Abstract
Combining autologous bone graft and recombinant human bone morphogenetic protein-7 (BMP-7) to treat long-bone fracture aseptic atrophic nonunions theoretically could promote bone healing at higher rates than each of these grafting agents separately. We retrospectively reviewed prospectively collected data on patient general characteristics, clinical outcomes, and complications over 3 years to determine the healing rates and the incidence of complications and adverse events of this “graft expansion rationale.” There were 45 patients (32 male) with a median age of 43 years (range, 19–76 years). Minimum followup was 12 months (mean, 24.5 months; range, 12–65 months). There were seven humeral, 19 femoral, and 19 tibial nonunions. The median number of prior operations was two (range, 1–7). All fractures united. Clinical and radiographic union occurred within a median of 5 months (range, 3–14 months) and 6 months (range, 4–16 months), respectively. Thirty-nine (87%) patients returned to their preinjury occupation at a mean of 4.2 months (range, 3–6 months). The median visual analog scale pain score was 0.9 (range, 0–2.8; maximum 10), and the median functional score was 86 (range, 67–95; maximum 100) at the final followup. BMP-7 as a bone-stimulating agent combined with conventional autograft resulted in a nonunion healing rate of 100% in these 45 patients.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Fracture nonunions continue to be challenging and occasionally have devastating complications despite ongoing advances in operative treatment and improvement in our understanding of fracture healing. Approximately 5% to 10% of the 6.2 million fractures occurring annually in the United States develop delayed unions or nonunions [35, 45]. Conventionally, nonunions are subdivided into hypertrophic or atrophic and septic or aseptic according to their clinical presentation and specific characteristics [3, 7].
Atrophic nonunions have proven most difficult to treat, often requiring multiple operations and long periods of recovery (in certain series more than three operations on average in periods extending over 10 years) [10, 37, 53]. Several authors have reported healing rates in over 80% of the cases, mostly with enhancement of the biologic substrate at the fracture site [11, 17, 29]. Autologous bone graft (ABG) is regarded as the gold standard among the many available alternatives of biologic augmentation with reported efficacy of 87% to 100% [3, 4, 34, 41]. Nevertheless, it is also associated with limited availability, longer operative time, and donor site morbidity [2]. For these reasons, other methods of biologic enhancement are currently the center of attention and the subject of a substantial amount of biomedical research [21, 30, 44, 46].
In 1965, Marshall Urist [6, 47] revolutionized the current understanding of fracture healing by hypothesizing the existence of bone morphogenetic proteins (BMPs) in the extracellular collagenous bone matrix. In 1988, Wozney et al. [49] identified the genetic sequences of BMPs. Since then, over 20 different BMP-homologous molecules have been described, 15 of which were identified in humans [43]. BMPs belong to the transforming growth factor-β superfamily, a large family of growth and differentiation factors, which are involved in several stages of embryonic organogenesis and in various stages of intramembranous and endochondral ossification during fracture healing [19, 36]. They have been tested in various experimental animal nonunion models [32, 39]. Since the FDA approval of BMP-7 for the treatment of recalcitrant tibial nonunions in 2001, its efficacy has been verified in a number of randomized trials [5, 18, 27] and large clinical series [12, 15, 23, 26, 33, 38] with healing rates ranging from 81% to 92%.
BMP-7 stimulates proliferation [51] and differentiation [52] of pluripotent mesenchymal cell lines as well as angiogenesis through osteoblast-derived vascular endothelial growth factor [14]. Vascular supply at the fracture site has been considered for years the main cause of atrophic nonunions [1, 7, 48]. Given BMP-7 enhances the inherent osteoinductive capacity of ABG, facilitating the proliferation and differentiation of osteoprogenitor cells to mature osteoblasts, combined use of the two could be considered under a “graft expansion” rationale. The synergistic application of BMP-7 and ABG under this rationale has been previously described [27, 38, 55], mostly in large series of nonunion cases with positive results (healing rates ranging from 88.8% to 92% in 6.5 to 7.9 months).
We therefore assessed the (1) healing rates and time to union and (2) complications and adverse events related to this combined treatment of ABG and BMP-7 in a series of patients with atrophic nonunions.
Materials and Methods
We retrospectively reviewed prospectively collected data on 45 patients with an equal number of atrophic aseptic nonunions of long-bone fractures that were treated with a combination of ABG and BMP-7 in two trauma centers between October 2003 and December 2006. All patients with atrophic upper or lower long-bone fracture nonunions were eligible to participate (Fig. 1). We required they had undergone at least one prior failed attempt at treatment (with revision of the fixation alone, autografting alone, or both) in which no recombinant human BMP-7 was used. The two centers treat on average 8000 musculoskeletal injuries annually; during the time of the study, we saw 157 patients with nonunions. We excluded 55 patients with hypertrophic or septic nonunions, eight patients with contraindication to administration of BMP-7 (rheumatoid arthritis or other systemic inflammatory disease process), four patients with pathologic fracture nonunions, and 45 patients treated with BMP-7 (20 patients) or ABG (25 patients) alone.
