Abstract
Bone morphogenic proteins (BMPs) may have neurotrophic functions but there is limited evidence of these functions in the peripheral nervous system. We therefore investigated the expression of BMPs and BMP receptors (BMPRs) in normal and injured peripheral nerves. In 10 of 15 Sprague-Dawley rats, a 3-mm segment of sciatic nerve was resected at the trifurcation in the thigh. One day (n = 5) and 7 days (n = 5) after transection, proximal and distal stumps were removed and immunohistochemically analyzed for BMP-2, -7, BMPR-1A, -1B, and -2. The other five animals served as normal controls. In normal nerves, BMP-2 expression was localized at Ranvier’s node, and BMP-7 and BMPR-1B were expressed in several axon-Schwann cell units, whereas other receptors were not expressed. After nerve transection, BMP-7 expression was upregulated at both proximal and distal stumps along with Schwann cell columns during Wallerian degeneration. BMPRs were also upregulated compared with the normal nerve. The upregulation in BMP expression after nerve transection suggests that BMPs may play a role in the healing response of the peripheral nerve.
Introduction
Unlike the central nervous system (CNS), axons can be regenerated by surgically suturing the ends of the proximal and distal stumps after a nerve injury [16]. Unfortunately, functional recovery has been incomplete because of obstacles such as axonal degeneration or scar tissue resulting from the injury or surgical intervention [17]. Moreover, regenerated axons may not function effectively even after reaching distal end organs unless they arrive close to their original site [11, 16, 17, 22]. To resolve these obstacles, a new strategy is needed for peripheral nerve repair after injury.
Neurotrophic factors influence and facilitate peripheral nerve regeneration [2, 25]. These molecules control the generation, survival, differentiation, and regeneration of neurons in the PNS and CNS [2, 25]. Until recently, although various neurotrophic factors were reported, nerve growth factor (NGF), glial cell-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3/4 (NT-3/4) were considered important molecules in these situations and decreased axonal degeneration and neuronal apoptosis [8, 18, 26]. Recent studies demonstrate recombinant human BMPs are capable of promoting regeneration in various tissues other than bone such as tendon or cartilage [7, 31] and that they also possess neurotrophic functions, such as regulation of neuronal survival and differentiation [21, 28, 29].
The BMPs constitute the largest group within the superfamily of transforming growth factors-β and are important during embryogenesis, neuronal commitment, and synapse formation [6, 9]. While there are some studies of the role of BMPs on neurons or glial cells [21, 28, 29], the distribution of BMPs in normal and injured peripheral nerves remains unknown. Helm et al. outlined potential clinical applications of recombinant human BMP-2 and BMP-7 in neurosurgery [7]. The administration of BMPs could also be useful as a new strategy for the peripheral nerve repair. Identifying and understanding the endogenous role of BMPs in nerve repair is necessary for successful therapeutic intervention in peripheral nerve injury.
Our purposes were therefore to determine (1) whether BMP-2, -7 and their receptors (BMPRs) are expressed in the normal sciatic nerves of rats; (2) if so, where they are distributed in transected nerves; and (3) whether BMPs and BMPRs are expressed in the vessels within the perineurium after nerve transection.
Materials and Methods
We performed experiments on 15 male Sprague-Dawley rats. To investigate expression of BMPs and BMPRs in normal and injured nerves, we performed immunohistochemical analyses with the sciatic nerves in normal (n = 5) and nerves 1 day (n = 5) and 7 days (n = 5) after nerve transection to observe the distribution in the early phase and during Wallerian degeneration. Animals were 8 weeks old and weighed approximately 300 g (SLC, Hamamatsu, Japan). Animals were housed in a temperature-controlled environment and maintained with a 12-hour light-dark cycle with food and water available ad libitum. The experimental protocol was approved by the committee of animal research at Mie University.
Animals were anesthetized deeply with an intramuscular injection of ketamine (100 mg/kg) and xylazine (3 mg/kg). Under aseptic conditions, we made a skin incision in the shaved right thigh, the overlying gluteal muscles were opened and retracted, and the nerve was exposed. We cut the right sciatic nerve with microsurgical scissors at the trifurcation of the thigh. The distal nerve was resected for a distance of 3 mm to prevent regeneration. We then closed the wound in anatomic layers using 5-0 nylon sutures. Buprenorphine at 0.1 mg/kg was administered with an intramuscular injection prior to recovery from anesthesia. No signs of pain, distress or skin problems were observed after surgery.
