Abstract
Glucocorticoids inhibit bone remodeling and fracture healing. We sought to determine whether osteogenic protein 1 (OP-1) can overcome this inhibition in a closed fracture model in the rat. Time-released prednisolone or placebo pellets were implanted subcutaneously; closed femoral fractures were created 2 weeks later in rats. Fractures received sham, OP-1 and collagen, or collagen-only implants. Femurs were harvested at 3, 10, 21, 28, and 42 days postfracture. Fractures were examined radiographically for amount of hard callus; mechanically for torque and stiffness (also expressed as a percentage of the contralateral intact femur); and histomorphometrically for amount of cartilaginous and noncartilaginous soft callus, hard callus, and total callus. Glucocorticoid administration inhibited fracture healing. The application of a devitalized Type I collagen matrix mitigated the inhibitory effects of prednisolone on fracture healing However, further increases in indices of fracture healing were observed when OP-1 was added to the collagen matrix compared with collagen alone. OP-1 and collagen was more effective than collagen alone.
Introduction
In 1965, Marshall Urist identified an extractable peptide in devitalized, decalcified bone capable of inducing de novo bone formation [49]. Subsequent work by Urist [50] and others [37, 39, 54] has identified up to 20 variants of this protein called “bone morphogenetic protein” (BMP), a member of the transforming growth factor β superfamily [36]. At present, only BMP-2 and BMP-7 are produced commercially as a therapy to enhance bone healing with BMP-7 called osteogenic protein 1 (OP-1). BMP-2 and OP-1 both promote bone formation in a variety of experimental models (rat [8, 13, 25, 46, 47, 56], rabbit [3, 10, 29, 48], sheep [15, 20], dog [9, 19, 32, 35, 40, 43]). Although these proteins are now used clinically, most of the information on their effects is known from these and other experimental studies.
In current human and veterinary orthopaedic practice, one of the most widely accepted means of enhancing the bone healing process is by grafting the defect with cancellous auto- or allograft [45]. However, patient morbidity from graft collection in human orthopaedics is well-documented [17, 40, 41]. Bone graft collection in veterinary orthopaedics also is associated with patient morbidity at the donor site [45]. The increasing availability of BMPs has made it possible to explore the use of these proteins to directly enhance bone healing as an alternative to bone grafting.
Glucocorticoids inhibit bone remodeling [18, 28, 30, 51, 52] and inhibit fracture healing in animal models [1, 22–24, 31, 33, 44, 53]. A recent study reported BMP-2 enhances osteotomy healing in glucocorticoid-treated rabbits [29]. If OP-1 enhances fracture healing under circumstances of prednisolone-induced inhibition, it may be used clinically to enhance fracture healing in patients receiving glucocorticoid therapy.
We therefore raised the following questions: (1) Do pharmacologic doses of glucocorticoid inhibit fracture healing in the rat closed femoral fracture model (as measured radiographically for amount of hard callus; mechanically for torque, stiffness, and amount of cartilaginous and noncartilaginous soft callus, hard callus, and total callus)? (2) Does OP-1 and a collagen carrier overcome the inhibition of fracture healing resulting from glucocorticoid administration in this model? (3) Does collagen alone overcome the inhibition of fracture healing resulting from glucocorticoid administration in this model?
Materials and Methods
One hundred seventy-four skeletally mature male Sprague-Dawley rats (Harlan SD, Madison, WI), 3 months of age, were housed two per cage and allowed to acclimate to the housing environment for 3 days. The 174 rats were divided into six groups: no OP-1 or collagen carrier applied to the fracture site with (treatment: Group 1; n = 34) or without (placebo: Group 2; n = 37) prednisolone; OP-1 and collagen carrier with (treatment: Group 3; n = 41) or without (placebo: Group 4; n = 40) prednisolone; or collagen carrier alone with (treatment: Group 5; n = 11) or without (placebo: Group 6; n = 11) prednisolone. Rats were anesthetized using intraperitoneal ketamine/xylazine at a dose of 40 to 60 mg/kg and 5 to 7.5 mg/kg, respectively, for subcutaneous implantation of 50-mg pellets of 60-day time-released prednisolone or placebo (Innovative Research of America, Sarasota, FL). This equates to 0.83 mg prednisolone released per rat per day. Rats weighed between 350 and 375 g at the initiation of this study; therefore, each rat received approximately 2.2 to 2.3 mg/kg prednisolone daily. This is equivalent to the dose administered clinically. The harvested femurs from the prednisolone treatment and placebo groups were compared at 10, 28, and 42 days for radiographic analysis; at 3, 10, 21, 28, and 42 days for histomorphometric analysis; and at 28 and 42 days for mechanical analysis (Table 1). An earlier study from our laboratory found a two-fold difference in radiographic callus between low and high concentrations of OP-1 in the presence of an acute bacterial infection [7]. In order to detect a 40% difference in radiographic callus area between treatment groups with an 80% power (alpha = 0.05), we determined that six animals would be required per group. However, group numbers varied as a result of unexpected mortality. This study received prior approval of the Institutional Animal Care and Use Committee of the University of Minnesota.
Table 1.
