Abstract
VEGF plays a role in bone remodeling. Ingrowth of reparative arterioles can be observed in late-stage osteonecrosis. To explore the reparative processes, we quantified the most important angiogenesis factor (VEGF) in different zones of late-stage glucocorticoid-induced osteonecrosis. We treated primary nonosteonecrosis osteoblasts with glucocorticoids in vitro as a model for the bone cells in early-stage steroid-related osteonecrosis. We obtained six late-stage (ARCO Stage IV) osteonecrosis femoral heads and six osteoarthritic femoral heads during THA. The expression of vascular endothelial growth factor was analyzed by reverse-transcription PCR, ELISA, or immunohistochemistry. Osteoblasts from the reactive interface (penumbra) of osteonecrosis femoral heads exhibited increased immunoreactivity to VEGF compared to those from the periphery. ELISA confirmed VEGF upregulation in the penumbra from osteonecrosis femoral heads. Primary osteoblasts derived from osteoarthritic femoral heads exhibited downregulation of VEGF after 24 hours of coincubation with glucocorticoids. The increase in VEGF in the reactive interface (penumbra) of osteonecrosis in late-stage femoral head may reflect a secondary phenomenon. The observed high amount of VEGF in later-stage osteonecrosis might stimulate the ingrowth of reparative arterioles into the femoral head.
Introduction
Femoral head osteonecrosis (ON) is a well-known complication after high-dose corticosteroid treatment in adults and children, with an incidence of 8% to 25% [15, 26, 32]. Disturbed blood supply is believed by some to be an early consequence [9, 12]. Glucocorticoids decrease cyclooxygenase-2 (COX-2) expression and subsequently prostaglandin E2 (PGE2) production [11]. The loss of PGE2 production may lead to a decrease of the angiogenesis factor VEGF. Since VEGF is an antiapoptotic factor for vessels, lower VEGF may lead to disturbed blood vessel remodeling. Recently, increased VEGF expression has been described in the epiphyseal cartilage after ischemic necrosis of the femoral head epiphysis in a pig model [14]. During early ON of the femoral head (ARCO Stage II [7]), new bone is formed on partly resorbed trabeculae within the reactive interface [20]. Since bone formation is coupled with angiogenesis, reparative arterioles grow into this reactive interface during this phase [18]. However, the reason for the neoangiogenesis in steroid-related ON is unclear. According to the adjacent area of stroke-affected brain parts, we called the ON surrounding the VEGF-producing area the penumbra. The therapeutic strategy of the ON penumbra should be similar to the stroke penumbra: a fast reconstitution of sufficient vessels.
VEGF (a potent angiogenic peptide, also termed vascular permeability factor) is reportedly an essential factor for endochondral ossification [2, 3, 8]. VEGF is a homodimeric, heavily glycosylated protein of 46 to 48 kDa. The effects of VEGF are mediated by two signaling receptors that belong to the Class III tyrosine kinase receptor family [4, 27], namely VEGFR-1 (flt-1, fms-like tyrosine kinase-1) and VEGFR-2 (kinase domain region/flk-1, fetal liver kinase-1). For signal transduction of VEGF in bone, VEGFR-2 is more important [33]. Interestingly, the angiogenic potency of VEGF is splice variant specific [24]. Therefore, the exact determination of splice variants in the investigated tissue is important for prognosis of VEGF effects. The 165-residue VEGF is the most potent variant of all VEGF isoforms [5]. The potency of VEGF also depends on its stability, although the minimum dose of dexamethasone producing an effect on VEGF expression in vitro is unknown. However, a therapeutic use of dexamethasone beyond the minimum dose could prevent a beneficial angiogenic response.
Harada et al. [10, 11] detected expression of VEGF in human osteoblasts and a PGE2-mediated increase in VEGF mRNA levels. Other authors have reported an age-related decrease in the secreted levels of VEGF in osteoblasts [16] and an increase in VEGF and a decrease in parathyroid hormone-related protein after vitamin D incubation. Since VEGF is a key factor of angiogenesis, we hypothesized a decrease in VEGF in the early stage of ON and an increase in the late stage of ON.
We therefore asked the following questions: (1) Is it possible to differentiate histologically distinct areas of ON femoral heads by VEGF and vessel specific markers? (2) What observations can be made concerning vessel ingrowth into the area of the penumbra? (3) Do osteoblasts produce small VEGF splice variants that have the potency to react over long distances and have good stability? And (4) is there a dose relationship of dexamethasone on VEGF expression in primary osteoblasts?
