Abstract
The menisci are intraarticular fibrocartilaginous structures essential to the normal function of the knee that lack the ability to self-repair. Human meniscal fibrochondrocytes may respond to beneficial genes like human insulin growth factor-1 (hIGF-1) and the meniscal cell may be a feasible donor for gene therapy. To explore this possibility, we amplified the hIGF-1 gene sequence in full length and cloned it into a bicistronic plasmid. This gene was then transfected into cultured human meniscal fibrochondrocytes by the liposome FuGene 6. Green fluorescence was expressed in part of the cells 6 hours after transfection and increased gradually, with a peak concentration of the hIGF-1 in the supernatants to 22.68 ng/mL 56 hours after transfection. Phenotypes of some cells changed and the proliferation accelerated after transfection. Flow cytometry analysis demonstrated upregulation of cell numbers in the G2 and S stages after hIGF-1 gene introduction. We conclude the hIGF-1 gene can be transfected into the human meniscal cell efficiently by liposome and it causes accelerated proliferation and differentiation. Within 10 days after transfection, the cytokine appears to be secreted into supernatants with the bioactivity and promotes the proliferation of the NIH 3T3 cell line.
Introduction
The menisci serve many important biomechanical functions, including load bearing, shock absorption, and load transmission. They also contribute to the stability of the knee [3, 15, 20]. Many reports demonstrate an early onset of degenerative changes in the knee after meniscectomy [1, 14, 26]. Because there is a greater understanding of the importance of the menisci, most surgeons attempt to preserve as much functional meniscal tissue as possible when treating meniscal injuries [10, 13]. Based on the blood and nutrition supply, the meniscus is divided into three zones: a red zone, a white zone, and a red-white zone [24]. Fibrochondrocytes in different zones of the menisci display different morphology correlating with their stress and nutritional microenvironment [28]. In primary monolayer cell cultures, the meniscal fibrochondrocytes are a mixture of phenotypes, oval- or spindle-shaped cells that normally exist in the superficial layer of the meniscus, and round-shaped cells which are found predominantly in the deep layer.
Human insulin-like growth factor-1 (hIGF-1) stimulates the synthesis and deposition of extracellular matrix components by chondrocytes as well as cell proliferation [5, 12, 16]. In cell culture, it suppresses chondrocyte dedifferentiation and stabilizes the chondrocyte phenotype [17]. Therefore, theoretically, the cytokine secreted by meniscal fibrochondrocytes transfected with the hIGF-1 gene could enhance cell metabolism while maintaining the differentiated phenotype. Using a cationic liposome for transfection helps avoid the limitations of the virus vectors including a risk of neoplastic transformation or infection-associated toxicity, so that might be clinically practical.
We therefore asked (1) whether the hIGF-1 gene could be transfected into human meniscal fibrochondrocytes by cationic liposome, and the hIGF-1 gene with marker gene could be expressed within cells and the cytokine be secreted into the supernatants, and (2) whether the proliferation of the cells could be accelerated under the circumstance of the gene expression.
Materials and Methods
We amplified the cDNA of human insulin-like growth factor-1 and cloned it into a bicistronic expression vector containing a marker gene and the internal ribosomal entry site. To test the expression of the target gene, we transfected the human meniscal fibrochondrocytes with cationic liposome FuGene6 in vitro and determined the efficiency by observation of the marker gene. Expression of bioactive hIGF-1 within and secreted from the cells was monitored by immunohistochemistry staining, Western blot, MTT chromatometry and RT-PCR as well as enzyme-linked immunosorbent assay. The changes of the cells after transfection were analyzed by the observation of the proliferation and population doubling times, and compared the variation of the cell cycle before and after gene intervention (Fig. 1).
Fig. 1.
Flow diagram of the procedure outlines the steps and the numbers of samples, cultures, and replicates at each step.