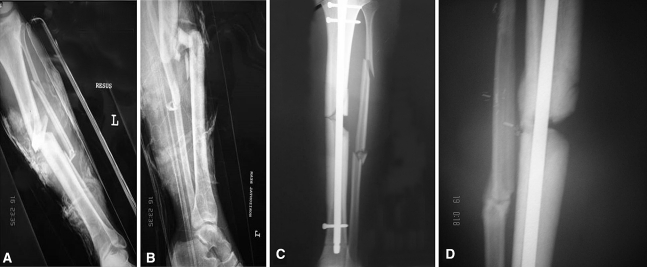
Fig. 1A–D.
Images illustrate the case of Patient 43 (Table 1) before combined treatment with autologous bone graft (ABG) and bone morphogenetic protein-7 (BMP-7). (A) Postinjury anteroposterior and (B) lateral radiographs show an open Grade IIIb tibial fracture. (C) A postsurgery anteroposterior radiograph shows the fracture after initial fixation (intramedullary nailing). (D) An anteroposterior radiograph taken 8 months postsurgery shows the evident atrophic nonunion.
There were 32 (71.1%) men and 13 (28.9%) women with a median age of 43 years (range, 19–76 years). We followed patients a minimum of 12 months (median, 21 months; mean, 24.8 months; range, 12–65 months) (Table 1). No patients were lost to followup. The ethics committees of both institutions approved the study. All patients included in the study signed the relevant informed consent form.
Table 1.
Epidemiologic and clinical profile of enrolled patients
| Patient number | Gender | Age (years) | Nonunion anatomic site | Initial injury | Initial treatment before index operation | Number of operations before index operation | Additional fixation or revision fixation | In-between period (months) | Followup (months) | Time to clinical union (months) | Time to radiographic union (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 28 | Femur | Closed | IMN | 2 | Revision IMN | 40 | 12 | 4 | 6 |
| 2 | Female | 43 | Femur | Closed | IMN | 4 | Revision IMN | 50 | 26 | 5 | 6 |
| 3 | Female | 37 | Femur | Closed. | IMN | 3 | Revision IMN | 39 | 26 | 3 | 4 |
| 4 | Female | 62 | Femur | Closed | ORIF | 3 | Ilizarov | 14 | 41 | 5 | 6 |
| 5 | Female | 37 | Femur | Closed | IMN | 2 | Revision IMN | 29 | 28 | 4 | 5 |
| 6 | Female | 76 | Femur | Closed | IMN | 3 | Revision IMN | 17 | 18 | 5 | 7 |
| 7 | Female | 57 | Humerus | Closed | ORIF | 1 | Revision ORIF | 29.5 | 12 | 3 | 4 |
| 8 | Female | 56 | Humerus | Closed | ORIF | 1 | Revision ORIF | 21.5 | 12 | 3 | 4 |
| 9 | Female | 69 | Humerus | Closed | IMN | 1 | No | 55 | 52 | 11 | 12 |
| 10 | Female | 50 | Humerus | Closed | ORIF | 3 | Revision ORIF | 40 | 12 | 5 | 6 |
| 11 | Female | 57 | Humerus | Closed | ORIF | 1 | Revision ORIF | 8 | 24 | 6 | 5 |
| 12 | Female | 47 | Tibia | Closed | ORIF | 1 | Revision ORIF | 10 | 30 | 4 | 5 |
| 13 | Female | 76 | Tibia | Open (IIIa)* | Ilizarov | 7 | Revision Ilizarov | 17 | 33 | 14 | 16 |
| 14 | Male | 36 | Femur | Open (IIIb) | ORIF | 1 | Revision ORIF | 13 | 18 | 4 | 8 |
| 15 | Male | 30 | Femur | Open (IIIa) | ORIF | 2 | No | 41.5 | 12 | 4 | 6 |
| 16 | Male | 43 | Femur | Open (IIIa) | IMN | 3 | Ilizarov | 108 | 65 | 5 | 6 |
| 17 | Male | 52 | Femur | Closed | IMN | 2 | Revision IMN | 52 | 12 | 4 | 5 |
| 18 | Male | 34 | Femur | Closed | IMN | 1 | Revision IMN | 20 | 14 | 4 | 4 |
| 19 | Male | 33 | Femur | Closed | IMN | 1 | Revision IMN | 84 | 22 | 6 | 7 |
| 20 | Male | 59 | Femur | Closed | External fixators | 1 | Ilizarov | 312 | 15 | 3 | 4 |
| 21 | Male | 34 | Femur | Closed | IMN | 1 | Revision IMN | 13 | 22 | 5 | 6 |
| 22 | Male | 69 | Femur | Open (IIb) | ORIF | 2 | No | 45 | 32 | 5 | 7 |
| 23 | Male | 39 | Femur | Closed | IMN | 3 | Revision IMN | 15 | 25 | 7 | 9 |
| 24 | Male | 45 | Femur | Closed | ORIF | 4 | Revision ORIF | 21 | 21 | 5 | 6 |
| 25 | Male | 58 | Femur | Closed | IMN | 1 | Revision IMN | 9 | 15 | 6 | 7 |
| 26 | Male | 55 | Femur | Closed | IMN | 3 | Revision IMN | 31 | 15 | 9 | 10 |
| 27 | Male | 57 | Humerus | Closed | Ilizarov | 6 | ORIF | 22 | 55 | 4 | 5 |
| 28 | Male | 23 | Humerus | Closed | ORIF | 1 | Revision ORIF | 11 | 24 | 6 | 6 |
| 29 | Male | 19 | Tibia | Open (IIIb) | ORIF | 4 | No | 19 | 18 | 4 | 6 |
| 30 | Male | 31 | Tibia | Closed | ORIF | 1 | Ilizarov | 19 | 22 | 6 | 8 |
| 31 | Male | 41 | Tibia | Closed | Ilizarov | 1 | No | 9 | 16 | 6 | 8 |
| 32 | Male | 54 | Tibia | Open (IIIb) | ORIF | 1 | No | 8 | 12 | 6 | 6 |
| 33 | Male | 28 | Tibia | Open (IIIb) | IMN | 3 | Ilizarov | 15 | 24 | 6 | 8 |
| 34 | Male | 35 | Tibia | Open (IIIb) | IMN | 1 | No | 8 | 18 | 4 | 6 |
| 35 | Male | 47 | Tibia | Open (IIIb) | ORIF | 2 | Revision ORIF | 38 | 18 | 4 | 6 |