Patients and Methods
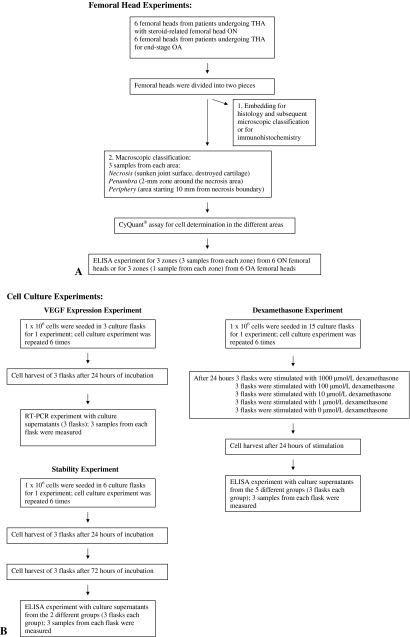
We quantified VEGF and immature vessel production in the necrotic area, the periphery, and the penumbra of six ON femoral heads and in six nonnecrotic osteoarthritic (OA) femoral heads using histology, immunohistochemistry, and ELISA (Fig. 1A). In vitro experiments with primary human osteoblasts using ELISA and reverse-transcription PCR (RT-PCR) evaluated VEGF potency, stability, and decrease at different concentrations of dexamethasone (Fig. 1B).
Fig. 1.
Flow diagrams illustrate the (A) femoral head experiments and (B) cell culture experiments.
The femoral heads came from six patients undergoing THA (aged 32–54 years) with steroid-related femoral head ON (Fig. 2) and six patients undergoing THA for end-stage OA. Patients who had undergone kidney transplantation were treated for 5 days with 1 g dexamethasone each day. The duration from cessation of dexamethasone usage to surgery was between 12 and 18 months. The indication for THA was late-stage ON (MRI and radiographically diagnosed ARCO Stage IV) when secondary arthritis of the hip caused a painful limitation of hip movement and reduced the patient’s ability to walk.
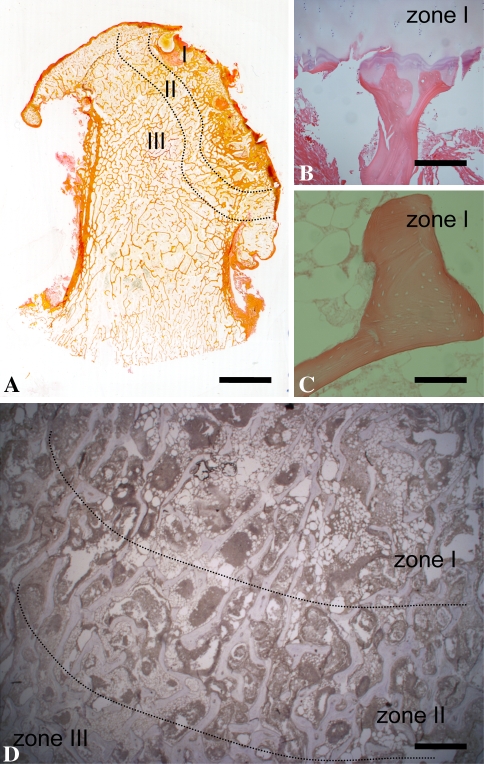
Fig. 2A–D.
(A) To detect different zones of ON of the femoral head, van Giesson staining was performed. Zone I represents the necrotic bone area, Zone II the penumbra, and Zone III the periphery. (B, C) Hematoxylin and eosin staining revealed the structure of the necrotic bone and the loss of osteocytes. (D) The density of fat cells is clearly visible with hematoxylin and eosin staining. (A) Bar = 1 cm; (B, C) bar = 100 μm; (D) bar = 500 μm.
For the femoral head experiments, we divided the ON and OA femoral heads into two pieces using a saw: one piece for histology, microscopic classification, and immunohistochemistry and the other piece for macroscopic classification, CyQuant® assay, and ELISA.
The first piece of both the ON and OA femoral heads was fixed in 3% paraformaldehyde, embedded in paraffin, sectioned, dewaxed, and irradiated at 750 W in a microwave oven in 0.01 mol/L sodium citrate buffer, pH 6.0 (twice for 5 minutes). The sections were first blocked with 3% hydrogen peroxide (endogenous peroxidases) and then with normal serum (1:5 in Tris-buffered saline) of the species in which the primary antibody was raised and then immunostained with anti-VEGF (1:40 in Tris-buffered saline) or anti-VEGFR-2 (1:40) for 60 minutes. We used sc7269 mouse monoclonal IgG2a (Santa Cruz Biotechnology, Santa Cruz, CA) for anti-VEGF and sc-6251 monoclonal IgG1 (Santa Cruz Biotechnology) for anti-VEGFR-2. To detect immature vessels, we used Qbend10 (AB8536; 1:40; Abcam, Cambridge, MA) followed by biotinylated secondary antibodies and a peroxidase-labeled streptavidin-biotin staining technique.
The femoral heads with ON were classified into three zones using histology (van Giesson [Fig. 2A] and hematoxylin and eosin [Fig. 2B–D] staining): Zone I was the necrotic area with empty osteocyte lacunas and a few alive fat cells in the bone marrow. Zone II was the reactive interface (“penumbra”). In this area, the osteoblasts and osteocytes were still alive, but the density of fat cells was decreased. Zone III, the nonnecrotic zone, contained normal bone trabeculae with intact osteoblasts and osteocytes and a few fat cells. Three blinded observers (MP, GG, WD) chose sections without any staining artifacts.