At 1 day or 7 days after surgery, deeply anesthetized animals were transcardially perfused with 1% heparin in phosphate-buffered saline (PBS; pH 7.2) and then with 4% paraformaldehyde in PBS, after which we harvested the 5 mm segment distal and proximal stumps of the sciatic nerve and immediately fixed them in 4% paraformaldehyde at 4ºC overnight. The other five animals served as normal controls and had their sciatic nerves removed according to the previously mentioned method.
We embedded the specimens in paraffin, and longitudinal sections were cut at 4 μm on a microtome. Six to 10 sections were dewaxed in xylene and rehydrated in graded ethanol (99% to 70% [v/v]) in distilled water. Endogenous peroxidase activity was quenched by 30-minute incubation with 0.3% (v/v) H2O2 in 99% methanol. Heat-induced antigen retrieval was applied with a pressure cooker (Delicio 6L; T-FAL, Rumily, France). The soaking solution used was 10 mM citrate buffer (pH 6.0). After pressure cooking, we left the sections to cool at room temperature in the soaking solution. Nonspecific staining was blocked by a solution of 1% bovine serum albumin (BSA) for 20 minutes at room temperature. BSA was then tipped off and specimens were incubated at room temperature overnight with each antibody (1:50). The primary antibodies used were against BMP-2, -7, BMPR-1A, -1B, and -2 (Santa Cruz Biotechnology, Santa Cruz, CA). Bound primary antibodies were detected by applying 1:100 secondary anti-goat antibodies conjugated with horseradish peroxidase (Dako Japan, Kyoto, Japan) for 1 hour at room temperature. Bound antibodies were visualized by reacting with 3–3′ diaminobenzidine. Between incubation steps, sections were dip immersion washed (3 × 5-minute wash) in PBS to eliminate excess nonbound antibody or reagent. Sections were counterstained using hematoxylin. For isotype control, the samples were incubated with isotype-matched IgGs (Dako Japan) in place at the primary antibodies. Ninety sections were examined using a microscope (BX50; Olympus, Tokyo, Japan) equipped with a digital camera. In all cases, control sections showed no evidence of staining or color reaction under microscopic examination.
Two of us (MT and KA) independently evaluated the staining level of the axon-Schwann cell units and vessel within the perineurium in the normal nerve, proximal stump, and distal stump after nerve transection on the following semiquantitative scale. The degree of positive staining for BMPs and BMPRs was evaluated by scoring intensity on a scale of 1–4 (I: none, mild, moderate, and strong), and distribution on a scale of 1–4 (D: none, focal patchy and diffuse). Tissue with I × D less than or equal to 4 were considered ‘weakly positive’, those with I × D greater than 4 were designated ‘strongly positive’ and those with no immunoreactivity were designated as ‘absent’ [27]. The intraobserver and interobserver variability studies were performed on 75 slides and by two observes. The kappa values for staining level at 2 times were 0.98, 0.90, respectively.
Results
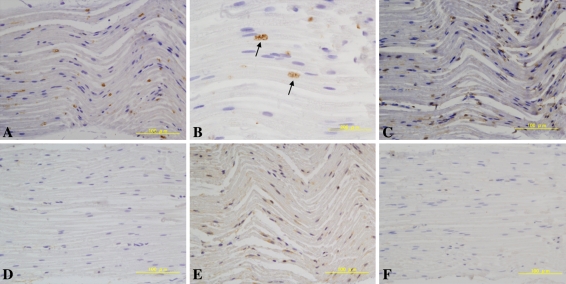
We observed BMP-2, -7 immunoreactivity and BMPR-1B in the normal sciatic nerve (Fig. 1). The expression of BMP-2 was localized at both distal and proximal edges of myelinating Schwann cells in the axon-Schwann cell units and seemed to occur at the nodes of Ranvier (Fig. 1A–B). BMP-7 was weakly expressed in the cytoplasm of several myelinated Schwann cells (Fig. 1C). BMPR-1B was weakly expressed in several axon-Schwann cell units, whereas there was no or little expression of BMPR-1A or -2 (Fig. 1D–F).
Fig. 1A–F.