Experimental animal number: treatment distribution
| Analysis | Treatment | Postfracture | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 Days | 10 Days | 21 Days | 28 Days | 42 Days | |||||||
| Placebo | Pred | Placebo | Pred | Placebo | Pred | Placebo | Pred | Placebo | Pred | ||
| Radiography | No OP-1 or collagen | 6 | 6 | 6 | 6 | 6 | 5 | ||||
| OP-1 and collagen | 6 | 7 | 6 | 6 | 6 | 6 | |||||
| Collagen only | 6 | 5 | |||||||||
| Histomorphometry | No OP-1 or collagen | 6 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | 5 | 5 |
| OP-1 and collagen | 6 | 6 | 6 | 7 | 4 | 5 | 6 | 6 | 6 | 4 | |
| Collagen only | 6 | 5 | |||||||||
| Mechanics | No OP-1 or collagen | 7 | 6 | ||||||||
| OP-1 and collagen | 6 | 5 | 6 | 6 | |||||||
| Collagen only | 5 | 6 | |||||||||
Pred = 2.2 mg/kg prednisolone slow-release subcutaneously; OP-1 = osteogenic protein 1.
Two weeks after implantation, each rat underwent surgery for intramedullary pin placement and subsequent fracture of either the right or left femur, alternating sides for each consecutive rat. Ketamine/xylazine (40–60 mg/kg and 5–7.5 mg/kg, respectively) was used for rat anesthesia and closed fracture performed as described previously [4]. For OP-1 treatment groups, OP-1 was reconstituted with normal saline; 25 μg was then mixed with 50 mg collagen carrier. In collagen-only groups, we mixed normal saline without OP-1 with 50 mg collagen carrier. We performed a limited cranial lateral approach to the femur at the diaphysis. A collagen carrier with OP-1 or saline (collagen only) was applied to the fracture site. The incision was closed in a routine fashion. At the end of the specified study interval, each rat was weighed and euthanized by intraperitoneal barbiturate overdose.
We made an effort to feed prednisolone treatment and placebo groups approximately the same amount of food. To control for differences in diet between prednisolone treatment and placebo groups, prednisolone-treated rats were fed ad libitum, whereas placebo rats were fed premeasured amounts based on approximate food consumption rates of prednisolone-treated rats.
At the end of specified study intervals, we harvested 72 fractured and contralateral intact hind limbs after euthanasia and performed high-resolution radiographic imaging using a Faxitron® xray system (Model 43805 N X-Ray System Faxitron® Series; Hewlett-Packard, McMinnville, OR) with slow-speed radiographic film. Lateral radiographs were digitized and scanned at a resolution and scale sufficient to ensure accurate analysis (ie, 300 × 300 dots per inch [dpi] at a 300% scale for an equivalent 900 dpi). We imported the scanned images into a personal computer for analysis by a semiautomated image analysis system (Bioquant Image Analysis; R&M Biometrics, Nashville, TN). Using the Bioquant Image Analysis program, lateral radiographic images were evaluated by one blinded investigator (RSG) by tracing the amount of hard (calcified) external callus at each time interval (pixels/area traced).
For mechanical testing, femurs were frozen in normal saline and stored at −20°C until the day of testing, at which time they were thawed at room temperature. The femurs were wrapped in saline-soaked gauze sponges to keep them moist until just before testing. Each femur was placed in a specially designed potting/alignment device and the distal and proximal ends of the femur were potted in aluminum sleeves filled with metal alloy potting medium. We then installed each femur in an axial-torsion test machine (Model AT 3045; Bose EnduraTEC Systems Corp, Eden Prairie, MN) with a low-capacity 50-in-lb torque cell (Transducer Techniques, Temecula, CA). The femurs were loaded in torsion to failure at a rate of 0.5°/sec. The torque versus angular displacement data were recorded (Wintest software; Bose EnduraTEC Systems Corp); maximum angular deformation to failure, maximum torque to failure, and torsional stiffness were recorded and these values were normalized to percentages of contralateral intact femurs.
Femurs with closed fractures were harvested for histomorphometry and placed in 10% buffered formalin for 24 hours. Specimens were decalcified at 25°C using a commercially available hydrochloric acid solution (Decalcifier II®; Surgipath Medical Industries Inc, Richmond, IL) for 24 to 36 hours to completely decalcify the specimens. They were transferred to 70% ethanol until histologic processing. Femurs were prepared for paraffin embedding using standard histologic techniques. Decalcified midsagittal sections 4 μm thick included the entire middiaphyseal fracture callus and adjacent proximal and distal femur. Sections were stained with hematoxylin and eosin.
Histomorphometric analyses were performed blinded by a single investigator (RSG) on a Bioquant workstation (R&M Biometrics) at 100 × magnification. One midsagittal section was examined for each fracture and measurements traced using the Bioquant Image Analysis program. Fracture callus measurements included noncartilaginous soft callus, cartilaginous soft callus, hard callus, and total external callus (the sum of these three histologic parameters). All animals used for histomorphometric analyses were first used for radiography.
We determined differences in fracture healing between prednisolone treatment (Group 1) and placebo (Group 2) groups with no OP-1 or collagen for all parameters using a two-way analysis of variance in which time and prednisolone treatment were the two main effects. A p value < 0.05 and < 0.2 for main effects and the interaction term, respectively, was considered significant. Post hoc pairwise comparisons were accomplished using the t-test. Significance levels for post hoc comparisons were adjusted for using the Bonferroni correction. Significance levels varied depending on the number of preplanned pairwise comparisons made; for five comparisons p < 0.01, for three comparisons p < 0.016, and for two comparisons p < 0.025. Differences in fracture healing between prednisolone treatment (Group 3) and placebo (Group 4) groups with OP-1 with collagen for all parameters were also determined using a two-way analysis of variance as described previously. Differences in fracture healing between prednisolone treatment (Group 5) and placebo (Group 6) groups with collagen only were evaluated at 28 days using the t-test for each measured parameter. We further determined differences in fracture healing between OP-1 and collagen (Groups 3 and 4) and collagen alone (Groups 5 and 6) using a two-way analysis of variance.