Two oligonucleotide primers of full-length hIGF-1 cDNA published in the GeneBank (X00173) were designed, each containing the sites of restriction enzyme EcoR I and Xho I, the start codon, and termination codon. The upstream primer sequence was 5′ GCCTCGAGGAAGATGCACACCATGTCCTC 3′, and the downstream primer sequence was 5′ GCGAATTCCTACATCCTGTAGTTCTTGTTTC 3′. The hIGF-1 cDNA was amplified from the human hepatocyte cDNA library (Clontech Company, Mountainview, CA) using polymerase chain reaction (PCR). The PCR products were analyzed by electrophoresis on 1% agarose gel. Then the PCR products were purified and subcloned into the PMD18-T vector. Nucleotide sequence analysis was carried out by the chain termination method using restriction fragments of the plasmid. The correct plasmid was selected out for cloning into the pIRES2-EGFP plasmid (Clontech Co). To construct the eukaryotic expression vector, the PMD18-T-hIGF-1 and pIRES2-EGFP were digested by Xho I and EcoR I and then the corresponding segments were retrieved and inserted into the multiple cloning site of the pIRES2-EGFP vector. This bicistronic expression vector contained enhanced green fluorescent protein (EGFP) as a marker for transfection efficiency. The hIGF-1 cDNA was inserted into the multiple cloning site located upstream of the encephalomyocarditis virus internal ribosomal entry site (IRES). The IRES sequence allows both the genes of interest and EGFP to be translated simultaneously from the same mRNA transcript. The sequence was consistent with the published sequence in GeneBank(X00173).
We retrieved menisci from the knees of 24 patients with torn or discoid menisci under arthroscopic surgery and cut each into small pieces. The cells were released from the meniscal fragments by sequential digestion with 0.2% (weight per volume) trypsin for 30 minutes and 0.2% (weight per volume) collagenase for 3 hours at 37°C and were then seeded into 48 25-cm2 tissue culture flasks that contained 4 mL of Dulbecco’s modified Eagle medium (DMEM; Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin (weight per volume). The cells were resuspended in the medium, passed through a nylon filter (70 μm), washed twice with the same medium, plated at a density of 1 × 106 on 144 35-mm diameter plastic culture dishes, and cultured in a monolayer at 37°C in a humidified atmosphere with 5% CO2 for 16 hours. During primary culture, adherent cells formed colonies that were removed when they had expanded to cover approximately 90% of the plate. The cells were then trypsinized, counted, and replated in a density of 2 × 105 cells per 35-mm dish for further culture. To expand cell numbers, we trypsinized dense cell plaques and replated them until they formed a confluent monolayer.
Trypan blue dye exclusion test was performed to test the cytoactivity since the live cells could not be stained by the reagent. Cells from all patients were tested twice for their cytoactivity. Population doubling times of cells were calculated based on the formula: PDT = {log 2/(log Nt − log No)} × t. We drew a growth curve to determine the cell propagation rate.
The cultured cells in 54 dishes were maintained in a mixture medium of DMEM containing 10% fetal bovine serum for 5 days. Then the cells were transfected with plasmid using FuGene6 transfect reagent (Roche Company, Basel, Switzerland) following the manufacturer’s instructions. Mixtures containing medium, FuGene6, and the eukaryotic expression plasmid pIRES2-EGFP-hIGF-1 at a ratio of 3:1 were added into the dishes and mixed uniformly. To increase the efficiency, the cells were treated with hyaluronidase at a density of 4 U/mL 12 hours before and at the time of the transfection procedure.
To test the possible influence of the empty vector and transfect reagent on cells, 30 dishes of cells were divided into three equal control groups: the empty vector group, FuGene 6 group, and blank group. The procedures applied on each group were the same. The tests on each control group were replicated three times.
The EGFP expression in 144 dishes of transfected cells was observed under the inverted fluorescent microscope in a dark room at different time points after transfection. The fluorescence was stimulated by ultraviolet light at 488-nm wavelength. To calculate the transfection efficiency, the EGFP-positive cells were counted in consecutive fields of vision and worked out based on the formula: transfection efficiency = EGFP-positive cell number/counted cell number × 100%.
The concentration of hIGF-1 in cell supernatant from 64 dishes of cultures was determined at different time points (0h, 4h, 8h, 12h, 24h, 36h, 48h, 56h, 7d, 10d) after transduction using a commercially available enzyme-linked immunosorbent assay (ELISA) kit and protocol (Diagnostics Laboratory Systems, Webster, TX). Each time point was replicated five times.
The purified hIGF-1 standard or sample containing hIGF-1 was preabsorbed onto 96-well plates by overnight incubation at 4°C. Ten plates were then washed thoroughly with phosphate-buffered saline (PBS), blocked by incubating with 3% (weight/volume) bovine serum albumin (BSA) for 4 hours at 4°C, and incubated overnight with 100 μL of primary antibody diluted 1:1000 in 1% BSA. Plates were then washed with PBS containing 0.05% Tween 20 and incubated with secondary antibody conjugated to alkaline phosphatase. After 2 hours incubation at room temperature, plates were washed, incubated with substrate, and the optical density at 405 nm was read on a plate reader.