Immunolabeling for bone morphogenetic protein (BMP) -2 and BMP receptors (BMPRs) in the normal sciatic nerve is shown. (A–B) BMP-2 was expressed at Ranvier’s nodes. (C, E) BMP-7 and BMPR-1B were expressed weakly in the cytoplasm of several myelinated Schwann cells. (D, F) BMPR-1A and BMPR-2 were not visualized. (A) BMP-2; original magnification, ×200, (B) BMP-2; original magnification, ×400, (C) BMP-7; original magnification, ×200, (D) BMPR-1A; original magnification, ×200, (E) BMPR-1B; original magnification, ×200, (F) BMPR-2; original magnification, ×200.
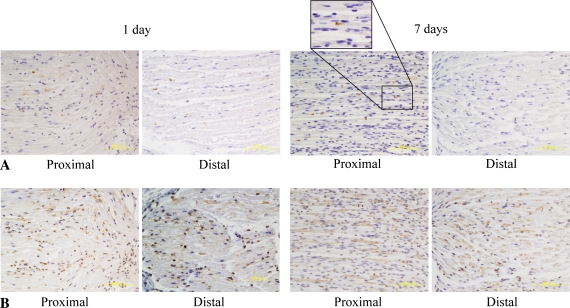
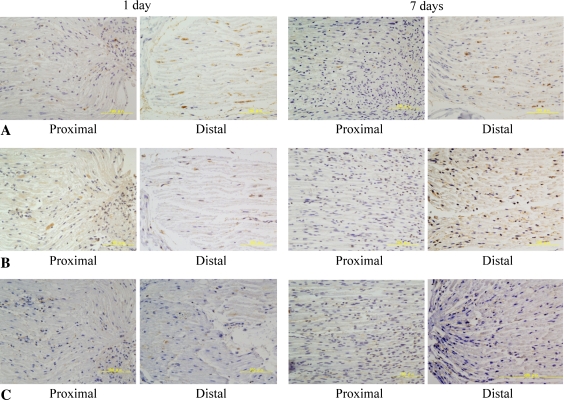
BMP-2 expression in the proximal stump was unchanged 1 day after nerve transection. However, by 7 days after transection, axonal degeneration of the proximal stump was observed to invade to the edge of the myelinating Schwann cells expressing BMP-2 (Fig. 2A; Table 1). These findings were consistent with the fact that the axonal degeneration of the proximal stump involved the most distal nodes of Ranvier. In contrast, the expression of BMP-2 in the distal stump was notably decreased (Fig. 2A; Table 1) when the myelinating Schwann cells forming the nodes changed to the proliferating type during Wallerian degeneration. The expression of BMP-7 was upregulated at the end of both proximal and distal stumps 1 day after the nerve transection. By 7 days, BMP-7 was strongly upregulated in the axon-Schwann cell units at the distal stump and at the distal end of the proximal stump (Fig. 2B; Table 1). The distribution was longitudinal, suggesting BMP-7 was expressed along with Schwann cells forming Bungner’s bands. BMPRs were also upregulated proximal and distal to the injury site (Fig. 3; Table 1). These findings suggest BMP signaling plays an important role in axonal regeneration.
Fig. 2A–B.
Immunolabeling for bone morphogenetic protein (BMP) -2 and -7 in axon-Schwann cell units after nerve transaction is shown. (A) There was no change of BMP-2 expression in the proximal stump. In contrast, the expression of BMP-2 was considerably decreased 7 days after nerve transection in the distal stump. The observation of proximal stumps under high magnification showed axonal degeneration invaded the most distal nodes of Ranvier, where BMP-2 was expressed. BMP-2; original magnification, ×200 (B) BMP-7 was upregulated at and distal to the nerve injury 7 days after transection. The distribution was longitudinally arranged, suggesting a relationship with Schwann cell columns during Wallerian degeneration. BMP-7; original magnification, ×200.
Table 1.
Immunoreactivity for BMPs and BMPRs in ASU and vessels within the perineurium
| Type | Location | Normal | Proximal stumps | Distal stumps | ||
|---|---|---|---|---|---|---|
| 1 day | 7 days | 1 day | 7 days | |||
| BMP-2 | ASU | Strong | Strong | Strong | Strong | Weak |
| Vessel | Weak | Weak | Weak | Weak | Weak | |
| BMP-7 | ASU | Weak | Strong | Strong | Strong | Strong |
| Vessel | Weak | Strong | Strong | Strong | Strong | |
| BMPR-1A | ASU | Absent | Weak | Weak | Strong | Strong |
| Vessel | Absent | Absent | Absent | Absent | Absent | |
| BMPR-1B | ASU | Weak | Weak | Weak | Weak | Strong |
| Vessel | Absent | Weak | Absent | Absent | Absent | |
| BMPR-2 | ASU | Absent | Weak | Weak | Weak | Weak |
| Vessel | Absent | Absent | Absent | Absent | Absent | |
BMP = bone morphogenetic protein; BMPR = bone morphogenetic protein receptor; ASU = axon-Schwann cell unit.