Results
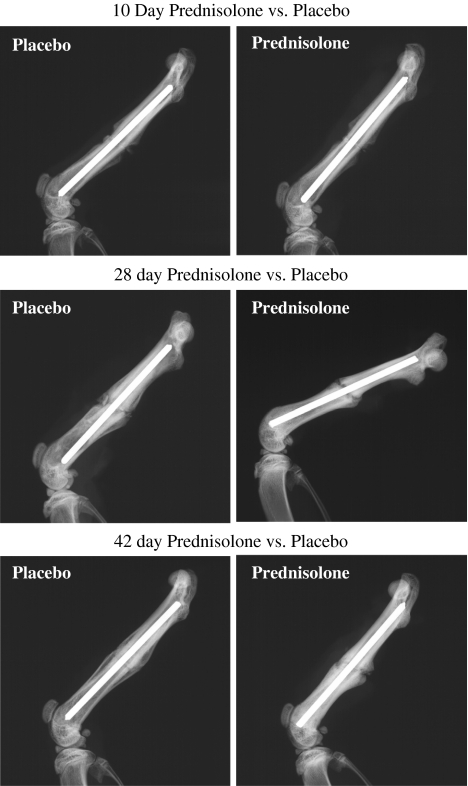
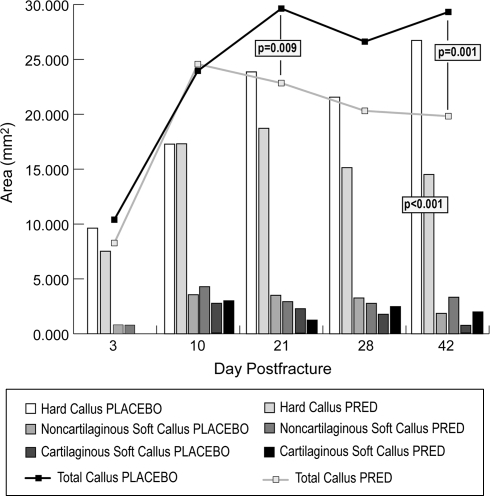
Pharmacologic doses of glucocorticoid inhibited fracture healing in prednisolone treatment compared with placebo groups. Radiographic analysis revealed less mineralized bone resulting from prednisolone treatment (p < 0.001) and this treatment effect differed over time (p = 0.028). Calcified external fracture callus formation was reduced at 28 (p < 0.001) and 42 (p = 0.002) days postfracture for prednisolone treatment when compared with placebo (Table 2; Fig. 1). Mechanical analyses at 28 days postfracture showed fracture torque to failure (p = 0.024), fracture stiffness (p = 0.025), percent of intact femur torque (p = 0.043), and percent of intact femur stiffness (p = 0.01) were decreased in prednisolone treatment compared with placebo groups (Table 3). Histomorphometric analyses revealed a decrease (p < 0.001) in hard callus area with prednisolone treatment compared with placebo. The treatment effect differed over time (p = 0.016) with a decrease (p < 0.001) in hard callus area at 42 days postfracture for prednisolone treatment compared with placebo (Fig. 2). Total callus area decreased with prednisolone treatment (p < 0.001). This treatment effect differed over time (p = 0.06) with reductions at 21 (p = 0.009) and 42 (p = 0.001) days with prednisolone treatment compared with placebo (Fig. 2).
Table 2.
Radiographic callus area (mm2) in prednisolone-treated and placebo fractured femurs at 10, 28, and 42 days postfracture
| Day | Treatment group | Mean | SD | p Value |
|---|---|---|---|---|
| 10-day | Prednisolone | 18.065 | 2.724 | 0.548 |
| Placebo | 20.144 | 3.801 | ||
| 28-day | Prednisolone | 20.573 | 7.475 | < 0.001* |
| Placebo | 35.914 | 7.888 | ||
| 42-day | Prednisolone | 20.292 | 4.119 | 0.002* |
| Placebo | 32.371 | 7.089 |
* Significant; SD = standard deviation.
Fig. 1.
Lateral radiographs of rat femurs show fracture healing for prednisolone and placebo groups at 10, 28, and 42 days after fracture. There was less calcified external fracture callus formation at 28 (p < 0.001) and 42 (p = 0.002) days postfracture for prednisolone treatment compared with placebo groups.
Table 3.
Fracture and percent of intact torque and stiffness in prednisolone-treated and placebo fractured femurs at 28 days postfracture
| Parameter | Treatment group | Mean | SD | p Value |
|---|---|---|---|---|
| Fracture torque (N-m) | Prednisolone | 0.144 | 0.072 | 0.024* |
| Placebo | 0.236 | 0.025 | ||
| Fracture stiffness (Nm/deg) | Prednisolone | 0.006 | 0.003 | 0.025* |
| Placebo | 0.018 | 0.011 | ||
| Percent of intact torque | Prednisolone | 0.456 | 0.19 | 0.043* |
| Placebo | 0.639 | 0.089 | ||
| Percent of intact stiffness | Prednisolone | 0.175 | 0.088 | 0.009* |
| Placebo | 0.479 | 0.225 |
* Significant; SD = standard deviation.
Fig. 2.
A graph shows the histomorphometric determination of callus areas for prednisolone treatment and placebo groups at 3, 10, 21, 28, and 42 days. A decrease in total callus area was seen at 21 (p = 0.009) and 42 (p = 0.001) days and in hard callus area at 42 (p < 0.001) days with prednisolone treatment compared with placebo. PRED = prednisolone.