We detected the biologic activity of secreted hIGF-1 in the supernatant of six dishes with MTT chromatometry. The supernatant was collected 48 hours and 72 hours after transfection, centrifuged, and diluted with RPMI-1640 culture medium at a different proportion. We diluted the purified hIGF-1 standards to a final concentration of 200 ng/mL to 6.25 ng/mL. The NIH3T3 cells were cultured onto six 96-well plates, 78 wells on each plate, at a density of 5 × 104 cells/mL and incubated for 12 hours, and then the diluted supernatant samples or hIGF-1 standards or culture medium were added onto the 96-well plates by overnight incubation. The plates were then washed thoroughly with PBS, adding 0.1 mL PBS and 10 μL MTT staining solution, and incubated for 5 hours. We then mixed the cultures with 0.1 mL 10% SDS, and read the optical density at 620 nm on a plate reader.
We performed immunohistochemical staining on the six samples of meniscal chondrocytes grown onto the coverslips using a standard indirect three-step immunoperoxidase technique. The cells were fixed with cold methanol for 1 minute. After rinsing with PBS, endogenous peroxidase was blocked by incubation with 3% hydrogen peroxide for 10 minutes. Serum-free blocking agent was used for blocking of nonspecific protein binding. The primary antibody (rabbit anti-hIGF-1 antibody; Boster Company, WH, China) was applied for overnight at 4°C, and then a biotinylated secondary antibody (Boster Company) was applied for 20 minutes. After washing with PBS four times, we performed DAB coloration at room temperature. Nonimmune rabbit serum was used as a negative control on each slide. Articular cartilage tissues for each antibody were also included in each procedure as a positive control.
We assessed supernatants from the four transfected adherent cell cultures at 48 hours and 72 hours (replicated twice at each point) for hIGF-1 protein. After centrifuging to remove all cells, the samples were loaded onto a 12% polyacrylamide gel, and the proteins were separated by polyacrylamide gel electrophoresis. We then transferred the separated proteins to a nitrocellulose membrane. The nitrocellulose was incubated with a blocking buffer (10% nonfat dry milk, 1% 1 M Tris [pH 7.4], and 3% 5 M NaCl) at room temperature for 30 minutes. The membrane was then incubated in the blocking buffer and 1 μg/mL of anti-hIGF-1 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. After three washes with a solution consisting of 1% 1 M Tris (pH 7.4), 10% 5 M NaCl, and 0.1% Tween 20, the filter was incubated with the secondary antibody (goat-antirabbit horseradish peroxidase-conjugated antibody; Santa Cruz Biotechnology) in the blocking solution. The filters were then washed three times in the wash solution. ECL Western blotting detection reagents (Amersham Biosciences, Buckinghamshire, UK) were applied to the nitrocellulose and exposed to radiographic film.
Total cellular total RNA was purified from cells transfected with hIGF-1 containing vector. We replicated the procedure twice. The cells were extracted with 4 mol/L guanidine thiocyanate and phenol (water-saturated). To eliminate any possible carryover genomic DNA contamination, the RNA was digested with RNase-free DNase I (RQI-DNase I; Promega Inc, Madison, WI) and extracted twice with phenol/chloroform/isoamyl alcohol (24:24:1 mixture) and precipitated in 2.5 volumes of 100% ice cold ethanol. We then performed reverse transcription (RT) using the SuperScriptTM preamplification system for first-strand cDNA synthesis (Invitrogen Inc, Carlsbad, CA) according to the manufacturer’s instructions. PCR amplification of the targets was performed in a 50 μL reaction mixture containing 2 μL of RT materials, 5 U/100 μL Taq DNA polymerase (Invitrogen Inc), and 1.5 mM MgCl2. The RT-PCR was performed using hIGF-1-specific primers (5′ GCCTCGAGGAAGATGCACACCATGTCCTC 3′; 5′ GCGAATTCCTACATCCTGTAGTTCTTGTTTC 3′) and internal ribosome entry site primers (5′ CGACGGATCTCCACGTGGCG 3′; 5′ GGGCCCAAGCTCCTATCCAA 3′). The primers of the internal ribosome entry site were designed according to the public sequence of the plasmid pIRES2-EGFP. The RT-PCR products were electrophoresed through a 1.2% agarose gel.
Meniscal fibrochondrocytes from eight dishes were harvested before analysis and counted, spun, and then washed with PBS. Cold 70% ethanol was added to the cell pellet. A minimum of 10,000 live cell events was analyzed on a flow cytometer (BD FACS Calibur, NJ).