To quantify VEGF staining, we used the whole area of Zones I, II, and III. Six sections of each femoral head stained against VEGF, VEGFR-2, and Qbend10 were assessed using densitometry. To avoid intraobserver variability concerning staining intensity, we performed densitometry using PC Bas 2.0 (Fuji LAS 1000; Berthold, Australia) [23]. For this purpose, sections were scanned and saved as 8-bit black and white TIFF files. The intensity of staining is proportional to the revealed dots per inch.
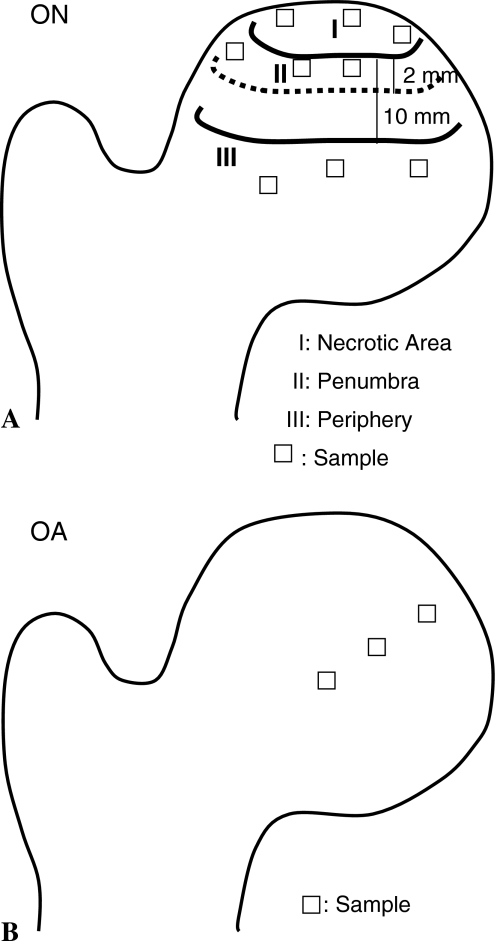
The second piece of the ON femoral heads was macroscopically divided into three areas: (1) a necrotic area with sunken joint surface and destroyed cartilage; (2) the penumbra, a 2-mm zone around the necrosis area; and (3) the periphery, an area starting 10 mm from the necrotic boundary. We dissected nine samples from each femoral head (three samples from each area) using a scalpel (Fig. 3A). From the second piece of the OA femoral heads, we dissected three samples using a scalpel: one from the center of the femoral head, one from the periphery, and one from the part in between (Fig. 3B). These samples were analyzed by a sandwich ELISA (R&D Systems, Minneapolis, MN) that detects all VEGF splice forms. The bone samples were crushed in an agate mortar under liquid nitrogen and homogenized in 150 mmol/L NaCl, 20 mmol/L Tris HCl buffer, pH 7.4, using a Polytron® homogenizer (Kinematica, Luzern, Switzerland). Human recombinant VEGF165 (PreproTech, Rocky Hill, NJ) served as standard. ELISAs were performed as described by Pufe et al. [22]. DNA levels were determined using the CyQuant® (Molecular Probes, Eugene, OR) technique [23].
Fig. 3A–B.
(A) A diagram illustrates the three zones and sampling sites of the ON femoral head. Three samples were taken from each zone for a total of nine samples from each femoral head. (B) A diagram illustrates the sampling sites of the OA femoral head. A total of three samples were taken from each femoral head: one from the center of the femoral head, one from the periphery, and one from the part in between.
Human osteoblasts from nonnecrotic OA femoral heads were obtained from Oligene (Berlin, Germany); after enzymatic digestion with collagenase, cells were seeded and sent proliferating in culture flasks to our laboratory. We first tested whether cultured primary osteoblasts expressed VEGF and its receptors and the potency and stability of this expression. For one experiment, 1 × 106 cells were incubated serum-free in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 containing phenol red in three 25-cm2 culture flasks for 24 hours; the experiment was repeated six times. To determine gene expression of VEGF and VEGFR-2, we used RT-PCR; RNA was isolated as described by the manufacturer (phenol-guanidinium thiocyanate method) (TRIzol®; Invitrogen, Karlsruhe, Germany). Crude RNA was purified by isopropanol and repeated ethanol precipitation, and contaminating DNA was destroyed by digestion with RNase-free DNase I (20 minutes, 25°C; Boehringer, Mannheim, Germany). After inactivating the DNase (15 minutes, 65°C), cDNA was generated with 1 μL (20 pmol) oligo(dT)15 primer (Amersham Pharmacia Biotech, Uppsala, Sweden) and 0.8 μL superscript RNase H− reverse transcriptase (Gibco, Paisley, UK) for 60 minutes at 37°C. For PCR, 4 μL cDNA was incubated with 30.5 μL water, 4 μL 25 mmol/L MgCl2, 1 μL dNTP, 5 μL 10× PCR buffer, and 0.5 μL (2.5 U) Platinum® Taq DNA polymerase (Gibco) and 2.5 μL (10 pmol) of each primer pair. The following primers/conditions were applied: VEGF splice variants, 5′-CCA-TGA-ACT-TTC-TGC-TGT-CTT-3′ (sense) and 5′-TCG-ATC-GTT-CTG-TAT-CAG-TCT-3′ (antisense) with 40 cycles performed at 55°C annealing temperature; VEGFR-1, 5′-GTA-GCT-GGC-AAG-CGG-TCT-TAC-CGG-CTC-3′ (sense) and 5′-GGA-TTT-GTC-TGC-TGC-CCA-GTG-GGT-AGA-GA-3′ (antisense) with 40 cycles at 55°C yielding a 316-bp product; and VEGFR-2, 5′-TAT-AGA-TGG-TGT-AAC-CCG-GA-3′ (sense) and 5′-TTT-GTC-HCT-GAG-ACA-GCT-TGG-3′ (antisense) with 40 cycles at 60°C yielding a 555-bp product.