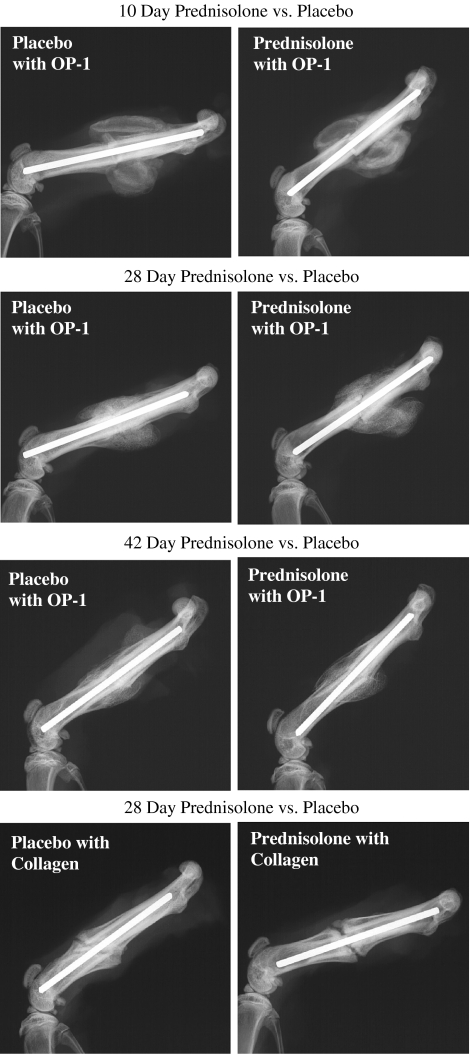
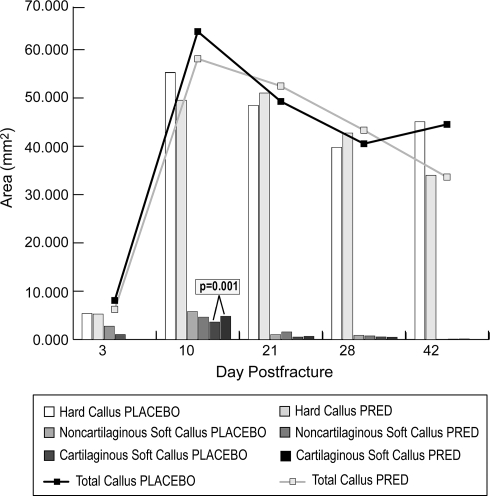
OP-1 and collagen application to the fracture site overcame the inhibition of fracture healing seen in prednisolone-treated rats. All rats with OP-1 and collagen application had exuberant callus at all time points (Fig. 3). Quantitative radiography revealed no difference (p = 0.97) in calcified external fracture callus between prednisolone treatment and placebo groups with OP-1 and collagen application (Table 4). The application of OP-1 with collagen carrier prevented the reductions in maximum torque to failure and torsional stiffness (also normalized to percent of contralateral intact femurs) observed with prednisolone treatment (Table 5). Histomorphometric analysis showed a treatment effect that differed over time (p = 0.06) with greater (p = 0.001) cartilaginous soft callus seen with prednisolone treatment compared with placebo at 10 days. However, we observed no differences for any other histomorphometric parameter between prednisolone treatment and placebo groups with OP-1 and collagen applied to the fracture site (Fig. 4).
Fig. 3.
Lateral radiographs of rat femurs show fracture healing for prednisolone and placebo groups at 10, 28, and 42 days after fracture with OP-1 in a collagen carrier applied to the fracture site and at 28 days for collagen only. No difference in calcified external fracture callus between prednisolone treatment and placebo groups was seen at any time. OP-1 = osteogenic protein 1.
Table 4.
Radiographic callus area (mm2) with OP-1 application in prednisolone-treated and placebo fractured femurs at 10, 28, and 42 days postfracture
| Days | Treatment group | Mean | SD | p Value |
|---|---|---|---|---|
| 10 | Prednisolone/OP-1 and collagen | 107.56 | 11.13 | 0.07 |
| Placebo/OP-1 and collagen | 123.7 | 14.68 | ||
| 28 | Prednisolone/OP-1 and collagen | 85.0 | 16.9 | 0.04 |
| Placebo/OP-1 and collagen | 69.7 | 12.1 | ||
| 42 | Prednisolone/OP-1 and collagen | 70.55 | 11.22 | 0.81 |
| Placebo/OP-1 and collagen | 72.32 | 8.64 |
OP-1 = osteogenic protein 1; SD = standard deviation.
Table 5.
Fracture and percent of intact torque and stiffness with OP-1 application in prednisolone-treated and placebo fractured femurs at 28 and 42 days postfracture
| Parameter (days) | Treatment group | Mean | SD | p Value |
|---|---|---|---|---|
| Fracture torque (Nm) (28 days) | Prednisolone/OP-1 and collagen | 0.40 | 0.067 | 0.71 |
| Placebo/OP-1 and collagen | 0.37 | 0.067 | ||
| Fracture stiffness (Nm/deg) (28 days) | Prednisolone/OP-1 and collagen | 0.03 | 0.010 | 0.70 |
| Placebo/OP-1 and collagen | 0.03 | 0.010 | ||
| Percent of intact torque (28 days) | Prednisolone/OP-1 and collagen | 120.6 | 38.7 | 0.11 |
| Placebo/OP-1 and collagen | 81.7 | 20.6 | ||
| Percent of intact stiffness (28 days) | Prednisolone/OP-1 and collagen | 117.8 | 81.4 | 0.35 |
| Placebo/OP-1 and collagen | 89.9 | 24.5 | ||
| Fracture torque (Nm) (42 days) | Prednisolone/OP-1 and collagen | 0.52 | 0.20 | 0.28 |
| Placebo/OP-1 and collagen | 0.61 | 0.12 | ||
| Fracture stiffness (Nm/deg) (42 days) | Prednisolone/OP-1 and collagen | 0.04 | 0.02 | 0.07 |
| Placebo/OP-1 and collagen | 0.05 | 0.02 | ||
| Percent of intact torque (42 days) | Prednisolone/OP-1 and collagen | 120.6 | 37.5 | 0.97 |
| Placebo/OP-1 and collagen | 119.9 | 50.6 | ||
| Percent of intact stiffness (42 days) | Prednisolone/OP-1 and collagen | 89.4 | 19.5 | 0.27 |
| Placebo/OP-1 and collagen | 120.9 | 50.6 |
OP-1 = osteogenic protein 1; SD = standard deviation.