Results
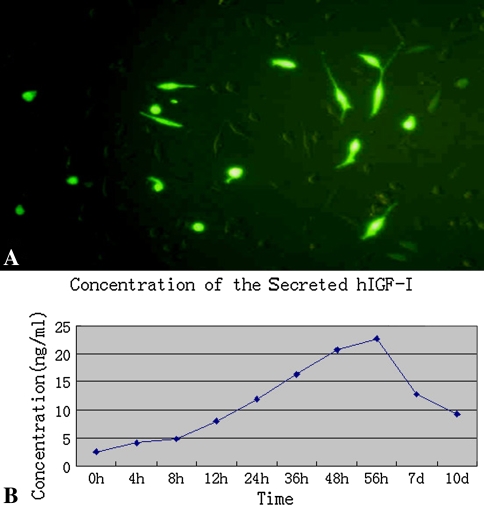
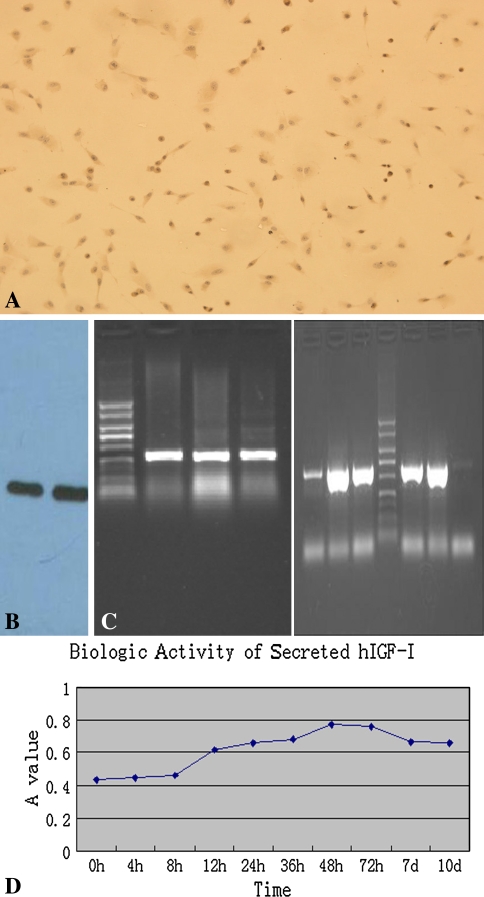
Six hours after transfection, green fluorescence was expressed in part of the cells with high intensity and increased gradually to peak at 56 hours, and then the intensity decreased; nevertheless, the newly expressed fluorescence was still bright (Fig. 2A). The positive cells were distributed unevenly but were relatively concentrated in many areas. The calculated transfect efficiency was approximately 16 ± 1.2%. The concentration of hIGF-1 in supernatants was detected at different time points to determine the secretion variation. The hIGF-1 in the supernatants of untransfected cells was 2.55 ± 0.8 ng/mL and increased up to 4.96 ± 0.7 ng/mL 6 hours after transfection. Increasing gradually with time, the concentration then reached the peak of 22.68 ± 1.4 ng/mL at 56 hours; then the secretion decreased to 9.22 ± 1.1 ng/mL 10 days after transfection (Fig. 2B). This changing tendency was similar with the fluorescent alteration under microscopic view. The empty vector, FuGene 6, and DMEM medium were also used to transfect the cells, which served as control groups, to detect any possible influence on cells. The hIGF-1 concentrations of these groups were similar with that of untransfected cells. No cytotoxicity was observed in control groups. In hIGF-1 immunohistochemistry staining, we observed multiple brown granules in cytoplasm; no coloration appeared in untransfected cells (Fig. 3A). Results of the Western blot demonstrated the bands appeared at approximately 7.6 kD after coloration(Fig. 3B). The electrophoresis revealed the amplified fragments at the corresponding sites was approximately 409 bp (for hIGF-1) and 614 bp (for IRES), which further demonstrated the existence of the genes of hIGF-1 and IRES in transfected cells (Fig. 3C). We determined the effectiveness of hIGF-1 to promote the proliferation and division of the NIH 3T3 cell line by assessing the biologic activity of the secreted cytokine. MTT chromatometry demonstrated the characteristics of the secreted hIGF-1 for enhancing proliferation (Fig. 3D).
Fig. 2A–B.
(A) After transfection, expression of the marker gene of EGFP was detected directly by monitoring the green fluorescence (enhanced green fluorescent protein or EGFP; original magnification, ×40). (B) Shown is the changing concentration of the hIGF-1 secreted in the supernatants over time; in normal meniscal fibrochondrocytes, it was 2.55 ± 0.8 ng/mL and increased gradually until the peak concentration at 22.68 ± 1.4 ng/mL.