To test the potency and stability of VEGF in vitro, 1 × 106 cells were incubated serum-free in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 containing phenol red in six culture flasks for 24 hours (three flasks) or 72 hours (three flasks); the experiment was repeated six times. Subsequently, VEGF production in cell supernatants was determined using ELISA as described above.
For the stimulation with dexamethasone experiments, in each flask (15 flasks; three flasks per condition), 1 × 106 cells were incubated serum-free in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 containing phenol red for 24 hours with dexamethasone (1, 10, 100, 1000 μmol/L) or without dexamethasone (control). The experiment was repeated six times. VEGF production in cell supernatants was determined using ELISA as described above.
We determined differences in VEGF staining intensity between the necrotic, penumbra, nonnecrotic areas of ON femoral heads and the bone of nonnecrotic OA femoral heads using Dunnett’s multiple comparison test. We determined differences in VEGF production between the necrotic, penumbra, nonnecrotic areas of ON femoral heads and the bone of nonnecrotic OA femoral heads using Dunnett’s multiple comparison test. We determined differences in VEGF production between 24- and 72-hour incubation using the t test from JMP® statistics package (SAS Institute Inc, Cary, NC). Finally, we determined differences in VEGF protein decrease between control and different concentrations of dexamethasone (1, 10, 100, 1000 μmol/L) using the t test from JMP® statistics package.
Results
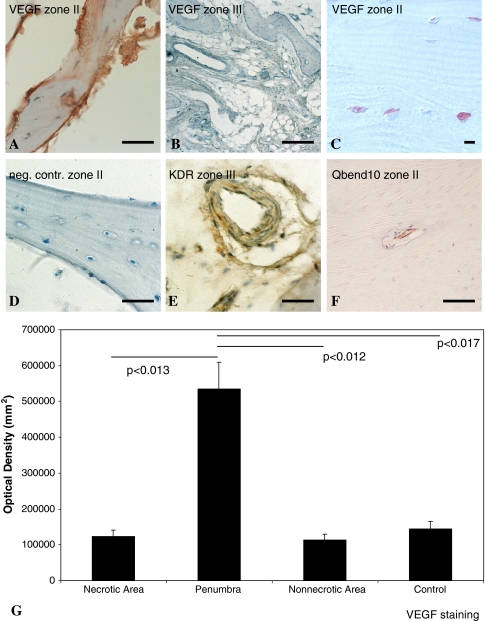
Within all sections of the penumbra and the periphery from ON femoral heads, VEGF could be immunostained within the intra- and pericellular matrix of osteoblasts and osteocytes (Fig. 4A–C). VEGF immunostaining was negative after preincubation of the primary antibody with recombinant VEGF or by omitting the primary antibody (Fig. 4D). Osteoblasts of the periphery were less immunoreactive to VEGF antibody (Fig. 4B). Osteoblasts and endothelial cells of the periphery were also immunostained for VEGFR-2 (Fig. 4E). VEGF expression appeared upregulated in the penumbra of steroid-related femoral head ON (Fig. 4A, C). In the penumbra, we detected immature vessels (Fig. 4F). In the periphery, we identified VEGFR-2-positive endothelial cells and osteoblasts (Fig. 4E). Using densitometry, immunohistochemistry revealed a fivefold upregulation of VEGF in osteoblasts and osteocytes from the penumbra of ON femoral heads compared to the necrotic area (p < 0.013) or nonnecrotic area (p < 0.012) or compared to nonnecrotic OA bone (p < 0.017) (Fig. 4G).
Fig. 4A–G.
Immunohistochemistry was performed to detect cells producing VEGF, VEGFR-2, or Qbend10. VEGF protein was immunostained in osteoblasts (A, C) from the penumbra and (B) from the periphery of necrotic femoral heads. (A, C) According to the staining intensity, VEGF was clearly upregulated in the case of steroid-related femoral head necrosis (ARCO Stage IV) in the penumbra of necrotic femoral heads. (D) Immunostaining is negative after preincubation of the primary antibody with recombinant human VEGF or after omission of the primary antibody (neg cont). (E) VEGFR-2 (KDR) could also be detected by immunohistochemistry in the periphery of necrotic femoral heads. (F) In the penumbra, Qbend10-positive immature vessels were detectable. (A, D, E, F) Bar = 20 μm; (C) bar = 100 μm (B); bar = 10 μm. (G) Densitometry of VEGF staining revealed strong upregulation of VEGF in the penumbra of ON femoral heads. Error bars = SD.
