Abstract
This Classic Article is a reprint of the original work by Marshall R. Urist and Basil S. Strates, Bone Morphogenetic Protein. An accompanying biographical sketch of Marshall R. Urist, MD is available at DOI 10.1007/s11999-009-1067-4; a second Classic Article is available at DOI 10.1007/s11999-009-1069-2; and a third Classic Article is available at DOI 10.1007/s11999-009-1070-9. The Classic Article is © 1971 by Sage Publications Inc. Journals and is reprinted with permission from Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971;50:1392–1406.
Introduction
This is a brief progress report on research on the molecular and biochemical characteristics of the bone morphogenetic proteins (BMP) in rat cortical bone matrix. The BMP guides modulation and differentiation of mesenchymal cells of muscle into bone and bone marrow cells. Previous communications deal with the histophysiology of the response of several vertebrate species to the BMP, and are concerned with temporal, spatial, and biologic features of the bone morphogenetic process in the living animal [1–14] and in systems in vitro [15, 16]. In the rodent, bioassay of BMP is made by implantation of a known weight of lyophilized bone matrix in muscle, fascia, or subcutaneous tissue [10]. Since the matrix demineralized in vitro does not recalcify, bone tissue produced by a given quantity of old matrix including BMP can be determined by measuring the ash weight per gram of dry weight of preimplanted matrix. The basis of the quantitative biochemical relationships between BMP in bone matrix and the yield of new bone is an embryonic induction system for osteogenesis in postfetal life. Little is known about the genetic control mechanism of bone cell differentiation. Terminology and theoretical concepts now being used are defined in the appendix, pages 1403–1404.
Preparations of Demineralized Matrix Producing Low Incidence and Yields of New Bone
Demineralized matrix of bones and teeth, as prepared by several research groups, has been reported to produce bone in rodents. Rat dentin matrix prepared by Huggins, Wiseman, and Reddi [17], and rabbit bone matrix prepared by Nade [18] produced bone in a significant number of implants. Preparations similar to those of Huggins, Wiseman, and Reddi and Nade tested by our research group produced bone, but the yield was relatively low, and at least half of the implants produced only fibrous tissue. Some demineralization procedures reduce the yield of new bone more than others [1]. Huggins, Wiseman, and Reddi [17], made coarse powders of dentin, and demineralized the matrix in 0.5 N HCl at room temperature for three hours. The powdered matrix was extracted with ethanol and sterilized in water-saturated phenol for 30 minutes. The sterile matrix was then washed in 70% ethanol in water, frozen in liquid nitrogen, dehydrated in ethanol and ether, dried overnight at 37 C, and sieved to a standard particle size. After extraction and denaturation of tissue proteins in this series of solutions, rat dentin matrix was implanted in xenogeneic guinea pigs and mice with little or no histoincompatibility reaction.
The unique physicochemical properties of dentin matrix must be taken into consideration in interpreting the observations of Huggins, Wiseman, and Reddi [17]. Dentin collagen is more highly cross-linked than bone collagen and more resistant to denaturation [19]. The highly cross-linked structure of dentin matrix is also more resistant than bone matrix to chemical and thermal denaturation, particularly if it is undemineralized when subjected to the heat of pulverization. When bone matrix is demineralized first and then ground to a particle size of a diameter less than 400 micrometers (μm) the protein structure is denatured sufficiently to eliminate all morphogenetic activity [20]. These observations suggest that the mineral protects the BMP from thermal denaturation. The mineral also insulates the BMP and prevents transmission of the morphogenetic property from bone matrix to proliferating mesenchymal cells [8].
Differences among species are notable in both responsiveness of allogeneic recipients to the BMP, and potency of BMP in xenogeneic recipients. Urist and Craven [9] noted that the stability of the BMP as determined from yield of bone in various species was as follows: rabbit > rat > guinea pig > mouse > , etc. Huggins, Wiseman, and Reddi [17], observed that the rat was the more responsive and produced bone in implants of allogeneic matrix and in implants of guinea pig and mouse dentin matrix. The rat also produced a small deposit of cartilage in implants of human bone. The mouse produced bone in response to mouse and rat dentin matrix but not to guinea pig dentin matrix. The guinea pig did not produce bone even in response to guinea pig dentin. In unpublished experiments, our research group obtained good yields of bone in implants of unpowdered guinea pig dentin in guinea pigs. Consequently, we consider the small particle size, and the chemical agents used by Huggins, Wiseman, and Reddi [17] for preparation of the demineralized powder, to be deleterious to a significant part of the morphogenetic activity in guinea pig dentin matrix. Nevertheless, we agree with them that allogeneic implants of bone matrix produce lower yields in the guinea pig than in either the rabbit or the rat [9].
Implants of ethylenediaminetetracetic acid (EDTA) demineralized bone matrix have no morphogenetic activity in guinea pigs and dogs. When the recipient is a rat or rabbit, species with bone morphogenetic property of high stability, bone will be produced in allogeneic recipients only if EDTA is used in low concentrations at neutral pH in the cold for a brief period of time [4]. Nade [18], for example, demineralized rabbit bone in EDTA at 4 C, and produced a matrix with some bone morphogenetic activity, but much greater activity is obtained if EDTA is avoided altogether and substituted with dilute HCl or H3PO4 at 2 C. To appreciate the chemical lability of the BMP, it is necessary to quantitate the reaction, eg, express the yield in terms either of milligrams of new bone ash per gram of preimplanted matrix [10, 12] or of alkaline phosphatase activity per microgram of deoxyribonucleic acid (DNA) [11, 14].
Nade [18] assumed that a pluripotential mesenchymal cell is responsible for osteogenesis. This assumption is difficult to reconcile with the concept of modern embryology, which holds that there are no undifferentiated cells in postfetal life in mammalian species. Even in the rabbit, a species with strong determination for osteogenesis in postfetal life, bone cell differentiation is restricted to special populations in limited regions of the body [6, 12, 13]. Mesenchymal cells, like other cells, are competent or incompetent to respond to the bone morphogenetic matrix, depending on whether implantation is made in visceral or somatic organs [6]. Huggins, Wiseman, and Reddi [17] presented a hypothesis of a fibroblast transforming factor (FTF). Reminiscent of the old idea of metaplasia of a fibroblast into a chondroblast or osteoblast, the FTF would act directly on ambient cells. However, bone cell differentiation, as observed by radio autographic 3H-thymidine labeling techniques, generally is preceded by a series of two or more preparatory mitotic divisions [5].
In the FTF hypothesis, the term fibroblast is used synonymously with the terms mesenchymal cell and connective tissue stem cell. We reserve the term fibroblast for a cell differentiated to produce only fibrous connective tissue, and assume that before a fibroblast can differentiate into a chondroblast, modulation and mitotic division must occur to generate chondroblasts and osteoprogenitor cells or preosteoblasts [21]. Direct transformation of a fibroblast (one differentiated cell) into an osteoblast (another differentiated cell) may be possible, but we have not observed it in cells labeled with 3H-thymidine in growing bone in implants of bone matrix [5]. Mitosis does not diminish the determination of the cells for osteogenesis. Instead, mitosis is required to assemble new populations of mesenchymal cells with preexisting osteogenetic competence for the osteogenetic process [5, 9]. The preparations of rat bone matrix that will be described invite immediate ingrowth of mesenchyme [9], promote transfer of bone morphogenetic activity within 6 days [14], and produce the maximum yield of new bone within 28 days [10].
Materials and Methods
The diaphyseal segment of long bones of 200 adult rats, Sprague-Dawley strain, were excised, cleaned of all muscle attachments, and washed until grossly free of bone marrow cells. The segments of mechanically cleaned cortical bone were demineralized in 0.6 N HCl at 2 C for two days, lyophilized, and stored in sterile packages. This preparation almost invariably produces new bone in quantities of 500 mg of ash per gm of preimplanted mineral-free matrix 30 days after implantation in muscle of allogeneic rats [10]. Segments of matrix untreated and treated in various ways to chemically extract or alter the structure of the protein were implanted for four weeks, and examined by radiographic, analytical (ash), and histological methods.
Ultrastructure.—Three samples of the preparation of demineralized bone matrix described were prepared for examination by electron microscopy. Samples less than 1 mm3 in volume were fixed in 3.0% glutaraldehyde buffered with 0.067 M s-collidine or 0.033 M sodium cacodylate, and postfixed in 1% osmium tetroxide. Each sample was aligned for examination in longitudinal and cross sections, dehydrated in solutions of alcohol of increasing concentrations, transferred to propylene oxide, impregnated with epoxy resin, and embedded in a curing agent (Epon). Polymerization was produced by heating at 60 C for 48 hours.
