Abstract
Bone morphogenic proteins (BMPs) are potent osteoinductive agents. Their use in fracture surgery is still being studied and the clinical indications are evolving. Heterotopic bone after BMP use in spine surgery is a known complication. While some literature describes the ability of BMP to enhance fracture healing, few articles describe complications of BMP. In tibial plateau fractures, after elevating the cartilage en mass, a subchondral void may be created in these fractures. Structural support provided by bone void-filling agents can be augmented with osteoinduction achieved by BMP. We asked whether heterotopic bone formation would occur more frequently with BMP-2 when used in tibial plateau fractures and whether BMP-2 enhanced the ability to maintain surgically restored subchondral bone integrity. Heterotopic bone developed more frequently in patients receiving BMP (10 of 17) than in patients not receiving BMP (one of 23). Four patients receiving BMP and no patients not receiving BMP underwent removal of heterotopic bone. Maintenance of subchondral bone integrity was similar without and with the use of BMP. BMP is a potent osteoinductive agent; however, when used for an off-label indication in periarticular situations, complications such as heterotopic bone are common and increase reoperation rates.
Level of Evidence: Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Bone morphogenic proteins (BMPs) are members of the transforming growth factor β superfamily. Some 20 BMPs have been identified so far, of which recombinant BMP-2 and BMP-7 are commercially available for treatment purposes. BMPs are probably the most important growth factors in bone formation and healing [45] and play critical roles in embryonic development and regeneration of skeletal tissue in adults [29]. BMP can be delivered to a surgical site by a variety of carrier and delivery systems. These carrier systems, which are absorbed over a period of time, maintain the concentration of the BMP at the treatment site [16, 39, 43]. They also provide temporary scaffolding for osteogenesis and are intended to prevent extraneous bone formation [14, 16, 27]. Carrier systems include inorganic material synthetic polymer [12, 16, 20, 50], natural polymers [16, 28, 39], and bone allograft [2, 18]. Different clinical applications require different doses of BMPs with different carrier systems. These carrier systems are important variables in the clinical use of BMPs.
The bone induced by BMP-2 appears normal (by histologic criteria), functions normally (by biomechanical criteria), and remodels normally (by radiographic and histologic criteria) [5–7, 15, 40]. BMP aids differentiation of mesenchymal cells into osteoblasts and chondroblasts and helps in vascular invasion [49]. Apart from its use in spinal fusion surgery, BMP-2 has multiple uses in fracture management, including use as an alternative to autograft in recalcitrant long bone nonunion and acute open fractures of the tibial shaft [26, 31, 36] and as an alternative or adjunct to bone grafting in other locations, such as craniomaxillofacial surgeries [46]. BMPs stimulate ectopic bone formation in vivo [35, 44, 51], probably by initiating the differentiation of mesenchymal stem cells into mature osteoblasts and chondroblasts. The clinical implication of the ectopic bone induced by BMP has been extensively studied. Incidence and clinical complications of ectopic bone have been reported in spinal fusion surgeries [8, 19, 22, 50]. With the benefits and complications of BMP-2 still being studied, the indications for its use are still evolving.
Surgical treatment of tibial plateau fractures can be challenging. Numerous studies demonstrate articular incongruity and joint instability are critical factors affecting the outcome of surgery and suggest these are the leading causes of posttraumatic osteoarthritis [1, 4, 23, 25, 34, 37, 38]. Maintaining the articular congruence until healing is vital for good functional outcomes. In patients with poor bone stock, maintaining articular congruity until healing without subchondral bone collapse can be a challenge, even with a stable biomechanical construct (Fig. 1). Osteoinductive and osteoconductive agents may produce more structural integrity and hasten healing. In 2005, we began using BMP-2 in tibial plateau fractures as an adjunct to structural subchondral bone void fillers as an off-label use.
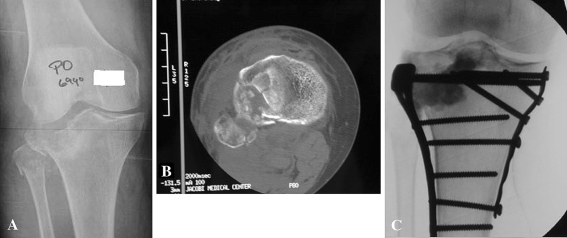
Fig. 1A–C.
(A) A plain radiograph of a tibial plateau fracture demonstrates the subchondral bone impaction. (B) An axial section of a computed tomography scan demonstrates the subchondral bone impaction in the tibial plateau fracture. (C) Intraoperative fluoroscopy demonstrates the anatomic reduction of the articular cartilage.