Fig. 3A–C.
Immunolabeling for bone morphogenetic proteins (BMPRs) in axon-Schwann cell units after nerve transaction is shown. (A–B) BMPR-1A and -1B were considerably upregulated by 7 days in the distal stump. (C) BMPR-2 was expressed weakly in the myelin at the injury site and distal to this after the nerve transection. (A) BMPR-1A; original magnification, ×200, (B) BMPR-1B; original magnification, ×200, (C) BMPR-2; original magnification, ×200.
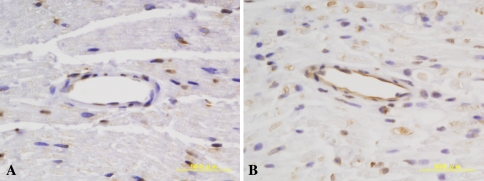
When compared with the normal nerve, the expression of BMP-7 qualitatively appeared upregulated by 7 days at the injured site (Fig. 4; Table 1). On the other hand, there was little or no change of the expression of BMP-2 and BMPRs in the vessels after the nerve transection.
Fig. 4.
Bone morphogenetic protein (BMP) -7 expression in the vessels within perineurium. Angiogenesis was also observed within the basal laminae during axonal regeneration. The expression of BMP-7 was clearly upregulated after nerve transection. BMP-7; original magnification, ×400.
Discussion
BMPs play an important role in the growth and development of numerous tissues, including bone, brain, and spinal cord [7]. In addition, although the literature contains many in vitro studies showing the neurotrophic function of BMPs [1, 3, 10, 12, 15], their distribution and the pattern of expression has not previously been demonstrated in vivo. We therefore determined (1) whether BMP-2, -7 and their receptors (BMPRs) are expressed in the normal sciatic nerves of rats; (2) if so, where they are distributed in the injured nerve; and (3) whether BMPs and BMPRs are expressed in the vessels within the perineurium after the nerve transection.
Our study has several major limitations in researching of the role of BMPs and BMPRs in the axonal regeneration of the PNS. First, we used only qualitative examination of immunohistochemical analyses but neither other quantitative analyses such as Western blotting or real-time polymerase chain reaction nor higher resolution assays such as in situ hybridization or transmission electron microscopy. Thus, we consider ours a preliminary study. Second, we used no markers of the expressing areas or cells (eg, node of Ranvier or Schwann cells). Third, we had no experimental groups of an axonal regeneration model. Thus, the details of the signaling pathway remain to be investigated, but expression of BMPs and BMPRs were clearly shown in the normal and the injured nerves, indicating that BMPs may play important role in axonal regeneration of PNS.
BMP-2 expression in the normal sciatic nerve was localized at the edges of myelinating Schwann cells. These areas, known as the nodes of Ranvier, incorporate voltage-gated ion channels, cell adhesion molecules, and cytoskeletal proteins, and they play active roles in regulating neuronal excitability and function in the PNS [23]. Although the underlying molecular mechanisms remain elusive, BMP-2 may play a crucial role in organizing membrane domains or axon-myelin contact at and near the nodes of Ranvier in the adult peripheral nerve [13].
Several studies suggest treatment with a combination of BMPs and other neurotrophic factors can substantially increase neurite outgrowth [1, 3, 15] and that the application of BMPs to Schwann cells increased the level of neurotrophic factors expressed [12]. Based on these results, Kinameri et al. speculated that the autocrine action of BMP-2 regulated expression of important neurotrophic factors in Schwann cells [12]. We observed decreased expression of BMP-2 in the distal stump, indicating their hypothesis is incorrect with neurotmesis. However, the exogenous application of BMP-2, which is expressed in reduced amounts after nerve transection, could promote axonal regeneration by regulating the main neurotrophic factors, NGF, or GDNF. In a rabbit model of crush injury of the facial nerve, Wang et al. reported local administration of recombinant human BMP-2 decreased pathologic changes, such as disappearance or degeneration of axons, in which neurotrophic factors were likely involved [30].