Thick sections were cut with glass knives and stained with toluidine blue to locate specific areas of interest with the light microscope. Thin sections were cut from selected blocks, and trimmed to less than 1.0 mm3 in volume. Staining procedures were used as follows: grids were floated on saturated uranyl acetate in aqueous solution for 3.5 minutes, followed by lead citrate for 3.5 minutes for impregnation of cell organelles and membranes, or exposed to 5% phosphotungstic acid (PTA) for impregnation of collagen fibrils of old matrix and cement lines, and/or stained with 0.5% bismuth nitrate in 0.1 N nitric acid for precipitation of proteoglycan [22].
Enzymedigestion.—α-Amylase-digested bone matrix.—Fifty samples of bone matrix, prepared as described, were transferred to a solution of crystalline amylase (α-amylase 840 U/mg).1 The solid to solution ratio was 0.1 gm of matrix to 10 ml of a solution of 35 mg amylase per liter of 0.2 M NaH2PO4 buffer, pH 5.4. Incubation was carried out for 90 hours at either 2 or 25 C. Digests and matrix residues were analyzed for hydroxyproline, hexosamines, amino nitrogen (Ninhydrin), uronic and sialic acids, and DNA. The matrix residues were washed repeatedly in cold distilled water for two hours and implanted into muscles of allogeneic rats.
Trypsin-digested bone matrix.—Fifty samples of rat bone matrix, demineralized as described, were transferred to a solution of trypsin (lyophilized trypsin, 180 U/mg),1 containing 2 mg of enzyme per ml of 0.1 M phosphate buffer, pH 7.6. The solid to solution ratio of the system was 0.1 gm of lyophilized bone matrix per 25 ml of enzyme-phosphate buffer solution. Controls for each set of samples of bone matrix were incubated in equivalent volumes of phosphate buffer solution without the enzyme. All samples, control and experimental, were incubated at 15 C, and five matrix segments from each group were removed and washed several times in cold distilled water and implanted in allogeneic adult rats. Aliquots of the enzyme solution were tested for activity with casein as a substrate. Matrix digests and residues were analyzed for hydroxyproline, hexosamines, amino nitrogen (Ninhydrin), uronic and sialic acids, and DNA. Space does not permit presentation of the complete chemical analyses. The biological implications and interpretations will be reported here.
Calciumchlorideextractionsofproteoglycans.—Six segments of the preparations of demineralized cortical bone described above were extracted for 24 hours with each of the solutions of calcium chloride (CaCl2) listed in Table 1. A 6.0 and 0.6 M solution of CaCl2 was used for the extraction of 1 mm3 diced fragments of demineralized cortical bone. Six segments were implanted in the anterior abdominal muscles of each recipient and excised after 28 days. Each recipient received one of each, including a saline control, of the preparations exposed to successively increasing concentrations of CaCl2 to test the biologic activity of matrix with variable residual quantities of proteoglycans. The basic assumption is that the effect of calcium ions is the same on bone as on cartilage, as described by Tsiganos and Muir [23], Gregory et al. [24] and Hallen [25].
Table 1.
Effect of CaCl2 extraction on bone morphogenesis in implants of bone matrix four weeks after implantation
| Solution | mm3 new bone/6 mm3 preimplanted matrix* | mg ash/gm preimplanted matrix |
|---|---|---|
| 0.9% NaCl | 5.0 | 520 ± 51 |
| 0.1 M CaCl2 | 5.0 | 550 ± 48 |
| 0.2 M CaCl2 | 5.0 | 495 ± 32 |
| 0.6 M CaCl2 | 5.0 | 500 ± 58 |
| 1.0 M CaCl2 | 4.5 | 480 ± 38 |
| 2.0 M CaCl2 | 3.0 | 325 ± 29 |
| 6.0 M CaCl2 | 2.0 | 150 ± 12 |
| 6.0 M CaCl2 (tissue diced, 1 day in solution) | 0 | 0 |
| 0.6 M CaCl2 (tissue diced, 1 day in solution) | 5.0 | 502 ± 45 |
| 0.6 M CaCl2 (tissue diced, 4 days in solution) | 4.5 | 490 ± 42 |
| 0.6 M CaCl2 (tissue diced, 9 days in solution) | 4.0 | 395 ± 40 |
| 0.6 M CaCl2 (tissue diced, 25 days in solution) | 0 | 0 |
* Based on correlated radiographic and quantitative histologic observations.
Chemicalmodificationoftheproteinsofbonematrix.—Glutaraldehyde cross-linking.—Three segments of demineralized rat bone matrix were treated in 100 ml of each of the following aqueous solutions of glutaraldehyde at 2 C for one hour: 1, 2, 5, 10, 100, 1,000 and 2,000 mM/liter before implantation.
Formaldehyde cross-linking.—Three segments of demineralized normal and lathyritic bone matrix were treated before implantation in 1, 1.8, 2.0, and 3.3 M solutions of formaldehyde to produce supernumerary intermolecular and intramolecular crosslinks as denned by Nold, Kang, and Gross [26].
Remazol black cross-linking.—Three segments of rat bone matrix were demineralized in 0.6 N HCl containing 1% Remazol black [27] for 48 hours, washed, lyophilized, and implanted in allogeneic host animals.
Borohydride reduction.—In six segments of demineralized bone matrix, the crosslinks were reduced before implantation in 100 ml of each of the following solutions of sodium borohydride (NaBH4) [28, 29] in carbonate buffer at pH 7.4: 0.5, 5, and 50 mM/liter.
Dinitrophenylation.—Three segments of rat bone matrix were exposed before implantation to solutions of l-fluoro-2,4-di-nitrobenzene (FDNB) [30] of the following concentrations: 3, 5, 10, 26, 53, and 268 mM/liter.
Deamination.—Three segments of fresh rat bone were demineralized and denatured [31] in 0.6 N HNO2 over a period of three days.
Results
Ultrastructure.—When rat bone was demineralized at 2 C in 0.6 N HCl with continuous stirring for not more than 48 hours, and immediately fixed by freeze-drying, the matrix consisted of a mesh of cross-banded collagen fibrils suspended in a lacework of noncollagenous constituents. Except for a small quantity of cell debris, the osteocyte lacunae and the canaliculi were empty. The collagen fibrils were well preserved, and arranged in bundles and layers. The direction of the collagen fibrils, alternating in longitudinal, oblique and transverse planes, produced a laminated structure. In longitudinal and oblique planes, electron micrographs of the laminated structure showed no space between fibrils. In cross sections, however, the structure was surprisingly different; there were large volumes of space between fibrils, constituting approximately a third of the matrix domain. These interfibrillar spaces were filled with a lacework of fine granules resembling electron microscope (EM) patterns of in vitro and in vivo preparations of proteoglycans. Although the lacework was imperfect in some regions, presumably from leaching of protein, the repeating pattern was constant in all sections and preparations (Figs. 1–3).
Fig. 2.
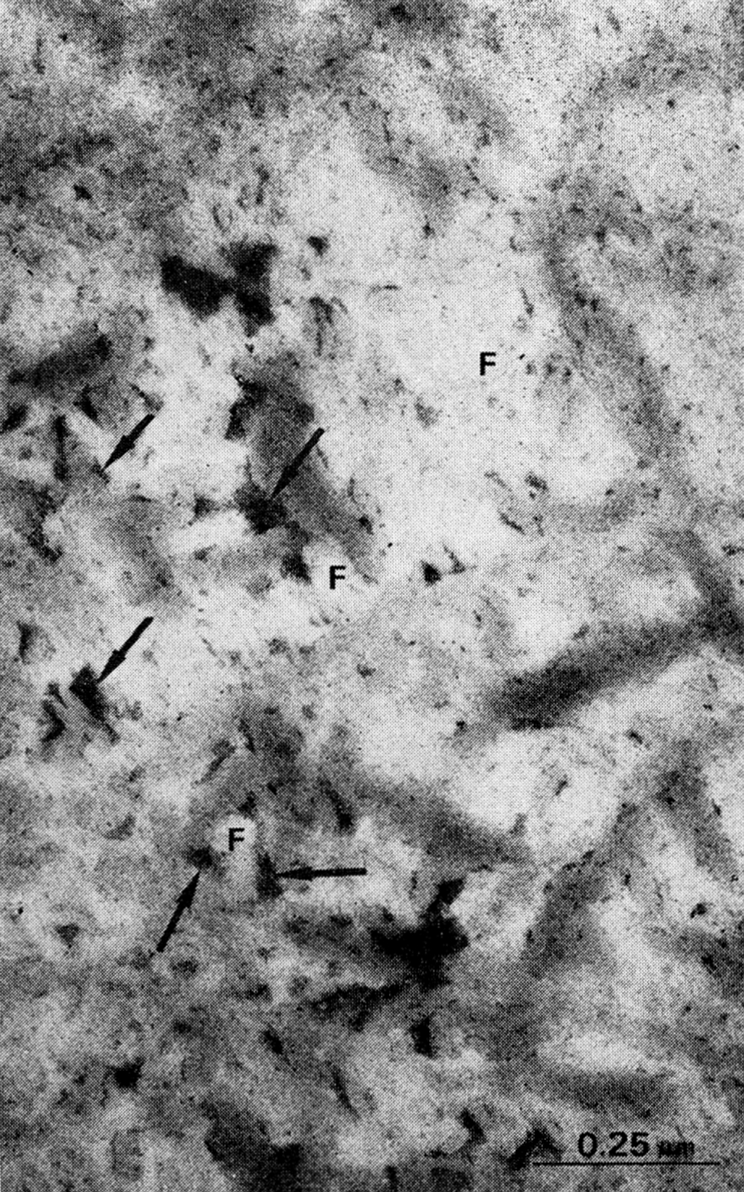
Rat demineralized cortical bone matrix, lyophilized and superfixed with 3% glutaraldehyde and 1.3% osmium tetroxide, cut in sections approximately 500 A thick and stained with bismuth nitrate 0.5% in 0.1 M nitric acid. Note electron-dense granules of bismuth-staining material presumably proteoglycans (arrow) between and on collagen fibrils (F).