| 36 | Male | 23 | Tibia | Closed | External fixators | 4 | Ilizarov | 29 | 36 | 6 | 7 |
| 37 | Male | 33 | Tibia | Open (IIIb) | IMN | 1 | Ilizarov | 156 | 42 | 13 | 14 |
| 38 | Male | 21 | Tibia | Open (IIIb) | ORIF | 3 | Ilizarov | 8 | 46 | 4 | 5 |
| 39 | Male | 39 | Tibia | Closed | ORIF | 4 | Ilizarov | 20 | 61 | 7 | 8 |
| 40 | Male | 52 | Tibia | Closed | ORIF | 4 | Ilizarov | 53 | 41 | 11 | 12 |
| 41 | Male | 41 | Tibia | Closed | Ilizarov | 3 | Revision Ilizarov | 15 | 12 | 4 | 4 |
| 42 | Male | 75 | Tibia | Closed | Ilizarov | 2 | No | 45 | 12 | 5 | 7 |
| 43 | Male | 62 | Tibia | Open (IIIb) | IMN | 1 | No | 8 | 12 | 4 | 5 |
| 44 | Male | 40 | Tibia | Open (IIb) | IMN | 2 | No | 11 | 18 | 5 | 6 |
| 45 | Male | 44 | Tibia | Closed | IMN | 1 | Revision IMN | 8 | 14 | 5 | 7 |
* Classified according to the system of Gustilo et al. [24]; index operation = the combined use of ABG and BMP-7; in-between period = period between fracture event and combined treatment (ABG and BMP-7); IMN = intramedullary nailing; ORIF = open reduction internal fixation; ABG = autologous bone graft; BMP-7 = bone morphogenetic protein-7.
Relevant clinical data referring to the original injury and the initial management of the long-bone fractures were collected retrospectively, and relevant clinical data until final discharge from the orthopaedic outpatient clinic were collected prospectively. We recorded demographic data, anatomic site of atrophic nonunion, type of initial fracture (closed or open; classified according to the system of Gustilo et al. [24]), initial treatment, subsequent procedures, additional revision stabilization, and postoperative complications. Seven nonunions (15.6%) were humeral, 19 (42.2%) femoral, and 19 (42.2%) tibial. Fourteen were originally open injuries: two (4.4%) Grade II, three (6.7%) Grade IIIa, and nine (20%) Grade IIIb. Of these 14 open injuries, 10 were tibial and four femoral nonunions. All nonunions were atrophic, and seven had bone defects of a median length of 2.5 cm (range, 2–4 cm). All intraoperative tissue cultures were negative and underlying infection was excluded clinically and microscopically. The median duration of the atrophic nonunion before recombinant human BMP-7 application was 20 months (mean, 36.4 months; range, 8–312 months). The median number of operations before the combined grafting was two (mean, 2.3; standard deviation, 1.4; range, 1–7). In 20 (44.4%) of the patients, iliac crest bone graft had been used previously at least once.
In 35 patients (77.8%), the fracture fixation was revised at the time of autograft and recombinant human BMP-7 application; 10 patients had open reduction and internal fixation (ORIF) (in one patient, ORIF replaced an Ilizarov external fixator; in nine, old implants were revised to new ones); 13 had exchange of the previous intramedullary nailing (IMN) to a new IMN; eight had conversion of the previous internal fixation to an Ilizarov frame (five IMN, three ORIF); and four had revision of their previous Ilizarov frame to a new one.
The revision strategy for treating all patients was based on providing optimal mechanical stability, when clinically needed, and enhancing the biologic substrate by applying the combination of the recombinant human BMP-7 (osteoinductive agent) and autograft (graft expander) at the same setting in all patients. All patients received prophylactic intravenous antibiotics perioperatively, and tissue samples were obtained in all patients during the revision surgery to exclude the presence of an underlying low-grade infection.
In all patients, we used recombinant human BMP-7 (Stryker Biotech, Hopkinton, MA) (Fig. 2). Each unit contained 3.5 mg recombinant human BMP-7 mixed with 1 g Type I bovine-derived collagen. The total volume per unit was approximately 4 mL. Autologous cancellous bone was harvested from the iliac crest of the pelvis of each patient. Per-Q-Graft® Instrumentation (Wright Cremascoli Ortho, Chester, UK) was used through small 1- to 2-mm skin incisions in one institution to minimize local trauma.