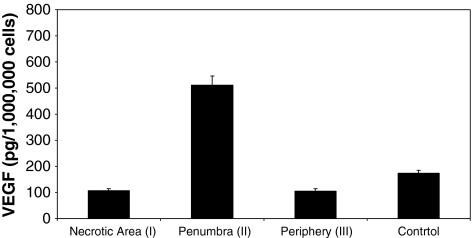
The strong increase of VEGF in the penumbra of ON femoral heads compared to the necrotic area, the periphery, or OA control bone could be attributed to the neovascularization of this area. ELISA experiments revealed an increase (p < 0.001) of VEGF in the penumbra (Fig. 3A, Zone II) of ON femoral head (510.32 pg/1,000,000 cells) compared to the necrotic area (Fig. 5). The VEGF amount per 1,000,000 cells in the necrotic area (107.18 pg) was similar (p < 0.537) to the VEGF amount per 1,000,000 cells in the periphery (106.47 pg) (Fig. 5). The VEGF amount in the nonnecrotic OA bone (173.72 pg/1,000,000 cells) was also similar to that in the periphery of ON area (p < 0.135) and the necrotic area (p < 0.132).
Fig. 5.
ELISA was used for the quantification of VEGF in ON (ARCO Stage IV) and OA femoral heads. VEGF protein is increased in the penumbra (Zone II) of ON femoral heads (510.32 pg/1,000,000 cells) compared to the necrotic area (Zone I) (107.18 pg/1,000,000 cells) and the periphery (Zone III) (106.47 pg/1,000,000 cells). Control OA femoral heads revealed VEGF amounts (173.72 pg/1,000,000 cells) similar to those of Zones I and III of the ON femoral heads. Error bars = SD.
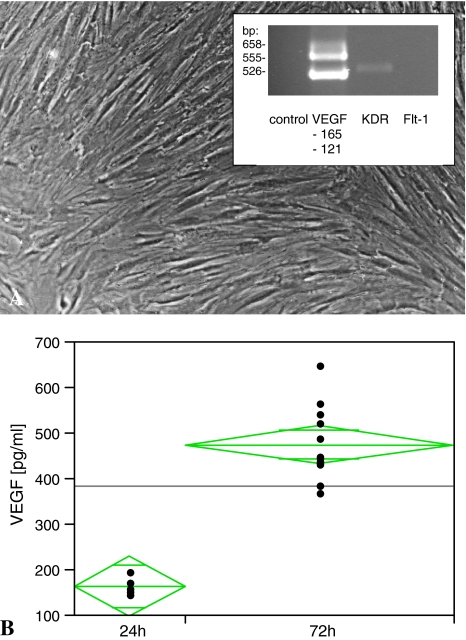
VEGF mRNA and VEGFR-2 were expressed in human non-ON osteoblasts. RT-PCR experiments revealed osteoblasts expressing the splice variants VEGF121 and VEGF165. RT-PCR experiments further revealed the existence of VEGFR-2 (555 kb; Fig. 6A) but not VEGFR-1. The 72-hour incubation revealed a nearly threefold increase (p = 0.013) in VEGF (from 164.45 ± 24 pg/mL to 476.5 ± 64.85 pg/mL [mean ± SD]) in culture supernatant (Fig. 6B).
Fig. 6A–B.
(A) RT-PCR experiments were performed to determine whether osteoblasts express strong small VEGF splice variants and the VEGF receptor. RT-PCR for VEGF splice variants revealed VEGF121 and VEGF165 and its receptor VEGFR-2 (KDR). (B) ELISA experiments demonstrated a stable production of osteoblast-derived VEGF under culture conditions for at least 72 hours. A 2.9-fold increase in VEGF protein concentration (from 164.45 ± 24 pg/mL to 476.5 ± 64.85 pg/mL) could be detected by ELISA after 72 hours’ culturing. Long green lines = mean value; short green lines = SD.
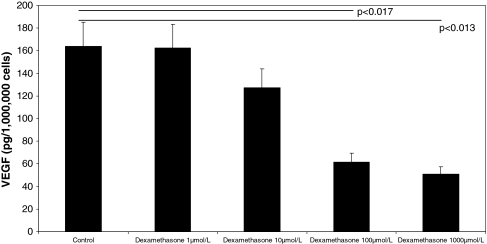
We observed decreasing amounts of VEGF after 24-hour incubation with increasing doses of dexamethasone. VEGF expression levels were decreased (p < 0.001) in the collected cell supernatants (control: 164.06 pg/mL; 100 μmol/L dexamethasone: 61.5 pg/mL) (Fig. 7). Stimulation with 1000 μmol/L dexamethasone did not further decrease (p = 0.5) VEGF expression (50.9 pg/mL) (Fig. 6).
Fig. 7.