Fig. 1.
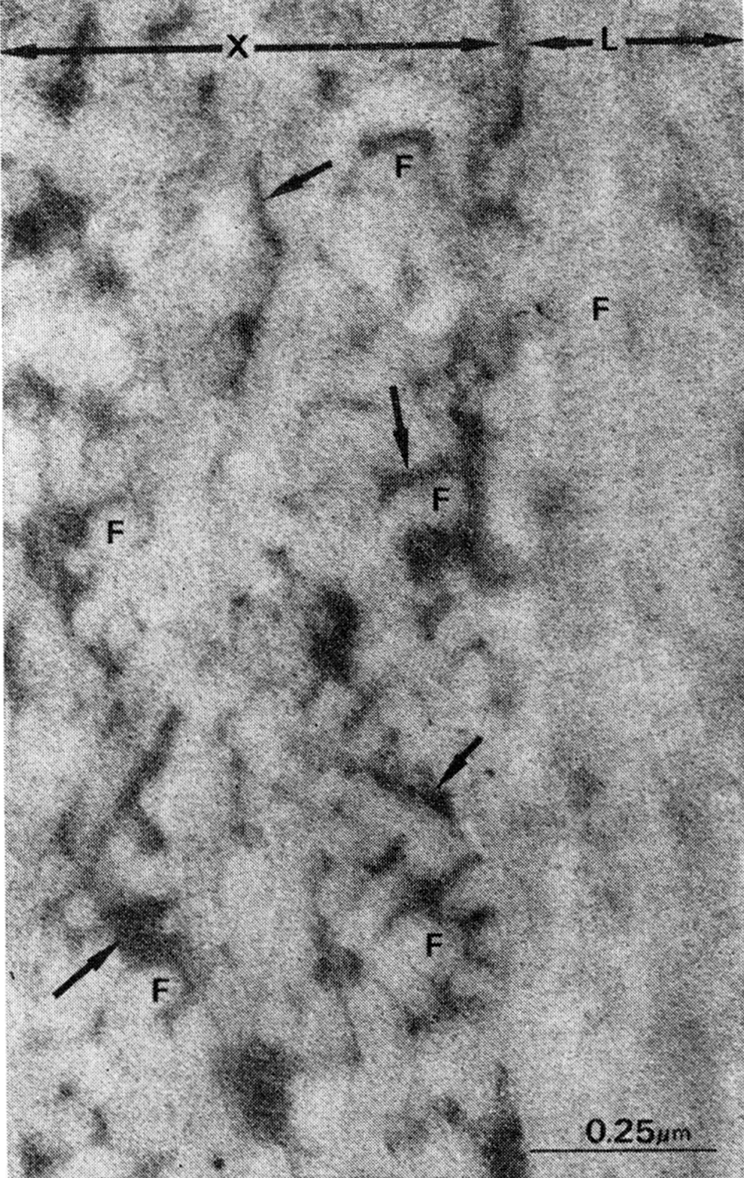
Demineralized cortical bone matrix, lyophilized and superfixed with 3% glutaraldehyde and 1.3% osmium tetroxide, cut in sections approximately 500 A thick and stained with uranyl acetate and lead citrate. Note large volume of space filled with interfibrillar electron-dense material, presumably proteoglycans (arrows) in the area cut in cross section (X). In longitudinal section (L) overlapping intertwined arrangement of collagen fibril (F) obscures large volume of interfibrillar noncollagenous material in bone matrix.
Fig. 3.
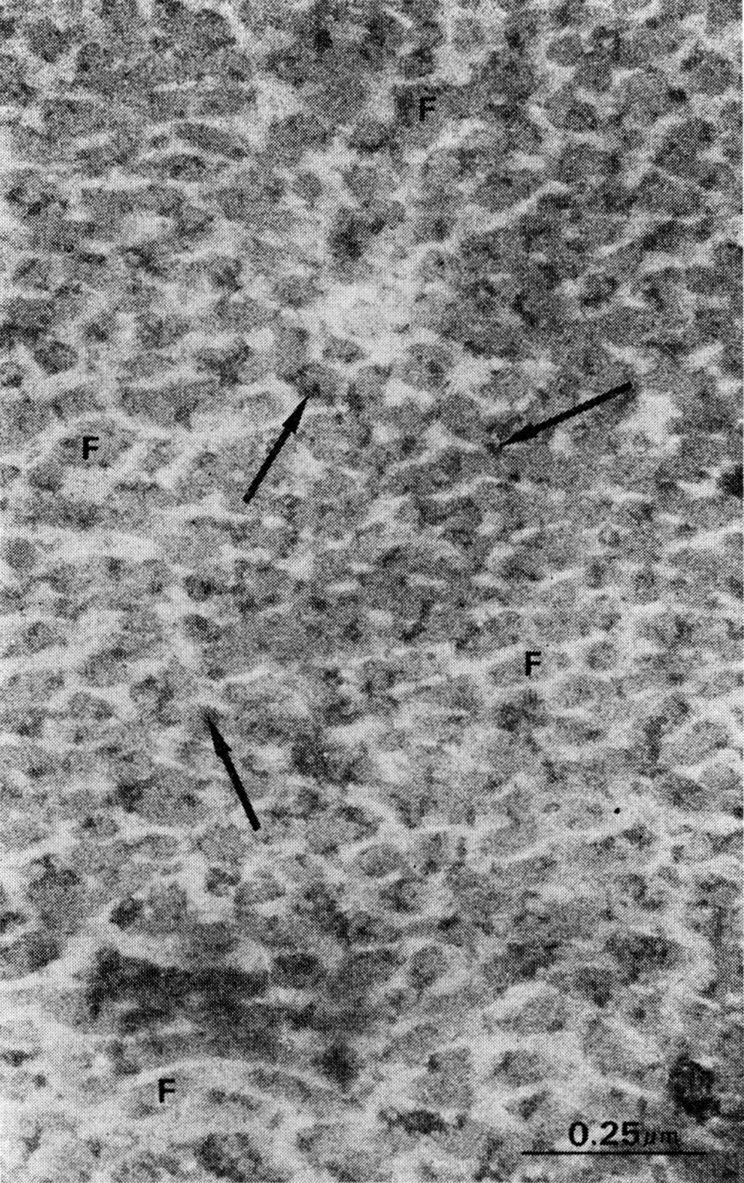
Rat bone matrix superfixed with glutaraldehyde and stained with 5% phosphotungstic acid and bismuth nitrate. Note bismuth-stained coat on collagen fibrils (arrows) and relatively large volume of interfibrillar space for proteoglycans.
Over a period of two weeks after implantation and exposure to body fluids and enzymes of the recipient muscle, the old matrix gradually lost the cross-banded structure of collagen fibrils and the interfibrillar lacelike conformation of proteoglycans. Although calcification occurred as soon as osteoblasts differentiated and new bone matrix was deposited on the surface of the old matrix, the bulk of the old bone collagen did not recalcify. The osteocytes of new bone were enclosed in a thin layer of uncalcified new collagen, but otherwise were completely enveloped in calcified bone matrix. The earliest deposits of apatite mineral appeared in clusters, which later coalesced. High power EM of the fibrils in cross section showed the microcrystallites formed between and on the surface of the collagen fibrils. A space of less than 1.0 μm remained between the plasma membrane and the calcified collagen, and continued along the extensions of the cell membranes of osteocytes (Fig. 4). Microfilaments filled the core of osteocyte extensions.
Fig. 4.
