies of the skeletal mass and bone formation of tibial fracture callus and femoral bone in growing osteopenic rats. Acta Chir Scand Suppl. 1974;449:37–42. [PubMed]
- 19.Maeda H, Sano A, Fujioka K. Controlled release of rhBMP-2 from collagen minipellet and the relationship between release profile and ectopic bone formation. Int J Pharm. 2004;275:109–122. [DOI] [PubMed]
- 20.Melhus G, Solberg LB, Dimmen S, Madsen JE, Nordsletten L, Reinholt FP. Experimental osteoporosis induced by ovariectomy and vitamin D deficiency does not markedly affect fracture healing in rats. Acta Orthop. 2007;78:393–403. [DOI] [PubMed]
- 21.Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;281:80–86. [DOI] [PubMed]
- 22.Nordsletten L, Madsen JE. The effect of bone morphogenetic proteins in fracture healing. Scand J Surg. 2006;95:91–94. [DOI] [PubMed]
- 23.Rosier RN, O’Keefe RJ, Hicks DG. The potential role of transforming growth factor beta in fracture healing. Clin Orthop Relat Res. 1998;355 Suppl:S294–S300. [DOI] [PubMed]
- 24.Royals MA, Fujita SM, Yevey GL, Rodriguez J, Schultheiss PC, Dunn RL. Biocompatibility of a biodegradable in situ forming implant system in Rhesus monkeys. J Biomed Mater Res. 1999;45:231–239. [DOI] [PubMed]
- 25.Schliephake H, Weich HA, Dullin C, Gruber R, Frahse S. Mandibular bone repair by implantation of rhBMP-2 in a slow release carrier of polylactic acid–an experimental study in rats. Biomaterials. 2008;29:103–110. [DOI] [PubMed]
- 26.Schmidmaier G, Schwabe P, Wildemann B, Haas NP. Use of bone morphogenetic proteins for treatment of non-unions and future perspectives. Injury. 2007;38-S4:S35–S41. [DOI] [PubMed]
- 27.Sciadini MF, Johnson KD. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. J Orthop Res. 2000;18:289–302. [DOI] [PubMed]
- 28.Sener M, Onar V, Kazimoglu C, Yagdi S. Mortality and morbidity in elderly patients who underwent partial prosthesis replacement for proximal femoral fractures. Joint Dis Relat Surg. 2009;20:11–17. [PubMed]
- 29.Swiontkowski MF, Aro HT, Donell S, Esterhai JL, Goulet J, Jones A, Kregor PJ, Nordsletten L, Paiement G, Patel A. Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am. 2006;88:1258–1265. [DOI] [PubMed]
- 30.Tang Y, Tang W, Lin Y, Long J, Wang H, Liu L, Tian W. Combination of bone tissue engineering and BMP-2 gene transfection promotes bone healing in osteoporotic rats. Cell Biol Int. 2008;32:1150–1157. [DOI] [PubMed]
- 31.Tou JC, Foley A, Yuan YV, Arnaud S, Wade CE, Brown M, Tou JC, Foley A, Yuan YV, Arnaud S, Wade CE, Brown M. The effect of ovariectomy combined with hindlimb unloading and reloading on the long bones of mature Sprague-Dawley rats. Menopause. 2008;15:494–502. [DOI] [PubMed]
- 32.Xu SW, Yu R, Zhao GF, Wang JW. Early period of fracture healing in ovariectomized rats. Chin J Traumatol. 2003;6:160–166. [PubMed]
- 33.Yang CY, Simmons DJ, Lozano R. The healing of grafts combining freeze-dried and demineralized allogeneic bone in rabbits. Clin Orthop Relat Res. 1994;298:286–295. [PubMed]