VEGF concentrations were strongly decreased in the supernatants of glucocorticoid (dexamethasone)-treated osteoblasts. Collected supernatants were investigated by ELISA detecting all VEGF splice variants. n = 6 for dexamethasone (1, 10, 100, 1000 μmol/L)-treated cells and n = 6 for controls. Error bars = SD. Horizontal bars = control versus dexamethasone 100 μmol/L (p < 0.017) or control versus dexamethasone 1000 μmol/L (p < 0.013).
Discussion
ON of the femoral head is a common complication after high-dose glucocorticoid treatment. The molecular mechanisms of the etiology of the ON are largely unknown. There are strong hints that a disturbed angiogenesis is involved. The hypothesis of our study was that VEGF increases in the penumbra of ON femoral heads to attract reparative arterioles. We further postulated glucocorticoid treatment leads to a disturbed vessel remodeling by reducing VEGF levels in non-ON bone cells.
We note a number of limitations. First is the limited number of ON femoral heads. However, even with this small number, we found differences between the tissue groups. Second, we investigated only ARCO Stage IV ON femoral heads. Nevertheless, the reactions in the penumbra suggest a therapeutic potential of this area. Third, we used nonnecrotic OA femoral heads as controls. We cannot exclude the possibility that some regions of an osteoarthritic hip also had necrotic areas since necrosis is also a feature of OA. However, the level of VEGF expression seems unaffected by any such necrotic bone. To minimize these effects, we did not use the subchondral bone but bone from the center of the femoral head. Finally, to evaluate the minimum dose of dexamethasone, we only performed in vitro studies by stimulating bone cells with different concentrations of dexamethasone.
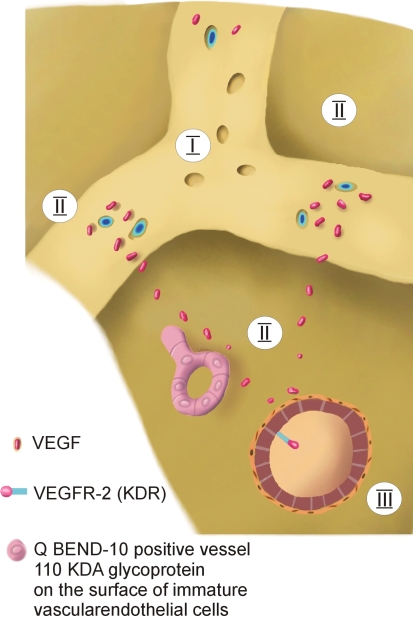
We observed increased expression of VEGF in osteocytes and osteoblasts from the penumbra of ON femoral heads, and this may be related to the process of revascularization and bone growth into the necrotic area, as the femoral heads were harvested in ARCO Stage IV ON [7]. Ohzono et al. [18] examined human femoral heads at Ficat [6] Stages 2 to 4 histopathologically and by microangiography. They described reparative arterioles growing on the trunk of the intracapital nutrient arteries. In our study, we could also observe QBend10-positive immature vessels in the zone of the reactive interface. They also described an avascular zone directly subchondrally, an underlying reparative vascular zone, and a normal vascular zone. In the reparative vascular zone, they described an advancing ingrowth of reparative vessels from Stage 2 to 4. These were described as sparse in Stage 2, a fine network in Stage 3, and a dense network in Stage 4. Our results showing an increased VEGF expression in the penumbra of ON femoral heads correlate well with the findings of Ohzono et al. [18]. VEGF is increased and stimulates the ingrowth of reparative vessels into the penumbra of ON femoral head (Fig. 8).
Fig. 8.
A schematic drawing shows the suggested role of VEGF in ON of the femoral head. In ARCO Stage IV, the cells of the penumbra secrete VEGF to induce angiogenesis and to attract reparative arterioles.
The expression of VEGF in normal bone may indicate VEGF plays a physiologic role in bone turnover. A crosstalk between osteoblasts and adjacent endothelial cells in view of altering the vascularity of the bone-forming site is also possible. The expression of VEGF can be enhanced by several growth factors, such as PGE2, FGF-2, TGF-β1, IGF-1, and BMP-4, -2, and -6 [25]. Basic fibroblast growth factor stimulates VEGF release through p42/p43 mitogen-activated protein (MAP) kinase in osteoblasts and negatively regulates it by p38 MAP kinase [28]. Harada et al. [10, 11] reported PGE2, which is synthesized by COX-2 [17], increased VEGF mRNA levels in osteoblastic RCT-3 cells and in rat calvaria-derived osteoblast-enriched cells. Migita et al. [17] examined the downregulation of COX-2 in response to glucocorticoids in human rheumatoid synovial fibroblasts and concluded glucocorticoids could reduce VEGF levels by downregulating COX-2 with subsequent reduction of PGE2.