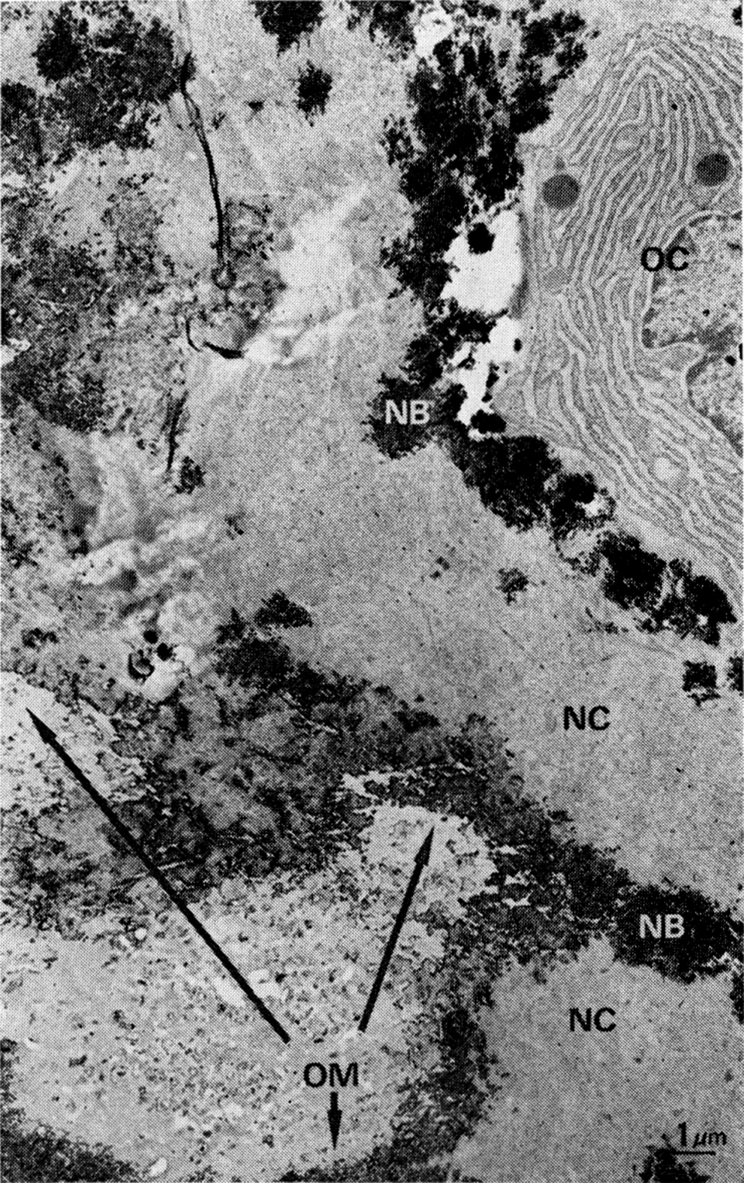
Amorphous structure of old bone matrix after two weeks of exposure to body fluids and apposition of new bone. OC, osteocyte; NB, calcified new bone matrix; NC, uncalcified and precalcified new bone matrix; OM, nonstaining old bone matrix with deposits of mineral at line of contact with new bone.
In glutaraldehyde-perfused implants, the deposits of mineral in new bone were chiefly crystalline. The microcrystals were rodlets, 400 × 50 × 50 A (Fig. 5). The new bone collagen, either in the precalcified or calcified state, had the same structure as the collagen of the old matrix before it was implanted and exposed to fluids of the body. In high power electron micrographs of the matrix cut in true cross section, there was always a large area, approximately a third of the space occupied by collagen fibrils, occupied by interfibrillar proteoglycans and ground substance.
Fig. 5.
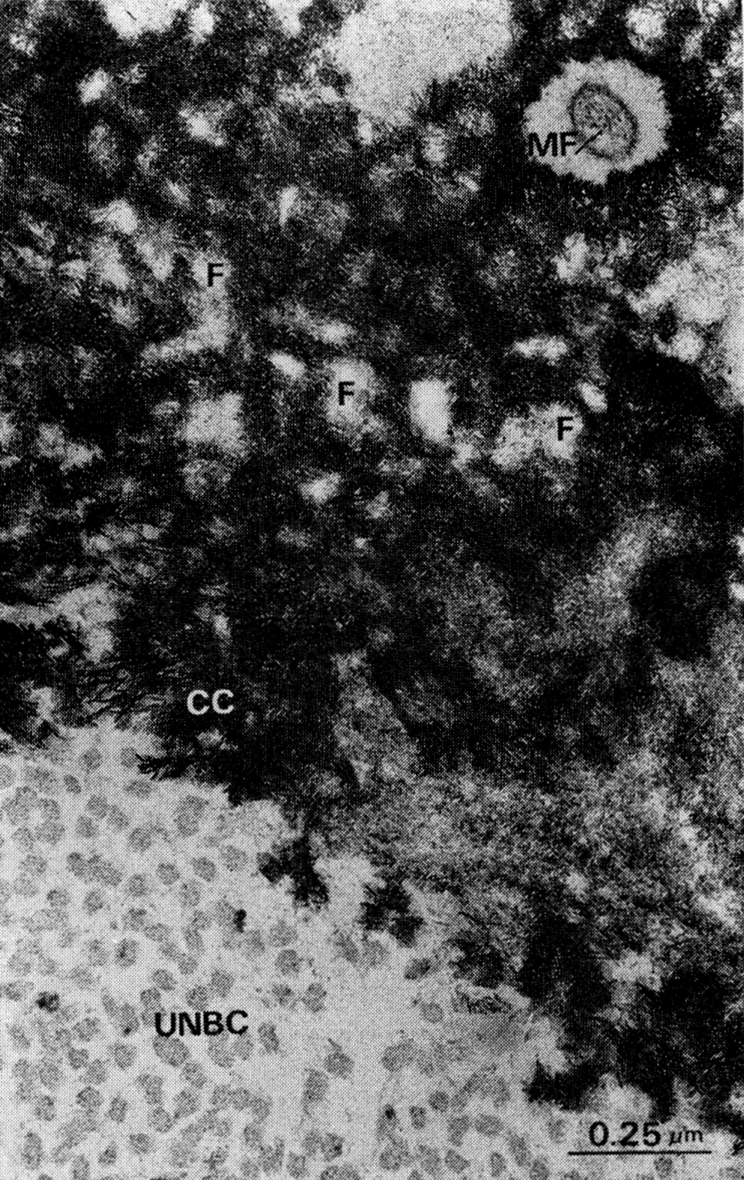
Structure of earliest deposits of mineral in new bone in another area of implant shown in Fig. 4. MF, osteocyte extension with microfilaments; F, uncalcified cores of collagen fibrils and groups of fibrils; CC, apatite deposits in spaces between and in surface of collagen fibrils; UNBC, uncalcified or precalcified new bone matrix. In cross section, because of interfibrillar space, at least a third of matrix domain is available for occupancy by ground substance including proteoglycans, shown in Figs. 1 to 3.
Enzymaticdigestionofnoncollagenousconstituents.—Segments of bone matrix were treated with α-amylase in a phosphate buffer solution at 25 C and at 2 C (using buffer solution without the enzyme for controls), for chemical and implantation studies. Chemical analysis of the residue showed that amylase-digested matrix lost 45 μg of hexosamine per gm of dry weight, compared to a loss of 10 μg by matrix treated only with the buffer solution for this enzyme system. Implants of the matrix residues after digestion with amylase produced almost as much bone as control preparations (Table 2).
Table 2.
Bone morphogenetic activity of demineralized matrix treated with enzymes
| Treatment of the preimplanted matrix | mm3 new bone/6 mm3 preimplanted matrix | mg bone ash/gm preimplanted matrix |
|---|---|---|
| Control (no treatment) | 5.0 | 520 ± 51 |
| α-Amylase in phosphate buffer, pH 5.4, at 25 C, 90 hr | 5.0 | 490 ± 40 |
| α-Amylase in phosphate buffer, pH 5.4, at 2 C, 90 hr | 5.0 | 500 ± 10 |
| Phosphate buffer without amylase, at 25 C, 90 hr | 5.0 | 510 ± 40 |
| Phosphate buffer without amylase, at 2 C, 90 hr | 5.0 | 515 ± 20 |
| Trypsin in phosphate buffer, pH 7.6 at 15 C, 8 hr | 0.5 | 50 ± 5 |
| Trypsin in phosphate buffer, pH 7.6, at 15 C, 24 hr | 0 | 0 |
| Trypsin in phosphate buffer, pH 7.6, at 15 C, 48 hr | 0 | 0 |
| Trypsin in phosphate buffer, pH 7.6, at 15 C, 96 hr | 0 | 0 |
| Phosphate buffer without trypsin, at 15 C, 96 hr | 0 | 485 ± 20 |
Experiments on samples of bone matrix treated with trypsin at 15 C for various periods of time before implantation are summarized in Table 2. This temperature is below the point of thermal denaturation of collagen but sufficiently high for proteolytic action. Analysis of the 24 hour digest revealed 21.3 μg of hexosamine and 680.8 μmol of amino acids per gm of lyophilized preimplanted matrix, but no hydroxyproline. After implantation of matrix residues digested for eight hours, new bone yield was only 10% of that of the control. In matrix digested for 24, 48, and 96 hours, the yield of new bone was zero.