Fig. 3A–D.
(A) The exogenous gene and protein within and outside of the transfected cells were determined by several methods. Immunohistochemistry stain for hIGF-1 in the transfected cells revealed multiple brown granules in the cytoplasm (no stain; original magnification, ×10). (B) Target cytokine in the supernatants of the transfected cells were detected by Western blot. Bands appeared at approximately 7.6 kD after coloration. (C) The electrophoresis showed the expected fragments at the corresponding sites representing the extraneous gene for hIGF-1 and internal ribosomal entry site in the transfected cells. (D) The biologic activity of the secreted cytokine was confirmed based on the characteristics of promoting the proliferation in the NIH 3T3 cell line. The changes of MTT chromatometry were similar to those of the concentration of the cytokine in the supernatants.
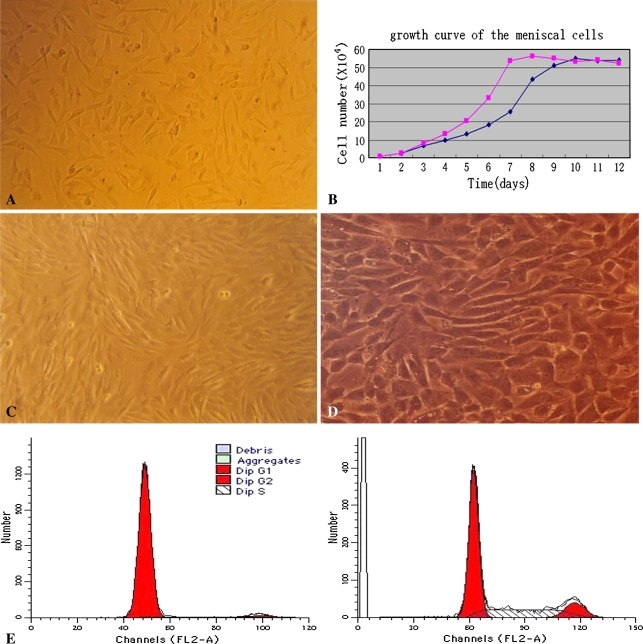
After transfection, proliferation of the meniscal fibrochondrocytes was accelerated with some morphologic changing, and the cell cycle was also altered. The meniscal fibrochondrocytes adhered to the plates after 40 hours and formed separate foci. After approximately 10 days, they grew into 60% to 70% confluence. Cells of the second generation proliferated faster and the cell phenotype was similar to the first generation. Morphologic analysis of the plated cells consistently distinguished different cell types such as triangular, polygonal, or a fibroblast-like shape (Fig. 4A). With hematoxylin and eosin stain, obvious mitosis could be seen in some of the cells. Trypan blue dye exclusion test demonstrated approximately 94% cells alive in primary and secondary generations. Population doubling time of the meniscal chondrocytes was calculated to be 52.6 hours. After transfection, the population doubling time of meniscal fibrochondrocytes was decreased from 52.6 hours to 40.2 hours (Fig. 4B). Mitosis was active in many cells with morphologic changes (Fig. 4C). The cells proliferated faster and enlarged after transfection with an increased percent of polygonal morphology (Fig. 4D). Before transfection, the majority of the fibrochondrocytes was in the G1 stage, but a percent of the S stage increased considerably after transfection with hIGF-1 containing vector (Fig. 4E).
Fig. 4A–E.
The proliferation and morphology of the cells changed after transfection. (A) Before gene intervention, the cells had varying cell morphologies (no stain; original magnification, ×40). (B) After transfection the growth curve of the cells migrated left, (C) the proliferation was accelerated with multiple cell aggregations (no stain; original magnification, ×40), and (D) the cells enlarged and appeared as slabstones after introduction of the hIGF-1 gene (no stain; original magnification, ×40). (E) Flow cytometry analysis demonstrated upregulation of cell number in G2 and S stages after hIGF-1 gene introduction.
Discussion
Several studies confirm human insulin-like growth factor-1 (hIGF-1) plays a key role in the regulation of chondrocyte proteoglycan metabolism and stimulates cell proliferation [5, 12, 16, 17]. Theoretically, then, human meniscal fibrochondrocytes cultured in vitro may present a positive response to liposome-mediated hIGF-1 gene transfection. We therefore tested the hypothesis that the hIGF-1 gene could be expressed within human meniscal fibrochondrocytes and the cytokine secreted, and that the proliferation and phenotype of the cells be changed after transfection.