Under pathologic conditions, VEGF is strongly expressed during the time course of fracture healing [21]. During the remodeling of the osseous fracture callus, VEGF is expressed by osteocytes while the receptor of VEGF, VEGFR-2, is expressed by osteoblasts, osteoclasts, and endothelial cells [21]. Thus, it appeared promising to us to investigate the role of angiogenic factors, specifically VEGF, in femoral head ON. VEGF expression is also influenced by physical factors. The exposure of osteoblasts to hypoxia induces VEGF expression via induction of the transcription factor hypoxia-inducible factor and transcriptional activation of the VEGF promoter [1]. This can be relevant during the ischemia initiating ON. Using ELISA techniques, we could detect strong differences in VEGF concentration between the three different zones of the ON femoral head. The expression of VEGF in the penumbra is stronger than in the nonnecrotic periphery. Since ELISA data are reliable and quantitative, these large VEGF amounts may be responsible for the attraction of reparative vessels. We would like to speculate that an earlier induction of these bone-specific stable splice variants in the area of the penumbra may have therapeutic potential. Parfitt [19] suggested this earlier induction may lead to an increase of remodeling and subsequently to a healing of ON and further postulated bone remodeling is coupled with angiogenesis. VEGF is capable of attracting the three cells types of bone remodeling: osteoclasts, osteoblasts, and endothelial cells [13, 24]. According to Parfitt [19], the endothelial cells are the ones orchestrating the remodeling process. Nevertheless, we cannot exclude the possibility that other cytokines such as IGF-1 may also be involved in the glucocorticoid effect.
Interestingly, the osteoblasts express the splice variants with the strongest angiogenic potency: VEGF121 and VEGF165. This is the same pattern of splice variants observed in fracture healing [21]. These small splice variants have the potency to react over long distances. Many growth factors have a short half-life. In contrast to other growth factors, the stability of VEGF for at least 72 hours is appropriate to survive these long distances.
The decrease in VEGF detected in osteoblasts incubated with glucocorticoid in our in vitro study may trigger the initiation of ON. However, the existence of phenol red in the stimulation medium may have influenced the expression of VEGF due to its estrogenlike character. Glucocorticoid impairs vessel ingrowth into the endarterial bed of the femoral head [31] and makes it more vulnerable to ischemia. Vessels are essential to osteogenesis [29, 30]. In our study of glucocorticoid-induced ON of the femoral head and glucocorticoid-induced VEGF decrease in osteoblasts, we indeed observed evidence suggesting the participation of VEGF in femoral head ON.
By a range of different experimental approaches, we have demonstrated VEGF is expressed by osteocytes and osteoblasts and is affected by dexamethasone in vitro. The in vitro data suggest VEGF loss may play a role in the initial stage of femoral head ON. Since VEGF is a potent angiogenic peptide, it is likely responsible for the neovascularization observed in the penumbra of ARCO Stage IV ON of the femoral head. Future studies have to elucidate the role of VEGF in early stages of ON of the femoral head in vivo and its possible application in prevention or early therapy of the ON.
Acknowledgments
We thank Susanne Echterhagen, Inka Geurink, Christiane Jaeschke, Patrycja Kozak, Sabine Sepke, Ursula Mundt, Michaela Nicolau, Angela Rüben, Sonja Seiter, and Kirsten Vosen for their expert technical assistance and Wolfgang Graulich for the drawing.
Footnotes
Deike Varoga and Wolf Drescher contributed equally to the present work.
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at RWTH Aachen University.
References
- 1.Akeno N, Czyzyk-Krzeska MF, Gross TS, Clemens TL. Hypoxia induces vascular endothelial growth factor gene transcription in human osteoblast-like cells through the hypoxia-inducible factor-2alpha. Endocrinology. 2001;142:959–962. [DOI] [PubMed]
- 2.Blumer MJ, Longato S, Fritsch H. Structure, formation and role of cartilage canals in the developing bone. Ann Anat. 2008;190:305–315. [DOI] [PubMed]
- 3.Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–950. [DOI] [PubMed]
- 4.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. [DOI] [PubMed]
- 5.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. [DOI] [PubMed]
- 6.Ficat RP. Idiopathic bone necrosis of the femoral head: early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. [DOI] [PubMed]
- 7.Gardeniers JW. A new international classification of osteonecrosis of the ARCO Committee on terminology and classification. J Jpn Orthop Assoc. 1992;66:18–20.