Fig. 4.
A graph shows the histomorphometric determination of callus areas for prednisolone treatment and placebo groups at 3, 10, 21, 28, and 42 days after OP-1 with collagen application. We observed an increase in cartilaginous soft callus at 10 days with prednisolone treatment compared with placebo (p = 0.001). No other differences were seen between groups at any time. PRED = prednisolone; OP-1 = osteogenic protein 1.
Collagen application to the fracture site alone improved fracture healing in prednisolone-treated rats at 28 days. At this time point, there were no differences seen in radiographic, mechanical, and histomorphometric analyses for prednisolone treatment compared with placebo groups (Tables 6, 7, 8; Fig. 3).
Table 6.
Radiographic callus area (mm2) with collagen application in prednisolone-treated and placebo fractured femurs at 28 days postfracture
| Treatment group | Mean | SD | p Value |
|---|---|---|---|
| Prednisolone/collagen | 33.88 | 4.32 | 0.60 |
| Placebo/collagen | 37.23 | 4.83 |
SD = standard deviation.
Table 7.
Fracture and percent of intact torque and stiffness after collagen application in prednisolone-treated and placebo fractured femurs at 28 days postfracture
| Parameter | Treatment group | Mean | SD | p Value |
|---|---|---|---|---|
| Fracture torque (Nm) | Prednisolone/collagen | 0.24 | 0.08 | 0.26 |
| Placebo/collagen | 0.29 | 0.04 | ||
| Fracture stiffness (Nm/deg) | Prednisolone/collagen | 0.01 | 0.005 | 0.88 |
| Placebo/collagen | 0.01 | 0.002 | ||
| Percent of intact torque | Prednisolone/collagen | 69.51 | 23.69 | 0.89 |
| Placebo/collagen | 67.91 | 10.92 | ||
| Percent of intact stiffness | Prednisolone/collagen | 30.30 | 11.17 | 0.88 |
| Placebo/collagen | 29.29 | 9.89 |
SD = standard deviation.
Table 8.
Histomorphometric analysis (mm2) with collagen application in prednisolone-treated and placebo fractured femurs at 28 days postfracture
| Parameter | Treatment group | Mean | SD | p Value |
|---|---|---|---|---|
| Noncartilaginous soft callus | Prednisolone/collagen | 3.64 | 2.41 | 0.12 |
| Placebo/collagen | 5.88 | 1.90 | ||
| Cartilaginous soft callus | Prednisolone/collagen | 3.16 | 1.08 | 0.95 |
| Placebo/collagen | 3.20 | 0.69 | ||
| Hard callus | Prednisolone/collagen | 27.07 | 4.71 | 0.64 |
| Placebo/collagen | 28.16 | 2.80 | ||
| Total external callus | Prednisolone/collagen | 33.88 | 4.32 | 0.26 |
| Placebo/collagen | 37.23 | 4.83 |
SD = standard deviation.
OP-1 and collagen applied to prednisolone treatment and placebo groups resulted in greater indices of healing compared with these groups implanted with collagen alone. Radiographic callus formation (p < 0.001), fracture torque (p < 0.001), torque as a percent of the contralateral intact femur (p = 0.009), fracture stiffness (p < 0.001), and stiffness as a percent of the contralateral intact femur (p < 0.001) were all greater with OP-1 and collagen compared with collagen alone. Although there was no difference in total external callus formation, histomorphometric analysis revealed greater hard callus (p < 0.001) and less cartilaginous (p < 0.001) and noncartilaginous soft callus (p < 0.001) for the fractures treated with OP-1 and collagen compared with the collagen-only group.
Discussion
Glucocorticoids are administered clinically to treat a variety of diseases and the medical benefits of glucocorticoid use are well-described [5, 28]. Despite this, glucocorticoids promote undesirable complications on many biologic processes [28]. One of the most important of these effects is the influence of glucocorticoids on the healing process of bone fractures [1, 22–24, 31, 33, 53]. If OP-1 experimentally enhances fracture healing under circumstances of prednisolone-induced inhibition, it may be used clinically to enhance fracture healing in patients receiving glucocorticoid therapy. Recent evidence supporting the use of BMP-2 to create new bone within a critical defect during glucocorticoid treatment [29] raises the following questions: Do pharmacologic doses of glucocorticoid inhibit fracture healing in the rat closed femoral fracture model? Do OP-1 and a collagen carrier overcome the inhibition of fracture healing resulting from glucocorticoid administration in this model? Does collagen alone overcome the inhibition of fracture healing resulting from glucocorticoid administration in this model?