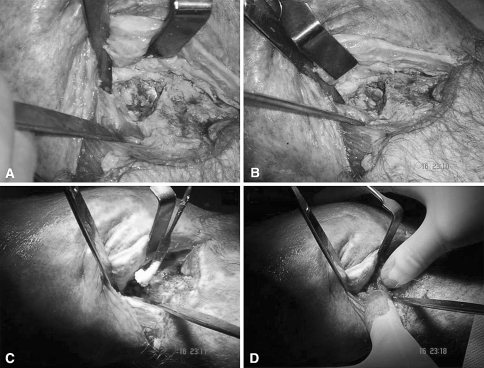
Fig. 2A–D.
Intraoperative photographs show the application of the combination of autologous bone graft (ABG) with bone morphogenetic protein-7 (BMP-7) at the site of the tibial nonunion (8 months after the original fracture fixation) in Patient 43 (Table 1). Photographs show (A–B) the nonunion site with the intramedullary nailing (IMN) in situ after the débridement of the nonunion tissues, (C) application of the BMP-7 putty at the nonunion site, and (D) application of the ABG at the nonunion site.
The postoperative protocol included initial support by a brace or a splint (ranging from 3 to 6 weeks when they were used for humeral and tibial nonunions) and immobilization or partial mobilization depending on the site of the nonunion and the fixation method. Early passive and active range of motion of the affected limb was encouraged at the immediate postoperative period and followed up by outpatient physiotherapy sessions. Weightbearing status evolved progressively as we clinically and radiographically judged bone healing. Decreases of local pain on loading and tenderness in deep palpation were the main clinical criteria and bridging callus in two or more cortexes the radiographic prerequisites to progress further on weightbearing and use of the affected limb.
Clinical and radiographic assessment was performed at 1, 2, 3, 6, 9, 12, and 18 months after surgery or until clinical and radiographic union was established. We (PVG, RJM) defined clinical union as painless full-weightbearing status or upper limb function for humeral nonunions. A visual analog scale (VAS) score [40] to assess related pain was administered by the treating surgeons (PVG, NKK, SJM) (scale from 0 to 10, where 0 is “no pain” and 10 the “worst pain”) as well as a scale similar to the EuroQol-5D general health questionnaire scale [16]. This was focused on the subjective functional status of the affected limb and not on the general health of the individual as in the EuroQol-5D. The patient was asked to draw a line from the cube representing the current functional status of his or her limb to cross a vertical scale from 0 (worst functional state) to 100 (best functional state). This was administered at the last followup appointment to quantify, respectively, residual pain and the functional outcome. The percentage of those patients returning to their preinjury occupation was used as an indicator of recovery. Any local or systemic allergic or anaphylactic reactions secondary to the BMP-7 administration were documented prospectively.
Two independent reviewers of each institution (CT, VS, RM, KV) assessed the radiographic union of the relative cases; radiographic union was defined as bridging callus on three of four cortices visible on both anteroposterior and lateral views (all necessary radiographs were available). The interrater agreement of their evaluation was assessed using Cohen’s kappa coefficient. The overall interrater agreement of the radiographic progress of healing was κ = 0.786. At 1, 2, 3 months, all reviewers agreed on the absence of radiographic healing; at 6 months, the kappa value was 0.862; at the end point of 9 and 18 months, all reviewers agreed about the progress of radiologic healing; at the 12-month end point, the kappa value was 0.789).
We used Microsoft® Access™ for Windows® (Microsoft Corp, Redmond, WA) for tracking all data. Descriptive statistics were used where needed for explanatory purposes.
Results
All 45 patients progressed to nonunion healing (Table 2). Clinical healing occurred at a median of 5 months (mean, 5.5 months; range, 3–14 months) and radiographic healing at a median of 6 months (mean, 6.8 months; range, 4–16 months) (Fig. 3). The median VAS for pain at the final followup appointment was 0.9 (range, 0–2.8). The median score of the functional scale at the final followup was 86 (range, 67–95). Thirty-nine patients (87%) returned to their preinjury occupations at a mean return-to-work time of 4.19 months (median, 4 months; range, 3–6 months). Six patients (13%), four tibial and two femoral nonunions, did not return to their preinjury occupation. These six patients all scored less than 70 on the function scale and more than 2 on the VAS for pain. Their persistent pain was attributed to soft tissue contractures (post-skin grafting) and joint stiffness.
Table 2.