- 8.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. [DOI] [PubMed]
- 9.Glueck CJ, Freiberg R, Tracy T, Stroop D, Wang P. Thrombophilia and hypofibrinolysis: pathophysiologies of osteonecrosis. Clin Orthop Relat Res. 1997;334:43–56. [DOI] [PubMed]
- 10.Harada S, Nagy JA, Sullivan KA, Thomas KA, Endo N, Rodan GA, Rodan SB. Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblasts. J Clin Invest. 1994;93:2490–2496. [DOI] [PMC free article] [PubMed]
- 11.Harada S, Rodan SB, Rodan GA. Expression and regulation of vascular endothelial growth factor in osteoblasts. Clin Orthop Relat Res. 1995;313:76–80. [PubMed]
- 12.Jones JP Jr. Intravascular coagulation and osteonecrosis. Clin Orthop Relat Res. 1992;277:41–53. [PubMed]
- 13.Kaku M, Kohno S, Kawata T, Fujita I, Tokimasa C, Tsutsui K, Tanne K. Effects of vascular endothelial growth factor on osteoclast induction during tooth movement in mice. J Dent Res. 2001;80:1880–1883. [DOI] [PubMed]
- 14.Kim HK, Bian H, Randall T, Garces A, Gerstenfeld LC, Einhorn TA. Increased VEGF expression in the epiphyseal cartilage after ischemic necrosis of the capital femoral epiphysis. J Bone Miner Res. 2004;19:2041–2048. [DOI] [PubMed]
- 15.Landmann J, Renner N, Gachter A, Thiel G, Harder F. Cyclosporin A and osteonecrosis of the femoral head. J Bone Joint Surg Am. 1987;69:1226–1228. [PubMed]
- 16.Martinez P, Esbrit P, Rodrigo A, Alvarez-Arroyo MV, Martinez ME. Age-related changes in parathyroid hormone-related protein and vascular endothelial growth factor in human osteoblastic cells. Osteoporos Int. 2002;13:874–881. [DOI] [PubMed]
- 17.Migita K, Tanaka H, Okamoto K, Yoshikawa N, Ichinose Y, Urayama S, Yamasaki S, Ida H, Kawabe Y, Kawakami A, Eguchi K. FK506 augments glucocorticoid-mediated cyclooxygenase-2 down-regulation in human rheumatoid synovial fibroblasts. Lab Invest. 2000;80:135–141. [DOI] [PubMed]
- 18.Ohzono K, Takaoka K, Saito S, Saito M, Matsui M, Ono K. Intraosseous arterial architecture in nontraumatic avascular necrosis of the femoral head: microangiographic and histologic study. Clin Orthop Relat Res. 1992;277:79–88. [PubMed]
- 19.Parfitt AM. The mechanism of coupling: a role for the vasculature. Bone. 2000;26:319–323. [DOI] [PubMed]
- 20.Plenk H Jr, Hofmann S, Breitenseher M, Urban M. [Pathomorphological aspects and repair mechanisms of femur head necrosis] [in German]. Orthopade. 2000;29:389–402. [DOI] [PubMed]
- 21.Pufe T, Claassen H, Scholz-Ahrens KE, Varoga D, Drescher W, Franke AT, Wruck C, Petersen W, Cellarius C, Schrezenmeir J, Glüer CC. Influence of estradiol on vascular endothelial growth factor expression in bone: a study in Göttingen miniature pigs and human osteoblasts. Calcif Tissue Int. 2007;80:184–191. [DOI] [PubMed]
- 22.Pufe T, Harde V, Petersen W, Goldring M, Tillmann B, Mentlein R. Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J Pathol. 2004;202:367–374. [DOI] [PubMed]
- 23.Pufe T, Scholz-Ahrens KE, Franke AT, Petersen W, Mentlein R, Varoga D, Tillmann B, Schrezenmeir J, Glüer CC. The role of vascular endothelial growth factor in glucocorticoid-induced bone loss: evaluation in a minipig model. Bone. 2003;33:869–876. [DOI] [PubMed]
- 24.Pufe T, Wildemann B, Petersen W, Mentlein R, Raschke M, Schmidmaier G. Quantitative measurement of the splice variants 120 and 164 of the angiogenic peptide vascular endothelial growth factor in the time flow of fracture healing: a study in the rat. Cell Tissue Res. 2002;309:387–392. [DOI] [PubMed]
- 25.Scholz-Ahrens KE, Acil Y, Schrezenmeir J. Effect of oligofructose or dietary calcium on repeated calcium and phosphorus balances, bone mineralization and trabecular structure in ovariectomized rats. Br J Nutr. 2002;88:365–377. [DOI] [PubMed]
- 26.Stern PJ, Watts HG. Osteonecrosis after renal transplantation in children. J Bone Joint Surg Am. 1979;61:851–856. [PubMed]
- 27.Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Böhlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. [DOI] [PubMed]
- 28.Tokuda H, Kozawa O, Uematsu T. Basic fibroblast growth factor stimulates vascular endothelial growth factor release in osteoblasts: divergent regulation by p42/p44 mitogen-activated protein kinase and p38 mitogen-activated protein kinase. J Bone Miner Res. 2000;15:2371–2379. [DOI] [PubMed]
- 29.Trueta J. The role of the vessels in osteogenesis. J Bone Joint Surg Br. 1963;45:402–418. [PubMed]
- 30.Trueta J. The normal vascular anatomy of the femoral head in adult man. 1953. Clin Orthop Relat Res. 1997;334:6–14. [PubMed]
- 31.Trueta J, Amato VP. The vascular contribution to osteogenesis. J Bone Joint Surg Br. 1960;42:571–587. [DOI] [PubMed]
- 32.Veenstra DL, Best JH, Hornberger J, Sullivan SD, Hricik DE. Incidence and long-term cost of steroid-related side effects after renal transplantation. Am J Kidney Dis. 1999;33:829–839. [DOI] [PubMed]
- 33.Yang Q, McHugh KP, Patntirapong S, Gu X, Wunderlich L, Hauschka PV. VEGF enhancement of osteoclast survival and bone resorption involves VEGF receptor-2 signaling and β3-integrin. Matrix Biol. 2008;27:589–599. [DOI] [PubMed]