There are several limitations in the current study. First, the transfection efficiency of the cationic liposome is relatively low compared to virus vector. Viral vectors have been the popular transgenic tool because of their higher efficiency [23, 29]. Madry et al. used recombinant adeno-associated viruses containing the reporter gene lacZ to transfer into the lapine and human meniscal cells in vitro and into lapine meniscal defects in vivo [18]. The maximal efficiency of gene transfer was about 81.6% for lapine and 87.2% for human meniscal cells in vitro. Expression of the transferred gene continued for 28 days. When the virus vector was injected into meniscal tears in a lapine meniscal tear model, transgene expression continued in meniscal cells adjacent to the tear for at least 20 days in vivo. However, the limitations of the virus vectors include induction of the host immune response, whereas retroviral vectors require dividing cells for integration. In addition, there is a small chance that viral vectors may integrate randomly into the host genome, posing a risk of neoplastic transformation or infection-associated toxicity [6, 8, 22]. To avoid such adverse effects, a shorter or lower-level transgene expression might be sufficient and thus more appropriate for these potent differentiation factors. Nonviral vectors could fulfill these requirements and might be more generally accepted in the treatment of nonlethal diseases, for example, cartilage and meniscal defects. Cationic liposomes and other lipid-based systems have the advantages of being easy and safe to prepare and do not restrict the size of the DNA that can be delivered [11]. When liposomes have been used for in vivo transfection of chondrocytes in normal rat knees, gene expression occurred in the superficial and middle zones and persisted for at least 3 weeks [19, 27]. Therefore, in gene therapy related to human tissues or cells, nonviral, lipid-mediated gene transfer may be a safer method. Second, the observation cycle of this study was relatively short (within 10 days) although the cytokine was still secreted. One of the characteristics of liposome-mediated gene therapy is the nonpermanent expression of the target gene since it does not integrate into the host genome. The concentration of the secreted hIGF-1 in the supernatants 10 days after transfection was still more than that of the untransfected cells. For clinical applications, this time period and concentration maybe suitable for the cytokine to play its role in joints. Third, the fluorescence quenching limited the EGFP observation. Some of the expressed EGFP diminished with time, although the target gene was still expressed. However, for the efficiency calculation and location of the transgenic cells, this quenching time is long enough.
Some researchers have introduced the gene of growth factors into meniscal tissue or cells to enhance the repair [2, 9]. In an in vitro repair of meniscal defects, growth factors could diffuse from a collagen sponge or a collagen gel into meniscal tissue surrounding defects and stimulate the formation of proteoglycan. This approach resulted in alignment and migration of meniscal cells toward the defect, which was not observed in untreated controls [2]. In other research related to gene-modified bovine meniscal cells, adenovirus-mediated hepatocyte growth factor gene therapy enhanced blood vessel formation in the subcutaneous pouch of athymic nude mice for 8 weeks [9]. The current research selected the hIGF-1 as the target cytokine because it plays a key role in the regulation of chondrocyte proteoglycan metabolism [21, 25]: it is an important anabolic modulator of cartilage metabolism whose action is mediated by high-affinity cell surface receptors. It also plays an important role in stimulating collagen and proteoglycan synthesis in cartilage through an autocrine feedback mechanism [25]. Since hIGF-1 is a major chondro-enhancing agent, notwithstanding its attenuated effect on aged cartilage, it would appear a logical choice for gene therapy approaches [4, 5, 7, 12, 16]. It is a relatively small gene of approximately 45 kb pairs with the transcribed region spanning less than 1 kb, and the DNA sequence is known in most species. These advantages enable this potent mitogenic cytokine to be a suitable target gene for cartilage gene therapy. Ye et al. [30] mixed hIGF-1 protein into the fetal meniscal fibrochondrocytes cultures in vitro to detect the possible influence on the cells. They reported the proliferating effects of the hIGF-1 (at a final concentration of 50 μg/L) on the third-passage cells at 48 h and 72 h were better than the control group. hIGF-1 combined with TGF-beta1 or bFGF can result in enhanced proliferating effects compared to the single growth factor [30]. We also found adult meniscal fibrochondrocytes are relatively sensitive to gene transfection in cell proliferation and morphologic changing, with an increased percent of cells in S and M stages, which indicating the vigorous function of synthesizing DNA and protein. The medium from transfected meniscal fibrochondrocyte cultures we tested contained a hIGF-1 level as high as 22.68 ng/mL, and several previous studies suggest hIGF-1 at 50 ng/mL optimally stimulates matrix elaboration and the development of a cartilage-specific matrix structure, whereas as low as 10 ng/mL is sufficient to produce considerable stimulation of the proliferative and metabolic actions of cultured chondrocytes [17, 21, 26].