Characteristics of the study population grouped per anatomic site
| Characteristic | Humeral nonunions | Femoral nonunions | Tibial nonunions |
|---|---|---|---|
| Number of patients | 7 | 19 | 19 |
| Males/females | 2/5 | 13/6 | 17/2 |
| Age (years)* | 57 (23–69) | 43 (28–76) | 41 (19–76) |
| Open/closed fractures | 0/7 | 4/15 | 10/9 |
| Initial operation | 1 IMN | 13 IMN | 6 IMN |
| 5 ORIF | 5 ORIF | 8 ORIF | |
| 1 External fixation—Ilizarov | 1 External fixation—Ilizarov | 5 External fixation—Ilizarov | |
| In-between period (months)* | 22 (8–55) | 31 (9–312) | 15 (8–156) |
| Number of previous operations* | 1 (1–6) | 2 (1–4) | 2 (1–7) |
| Type of revision fixation | 5 Revision ORIF | 2 Revision ORIF | 2 Revision ORIF |
| 1 ORIF | 3 Ilizarov | 7 Ilizarov | |
| 1 None | 12 Exch. IMN | 2 Revision Ilizarov | |
| 2 None | 1 Exch. IMN | ||
| 7 None | |||
| Followup (months)* | 24 (12–55) | 21 (12–65) | 18 (12–61) |
| Time to clinical union (months)* | 5 (3–11) | 5 (3–9) | 5 (4–14) |
| Time to radiographic union (months)* | 5 (4–12) | 6 (4–10) | 7 (4–16) |
| VAS for pain* | 0.4 (0–1.2) | 0.8 (0–2.8) | 1.7 (0–2.7) |
| 0–100 functional scale* | 89 (72–95) | 87 (68–95) | 78 (67–93) |
* Values expressed as median with range in parentheses; IMN = intramedullary nailing; ORIF = open reduction plate fixation, in-between period = period between fracture event and combined treatment (ABG and BMP-7); Exch. IMN = exchange of nail; VAS = visual analog scale.
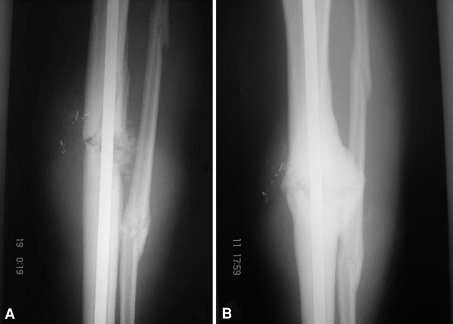
Fig. 3A–B.
Images illustrate the case of Patient 43 (Table 1) after combined treatment with autologous bone graft (ABG) and bone morphogenetic protein-7 (BMP-7). (A) An anteroposterior radiograph taken on the first postoperative day after the combined grafting of the tibial nonunion shows the grafting material (ABG and BMP-7) as applied at the nonunion site. (B) An anteroposterior radiograph taken 5 months after the combined ABG and BMP-7 grafting shows the healed tibial nonunion.
The skin healed in all 45 patients after the final revision procedure. There were no deep infections, no deep venous thromboses, and no neurologic deficits attributed to the operative treatment or the grafting procedure. Six patients (13.3%) had hematoma and pain over the iliac crest where the ABG was harvested; all resolved within 4 to 12 weeks (average, 6 weeks) from the grafting procedure. Three patients postoperatively developed superficial wound infections, which resolved with oral antibiotics for 2 weeks. No systemic or allergic reactions or adverse effects were encountered after the application of recombinant human BMP-7.
Discussion
The methods of biologic enhancement of bone healing are evolving, guided by the progress of our understanding and the advances of basic sciences [4, 29]. However, especially in the clinical setting of recalcitrant atrophic nonunions, the treatment of these severe complications is often unsuccessful, time- and large asset-consuming. The combination of different grafting materials and agents has been suggested in the past as an alternative to treating nonunions resistant to standard methods [27, 38, 55]. Following this rationale of “graft expansion,” we evaluated the efficacy and safety of the simultaneous use of ABG and recombinant human BMP-7 as an approach of biologic enhancement to atrophic aseptic long-bone fracture nonunions resistant to conventional means.
Our descriptive study bears certain limitations, because no randomization was performed and no control groups were used. Further evaluation of this rationale is desirable and should originate from a randomized, controlled study of adequate power, thus avoiding any confounding variables and selection bias. A study accounting for these issues will have to be a multicenter one; a power calculation analysis revealed at least 180 patients would be required to achieve 80% power with an effect size of an increase of the rate of nonunion healing of 10%. A statistical validation of the interrater agreement between the radiograph reviewers was performed to attain a consensus on the time to nonunion healing with positive results (κ = 0.76). The inclusion of three different anatomic sites (humerus, tibia, and femur) and the small number of the patients per anatomic site (7, 19, and 19, respectively) do not allow comprehensive statistical analysis for further evaluation of our results (Table 2). The absence of comparative functional outcome results during the period of followup of the 45 enrolled patients, as a result of the inadequacy of the retrospective part of our data, did not allow complete assessment of the progress of symptoms of the studied patients. However, the recorded level of final functional recovery and pain score was satisfactory and comparable to other series [27, 54]. Nevertheless, this case series offers a specific example of the combined application of BMP-7 and ABG for the most common long-bone nonunion sites and describes a 100% healing rate of a group of nonunions resistant to standard treatment means. Furthermore, despite recently raised concerns over local and systemic adverse effects (osteolysis, bone resorption, heterotopic calcification, allergic reactions) after BMP use [8, 22, 25], no such complications were encountered over a minimum followup period of 1 year. The full hematologic, liver, and renal function profile was normal in the followup appointments. We did not, however, perform immunologic studies for antibodies to recombinant human BMP-7 or to Type I collagen carrier to exclude sensitization to human recombinant osteogenic protein-1 administration; thus, the absence of subclinical immunologic adverse effects cannot be excluded.