Our dose of glucocorticoid was based on the effects reported to produce osteoporosis in the rat [16, 26, 27, 42]. Although this dose is on the high end of the range administered clinically, we believe these higher doses are reasonable, because the goal was to elicit a bone response; this response parallels that seen clinically. We used a small number of rats at only one time point to test for the effects of collagen alone on fracture healing with glucocorticoid administration. The use of only one time point at 28 days could have limited the ability to detect major differences between prednisolone treatment and placebo with collagen alone. This possibility is suggested by the histomorphometric evaluation for Groups 1 and 2. At the 28-day time point, we observed no difference for all parameters of fracture callus measurement with prednisolone treatment compared with the placebo group (Fig. 2). This is similar to the results of collagen used alone with prednisolone treatment (Table 8). It is unknown whether the inclusion of other time points may have revealed a difference, because we observed a decrease at 21 and 42 days with prednisolone treatment (Fig. 2). Thus, further study is needed to evaluate collagen at different time points to confirm that collagen alone reverses the inhibition of fracture healing during glucocorticoid therapy, as shown at multiple time points with OP-1 and collagen.
Our data suggest OP-1 overcomes the inhibition of fracture healing resulting from glucocorticoid administration in this model. Other investigations also suggest BMPs enhance bone formation in a compromised clinical environment. OP-1 overcomes the inhibitory effect of nicotine in a rabbit spinal fusion model [34] and induces bone formation in an infected segmental defect in the rat femur [7]. BMP-2 enhances osteotomy healing in rabbits under the inhibitory effects of glucocorticoid treatment [29]. Thus, the current work in conjunction with these previously reported studies suggest the clinical application of exogenous BMP may be of particular value when there is inhibition to the normal bone healing process.
The data suggest, at Day 28, collagen alone overcomes the inhibition of fracture healing resulting from prednisolone administration. This was unexpected because the collagen carrier lacks the necessary osteoinductive peptides necessary for bone healing [9, 10]. The purified bovine Type I collagen used as a carrier for OP-1 in this study was osteoconductive only [38]. Thus, some contribution to bone healing may result from the collagen matrix alone. However, these findings for collagen alone could be by chance because of multiple uncorrected comparisons. We chose the 28-day time point because healing was expected to be near complete and it was a time when comparisons could be made for all measured parameters in this study. Despite the improvement matrix alone appeared to provide, adding OP-1 to the same collagen carrier produced greater radiographic and histologic hard callus, and the callus formed was stronger in torsion than if collagen was used alone. Thus, OP-1 was more effective at healing fractures than collagen alone in this model.
The reason collagen contributed to fracture healing in this study may be related to events occurring during the inflammation stage of fracture healing, which is important in the fracture repair process [2, 14]. Application of a foreign collagen may cause inflammation at the fracture site and migration of inflammatory cells to the fracture. Cells involved with inflammation during fracture healing release inflammatory mediators and cytokines [12, 14]. Many of these cytokines are important to the maturation and proliferation of osteoprogenitor cells [14]. Thus, collagen may induce the release of cytokines important to fracture healing from cells participating in this process.
The role of BMP in fracture healing has been well-described [2, 12, 49]. However, the details regarding the underlying molecular mechanisms involved in exogenous BMP enhancement of fracture healing continue to be elucidated. The isolation of osteoblast-specific BMP receptors during bone formation and fracture healing reinforces the theory that BMPs are important in the differentiation of osteoprogenitor cells to mature osteoblasts [36]. For example, BMP upregulates the expression of the transcriptional factor Cbfa1 [11, 55] which is essential to osteoblast and chondrocyte differentiation and proliferation [11, 21, 55], whereas glucocorticoids downregulate the expression of this protein [6]. Considering this, OP-1 may be offsetting the inhibitory action of glucocorticoid in part through counteracting the downregulation of Cbfa1. This theory has been proposed previously [29]; however, the findings in the current investigation provide further support for the ability of BMP to overcome the inhibitory effects of glucocorticoid administration in a fracture healing model.
Our data suggest the local application of OP-1 to the fracture site overcomes the inhibitory effects of glucocorticoids on fracture healing. These results support the hypotheses that radiographic callus, mechanical strength of the fracture, and histomorphometric measurements of callus formation are the same in prednisolone-treated and placebo rats when OP-1 is applied to the fracture site. The fundamental questions of how OP-1 is working to reverse the inhibitory effects of glucocorticoids must be examined hand in hand with how the glucocorticoid is affecting cellular metabolic pathways. Thus far, these effects are incompletely understood. Investigations using molecular biologic and nuclear magnetic resonance techniques will provide insight into these questions. Despite the present gap in mechanistic information, our data suggest OP-1 has potential for clinical use in patients treated with glucocorticoids.
Acknowledgments
We thank Dr. Mark Bolander (Mayo Clinic, Rochester, MN) for his assistance with this model, Dr. Frances S. Shofer (University of Pennsylvania, Philadelphia, PA) for statistical analysis, and William Lew, MS (Midwest Orthopaedic Research Foundation, Minneapolis, MN) for contributions to the overall project. We also thank Nancy M. Roget, RN, for financial support and Stryker Biotech, Hopkinton, MA, for the generous donation of OP-1 and collagen carrier used in this study.
Footnotes
One or more of the authors (RSG, LJW) have received funding from Hennepin Faculty Associates with support from the Orthopaedic Biomechanics Laboratory, Midwest Orthopaedic Research Foundation.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the College of Veterinary Medicine, University of Minnesota, St Paul, MN.