We found the expression of the marker gene EGFP increased gradually and reached a peak at 56 hours, with the transfection efficiency at about 16 ± 1.2%. Another transgenic study compared the efficiencies of different commercially available cationic liposomes including Cellfectin, DMRIE-C, LipofectAmine, Lipofectin, LipoTaxi, TransFast, and the lipid-based reagent FuGENE 6 [19]. Transfection conditions were determined for primary cultures of normal human articular, osteoarthritic human articular, and normal bovine articular chondrocytes using a lacZ reporter gene construct. Results demonstrated the presence of hyaluronidase could enhance the transfection efficiency. FuGene6 transfection produced the maximum levels of transgene expression. Analysis of X-gal staining demonstrated an efficiency of 41% in normal bovine articular chondrocytes, 20.7% in normal human articular chondrocytes, and 7.8% in osteoarthritic human chondrocytes. Compared to this research using the same transgenic agent, the transgenic efficiency of human meniscal fibrochondrocytes was relatively lower than that of the cells from hyaline cartilage.
Our preliminary data suggest that hIGF-1 gene can be transfected into adult meniscal fibrochondrocytes by cationic liposome, and the expression of the gene may be useful to enhance the proliferation of the cells.
Acknowledgments
We thank Dr. Xiaokui Hou for assistance with the manuscript. We also thank Dr. Guanzhen Yang and Dr. Xiangfu Wu for technical assistance.
Footnotes
Three of the authors (Hai-ning Zhang, Ping Leng and Ying-zhen Wang) received funding from the National Natural Science Foundation of China (No. 30801167).
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at The Affiliated Hospital of Tsingtao University, China.
References
- 1.Aagaard H, Verdonk R. Function of the normal meniscus and consequences of meniscal resection. Scan J Med Sci Sports. 1999;9:134–140. [DOI] [PubMed]
- 2.Bhargava MM, Hidaka C, Hannafin JA, Doty S, Warren RF. Effects of hepatocyte growth factor and platelet-derived growth factor on the repair of meniscal defects in vitro. In Vitro Cell Dev Biol Anim. 2005;41:305–310. [DOI] [PubMed]
- 3.Brindle T, Nyland J, Johnson DL. The meniscus: review of basic principles with application to surgery and rehabilitation. J Athl Train. 2001;36:160–169. [PMC free article] [PubMed]
- 4.Capito RM, Spector M. Collagen scaffolds for nonviral IGF-1 gene delivery in articular cartilage tissue engineering. Gene Ther. 2007;14:721–732. [DOI] [PubMed]
- 5.Dupont J, Holzenberger M. Biology of insulin-like growth factors in development. Birth Defects Res C Embryo Today. 2003;69:257–271. [DOI] [PubMed]
- 6.Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC. Osteoarthritis gene therapy. Gene Ther. 2004;11:379–389. [DOI] [PubMed]
- 7.Garcia AM, Szasz N, Trippel SB, Morales TI, Grodzinsky AJ, Frank EH. Transport and binding of insulin-like growth factor I through articular cartilage. Arch Biochem Biophys. 2003;415:69–79. [DOI] [PubMed]
- 8.Gelse K, von der Mark K, Schneider H. Cartilage regeneration by gene therapy. Curr Gene Ther. 2003;3:305–317. [DOI] [PubMed]
- 9.Hadaka C, Ibarra C, Hannafin JA, Torzilli PA, Quitoriano M, Jen SS. Warren RF, Crystal RG. Formation of vascularized meniscal tissue by combining gene therapy with tissue engineering. Tissue Eng. 2002;8:93–105. [DOI] [PubMed]
- 10.Heckmann TP, Barber-Westin SD, Noyes FR. Meniscal repair and transplantation: indications, techniques, rehabilitation, and clinical outcome. J Orthop Sports Phys Ther. 2006;36:795–814. [DOI] [PubMed]