Our treatment approach was based on current evidence that autograft contains osteoprogenitor cells and osteoinductive factors and also provides some osteoconductive scaffolding properties [17, 31] and on the hypothesis that BMP-7 may enhance the osteoinductive capacity of the ABG [9, 23, 27, 38]. In patients with bone defects, the concept was to use BMP-7 as a powerful osteoinductive agent and the ABG as a graft expander. Previous authors have reported the results of either ABG [20, 41, 50] or recombinant human BMP-7 [15, 18, 23, 27, 33, 54] in isolation. Currently the existing clinical reports regarding the healing of atrophic nonunions with the use of BMPs have provided substantial arguments in favor of these agents (Table 3). All of our 45 patients achieved union after implantation of autograft with recombinant human BMP-7. In the landmark study of Friedlaender et al. [18], 82% of all patients receiving recombinant human BMP-7 or autograft achieved satisfactory healing. However, 3% of the patients implanted with recombinant human BMP-7 and 21% of patients receiving autograft alone developed osteomyelitis postoperatively. The demographics in our study group were similar to those in the study of Friedlaender et al. [18], including age, gender ratio, and number of prior surgical interventions; however, the mean duration of atrophic nonunion was higher in our series. Our findings confirm that recombinant human BMP-7 is a clinically safe osteoinductive implant, which is at the same time associated with substantial clinical and radiographic healing rates when it is implanted in conjunction with ABG for the treatment of atrophic nonunions.
Table 3.
Clinical studies on the application of BMP-7, ABG, or their combination for the treatment of long-bone fracture nonunions
| Study (year) | Type of study, LOE | Anatomic site of nonunions | Number of cases | Age (years)* | Time from injury (months)* | Number of previous operations* | Union rates | Time to nonunion healing (months)* | Reoperations |
|---|---|---|---|---|---|---|---|---|---|
| Souter [42] (1969) | Retrospective observational (ABG), IV | Tibia, femur, humerus, forearm | 102 ABG | 40 (15–77) | 6 (2–84) | NA | 86% | 4 | NA |
| Friedlaender et al. [18] (2001) | Prospective randomized, controlled (BMP-7 versus ABG), II | Tibia | 63 BMP-7 | Median 38 ± 16 | Median 27 ± 26 | NA | 81% | 9 | 5% |
| 61 ABG | Median 34 ± 14 | Median 33 ± 46 | 85% | 10% | |||||
| Babhulkar and Pande [3] (2005) | Retrospective observational (ABG), IV | Tibia, femur, humerus, forearm atrophic | 52 ABG | NA | NA | NA | 77% | 6 (4–8) | NA |
| Ronga et al. [38] (2006) | Retrospective observational (BMP-7), IV | Tibia, femur, humerus | 38 BMP-7 | 42.2 (15–78) | 27.3 ± 39.9 | 1.6 ± 0.8 | 86% | 7.9 (2–21) | 15.2% |
| 50 BMP-7 + ABG | 31.2 ± 59.0 | 2.7 ± 2.1 | 86.8% | ||||||
| Calori et al. [9] (2006) | Prospective randomized, controlled (BMP-7 versus PRP), II–III | Tibia, femur, humerus, forearm | 16 BMP-7 | 47.4 ± 2.56 | 15.2 ± 2.46 | 2.5 ± 0.57 | 94% | Radiographic 8 ± 0.43 | 6.2% |
| Zimmermann et al. [55] (2007) | Prospective comparative (BMP-7 versus ABG), III | Tibia | 18 BMP-7 | 44 (19–68) | 11 (NA) | 4 (NA) | 94.5% | 5 | 5.5% |
| 8 BMP-7 + ABG | 87.5% | 12.5% | |||||||
| 82 ABG | 0 | 72% | 31.7% | ||||||
| Kanakaris et al. [27] (2008) | Prospective observational (BMP-7), IV | Tibia | 43 BMP-7 | 42.6 (19–78) | 23 (9–317) | 2 (0–11) | 89.7% | 6.5 (3–15) | 6% |
| 25 BMP-7 + ABG | |||||||||
| Giannoudis et al. [current study] | Prospective observational (BMP-7 + ABG), IV | Tibia, femur, humerus | 45 BMP-7 + ABG | Median 43 (19–76) | Median 30 (6–312) | Median 2 (1–7) | 100% | Median 6 (4–16) | 0% |
* Values are expressed as mean (or median as noted) ± standard deviation with range in parentheses; BMP-7 = bone morphogenetic protein-7; ABG = autologous bone graft; LOE = level of evidence; NA = not available.
Given the ineffective prior use of ABG alone (gold standard) in 44.4% of our series, the high number (average 2.3) of previous unsuccessful surgical interventions and the long period of incapability and its economic implications (median time before combined treatment plus median time to union was 26 months), the highly effective strategy of combined ABG and BMP-7 use could have potential economic benefits. Recent publications have identified the importance of this aspect of contemporary medicine as well as the lack of existing evidence regarding the financial burden of treating nonunions of fractures [13, 28]. A gross comparison of the reported efficacy of ABG or BMP-7 of several published large series (Table 3) to our sample of the combined use of ABG and BMP-7 supports the existence of a potential synergism between recombinant human BMP-7 and ABG that may promote better healing rates in atrophic nonunions. A larger-scale randomized, controlled, blinded, prospective clinical study, comparing the efficacy of the latter combination against ABG and BMP-7 alone for the treatment of atrophic nonunion, will enlighten our hypothesis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Leeds General Infirmary and Middlesbrough General Hospital.
Contributor Information
Peter V. Giannoudis, Email: pgiannoudi@aol.com.