References
- 1.Blunt JW, Plotz CM, Lattes R, Howes EL, Meyer K, Ragan C. Effects of cortisone on experimental fractures in the rabbit. Proc Soc Exp Biol Med. 1950;73:678–681. [DOI] [PubMed]
- 2.Bolander ME. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992;200:165–170. [DOI] [PubMed]
- 3.Bolander ME, Balian G. The use of demineralized bone matrix repair of segmental defect: augmentation with extracted matrix proteins and a comparison with autologous grafts. J Bone Joint Surg Am. 1986;68:1264–1274. [PubMed]
- 4.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. [DOI] [PubMed]
- 5.Buttgereit F, da Silva JA, Boers M, Burmester GR, Cutolo M, Jacobs J, Kirwan J, Kohler L, Van Riel P, Vischer T, Bijlsma JW. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis. 2002;61:718–722. [DOI] [PMC free article] [PubMed]
- 6.Chang DJ, Ji C, Kim K, Casinghino S, McCarthy TL, Centrella M. Reduction in transforming growth factor beta receptor I expression and transcription factor CBFa1 on bone cells by glucocorticoid. J Biol Chem. 1998;273:4892–4896. [DOI] [PubMed]
- 7.Chen X, Kidder LS, Lew WD. Osteogenic protein-1 induced bone formation in an infected segmental defect in the rat femur. J Orthop Res. 2002;20:142–150. [DOI] [PubMed]
- 8.Cochran DL, Nummikoski PV, Jones AA, Makins SR, Turek TJ, Buser D. Radiographic analysis of regenerated bone around endosseous implants in the canine using recombinant human bone morphogenetic protein-2. Int J Oral Maxillofac Implants. 1997;12:739–748. [PubMed]
- 9.Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC. Recombinant human bone morphogenetic protein-7 induces healing in a canine long-bone segmental defect model. Clin Orthop Relat Res. 1994;301:302–312. [PubMed]
- 10.Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC, Whitecloud TS 3rd. The effect of recombinant human osteogenic protein-1 on healing of large segmental bone defects. J Bone Joint Surg Am. 1994;76:827–838. [DOI] [PubMed]
- 11.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. [DOI] [PubMed]
- 12.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;355(Suppl):S7–S21. [DOI] [PubMed]
- 13.Einhorn TA, Lane JM, Burnstein AH, Kopman CR, Vigorita VJ. The healing of segmental bone defects induced by demineralized bone matrix. J Bone Joint Surg Am. 1984;66:274–278. [PubMed]
- 14.Einhorn TA, Majeska RJ, Rush EB, Levine PM, Horowitz MC. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10:1272–1281. [DOI] [PubMed]
- 15.Gerhart TN, Kirker-Head CA, Kriz MJ, Holtrop ME, Hennig GE, Hipp J, Schelling SH, Wang E. Healing segmental femoral defects in sheep using recombinant human bone morphogenetic protein. Clin Orthop Relat Res. 1993;293:317–326. [PubMed]
- 16.Goulding A, Gold E. Effects of chronic prednisolone treatment on bone resorption and bone composition in intact and ovariectomized rats receiving B-estradiol. Endocrinology. 1988;122:482–487. [DOI] [PubMed]
- 17.Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:766–781. [DOI] [PubMed]
- 18.Ishida Y, Heersche JN. Glucocorticoid-induced osteoporosis: both in vivo and in vitro concentration of glucocorticoids higher than physiologic levels attenuate osteoblast differentiation. J Bone Miner Res. 1998;13:1822–1826. [DOI] [PubMed]
- 19.Itoh T, Mochizuki M, Nishimura R, Matsunaga S, Kadosawa T, Kokubo S, Yokota S, Sasaki N. Repair of ulnar segmental defect by recombinant human bone morphogenetic protein-2 in dogs. J Vet Med Science. 1998;60:451–458. [DOI] [PubMed]
- 20.Kirker-Head CA, Gerhart TN, Schelling SH, Hennig GE, Wang E, Holtrop ME. Long term healing of bone using recombinant human bone morphogenetic protein 2. Clin Orthop Relat Res. 1995;318:222–230. [PubMed]
- 21.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrests of osteoblasts. Cell. 1997;89:755–764. [DOI] [PubMed]
- 22.Kostenszky KS, Olah EH. Effect of prednisolone on callus formation. Acta Biol Acad Sci Hung. 1974;25:49–60. [PubMed]
- 23.Kowalewski K. Comparison of the effects of cortisone and certain anabolic androgenic steroids on the uptake of radiosulfur in a healing fractured bone. Endocrinology. 1958;62:463–497. [DOI] [PubMed]
- 24.Kowalewski K, Gort J. An anabolic androgen as a stimulant of bone healing in rats treated with cortisone. Acta Endocrinol. 1959;30:273–276. [DOI] [PubMed]
- 25.Lee SC, Shea M, Battle MA, Kozitza K, Ron E, Turek T, Schaub RG, Hayes WC. Healing of large segmental defects in rat femurs is aided by rhBMP-2 in PLGA matrix. J Biomed Mater Res. 1994;28:1149–1156. [DOI] [PubMed]
- 26.Lindgren JU, Johnell O, Deluca HF. Studies of bone tissue in rats treated by prednisolone and 1,25-(OH)2D3. Clin Orthop Relat Res. 1983;181:264–268. [PubMed]
- 27.Lindgren JU, Merchant CR, Deluca HF. Effect of 1,25-dihydroxyvitamin D3 on osteopenia induced by prednisolone in adult rats. Calcif Tissue Int. 1982;34:253–257. [DOI] [PubMed]
- 28.Lukert B, Kream B. Clinical and basic aspects of glucocorticoid action on bone. In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of Bone Biology. San Diego, CA: Academic Press; 1996:533–548.