- 11.Hellgren I, Drvota V, Pieper R, Enoksson S, Blomberg P, Islam KB, Sylvén C. Highly efficient cell-mediated gene transfer using non-viral vectors and FuGene6: in vitro and in vivo studies. Cell Mol Life Sci. 2000;57:1326–1333. [DOI] [PMC free article] [PubMed]
- 12.Itoh K, Suzuki S, Kuroda T. Effects of local administration of insulin-like growth factor-I on mandibular condylar growth in rats. J Med Dent Sci. 2003;50:79–85. [PubMed]
- 13.Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16:254–261. [DOI] [PubMed]
- 14.Kessner K, Fahlgren A, Ross I, Andersson B. Simultaneous changes in bone mineral density and articular cartilage in a rabbit meniscectomy model of knee osteoarthrosis. Osteoarthritis Cartilage. 2000;8:197–206. [DOI] [PubMed]
- 15.Kollias SL, Fox JM. Meniscal repair. Where do we go from here? Clin Sports Med. 1996;15:621–630. [PubMed]
- 16.Lee PD, Giudice LC, Conover CA, Powell DR. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc Soc Exp Biol Med. 1997;216:319–357. [DOI] [PubMed]
- 17.Loeser RF, Shanker G. Autocrine stimulation by insulin-like growth factor 1 and insulin-like growth factor 2 mediates chondrocyte survival in vitro. Arthritis Rheum. 2000;43:1552–1559. [DOI] [PubMed]
- 18.Madry H, Cucchiarini M, Kaul G, Kohn D, Terwilliger EF, Trippel SB. Menisci are efficiently transduced by recombinant adeno-asociated virus vectors in vitro and in vivo. Am J Sports Med. 2004;32:1860–1865. [DOI] [PubMed]
- 19.Madry H, Trippel SB. Efficient lipid-mediated gene transfer to articular chondrocytes. Gene Ther. 2000,7:286–291. [DOI] [PubMed]
- 20.McDermott ID, Amis AA. The consequences of meniscectomy. J Bone Joint Surg Br. 2006;88:1549–1556. [DOI] [PubMed]
- 21.Nixon AJ, Saxer RA, Brower-Toland BD. Exogenous insulin-like growth factor-I stimulates an autoinductive IGF-1 autocrine/paracrine response in chondrocytes. J Orthop Res. 2001;19:26–32. [DOI] [PubMed]
- 22.Ohan J, Gilbert MA, Leseche G, Panis Y, Midoux P, Drouet L. Nonviral gene transfer into primary cultures of human and porcine mesothelial cells. In VitroCell Dev Biol Anim. 2001;37:402–407. [DOI] [PubMed]
- 23.Phillips JE, Gersbach CA, Garcia AJ. Virus-based gene therapy strategies for bone regeneration. Biomaterials. 2007;28:211–229. [DOI] [PubMed]
- 24.Roeddecker K, Nagelschmidt M, Koebke J, Guensche K. Meniscal healing: a histological study in rabbits. Knee Surg Sports Traumatol Arthrosc. 1993;1:28–33. [DOI] [PubMed]
- 25.Rogachefsky RA, Dean DD, Howell DS, Altman RD. Treatment of canine osteoarthritis with insulin-like growth factor-1 (IGF-1) and sodium pentosan polysulfate. Osteoarthritis Cartilage. 1993;1:105–114. [DOI] [PubMed]
- 26.Takahashi T, Ogasawara T, Kishimoto J, Liu G, Asato H, Nakatsuka T, Uchinuma E, Nakamura K, Kawaguchi H, Chung UI, Takato T, Hoshi K. Synergistic effects of FGF-2 with insulin or IGF-1 on the proliferation of human auricular chondrocytes. Cell Transplant. 2005;14:683–693. [DOI] [PubMed]
- 27.Tomita T, Hashimoto H, Tomita N, Morishita R, Lee SB, Hayashida K, Nakamura N, Yonenobu K, Kaneda Y, Ochi T. In vivo direct gene transfer into articular cartilage by intraarticular injection mediated by HVJ (Sendai virus) and liposomes. Arthritis Rheum. 1997;40:901–906. [DOI] [PubMed]
- 28.Tumia NS, Johnstone AJ, Regional regenerative potential of meniscal cartilage exposed to recombinant insulin-like growth factor-1 in vitro. J Bone Joint Surg Br. 2004;86:1077–1081. [DOI] [PubMed]
- 29.Witlox MA, Lamfers ML, Wuisman PI, Curiel DT, Siegal GP. Evolving gene therapy approaches for osteosarcoma using viral vectors: review. Bone. 2007;40:797–812. [DOI] [PMC free article] [PubMed]
- 30.Ye C, Deng Z, Li B. Effect of three growth factors on proliferation and cell phenotype of human fetal meniscal cells [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21:1137–1141. [PubMed]