Nikolaos K. Kanakaris, Email: nikolaoskanakaris@yahoo.co.uk.
References
- 1.Arany L, Baranyai T, Mandi A, Kunkli F. Arteriographic studies in delayed-union and non-union of fractures. Radiol Diagn (Berl). 1980;21:673–681. [PubMed]
- 2.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. [DOI] [PubMed]
- 3.Babhulkar S, Pande K. Nonunion of the diaphysis of long bones. Clin Orthop Relat Res. 2005;431:50–56. [DOI] [PubMed]
- 4.Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10–27. [DOI] [PubMed]
- 5.Bilic R, Simic P, Jelic M, Stern-Padovan R, Dodig D, van Meerdervoort HP, Martinovic S, Ivankovic D, Pecina M, Vukicevic S. Osteogenic protein-1 (BMP-7) accelerates healing of scaphoid non-union with proximal pole sclerosis. Int Orthop. 2006;30:128–134. [DOI] [PMC free article] [PubMed]
- 6.Brand RA. 50 years ago in CORR: physiologic basis of bone-graft surgery Marshall R. Urist MD CORR 1953;1:207-216. Clin Orthop Relat Res. 2008;466:2015–2016. [DOI] [PMC free article] [PubMed]
- 7.Brinker MR. Nonunions: evaluation and treatment. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, eds. Skeletal Trauma Basic Science Management and Reconstruction. 3rd Ed. Vol I. Philadelphia, PA: WB Saunders; 2003:507–604.
- 8.Brower RS, Vickroy NM. A case of psoas ossification from the use of BMP-2 for posterolateral fusion at L4-L5. Spine. 2008;33:E653–E655. [DOI] [PubMed]
- 9.Calori GM, D’Avino M, Tagliabue L, Albisetti W, d’Imporzano M, Peretti G. An ongoing research for evaluation of treatment with BMPs or AGFs in long bone non-union: protocol description and preliminary results. Injury. 2006;37(Suppl 3):S43–S50. [DOI] [PubMed]
- 10.Cavadas PC, Landin L. Treatment of recalcitrant distal tibial nonunion using the descending genicular corticoperiosteal free flap. J Trauma. 2008;64:144–150. [DOI] [PubMed]
- 11.Connolly JF. Injectable bone marrow preparations to stimulate osteogenic repair. Clin Orthop Relat Res. 1995;313:8–18. [PubMed]
- 12.Cook SD. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics. 1999;22:669–671. [PubMed]
- 13.Dahabreh Z, Dimitriou R, Giannoudis PV. Health economics: a cost analysis of treatment of persistent fracture non-unions using bone morphogenetic protein-7. Injury. 2007;38:371–377. [DOI] [PubMed]
- 14.Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, Lowik CW. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. [DOI] [PubMed]
- 15.Dimitriou R, Dahabreh Z, Katsoulis E, Matthews SJ, Branfoot T, Giannoudis PV. Application of recombinant BMP-7 on persistent upper and lower limb non-unions. Injury. 2005;36(Suppl 4):S51–S59. [DOI] [PubMed]
- 16.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed]
- 17.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84:454–464. [DOI] [PubMed]
- 18.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83(Suppl 1):S151–S158. [PMC free article] [PubMed]
- 19.Gamradt SC, Abe N, Bahamonde ME, Lee YP, Nelson SD, Lyons KM, Lieberman JR. Tracking expression of virally mediated BMP-2 in gene therapy for bone repair. Clin Orthop Relat Res. 2006;450:238–245. [DOI] [PMC free article] [PubMed]
- 20.Gerber A, Marti R, Jupiter J. Surgical management of diaphyseal humeral nonunion after intramedullary nailing: wave-plate fixation and autologous bone grafting without nail removal. J Shoulder Elbow Surg. 2003;12:309–313. [DOI] [PubMed]
- 21.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–S27. [DOI] [PubMed]
- 22.Giannoudis PV, Kanakaris NK, Einhorn TA. Interaction of bone morphogenetic proteins with cells of the osteoclast lineage: review of the existing evidence. Osteoporos Int. 2007;18:1565–1581. [DOI] [PubMed]
- 23.Giannoudis PV, Psarakis S, Kanakaris NK, Pape HC. Biological enhancement of bone healing with bone morphogenetic protein-7 at the clinical setting of pelvic girdle non-unions. Injury. 2007;38(Suppl 4):S43–48. [DOI] [PubMed]
- 24.Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24:742–746. [DOI] [PubMed]
- 25.Harwood PJ, Giannoudis PV. Application of bone morphogenetic proteins in orthopaedic practice: their efficacy and side effects. Expert Opin Drug Saf. 2005;4:75–89. [DOI] [PubMed]
- 26.Johnson EE, Urist MR. Human bone morphogenetic protein allografting for reconstruction of femoral nonunion. Clin Orthop Relat Res. 2000;371:61–74. [DOI] [PubMed]
- 27.Kanakaris NK, Calori GM, Verdonk R, Burssens P, De Biase P, Capanna R, Vangosa LB, Cherubino P, Baldo F, Ristiniemi J, Kontakis G, Giannoudis PV. Application of BMP-7 to tibial non-unions: a 3-year multicenter experience. Injury. 2008;39(Suppl 2):S83–S90. [DOI] [PubMed]