Neutralsalt-solubleproteinsofbonematrix.—Table 1 summarizes data obtained from experiments with matrix that was first demineralized and then extracted with solutions of CaCl2 of increasing concentrations before implantation. The samples were exposed to the extractant solution either as uncut diaphyses or 1 mm3 diced fragments. Chemical extraction of whole diaphyses with 0.1 to 0.6 M CaCl2 for 24 hours did not reduce the yield of new bone after implantation of the residual matrix in muscle. Extraction of diced samples with 0.6 M CaCl2 for periods of two to four days did not reduce the bone morphogenetic activity of rat bone matrix. However, extraction of diced samples for a period of nine days reduced the yield to 80% of controls; extraction for 25 days eliminated all the bone-forming potential from the residue.
Extraction with 6 M CaCl2 over a period of only 24 hours removed as much bone-forming potential from whole, uncut segments of matrix as 0.6 M solutions from diced matrix over long periods of time. With intermediate concentrations, the yields declined approximately 5% after extraction with 1 M CaCl2, 40% with 2 M CaCl2, and 70% with 6 M CaCl2. Diced matrix extracted with 6 M CaCl2 for only 24 hours produced no new bone after implantation in muscle. The components of the matrix, extracted by these solutions, were analyzed chemically for hydroxyproline and uronic acid. No hydroxyproline was found in any measurable quantity after any of the extraction procedures carried out at 2 C. Uronic acid, however, was extracted by all solutions in the following quantities, expressed in μmol/gm of lyophilized matrix: 0.6 M CaCl2 extracted 4.2; 2 M CaCl2, 3.6; 6 M CaCl2, 3.5. How much BMP is unaltered, extracted, precipitated, or denatured by each concentration of CaCl2 is under investigation.
Denaturedproteins.—Table 3 summarizes observations on demineralized bone matrix treated with denaturants with nonspecific and specific chemical action on the molecular configuration of proteins. Glutaraldehyde, a cross-linking agent, reduced the yield of new bone only slightly when concentrations of 1 to 5 mM/liter were used; concentrations of 10 mM/liter reduced the yield by 40%, and concentrations of 100 mM/liter or above reduced the yield to zero. Formaldehyde in concentrations of 1 to 2 M also reduced the yield to zero in 28-day-old implants. In 12-week-old implants of matrix treated with concentrations of formaldehyde ranging between 1 and 3.3 M, there were a few small islands of cartilage and occasionally a scanty deposit of bone. After shorter periods of implantation, eg four weeks, samples treated with such high concentrations of formaldehyde contained only fibrous tissue.
Table 3.
Yield of new bone in implants of demineralized matrix treated with protein denaturants (four weeks after implantation)
| Agent | mM/liter | mg bone ash/gm preimplanted matrix |
|---|---|---|
| Glutaraldehyde | 0 | 500 |
| 1 | 505 | |
| 2 | 510 | |
| 5 | 450 | |
| 10 | 300 | |
| 100 | 0 | |
| 1000 | 0 | |
| 2000 | 0 | |
| Formaldehyde, (4 weeks after implantation) | 1000 | 0 |
| Formaldehyde, (4 weeks after implantation) | 1800 | 0 |
| Formaldehyde, (4 weeks after implantation) | 2000 | 0 |
| Formaldehyde, (12 weeks after implantation) | 3300 | 10 |
| Formaldehyde-treated lathyritic matrix (4 weeks after implantation) | 1800 | 0 |
| Remazol black | (1% w/v) | 0 |
| Borohydride | 0.5 | 500 |
| 5 | 450 | |
| 50 | 490 | |
| FDNB | 0 | 490 |
| 3 | 460 | |
| 5 | 450 | |
| 10 | 470 | |
| 26 | 210 | |
| 53 | 200 | |
| 268 | 20 | |
| HNO3 | 600 | 0 |
| HNO2 | 600 | 0 |
When demineralized bone matrix was treated with 1% Remazol black, a vital dye that binds to collagen, to an interfibrillar component, or to both, there is a deleterious effect on the bone morphogenetic protein. Borohydride, in concentrations of 0.5 to 50 mM/liter had no effect on the bone morphogenetic activity of the matrix. FDNB reduced the yield of new bone in concentrations higher than 10 mM/liter but did not eliminate all morphogenetic activity until the matrix was exposed to concentrations in excess of 268 mM/liter. Nitrous and nitric acid, in the same concentrations and under the same conditions of time and temperature used for demineralization of bone with HCl, destroyed all morphogenetic activity.
Discussion
The space occupied by collagen fibrils represents about two thirds of the total volume of bone matrix. The remaining third is occupied by interfibrillar noncollagenous proteins and ground substance.
Although 97% of the weight of the dry fat-free demineralized bone is collagen, about 30% of the volume of fresh bone matrix is occupied by noncollagenous constituents. Electron micrographs of the matrix cut in cross section reveal that this 30% contains the remaining 3% of the dry weight. The same 3% includes large molecules of proteoglycans that weigh much less than collagen molecules, but are spread out over much larger areas of the matrix domain. The area occupied by proteoglycans is identified by bismuth-stained EM sections [22] as a lacework of small electron-dense granules.
The intertwined and helical weave pattern of the collagen fibers obscures the large volumes of space between the collagen fibrils. Consequently, the weight relationships between collagenous and non-collagenous proteins are deceiving, and tend to obscure the spatial characteristics of the matrix that are responsible for its bone morphogenetic activity.
Boneproteoglycans.—Bone morphogenetic activity in implants of residues of enzymatically digested matrix indicates that BMP resides in or on quaternary or higher structures of the collagen fibrils. Alpha-amylase removes sugars and small quantities of amino acid residues without altering the 640 A striations on the collagen fibrils or reducing the bone morphogenetic activity. As α-amylase removes significant quantities of polysaccharide but only insignificant quantities of protein [32, 33], its action on bone matrix suggests that the polysaccharide moiety is not a part of the BMP.
EDTA mobilizes some of the same components as α-amylase, but extracts larger quantities of protein, sialoprotein, proteoglycans, and other interfibrillar constituents [32–34]. In concentrations used for decalcification of bone at pH 7.4 at room temperature, EDTA extracts proteoglycans but in addition removes or denatures BMP. These observations point to the protein core of proteoglycans or a component of the collagen fibril [29, 30, 34] as the locus of BMP.
Trypsin-labileproteinsofbonematrix.—The absence of osteogenetic activity in implants of bone matrix digested with trypsin at 15 C suggests that either the protein core of bone proteoglycans or the telopeptide terminal, nonhelical portion of the collagen molecule, or the link between these two structures, imposes the bone morphogenetic pattern on mesenchymal cells. Evidence of enzymatic cleavage of telopeptides of collagen comes from amino acid analyses. The trypsin-labile region of bone collagen does not include hydroxyproline, and therefore is different from the trypsin-resistant triple helical portion of the collagen macromolecules [35, 36]. Rubin et al. [35] cleaved the peptides of the N-terminal region of collagen with pepsin and noted that hydroxyproline and hydroxylysine were missing and glycine was low, whereas tyrosine, aspartic acid, and glutamic acid were high. Pepsin also reduced the viscosity of the solutions of tropocollagen and prevented reconstitution of the fibrillar form, but the triple helix structure that represented 95% of the total macromolecule was unaffected. Bornstein, Kang, and Piez [37] claimed that trypsin is less specific than pepsin and may not attack the iV-terminal region of many preparations of native collagen. The telopeptide is a part of the 10% of the length of the collagen molecule entering into overlap region, and is not detectable by EM. Davison and Schmitt [36] reviewed evidence for and against the theory of the terminal peptides of the collagen molecules, and proposed that the extrahelical portion of the collagen molecule is responsible for immunologic properties, pathologic disorganization, and aging processes.
Inasmuch as enzymes can act on insoluble bone collagen to yield amino acids but not hydroxyproline, and insofar as the residual matrix produces no new bone following implantation in muscle, it is reasonable to assume that telopeptides of the nonhelical region of the collagen molecule could be the binding site, the site itself, or a part of the site of the bone morphogenetic activity. Another observation to support this assumption comes from implants of lathyritic bone, which are similarly nonosteogenetic [7]. Limited proteolysis of soluble collagens by pepsin and trypsin produces monomers physicochemically similar to those obtained from lathyritic collagen [38]. Amino acid analyses now in progress may answer the question of whether telopeptides of insoluble collagens are cleaved when BMP is destroyed by the action of limited proteolysis of bone matrix.