We asked (1) whether heterotopic ossification (HO) would be increased with the use of BMP-2 in tibial plateau fractures; and (2) whether BMP-2 would enhance maintenance of subchondral bone integrity after restoration.
Patients and Methods
We retrospectively reviewed the charts and radiographs of 44 patients who underwent surgery for acute traumatic tibial plateau fractures between September 2005 and June 2006. We excluded four patients who were lost to followup, leaving 40 patients for the study cohort. Surgery was performed within 7 days of the injury in all cases by a fellowship-trained trauma surgeon (DGL). The average age of the patients was 53 years (range, 17–83 years). Nineteen patients were younger than 60 years old. There were 22 males and 18 females in the study. We classified the patients into two groups: those who received intraoperatively the osteoinductive agent BMP-2 (BMP-positive group; n = 17) and those who did not (BMP-negative group; n = 23). The minimum followup in the BMP-positive group was 12 months (average, 18 months; range, 12–25 months). The minimum followup in the BMP-negative group was 13 months (average, 18 months; range, 13–26 months). All information was obtained from the medical records; no patients were recalled specifically for this study. We had prior approval from our Institutional Review Board.
We used a Type I collagen sponge (Medtronic, Sofamor Danek, Memphis, TN) as the carrier of the recombinant human BMP-2 for insertion into the fracture site per the manufacturer’s guidelines. A 7.5- × 10-mm absorbable collagen sponge of Type 1 derived from bovine Achilles tendon was used a surgical matrix. A standardized manufacturer’s protocol was used to constitute BMP with sterile water. Freeze-dried, cancellous fibular allograft was trimmed to the required shape and was placed in the metaphyseal bony defect. After hemostasis was achieved, the collagen sponge with BMP was used as an onlay, covering the allograft and bridging the fracture fragments. Early on in the series, we did not use BMP in the treatment of tibial plateau fractures. Rather, after en bloc elevation of the articular cartilage, we used subchondral bone void fillers. The choice of the bone void filler depended on the quality of the metaphyseal bone and the size of the defect after elevation of the articular cartilage. Allograft fibula was our void filler of choice when a sizable structural defect was present. Norian bone cement (Norian Corp, West Chester, PA) and demineralized bone matrix were used more frequently early on in the series. Since the indications of different bone void fillers evolved over the course of the study period, it is difficult to assess the true isolated effect of BMP after controlling other variables, including the choice of other void fillers and the quality of the bone; however, we have reported the subchondral bone integrity when BMP was used. Of the 17 patients in the BMP-positive group, four patients received demineralized bone matrix, two patients received calcium phosphate bone cement, and 11 patients received fibular allograft for structural support.
All patients were followed up at 1, 3, 6, and 12 months and yearly thereafter. Radiographs were made at every followup visit. We recorded union, additional surgeries, and other complications, including reoperation rates for removal of HO around the knee, from the medical charts. We also noted the use of other bone-grafting agents, such as calcium phosphate bone cement, demineralized bone matrix, allograft fibula, and autografts.
Three of us (SB, DH, MW) independently assessed radiographs for the presence and site of HO and subchondral bone settling. The amount was quantified using length and breadth measurements of the fragments on magnification-standardized anteroposterior radiograph film. We noted fragments of HO located on the lateral side in the iliotibial band area but not on the medial side of the tibia because the surgical approach through which the collagen-soaked BMP sponge was placed was from the lateral side and any HO there would not likely relate to the sponge. We deemed this appropriate because there was very little chance, if any, the BMP would have seeped to the medial side. To identify postoperative subchondral bone settling we used joint line congruity as a marker. That is, we compared the articular surface depression between the immediate postoperative radiographs and the radiographs at the most recent followup. A line was drawn along the tibial shaft axis. Two more lines were drawn along the articular surface of the fractured condyle and the unfractured condyle to intersect the first line. In the immediate postoperative radiographs, the latter two lines typically overlapped. These lines were then drawn on the late postoperative radiographs. The subchondral bone settling was measured by the difference between the two lines along the articular surface for each condyle. In case of bicondylar fractures, the preoperative and postoperative radiographs were overlaid to generate the readings.
We used Fisher’s exact test to compare the differences in HO formation, reoperation rates, and the maintenance of articular congruity between the BMP-positive and BMP-negative groups. To determine whether the age, use of BMP-2, surgical approach, and use of other bone-grafting agents predicted HO, we performed multivariable log-binomial regressions.