- 28.Kanakaris NK, Giannoudis PV. The health economics of the treatment of long-bone non-unions. Injury. 2007;38(Suppl 2):S77–S84. [DOI] [PubMed]
- 29.Kanakaris NK, Giannoudis PV. Clinical applications of bone morphogenetic proteins: current evidence. J Surg Orthop Adv. 2008;17:133–146. [PubMed]
- 30.Kanakaris NK, Paliobeis C, Manidakis N, Giannoudis PV. Biological enhancement of tibial diaphyseal aseptic non-unions: the efficacy of autologous bone grafting, BMPs and reaming by-products. Injury. 2007;38(Suppl 2):S65–S75. [DOI] [PubMed]
- 31.Khan SN, Cammisa FP Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13:77–86. [PubMed]
- 32.Makino T, Hak DJ, Hazelwood SJ, Curtiss S, Reddi AH. Prevention of atrophic nonunion development by recombinant human bone morphogenetic protein-7. J Orthop Res. 2005;23:632–638. [DOI] [PubMed]
- 33.Pecina M, Haspl M, Jelic M, Vukicevic S. Repair of a resistant tibial non-union with a recombinant bone morphogenetic protein-7 (rh-BMP-7). Int Orthop. 2003;27:320–321. [DOI] [PMC free article] [PubMed]
- 34.Phieffer LS, Goulet JA. Delayed unions of the tibia. J Bone Joint Surg Am. 2006;88:206–216. [DOI] [PubMed]
- 35.Praemer A, Furner S, Rice DP, eds. Musculoskeletal Conditions in the United States. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1999.
- 36.Reddi AH. Bone morphogenetic proteins: from basic science to clinical applications. J Bone Joint Surg Am. 2001;83(Suppl 1):S1–S6. [DOI] [PubMed]
- 37.Ring D, Barrick WT, Jupiter JB. Recalcitrant nonunion. Clin Orthop Relat Res. 1997;340:181–189. [DOI] [PubMed]
- 38.Ronga M, Baldo F, Zappala G, Cherubino P. Recombinant human bone morphogenetic protein-7 for treatment of long bone non-union: an observational, retrospective, non-randomized study of 105 patients. Injury. 2006;37(Suppl 3):S51–S56. [DOI] [PubMed]
- 39.Salkeld SL, Patron LP, Barrack RL, Cook SD. The effect of osteogenic protein-1 on the healing of segmental bone defects treated with autograft or allograft bone. J Bone Joint Surg Am. 2001;83:803–816. [DOI] [PubMed]
- 40.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2:175–184. [DOI] [PubMed]
- 41.Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38(Suppl 1):S75–S80. [DOI] [PubMed]
- 42.Souter WA. Autogenous cancellous strip grafts in the treatment of delayed union of long bone fractures. J Bone Joint Surg Br. 1969;51:63–75. [PubMed]
- 43.Termaat MF, Den Boer FC, Bakker FC, Patka P, Haarman HJ. Bone morphogenetic proteins. Development and clinical efficacy in the treatment of fractures and bone defects. J Bone Joint Surg Am. 2005;87:1367–1378. [DOI] [PubMed]
- 44.Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38(Suppl 1):S11–S25. [DOI] [PubMed]
- 45.Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury. 2007;38(Suppl 2):S3–S9. [DOI] [PubMed]
- 46.Urban RM, Turner TM, Hall DJ, Inoue N, Gitelis S. Increased bone formation using calcium sulfate-calcium phosphate composite graft. Clin Orthop Relat Res. 2007;459:110–117. [DOI] [PubMed]
- 47.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. [DOI] [PubMed]
- 48.Weber BG, Brunner C. The treatment of nonunions without electrical stimulation. Clin Orthop Relat Res. 1981;161:24–32. [PubMed]
- 49.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. [DOI] [PubMed]
- 50.Wu CC. Bone grafting techniques in treating fracture nonunion. Chang Gung Med J. 2000;23:319–330. [PubMed]
- 51.Yeh LC, Adamo ML, Olson MS, Lee JC. Osteogenic protein-1 and insulin-like growth factor I synergistically stimulate rat osteoblastic cell differentiation and proliferation. Endocrinology. 1997;138:4181–4190. [DOI] [PubMed]
- 52.Yeh LC, Tsai AD, Lee JC. Osteogenic protein-1 (OP-1, BMP-7) induces osteoblastic cell differentiation of the pluripotent mesenchymal cell line C2C12. J Cell Biochem. 2002;87:292–304. [DOI] [PubMed]
- 53.Zaslav KR, Meinhard BP. Management of resistant pseudarthrosis of long bones. Clin Orthop Relat Res. 1988;233:234–242. [PubMed]
- 54.Zimmermann G, Moghaddam A, Wagner C, Vock B, Wentzensen A. Clinical experience with bone morphogenetic protein 7 (BMP 7) in nonunions of long bones [in German]. Unfallchirurg. 2006;109:528–537. [DOI] [PubMed]
- 55.Zimmermann G, Muller U, Loffler C, Wentzensen A, Moghaddam A. Therapeutic outcome in tibial pseudarthrosis: bone morphogenetic protein 7 (BMP-7) versus autologous bone grafting for tibial fractures [in German]. Unfallchirurg. 2007;110:931–938. [DOI] [PubMed]