- 29.Luppen CA, Blake CA, Ammirati KM, Stevens ML, Wozney JM, Bouxsein ML. Recombinant human bone morphogenetic protein-2 enhances osteotomy healing in glucocorticoid treated rabbits. J Bone Miner Res. 2002;17:301–310. [DOI] [PubMed]
- 30.Manolagas SC, Weinstein RS. New development in the pathogenesis and treatment of steroid-induced osteoporosis [Editorial]. J Bone Miner Res. 1999;14:1061–1066. [DOI] [PubMed]
- 31.Murakami H, Kowalewski K. Effects of cortisone and an anabolic androgen on the fractured humerus in guinea pigs: clinical and histological study over a six-week period of fracture healing. Can J Surg. 1966;9:425–434. [PubMed]
- 32.Nilsson OS, Urist MR, Dawson EG, Schmalzried TP, Finerman GA. Bone repair induced by morphogenetic protein in ulnar defects in dogs. J Bone Joint Surg Am. 1986;68:635–642. [DOI] [PubMed]
- 33.Oloein LJ, Kowalewski K. Some effects of cortisone and an anabolic steroid on healing of experimental fractures. Can J Surg. 1962;5:108–117. [PubMed]
- 34.Patel TC, Erulkar JS, Grauer JN, Troiano NW, Panjabi MM, Friedlaender GE. Osteogenic protein-1 overcomes the inhibitory effect of nicotine on posterior lateral lumber fusion. Spine. 2001;26:1656–1661. [DOI] [PubMed]
- 35.Pluhar GE, Manley PA, Heiner JP, Vanderby R Jr, Seeherman HJ, Markel MD. The effect of recombinant bone morphogenetic protein-2 on femoral reconstructions with an intercalary allograft in a dog model. J Orthop Res. 2001;19:308–317. [DOI] [PubMed]
- 36.Rosen V, Cox K, Hattersley G. Bone morphogenetic protein. In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of Bone Biology. San Diego, CA: Academic Press; 1996:661–671.
- 37.Samartzis D, Khanna N, Shen FH, An HS. Update on bone morphogenetic proteins and their application in spine surgery. J Am Coll Surg. 2005;200:236–248. [DOI] [PubMed]
- 38.Sampath TK, Reddi AH. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci USA. 1981;78:7599–7603. [DOI] [PMC free article] [PubMed]
- 39.Sampath TK, Reddi AH. Homology of bone inductive proteins from human, monkey, bovine, and rat extracellular matrix. Proc Natl Acad Sci USA. 1983;80:6591–6595. [DOI] [PMC free article] [PubMed]
- 40.Sandhu HS, Kanim LE, Kabo JM, Toth JM, Zeegen EN, Liu D, Delamarter RB, Dawson EG. Effective doses of recombinant bone morphogenetic protein in experimental spinal fusion. Spine. 1996;21:2115–2122. [DOI] [PubMed]
- 41.Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255–265. [DOI] [PubMed]
- 42.Shen V, Birchman R, Liang XG, Wu DD, Linsay R, Dempster DW. Prednisolone alone, or in combination with estrogen or dietary calcium deficiency or immobilization, inhibits bone formation but does not induce bone loss in mature rats. Bone. 1997;21:345–351. [DOI] [PubMed]
- 43.Sigurdsson TJ, Nygaard L, Tatakis DN, Fu E, Turek TJ, Jin L, Wozney JM. Periodontal repair in dogs: evaluation of rhBMP-2 carriers. Int J Periodontics Restorative Dent. 1996;16:524–537. [PubMed]
- 44.Sissons H, Hadfield G. The influence of cortisone on the repair of experimental fractures in the rabbit. Br J Surg. 1951;39:172–178. [DOI] [PubMed]
- 45.Stevenson S. Bone grafting. In: Slatter DH, ed. Textbook of Small Animal Surgery. Philadelphia, PA: WB Saunders; 1993:1694–1703.
- 46.Stevenson S, Cunningham N, Toth NJ, Davy D, Reddi AH. The effect of osteogenin (a bone morphogenetic protein) on the formation of bone in orthotopic segmental defects in rats. J Bone Joint Surg Am. 1994;76:1676–1687. [DOI] [PubMed]
- 47.Tagagi K, Urist MR. The role of bone marrow in bone morphogenetic protein-induced repair of femoral massive defects. Clin Orthop Relat Res. 1982;171:224–231. [PubMed]
- 48.Teixeira JO, Urist MR. Bone morphogenetic protein induced repair of compartmentalized segmental diaphyseal defects. Arch Orthop Trauma Surg. 1998;117:27–34. [DOI] [PubMed]
- 49.Urist MR. Bone formation by autoinduction. Science. 1965;150:893–899. [DOI] [PubMed]
- 50.Urist MR, Huo YK, Brownell AG, Hohl WM, Buyske J, Lietze A, Tempst P, Hunkapillar M, Delange RJ. Purification of bovine bone morphogenetic protein by hydroxyapatite chromatography. Proc Natl Acad SciUSA. 1984;81:371–375. [DOI] [PMC free article] [PubMed]
- 51.Weinstein RS, Jilka RL, Parfitt AM, Manolasgas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on the bone. J Clin Invest. 1998;102:274–282. [DOI] [PMC free article] [PubMed]
- 52.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. [DOI] [PubMed]
- 53.Wiancko KB, Kowalewski K. Strength of callus in fractured humerus of rat treated with anti-anabolic and anabolic compounds. Acta Endocrinol. 1961;36:310–318. [DOI] [PubMed]
- 54.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. [DOI] [PubMed]
- 55.Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocrine Rev. 2000;21:393–411. [DOI] [PubMed]
- 56.Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). J Bone Joint Surg Am. 1992;74:659–670. [PubMed]