In inflammation, tissue repair/regeneration, or tumor invasion, there is reexpression of proteins abundantly expressed during development. In the CNS, BMP ligands and their receptor subunits are expressed throughout development, and, among other functions, they help regulate cellular proliferation, survival, differentiation, apoptosis, and lineage commitment [5, 32]. Thus, BMPs play an important role during neurogenesis, whereas the expression of BMP-7 and BMP receptors is absent or minimal in the adult normal nerve as suggested by our data. However, the expression of BMP-7 and their receptors increased at the injury site after nerve transection. Okuyama et al. [19] demonstrated several Smad family members, which are downstream molecules of BMP signaling, were upregulated at the injury site and the neuron after nerve resection of the hypoglossal nerve of the rats. Taken together, these findings suggest BMP signaling is involved in axonal regeneration [19].
In the CNS, treatment with BMP-7 before generalized hypoxia or ischemia reduced brain infarction volume and mortality in rats [14, 20]. Additionally, administration of BMP-7 after focal cerebral stroke enhanced recovery of sensorimotor function in the impaired limbs, decreased body asymmetry, and increased locomotor activity [4, 24]. We found BMP-7 was upregulated in the vessels and axon-Schwann cell units within the basal laminae of the nerve. Longitudinal arrangement of BMP-7 expression was observed at and distal to the injured nerve when the Schwann cells formed columns. BMP-7 may promote axonal growth by guiding or maintaining Schwann cell columns because these are a very favorable environment for regenerating axons. Application of BMP-7 may result in neuroprotective and neuroregenerative effects in the PNS as well as the CNS.
Our preliminary data demonstrate the expression of BMP-2, -7, and BMPRs in normal and injured peripheral nerves. In the normal nerve, BMPs, particularly BMP-2, may regulate neuronal function and excitability by maintaining and regulating axon-myelin contact. After transection of the nerve there was activation of BMP signaling, which may suggest these molecules may play a role in the peripheral nerve’s response to injury.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Althini S, Usoskin D, Kylberg A, ten Dijke P, Ebendal T. Bone morphogenetic protein signalling in NGF-stimulated PC12 cells. Biochem Biophys Res Commun. 2003;307:632–639. [DOI] [PubMed]
- 2.Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. [DOI] [PubMed]
- 3.Bengtsson H, Soderstrom S, Kylberg A, Charette MF, Ebendal T. Potentiating interactions between morphogenetic protein and neurotrophic factors in developing neurons. J Neurosci Res. 1998;53:559–568. [DOI] [PubMed]
- 4.Chang CF, Lin SZ, Chiang YH, Morales M, Chou J, Lein P, Chen HL, Hoffer BJ, Wang Y. Intravenous administration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–564. [DOI] [PubMed]
- 5.Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. [DOI] [PubMed]
- 6.Ebendal T, Bengtsson H, Soderstrom S. Bone morphogenetic proteins and their receptors: potential functions in the brain. J Neurosci Res. 1998;51:139–146. [DOI] [PubMed]
- 7.Helm GA, Alden TD, Sheehan JP, Kallmes D. Bone morphogenetic proteins and bone morphogenetic protein gene therapy in neurological surgery: a review. Neurosurgery. 2000;46:1213–1222. [DOI] [PubMed]
- 8.Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987;104:1623–1631. [DOI] [PMC free article] [PubMed]
- 9.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. [DOI] [PubMed]
- 10.Ishibe T, Nakayama T, Aoyama T, Nakamura T, Toguchida. Neuronal differentiation of synovial sarcoma and its therapeutic application. Clin Orthop Relat Res. 2008;466:2147–2155. [DOI] [PMC free article] [PubMed]