Results
The risk of developing HO was higher (relative risk, 14; 95% confidence interval, 2, 96; p < 0.001) in the patients receiving BMP (10 of 17) than in those not receiving BMP (one of 23) (Table 1). The average length and breadth of the HO fragments were 13.6 mm and 9.9 mm, respectively, on magnification-standardized anteroposterior radiographs. Also, more (p = 0.026) patients in the BMP-positive group (four of 17) underwent removal of ectopic bone (including three arthroscopic arthrolysis and one open surgery) than patients in the BMP-negative group (none) (Table 1). We found no association (p < 0.01) between the variables other than the use of BMP (ie, age, gender, and use of other bone-grafting agents) and the development of HO.
Table 1.
Comparison between the two groups
| Parameter | BMP-positive | BMP-negative | p Value |
|---|---|---|---|
| Total number of patients | 17 | 23 | |
| Number of patients who developed HO | 10 (59%) | 1 (4%) | < 0.001 |
| Number of patients who underwent excision of HO | 4 (24%) | 0 (0%) | 0.026 |
| Average subchondral bone settlement during healing (mm) | 1 | 2.3 | 0.78 |
BMP = bone morphogenetic protein; HO = heterotopic ossification.
There was no difference (p = 0.78) in maintenance of articular congruence between the two groups (1-mm margin in the BMP-positive group versus 2.3-mm margin in the BMP-negative group) (Table 1).
Discussion
BMPs are potent agents in inducing new bone formation. They bind to receptors on the pluripotent mesenchymal cells and cause them to differentiate into osteoblasts and chondroblasts. BMP may form bone by an endochondral (through a cartilage intermediate) or intramembranous (direct) bone formation pathway, but bone formation by BMP is a self-limiting process [49]. BMP is the major physiologic factor in fracture healing and its expression increases during fracture healing; it has been widely studied as a therapeutic bone-healing agent. We asked whether the likelihood of HO would be increased with the use of BMP-2 in tibial plateau fractures and whether BMP-2 would enhance our ability to maintain subchondral bone integrity after surgical restoration.
There are limitations to our study. First, we used BMP only later in the series. The choice of the bone void filler evolved over the study period. The use of BMP with or without other adjunct void fillers has not been randomized. Second, the fracture patterns and the patient population were heterogeneous. This heterogeneity may mask the benefits of BMP in maintaining the subchondral bone integrity; however, our main purpose was to report the complications of its use. When extrapolating this clinical result, the reader should be mindful of this limitation. Third, since the study sample is small, the power of the study is limited. A prospective randomized study using a carrier system that contains the BMP to the area of interest, after controlling for demographics and fracture pattern, is needed to outline the benefits and complications of using a potent osteoinductive agent in a periarticular fracture situation.
Early clinical experience suggested high fusion rates in vertebral bodies with the use of BMP [9–11]. Literature also exists describing the benefits of using BMP in diaphyseal fractures. A combination of allograft and recombinant BMP-2 is reportedly as safe and effective as traditional bone graft [17, 24]. BMP-2, when implanted in a collagen carrier, is also reportedly effective for treating tibial nonunions [13]. BMP apparently results in higher union rates and decreased infection rates with open tibial fractures [42].
Although multiple benefits are being ascribed to the use of BMP in spinal and trauma surgery, its use is not free of complications. A recent study reported formation of ectopic epidural bone formation; however, the authors reported no clinical implications [19]. Wong et al. [50], in contrast, reported association of neurologic impairment with use of BMP in anterior interbody fusion surgery. Other complications, including bone resorption or remodeling at the graft site, hematoma, neck swelling, and painful seroma, have been reported in the spine literature [3, 8, 19, 30, 33, 47, 48]. Shields et al. [41] reported about 23.2% incidence of complications in the postoperative period ranging from hematoma to large swellings in the neck in patients who underwent anterior cervical spine fusion. All these patients required either hospital readmission or a longer stay in the hospital. Respiratory distress and dysphagia may result from BMP-induced anterior neck swelling [32]. Katsuno et al. [21] reported a possible association of BMP use with increased invasiveness of breast cancer through the enhancing of invasion and bone metastasis through the SMAD pathway.
Off-label use of BMP in a periarticular fracture situation, particularly in tibial plateau fractures, might lead to higher reoperation rates as a result of the symptoms associated with HO formation. Surgeons must exercise caution and weigh the risks and benefits of these products before using them in their patients.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Hospital for Special Surgery.