Neutralsalt-solublenoncollagenousandcollagenouscomponentsofbonematrix.—At 2 C, chemical extraction with solutions of CaCl2, in successively increasing concentrations, removes significant quantities of uronic acid without solubilizing any of the collagen of bone matrix. Solutions of 0.5 to 1.0 M CaCl2, which extract 25 to 30% of the polysaccharides from cartilage [23–25, 39], also extract polysaccharides from bone matrix, but implants of the residue produce high yields of new bone. Concentrations of 2 M CaCl2, which remove 80% of the polysaccharide of cartilage matrix, extract slightly less polysaccharide than 0.6 M CaCl2, yet reduce the bone yield by more than 40%. In solutions of 4 M CaCl2, the crossbanding disappears from the collagen [40], and in solutions of 6 M CaCl2, the entire structure of collagenous and noncollagenous matrix is denatured or precipitated. These solutions eliminate all of the bone morphogenetic activity. The reduction of bone morphogenetic activity in neutral salt solutions of high but not low concentrations, suggests that BMP is associated intimately with proteoglycans and the native structure of bone collagen.
Blockingandcross-linkingreagents.—Experiments with blocking and cross-linking agents suggest that the BMP becomes nonosteogenetic after dinitrophenylation, but this adverse effect may reflect denaturation as well as blocking of amino groups of telopeptides. At low concentrations, FDNB had slight or no effect, but at concentrations of 26 mM/liter and greater the yield of new bone after dinitrophenylation declined precipitously.
Free ε-amino groups that react with FDNB also may react with glutaraldehyde and produce supernumerary cross-links. Cross-linking with glutaraldehyde forms intermolecular bonds highly resistant to degradation by proteolytic enzymes [41]. In concentrations below 5 mM/liter, glutaraldehyde reduces the yield of bone little or not at all, whereas in concentrations of 5 to 10 mM/liter, the reduction is from 10 to 40%. All morphogenetic activity is destroyed by concentrations above 100 mM/liter. Thus, data obtained from glutaraldehyde cross-linked matrix, like data obtained from dinitrophenylated matrix, suggest that the bone morphogenetic activity depends on the undenatured native protein structure. The cross-linked structure alone does not account for bone morphogenesis. Lathyritic matrix lacking osteogenetic activity [7] did not produce new bone after cross-linking proteins with formaldehyde by the method of Veis and Drake [42].
Implants of bone matrix with proteins cross-linked by high concentrations of formaldehyde retain a low level of biologic activity, demonstrable only after long periods of time in muscle. The formaldehyde cross-linked matrix is resorbed by the proteolytic action of giant cells slowly or with difficulty, but eventually produces small islands of cartilage and sometimes also scanty deposits of bone. This observation suggests that the introduction of supplementary cross-links suppresses but does not destroy all of the biologic activity enclosed in bone matrix.
Remazol black, a cross-linking matrix-seeking vital dye [43], is a powerful inhibitor of bone morphogenetic activity. It is not known whether the dye cross-links the terminal region of the collagen molecule, the whole molecule, or the noncollagenous proteins. The Remazol black reaction is important insofar as the dye is selectively bound to an organic component of calcifying but not noncalcifying connective tissues. If Remazol black cross-links calcifiable collagenous protein and protein polysaccharide complexes, the dye may constitute a specific label for BMP.
Borohydridestabilizationofintermolecularbondsofcollagen.—Reduction with borohydride stabilizes the intermolecular structure of soluble collagen [28, 44]. The three dimensional structure of insoluble bone collagen may be stabilized similarly and made chemically less reactive than native collagen. Borohydride reduction of bone collagen by means of the procedure of Bailey [28], appears to stabilize bone collagen without affecting the bone morphogenetic property. How complete borohydride reduction may be in systems containing insoluble bone collagen and the BMP presently is not known.
Deamination.—Nitrous acid deamination replaces the ε-amino group of the lysine and hydroxylysine side chains with a hydroxyl group, without any change in the crossbanded pattern of the collagen fibrils [31]. Unfixed bone matrix demineralized in either nitrous or nitric acid, under exactly the same conditions as bone matrix demineralized in HCl, may be deaminated similarly. Implantation of bone demineralized by nitrous or nitric acid produced no bone. Consequently, deamination, like FDNB blocking, suggests that lysine and hydroxylysine may be a part of the bone matrix morphogenetic pattern.
Problemofcharacterizationofthebmp.—The intercellular matrix of hard tissue, composed of molecules of protein, lipid, carbohydrate, salt, and water, has many biological and mechanical-functional components intrinsic to its structure. Nearly all of the protein is structural, but a small quantity is enzymatic. If it is assumed that a single molecular species of structural protein is responsible for bone morphogenesis, the characterization of the BMP and the determination of its relationship to the collagen, as well as to other components of calcified tissue, now seems feasible. This would provide an idea of the way molecules are put together to produce both bone morphogenetic activity and calcifiability of hard tissues.
As noted previously, BMP can be identified in preparations of matrix in which proteoglycans may be covalently bonded to the telopeptide region of the collagen, and part of a specific complex protein macromolecule. Such bonds exist between the protein backbone of proteoglycans and the collagen, and these are sufficiently stable to preserve the native, three dimensional structure of the matrix even after the lipids have been extracted and part of the polysaccharide has been digested with amylase or extracted with calcium chloride [4]. Such chemical bonds could determine the structural characteristics of all of the various calcified matrixes of specialized tissues such as dentin, bone, calcified cartilage, enamel, and so forth, and without any significant involvement of such ubiquitous constituents as sialic acid, hexosamines, phosphoproteins, and phospholipids [45, 46].
There is as yet no clue to the molecular component that calculable tissues have in common. On the basis of correlated observations of systems in vitro and in vivo, we have postulated a tripartite protein-calcium-phosphate complex secreted by bone cells along with other components of bone matrix [47–49]. The tripartite complex is formed by the uptake of calcium by carboxylate groups, followed by a second phase of phosphate ion association and a third phase of apatite crystal formation within the folds or interfibrillar spaces of the extracellular matrix [49]. Removal of the mineral in the cold prevents thermal denaturation of the protein, and preserves the bone morphogenetic activity of the previously calcified matrix. The folded structure of the native calcified proteins can be visualized as being held together predominately by salt linkages between charged groups of the protein back-bone of the proteoglycans. Once deposited in bone matrix, chemical extraction of polysaccharides, chiefly chondroitin sulfates A and C with 0.6 M CaCl2, or digestion with amylase, would not alter the folded structure of the BMP.
It is not known whether the integrity of the BMP requires the helical portion of the collagen molecule or whether it is self-assembled into a three dimensional structure by virtue of some of the intrinsic properties of the BMP molecules themselves. To find the answer, it may be necessary to add the protein backbone of the proteoglycan molecule back to the collagen framework in vitro. This procedure presents the difficult problem of taking the molecules apart and putting them back together.
There is some reason to believe that the chemical components of the BMP are not synthesized and deposited into the extracellular space at the same time and under the same conditions. For example, some species store BMP in the matrix in greater quantities and in a more active form than other species. The rat has comparatively more BMP than the guinea pig [9, 17]. In subjects with lathyrism, penicillaminism, rickets, and adrenal hypercorticoidism, BMP is missing, and therefore not an obligatory part of the structure of the bone matrix. Thus, we may visualize bone matrix performing the function of a molecular crate or framework of collagenous partitions containing BMP and various accessory chemical components, synthesized in the course of the long process of cell differentiation, tissue development, and disease.
Much work must be done before it will be possible to isolate and define the BMP in physicochemical terms. For the present the BMP is considered a highly plastic rather than static component of the bone matrix. Hopefully, it is also a constantly renewable component. If the structure of the intercellular matrix is thought of as a crate for a large array of substances with different properties and functions, its size and shape would be determined by specialized connective tissue cells as well as by macromolecular configurations. Although the crate is made to hold molecules of a variety of sizes and shapes, the pigeonholes are not all filled at any one time. When filled with the BMP, cells that come in contact with the matrix change the pathway of differentiation and all further tissue development.
The mode of action of the BMP on mesenchymal cells is not known. Viewed as a “fibroblast transforming factor” postulated by Huggins, Wiseman, and Reddi [17], BMP could be degraded to promote diffusion through the cytoplasm, across the nuclear membrane, and transferred onto a selection of genes for bone biosynthesis. The BMP macromolecule also may be the substrate for an enzyme in the plasma membrane permeability is envisioned by Fell [52] as derived from telopeptides, at the interface between old matrix and young mesenchymal cells [50, 51]. Before degradation, the BMP macromolecule may come into direct contact with the structure of the cell membrane, and impose a bone morphogenetic change in permeability of mesenchymal cell membranes; in systems in vitro, a change in membrane permeability is envisioned by Fell [52] as the initial step in the process of cell differentiation. In any event, the disappearance of interfibrillar and fibrillar protein ultrastructure of old matrix, preceding and during osteogenesis, is circumstantial evidence of transfer of BMP macromolecules from implant to cell populations with osteogenetic competence.