- 11.Kalbermatten DF, Erba P, Mahay D, Wiberg M, Pierer G, Terenghi G. Schwann cell strip for peripheral nerve repair. J Hand Surg Eur Vol. 2008;33:587–594. [DOI] [PubMed]
- 12.Kinameri E, Matsuoka I. Autocrine action of BMP2 regulates expression of GDNF-mRNA in sciatic Schwann cells. Brain Res Mol Brain Res. 2003;117:221–227. [DOI] [PubMed]
- 13.Lara-Ramirez R, Segura-Anaya E, Martinez-Gomez A, Dent MAR. Expression of interleukin-6 receptor α in normal and injured rat sciatic nerve. Neuroscience. 2008;152:601–608. [DOI] [PubMed]
- 14.Lin SZ, Hoffer BJ, Kaplan P, Wang Y. Osteogenic protein-1 protects against cerebral infarction induced by MCA ligation in adult rats. Stroke. 1999;30:126–133. [DOI] [PubMed]
- 15.Lonn P, Zaia K, Israelsson C, Althini S, Usoskin D, Kylberg A, Ebendal T. BMP enhances transcriptional responses to NGF during PC12 cell differentiation. Neurochem Res. 2005;30:753–765. [DOI] [PubMed]
- 16.Lundborg G, Rosén B. Hand function after nerve repair. Acta Physiol (Oxf). 2007;189:207–217. [DOI] [PubMed]
- 17.Maki Y, Yoshizu T, Tsubokawa N. Selective regeneration of motor and sensory axons in an experimental peripheral nerve model without endorgans. Scand J Plast Reconstr Surg Hand Surg. 2005;39:257–260. [DOI] [PubMed]
- 18.Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119:45–54. [DOI] [PMC free article] [PubMed]
- 19.Okuyama N, Kiryu-Seo S, Kiyama H. Altered expression of Smad family members in injured motor neurons of rat. Brain Res. 2007;1132:36–41. [DOI] [PubMed]
- 20.Perides G, Jensen FE, Edgecomb P, Rueger DC, Charness ME. Neuroprotective effect of human osteogenic protein-1 in a rat model of cerebral hypoxia/ischemia. Neurosci Lett. 1995;187:21–24. [DOI] [PubMed]
- 21.Reissmann E, Ernsberger U, Francis-West PH, Rueger D, Brickell PM, Rohrer H. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development. 1996;122:2079–2088. [DOI] [PubMed]
- 22.Saito H, Dahlin LB. Expression of ATF3 and axonal outgrowth are impaired after delayed nerve repair. BMC Neurosci. 2008;9:88. [DOI] [PMC free article] [PubMed]
- 23.Schafer DP, Rasband MN. Glial regulation of the axonal membrane at nodes of Ranvier. Curr Opin Neurobiol. 2006;16:508–514. [DOI] [PubMed]
- 24.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. [DOI] [PubMed]
- 25.Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170. [DOI] [PubMed]
- 26.Trupp M, Rydén M, Jörnvall H, Funakoshi H, Timmusk T, Arenas E, Ibáñez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. [DOI] [PMC free article] [PubMed]
- 27.Turgut M, Oktem G, Uysal A, Yurtseven ME. Immunohistochemical profile of transforming growth factor-beta1 and basic fibroblast growth factor in sciatic nerve anastomosis following pinealectomy and exogenous melatonin administration in rats. J Clin Neurosci. 2006;13:753–758. [DOI] [PubMed]
- 28.Varley JE, Maxwell GD. BMP-2 and BMP-4, but not BMP-6, increase the number of adrenergic cells which develop in quail trunk neural crest cultures. Exp Neurol. 1996;140:84–94. [DOI] [PubMed]
- 29.Varley JE, Wehby RG, Rueger DC, Maxwell GD. Number of adrenergic and islet-1 immunoreactive cells is increased in avian trunk neural crest cultures in the presence of human recombinant osteogenic protein-1. Dev Dyn. 1995;203:434–447. [DOI] [PubMed]
- 30.Wang YL, Wang DZ, Nie X, Lei DL, Liu YP, Zhang YJ, Suwa F, Tamada Y, Fang YR, Jin Y. The role of bone morphogenetic protein-2 in vivo in regeneration of peripheral nerves. Br J Oral Maxillofac Surg. 2007;45:197–202. [DOI] [PubMed]
- 31.Yamada M, Akeda K, Asanuma K, Thonar EJ, An HS, Uchida A, Masuda K. Effect of osteogenic protein-1 on the matrix metabolism of bovine tendon cells. J Orthop Res. 2008;26:42–48. [DOI] [PubMed]
- 32.Zhang D, Mehler MF, Song Q, Kessler JA. Development of bone morphogenetic protein receptors in the nervous system and possible roles in regulating trkC expression. J Neurosci. 1998;18:3314–3326. [DOI] [PMC free article] [PubMed]