References
- 1.Apley AG. Fractures of the tibial plateau. Orthop Clin North Am. 1979;10:61–74. [PubMed]
- 2.Baas J, Elmengaard B, Jensen TB, Jakobsen T, Andersen NT, Soballe K. The effect of pretreating morselized allograft bone with rhBMP-2 and/or pamidronate on the fixation of porous Ti and HA-coated implants. Biomaterials. 2008;29:2915–2922. [DOI] [PubMed]
- 3.Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008;62(5 suppl 2):ONS423–ONS431; discussion ONS431. [DOI] [PubMed]
- 4.Blokker CP, Rorabeck CH, Bourne RB. Tibial plateau fractures: an analysis of the results of treatment in 60 patients. Clin Orthop Relat Res. 1984;182:193–199. [PubMed]
- 5.Bouxsein ML, Turek TJ, Blake CA, D’Augusta D, Li X, Stevens M, Seeherman HJ, Wozney JM. Recombinant human bone morphogenetic protein-2 accelerates healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2001;83:1219–1230. [DOI] [PubMed]
- 6.Boyne PJ. Animal studies of application of rhBMP-2 in maxillofacial reconstruction. Bone. 1996;19(1 suppl):83S–92S. [DOI] [PubMed]
- 7.Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, Lilly LC, Alder M, Nummikoski P. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent. 1997;17:11–25. [PubMed]
- 8.Brower RS, Vickroy NM. A case of psoas ossification from the use of BMP-2 for posterolateral fusion at L4–L5. Spine. 2008;33:E653–E655. [DOI] [PubMed]
- 9.Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28:372–377. [DOI] [PubMed]
- 10.Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396–2408. [DOI] [PubMed]
- 11. Dimar JR, Glassman SD, Burkus KJ, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine. 2006;31:2534–2539; discussion 2540. [DOI] [PubMed]
- 12.Downes S, Patel M, Di Silvio L, Swai H, Davy K, Braden M. Modifications of the hydrophilicity of heterocyclic methacrylate copolymers for protein release. Biomaterials. 1995;16:1417–1421. [DOI] [PubMed]
- 13.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83(suppl 1 pt 2):S151–S158. [PMC free article] [PubMed]
- 14.Fu YC, Nie H, Ho ML, Wang CK, Wang CH. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/HAp composite scaffolds loaded with BMP-2. Biotechnol Bioeng. 2008;99:996–1006. [DOI] [PubMed]
- 15.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84:2123–2134. [DOI] [PubMed]
- 16.Issa JP, Bentley MV, Iyomasa MM, Sebald W, De Albuquerque RF. Sustained release carriers used to delivery bone morphogenetic proteins in the bone healing process. Anat Histol Embryol. 2008;37:181–187. [DOI] [PubMed]
- 17.Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, Pollak AN, Golden JD, Valentin-Opran A. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects: a randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1431–1441. [DOI] [PubMed]
- 18.Jones CB, Sabatino CT, Badura JM, Sietsema DL, Marotta JS. Improved healing efficacy in canine ulnar segmental defects with increasing recombinant human bone morphogenetic protein-2/allograft ratios. J Orthop Trauma. 2008;22:550–559. [DOI] [PubMed]
- 19.Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32:2885–2890. [DOI] [PubMed]
- 20.Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. 2005;26:1857–1875. [DOI] [PubMed]
- 21.Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K, Imamura T. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27:6322–6333. [DOI] [PubMed]
- 22.Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJ. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–2825. [DOI] [PubMed]
- 23.Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop Relat Res. 1988;234:159–169. [PubMed]
- 24.Krause F, Younger A, Weber M. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. J Bone Joint Surg Am. 2008;90:1168; author reply 1168–1169. [PubMed]
- 25.Lansinger O, Bergman B, Korner L, Andersson GB. Tibial condylar fractures: a twenty-year follow-up. J Bone Joint Surg Am. 1986;68:13–19. [PubMed]
- 26.Lee FY, Sinicropi SM, Lee FS, Vitale MG, Roye DP Jr, Choi IH. Treatment of congenital pseudarthrosis of the tibia with recombinant human bone morphogenetic protein-7 (rhBMP-7): a report of five cases. J Bone Joint Surg Am. 2006;88:627–633. [DOI] [PubMed]
- 27.Lee SH, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59:339–359. [DOI] [PubMed]