Conclusions
Electron micrographs of rat cortical bone, demineralized in cold diluted HCl within 48 hours to conserve the interfibrillar structure, reveal that constituents representing 3% of the dry fat-free weight of the tissue occupy nearly a third of the matrix domain. These constituents include large molecules of bismuth nitrate-staining proteoglycans with attachments to collagen fibrils.
The development of bone and bone marrow in implants of residues of matrix derived from enzymatic cleavage, chemical extraction, or organic blocking procedures, suggests bone morphogenetic activity resides in an interfibrillar macromolecular protein complex. Noncollagenous proteins of bone matrix or the portions of the collagen molecules that are digestible with trypsin are essential for bone morphogenetic activity. Portions of the proteoglycans cleaved enzymatically with amylase are not essential for osteogenesis in implants of the residual bone matrix. Extraction with dilute solutions of calcium chloride does not remove constituents essential for bone morphogenesis; high concentrations of CaCl2, which denature proteins, destroy bone morphogenetic activity. High concentrations of blocking and cross-linking reagents reduce or eliminate bone morphogenetic activity. Reduction of bone matrix proteins with borohydride is relatively innocuous, but nitrous acid deamination eliminates all bone morphogenetic activity. These observations suggest that the hypothetical bone morphogenetic protein (BMP) is structurally related either to the telopeptides of the collagen molecule or to the protein moiety of bone proteoglycans, or may constitute the bonding site between the two.
The authors thank G. L. Molson for electron micrographs.
Appendix
Induction: The process of tissue differentiation initiated by close contact and mutual interaction of cell populations of diverse origins is called induction. The mechanism presently is unknown and uncharacterized in physicochemical terms.
Cellcontact: The contact between inducing and responding cells is mediated by intercellular substances and is dynamic rather than static in nature.
Inducer: Diffusible chemical substances of a wide variety, including inorganic ions, chemical compounds, vitamins, and hormones capable of stimulating cell differentiation in the early stages of embryonic development, are commonly referred to as primary embryonic inducers.
Inductors: Nondiffusible solid structures, generally consisting of a complex arrangement of many different substances, leading to differentiation of various tissues and organs in fetal life are known as inductors [13].
Inductivematrix: The organic framework produced by demineralization of bones, teeth, and other calcified tissues serves as an inductive matrix for differentiation of mesenchymal cell populations with determination or competence to develop into cartilage, bone, and bone marrow.
Bonehistogeneticsubstratum: The demineralized organic matrix of enamel is a substratum for differentiation of mesenchymal cells into bone tissue of a heteromorphic type resembling that of teleost fishes.
Bonemorphogeneticsubstratum: The structure of the demineralized matrix is a substratum for differentiation of mesenchymal cells, not only into bone as a tissue, but also bone as an organ consisting of a shell of lamellar bone with a marrow cavity filled with hematopoietic bone marrow.
Demineralizedbonematrix: Bone divested of inorganic salts, with minimal leaching or denaturation of the organic components of the matrix, is demineralized bone matrix. Demineralization is carried out in the cold at 2 C, avoiding protein-polysaccharide extractants such as EDTA. Acids such as hydrochloric acid, phosphoric acid, or citric acid, over a period of not more than three to five days, at concentrations of not more than 0.6 N, are preferable. Higher temperatures, higher concentration, and most organic acids extract variable quantities of the organic components of bone matrix and destroy bone morphogenetic proteins. The highly cross-linked matrix of dentin, although highly permeable, protects the BMP against the deleterious actions of organic acids, high temperatures, and long periods of exposure.
Bonemorphogeneticprotein (BMP): The osteogenetic chemical components of the matrix of bone, dentin, and other hard tissues that are deinsulated by demineralization and associated intimately with collagen fibrils, are termed the bone morphogenetic proteins.
Boneinductionprinciple (BIP): The product of the interaction of the mesenchymal cells with the surface of the implanted matrix is referred to as the bone induction principle (BIP). Since the BIP occupies the space between the implanted matrix and the extracellular substances secreted by the mesenchymal cells, it has a three-dimensional structure. The BIP fills the pores of a millipore membrane, 125 μm, with a pore size of 0.45 μm [3].
Transfilterboneinduction: The demonstration of mutual interaction of tissues across a millipore membrane suggests that the bone induction principle is transmissible. Inasmuch as smaller pore sizes are filled with extracellular substances and filopodial extensions of plasma membranes, transfilter bone induction cannot be offered as evidence of action of a diffusible molecular species. Instead, the evidence is more correctly interpreted as extension of a complex structure intimately related to the surfaces of interacting cells.
Filopodialextensionsofcell: Tubular extensions of the plasma membranes of mesenchymal cells, containing micro filaments, free of other cell organelles, averaging about 1 μm in length and 0.3 μm in diameter, are termed filopodial extensions. Comparable to filopods of migratory ameboid cells, filopods of mesenchymal cells are contractile organs that probe the microcanalicular system, vascular channels, and other intersticies of an implant of bone matrix [13].
Matrixclasts: The process of multinucleation occurring by fusion of proliferating mesenchymal cells, produces matrixclasts. Comparable to osteoclasts, which resorb undemineralized bone, matrixclasts are multinucleated cells having an undulating membrane and are found in excavation chambers in the surfaces of an implant of demineralized bone matrix [9].
Determinationforbonedevelopment: Mesenchymal cells have osteogenetic determination when genes coding for synthesis of calcifiable collagenous matrix are present. Osteogenetic competence or determination is the state of readiness but not yet activated capacity to differentiate into bone cells. The basis of osteogenetic competence is a group of gene-action systems for production of cartilage, bone, and bone marrow cells. Determination for bone cell specialization is therefore a heritable or genetic property. Osteogenetic determination is present in somatic mesenchyme, but its expression may be held in abeyance throughout all of postfetal life. Based on a broad selection of genes, osteogenetic determination develops in somatic mesenchyme early in embryonic development, possibly as early as the gastrula stage [6, 13].
Footnotes
Worthington Biochemical Corp., Freehold, NJ.
This study was aided by a grant-in-aid from USPHS, National Institute of Dental Research (DE-02103-07), National Institutes of Health, Bethesda, Md, The John A. Hartford Foundation, Inc., Orthopaedic Research and Education Foundation, and a contract between the US Army Research and Development Command (DA-49-193-MD-2556) and the University of California.
Richard A. Brand MD (✉) Clinical Orthopaedics and Related Research, 1600 Spruce Street, Philadelphia, PA 19103, USA e-mail: dick.brand@clinorthop.org
References
- 1.Urist, M.R.; Silverman, B.F.; Buring, K.; Dubuc, F.L.; and Rosenberg, J.M.: The Bone Induction Principle, Clin Orthop 53: 243-283, 1967. [PubMed]
- 2.Yeomans, J.D., and Urist, M.R.: Bone Induction by Decalcified Dentin Implanted into Oral Osseous and Muscle Tissues, Arch Oral Biol 12:999-1008, 1967. [DOI] [PubMed]
- 3.Buring, K., and Urist, M.R.: Transfilter Bone Induction, Clin Orthop 54:235-242, 1967. [PubMed]
- 4.Urist, M.R.; Dowell, T.A.; Hay, P.H.; and Strates, B.S.: Inductive Substrates for Bone Formation, Clin Orthop 59:59-96, 1968. [PubMed]
- 5.Urist, M.R.: Mesenchymal Cell Reactions to Inductive Substrates for Bone Formation, in Wound Healing, New York: McGraw-Hill, 1969, pp 229-259.