- 28.Li RH, Wozney JM. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001;19:255–265. [DOI] [PubMed]
- 29.Lyons KM, Pelton RW, Hogan BL. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes Dev. 1989;3:1657–1668. [DOI] [PubMed]
- 30.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop. 2007;31:729–734. [DOI] [PMC free article] [PubMed]
- 31.Nordsletten L. Recent developments in the use of bone morphogenetic protein in orthopaedic trauma surgery. Curr Med Res Opin. 2006;22(suppl 1):S13–S17; S23. [DOI] [PubMed]
- 32.Perri B, Cooper M, Lauryssen C, Anand N. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J. 2007;7:235–239. [DOI] [PubMed]
- 33.Poynton AR, Lane JM. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine. 2002;27(16 suppl 1):S40–S48. [DOI] [PubMed]
- 34.Rasmussen PS. Tibial condylar fractures: impairment of knee joint stability as an indication for surgical treatment. J Bone Joint Surg Am. 1973;55:1331–1350. [PubMed]
- 35.Reddi AH, Cunningham NS. Bone induction by osteogenin and bone morphogenetic proteins. Biomaterials. 1990;11:33–34. [PubMed]
- 36.Ristiniemi J, Flinkkila T, Hyvonen P, Lakovaara M, Pakarinen H, Jalovaara P. RhBMP-7 accelerates the healing in distal tibial fractures treated by external fixation. J Bone Joint Surg Br. 2007;89:265–272. [DOI] [PubMed]
- 37.Sarmiento A, Kinman PB, Latta LL, Eng P. Fractures of the proximal tibia and tibial condyles: a clinical and laboratory comparative study. Clin Orthop Relat Res. 1979;145:136–145. [PubMed]
- 38.Schatzker J, McBroom R, Bruce D. The tibial plateau fracture: the Toronto experience 1968–1975. Clin Orthop Relat Res. 1979;138:94–104. [PubMed]
- 39.Schmidmaier G, Schwabe P, Strobel C, Wildemann B. Carrier systems and application of growth factors in orthopaedics. Injury. 2008;39(suppl 2):S37–S43. [DOI] [PubMed]
- 40.Sciadini MF, Johnson KD. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. J Orthop Res. 2000;18:289–302. [DOI] [PubMed]
- 41.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, Shields CB. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542–547. [DOI] [PubMed]
- 42.Swiontkowski MF, Aro HT, Donell S, Esterhai JL, Goulet J, Jones A, Kregor PJ, Nordsletten L, Paiement G, Patel A. Recombinant human bone morphogenetic protein-2 in open tibial fractures: a subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am. 2006;88:1258–1265. [DOI] [PubMed]
- 43.Uludag H, Gao T, Porter TJ, Friess W, Wozney JM. Delivery systems for BMPs: factors contributing to protein retention at an application site. J Bone Joint Surg Am. 2001;83(suppl 1 pt 2):S128–S135. [PubMed]
- 44.Urist MR, Mikulski A, Lietze A. Solubilized and insolubilized bone morphogenetic protein. Proc Natl Acad Sci USA. 1979;76:1828–1832. [DOI] [PMC free article] [PubMed]
- 45.Urist MR, Silverman BF, Buring K, Dubuc FL, Rosenberg JM. The bone induction principle. Clin Orthop Relat Res. 1967;53:243–283. [DOI] [PubMed]
- 46.Vaccaro AR, Anderson DG, Patel T, Fischgrund J, Truumees E, Herkowitz HN, Phillips F, Hilibrand A, Albert TJ, Wetzel T, McCulloch JA. Comparison of OP-1 putty (rhBMP-7) to iliac crest autograft for posterolateral lumbar arthrodesis: a minimum 2-year follow-up pilot study. Spine. 2005;30:2709–2716. [DOI] [PubMed]
- 47.Vaccaro AR, Lawrence JP, Patel T, Katz LD, Anderson DG, Fischgrund JS, Krop J, Fehlings MG, Wong D. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis: a long-term (>4 years) pivotal study. Spine. 2008;33:2850–2862. [DOI] [PubMed]
- 48.Vaccaro AR, Whang PG, Patel T, Phillips FM, Anderson DG, Albert TJ, Hilibrand AS, Brower RS, Kurd MF, Appannagari A, Patel M, Fischgrund JS. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft for posterolateral lumbar arthrodesis: minimum 4-year follow-up of a pilot study. Spine J. 2008;8:457–465. [DOI] [PubMed]
- 49.Valentin-Opran A, Wozney J, Csimma C, Lilly L, Riedel GE. Clinical evaluation of recombinant human bone morphogenetic protein-2. Clin Orthop Relat Res. 2002;395:110–120. [DOI] [PubMed]
- 50.Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8:1011–1018. [DOI] [PubMed]
- 51.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. [DOI] [PubMed]