- 6.Urist, M.R.; Hay, P.H.; Dubuc, F.; and Buring, K.: Osteogenetic Competence, Clin Orthop 64:194-220, 1969. [PubMed]
- 7.Strates, B.S., and Urist, M.R.: The Origin of the Inductive Signal in Implants of Normal and Lathyritic Bone Matrix, Clin Orthop 66:226-240, 1969. [PubMed]
- 8.Urist, M.R., and Strates, B.S.: Bone Formation in Implants of Partially and Wholly Demineralized Bone Matrix, Clin Orthop 71:271-278, 1970. [PubMed]
- 9.Urist, M.R., and Craven, P.L.: Bone Cell Differentiation in Avian Species; Including Comments on Multinucleation and Morphogenesis, Fed Proc 29:1680-1693, 1970. [PubMed]
- 10.Urist, M.R.; Jurist, J.M.; Dubuc, F.L.; and Strates, B.S.: Quantitation of New Bone Formation in Intramuscular Implants of Bone Matrix in Rabbits, Clin Orthop 68:279-293, 1970. [PubMed]
- 11.Huggins, C.B., and Urist, M.R.: Dentin Matrix Transformation: Rapid Induction of Alkaline Phosphatase and Cartilage, Science 167:896-898, 1970. [DOI] [PubMed]
- 12.Urist, M.R.: Bone Histogenesis and Morphogenesis in Implants of Demineralized Enamel and Dentin, J Oral Surg 29:88-102, 1971. [PubMed]
- 13.Urist, M.R.: The substratum for Bone Morphogenesis, Develop Biol (suppl), pp 125-163, 1970. [PubMed]
- 14.Firschein, H.E., and Urist, M.R.: The Induction of Alkaline Phosphatase by Extraskeletal Implants of Bone Matrix, Cal Tissue Res 7:108-131, 1971. [DOI] [PubMed]
- 15.Urist, M.R., and Nogami, H.: A Morphogenetic Substratum for Differentiation of Cartilage in Tissue, Nature 225:1051-1052, 1970. [DOI] [PubMed]
- 16.Nogami, H., and Urist, M.R.: A Substratum of Bone Matrix for Differentiation of Mesenchymal Cells into Chondro-osseous Tissues in vitro, Exp Cell Res 63:404-410, 1970. [DOI] [PubMed]
- 17.Huggins, C; Wiseman, S.; and Reddi, A.H.: Transformation of Fibroblasts by Allogeneic and Xenogeneic Transplants of Demineralized Tooth and Bone, J Exp Med 132:1250-1258, 1970. [DOI] [PMC free article] [PubMed]
- 18.Nade, N.: Bone Graft Surgery Reappraised: The Contribution of the cell to Ultimate Success, Brit J Surg 57:752-756, 1970. [DOI] [PubMed]
- 19.Veis, A.; Spector, A.R.; and Carmichael, D.J.: The Organization and Polymerization of Bone and Dentin Collagens, Clin Orthop 66:188-211, 1969. [PubMed]
- 20.Urist, M.R., and Dowell, T.A.: The Inductive Substratum for Osteogenesis in Pellets of Particulate Bone Matrix, Clin Orthop 61:61-78, 1968. [PubMed]
- 21.Owen, M.: The Origin of Bone Cells, Intern Rev Cyt 28:213-238, 1970. [DOI] [PubMed]
- 22.Smith, J.W.: The Disposition of Protein Polysaccharides in the Epiphyseal Plate of the Young Rabbit, J Cell Sci 6:843-864, 1970. [DOI] [PubMed]
- 23.Tsiganos, CP., and Muir, H.: The Natural Heterogeneity of Proteoglycans of Porcine and Human Cartilage, in Balazs, E.A. (ed): Chemistry and Molecular Biology of the Intercellular Matrix, Vol 2, New York: Academic Press, 1970, pp 859-866.
- 24.Gregory, J.D.; Sajdera, S.W.; Hascoll, V.C.; and Dziewiatkowski, D.D.: The Proteoglycans of Bovine Nasal Cartilage: Dissociative Extraction, in Balazs, E.A. (ed): Chemistry and Molecular Biology of the Intercellular Matrix, Vol 2, New York: Academic Press, 1970, pp 843-849.
- 25.Hallen, A.A.: On Differences in Extractability of the Proteoglycans in Balzas, E.A. (ed): Chemistry and Molecular Biology of the Intercellular Matrix, Vol 2, New York: Academic Press, 1970, pp 903-906.
- 26.Nold, J.G.; Kang, A.H.; and Gross, J.: Collagen Molecules: Distribution of Alpha Chains, Science 170:1096-1098, 1970. [DOI] [PubMed]
- 27.Seiton, E.C., and Engel, M.B.: Reactive Dyes as Vital Indicators of Bone Growth, Amer J Anat 126:373-392, 1969. [DOI] [PubMed]
- 28.Bailey, A.J.: Intermediate Labile Intermolecular Crosslinks in Collagen Fibres, Biochim Biophys Acta 160:447-453, 1968. [DOI] [PubMed]
- 29.Mechanic, G., and Tanzer, M.L.: Biochemistry of Collagen Crosslinking: Isolation of a New Cross-link, Hydroxylysino-hydroxyleucine and its Reduced Precursor, Dihydroxynorleucine from Bovine Tendon, Biochem Biophys Res Comm 41:1597-1603, 1970. [DOI] [PubMed]
- 30.Hughes, R.C., and Thurman, P.F.: Cross-linking of Bacterial Cell Walls with Glutaraldehyde, J Biochem 119:925-926, 1970. [DOI] [PMC free article] [PubMed]
- 31.Grant, R.A.; Cox, R.W.; and Kent, CM.: Electron Microscope Studies of Deaminated and Cross-linked Collagen, J Microscopy 92:27-30, 1970. [DOI] [PubMed]
- 32.Veis, A.; Bhatnagar, R.S.; Shuttleworth, C.A.; and Mussel, S.: The Solubilization of Mature Polymeric Collagen Fibrils by Lyotropic Relaxation, Biochim Biophys Acta 200:97-112, 1970. [DOI] [PubMed]
- 33.Di Ferrante, N., and Neri, G.: The Action of Crude α-amylase from B. Subtilis on Cartilage Glycosaminoglycans, J Lab Clin Med 77:7-13, 1971. [PubMed]
- 34.Lapiere, Ch.M.; and Nusgens, B.V.: Maturation Related Changes of the Protein Matrix of Bone, in Balazs, E.A. (ed): Chemistry and Molecular Biology of the Intercellular Matrix, Vol 1, New York: Academic Press, 1970, pp 55-79.
- 35.Rubin, A.L.; Pfahl, D.; Speakman, P.T.; Davison, P.F.; and Schmitt, F.O.: Tropocollagen: Significance of Protease-induced Alterations, Science 139:37-38, 1963. [DOI] [PubMed]
- 36.Davison, P.F., and Schmitt, F.O.: The Enzymatic Dissection of Tropocollagen, Hoppe Seylefs Z Physiol Chem 349:119-124, 1968. [DOI] [PubMed]
- 37.Bornstein, P.; Kang, A.H.; and Piez, K.A.: The Limited Cleavage of Native Collagen with Chymotrypsin, Trypsin, and Cyanogen Bromide, Biochemistry 5:3803-3812, 1966. [DOI]
- 38.Tanzer, M.: Experimental Lathyrism, Int Rev Conn Tissues Res 3:91-112, 1965. [DOI] [PubMed]
- 39.Mathews, M.B.: The Interactions of Proteoglycans and Collagen—Model Systems, in Balazs, E.A. (ed): Chemistry and Molecular Biology of the Intercellular Matrix, Vol 2, New York: Academic Press, 1970, pp 1155-1169.
- 40.Anderson, H.C., and Sajdera, S.W.: Extraction as a Technique for the Electron Microscopic Study of Protein Polysaccharides and Collagen in Cartilage Matrix, abstracted, Fed Proc 29, March-April, 1970.
- 41.Haynes, R., and Walsh, K.A.: Enzyme Envelopes on Colloidal Particles, Biochem Biophys Res Comm 36:235-242, 1969. [DOI] [PubMed]
- 42.Veis, A., and Drake, M.P.: The Introduction of Intramolecular Covalent Cross-Linkages into Ichthyocol Tropocollagen with Monofunctional Aldehydes, J Biol Chem 238:2003-2011, 1963. [PubMed]
- 43.Goland, P., and Engel, M.: Fixation of Cells and Tissues by Chloro-S-Triazines, J Histochem Cytochem 11:751-762, 1963.
- 44.Tanzer, M.L.: Intermolecular Cross-links in Reconstituted Collagen Fibrils, J Biol Chem 243:4045-4054, 1968. [PubMed]
- 45.Miller, E.J.; Martin, G.R.; Piez, K.A.; and Powers, M.J.: Characterization of Chick Bone Collagen and Compositional Changes Associated with Maturation, J Biol Chem 242:5481-5489, 1967. [PubMed]
- 46.Glimcher, M.J., and Katz, E.P.: The Organization of Collagen in Bone, J Ultrastruct Res 12:705-729, 1965. [DOI] [PubMed]
- 47.Urist, M.R., and Adams, J.M., Jr.: Effects of Various Blocking Reagents upon Local Mechanisms of Calcification, Arch Path 81:325-342, 1966. [PubMed]
- 48.Urist, M.R.: Biologic Initiators of Calcification, in Zipkin, I. (ed): Biological Mineralization, New York: John Wiley & Sons, in press.
- 49.Kashiwa, H.K.: Mineralized Spherules in Cartilage of Bone Revealed by Cytochemical Methods, Amer J Anat 129:459-466, 1970. [DOI] [PubMed]
- 50.Hodge, A.J., and Schmitt, F.O.: The Charge Profile of the Tropocollagen Macromolecule and the Packing Arrangement in Native-type Collagen Fibrils, Proc Nat Acad Sci USA 46:186-197, 1960. [DOI] [PMC free article] [PubMed]
- 51.Schmitt, F.O.: Telopeptide Control of Tropocollagen Interaction Dynamics, Fed Proc 23:618-622, 1964. [PubMed]
- 52.Fell, H.B.: Role of Biological Membranes in Some Skeletal Reactions, Ann Rheum Dis 28:213-227, 1969. [DOI] [PMC free article] [PubMed]


