Abstract
The ideal treatment of osteochondral lesions is debatable. Although autologous chondrocyte implantation provides pain relief, the need for two operations and high costs has prompted a search for alternatives. Bone marrow-derived cells may represent the future in osteochondral repair. Using a device to concentrate bone marrow-derived cells and collagen powder or hyaluronic acid membrane as scaffolds for cell support and platelet gel, a one-step arthroscopic technique was developed for cartilage repair. We performed an in vitro preclinical study to verify the capability of bone marrow-derived cells to differentiate into chondrogenic and osteogenic lineages and to be supported onto scaffolds. In a prospective clinical study, we investigated the ability of this technique to repair talar osteochondral lesions in 48 patients. Minimum followup was 24 months (mean, 29 months; range, 24–35 months). Clinical results were evaluated using the American Orthopaedic Foot and Ankle Society (AOFAS) score and the influence of scaffold type, lesion area, previous surgeries, and lesion depth was considered. MRI and histologic evaluation were performed. The AOFAS score improved from 64.4 ± 14.5 to 91.4 ± 7.7. Histologic evaluation showed regenerated tissue in various degrees of remodeling although none showed entirely hyaline cartilage. These data suggest the one-step technique is an alternative for cartilage repair, permitting improved functional scores and overcoming the drawbacks of previous techniques.
Level of Evidence: Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteochondral lesions of the talus frequently occur after inversion or eversion ankle sprains in young patients participating in sports activities [7, 53, 56]. Chondral tissue has poor healing abilities [1], therefore, the damage may be irreversible [12] and lead to chronic symptoms and early osteoarthritis, with a reported frequency ranging from 17% to 50% [3, 13, 14, 33, 49].
Various surgical options have been proposed to restore an adequate cartilaginous layer on the talar dome [2, 37, 45, 58], but among them, only osteochondral grafting and autologous chondrocyte implantation have shown the ability to provide repair of the lesion site with hyaline cartilage [9, 10]. Owing to tissue engineering, biodegradable scaffolding for cell support and proliferation such as Hyalograft® C (Fidia Advanced Biopolymers, Abano Terme, Italy) or matrix-induced autologous chondrocyte implantation has been proposed, permitting entirely arthroscopic autologous chondrocyte implantation with a substantial reduction in surgical morbidity [4, 19, 22].
Nevertheless, the need for two operations and high costs remain drawbacks of autologous chondrocyte implantation [10, 24, 25, 29, 30, 52, 61]. Otherwise, the limited donor area available, the risk of damage to a healthy joint during the plug harvesting phase, discontinuous cartilage repair obtained in the recipient site owing to slight discrepancies in plug orientation, and the need for open surgery of the donor and recipient sites, more frequently with a malleolar osteotomy, are major disadvantages of osteochondral grafting [28, 36].
These considerations have prompted the search for new cartilage repair methods able to overcome these disadvantages. Recently, mesenchymal stem cells (MSCs) were proposed as a new option for treatment of articular cartilage defects [6] because of their ability to differentiate into various lineages, including osteoblasts and chondrocytes [21, 51]. MSC differentiation in the desired direction may be achieved as a result of environment, mechanical stimulation, and growth factors present in the platelet gel able to stimulate cells toward osteogenesis and chondrogenesis [8, 20, 39, 40, 43, 55]. Although the use of MSCs as a pure cell lineage has been advocated [6], after selective elimination of cells that do not express the typical markers of MSCs, the basis of the described technique lies in the key role of the surrounding microenvironment (or niche). The potential of a multipotent cell may be considered not only an intrinsic capability of the cell alone but also the interaction between a cell with its physiologic niche that provides a signaling network (ie, the extracellular matrix, adhesion molecules, growth factors, cytokines, and chemokines secreted by the resident cells) [16, 18, 34, 38, 50, 59]. Autologous bone marrow contains not only stem cells and precursor cells as a source of regeneration tissue, but also accessory cells that support angiogenesis and vasculogenesis by producing several growth factors. This suggests no cell selection and expansion in the laboratory may be required (as with autologous chondrocyte implantation), and consequently the transplant can be performed in one operative procedure.
Using a kit to concentrate bone marrow-derived cells in the operating room and collagen powder or hyaluronic acid membrane as scaffolds for cell support and platelet gel, a one-step arthroscopic technique was developed [20, 40, 43, 44, 54]. As both scaffolds used in the study were projected for purposes different from the support of bone marrow-derived cells, we performed a preclinical laboratory study on five samples to verify (1) the in vitro chondrogenic and osteogenic potential of bone marrow-derived cells and (2) the in vitro capability of the biomaterials used in the study to support bone marrow-derived cells.
We then asked whether the one-step arthroscopic technique using bone marrow-derived cells supported by different scaffolds and platelet gel to treat osteochondral lesions of the talus would improve the patients’ functional scores. Finally, using MRI, second-look arthroscopies, and histologic analysis, we ascertained the influence of type of scaffold used, area of the lesion, presence of previous surgeries at the same lesion site, and lesion depth; and the ability to regenerate osteochondral tissue.
Materials and Methods
From October 2005 to September 2006, we enrolled 48 patients with focal osteochondral lesions of the talar dome to be treated with arthroscopic bone marrow-derived cell transplantation using a one-step technique and one of two scaffolds. The treatment was indicated for focal osteochondral lesions of the talar dome classified as chronic Type II (> 1.5 cm2 in area, < 5 mm deep) [23]. We excluded patients younger than 15 years or older than 50 years, patients with osteoarthritis or kissing lesions of the ankle, and patients with rheumatoid arthritis. Malalignment of the lower limb and the presence of joint laxity were considered relative contraindications to be corrected if present. The ethical committee of our institution approved the human protocol for this investigation. There were 27 men and 21 women with a mean age of 28.5 ± 9.5 years. All the patients were affected by posttraumatic Type II lesions [23]. The mean size of the lesions was 2.07 ± 0.48 cm2, and the mean depth of the lesions was 4.0 ± 0.9 mm. The lesion involved the right ankle in 21 patients and the left ankle in 27; the lesion was located medially in 39 and laterally in nine. In 35 patients, the lesion had a definite posttraumatic origin. Four patients had a previous ankle fracture and 31 had a previous ankle sprain without fracture (mean time from the trauma, 35.2 ± 52.4 months), whereas 13 were not able to recall a specific major traumatic event, but different minor traumas at the affected ankle. Fifteen patients had been treated previously by microfractures (eight), arthroscopic ankle débridement (five), and autologous chondrocyte implantation (two). Both patients who were treated by autologous chondrocyte implantation that failed had been treated previously by microfracture (one) and arthroscopic débridement (one); therefore, bone marrow-derived cell transplantation was their third operation. The minimum followup was 24 months (mean, 29 months; range, 24–35 months). No patients were lost to followup. All investigations were conducted in conformity with ethical principles of research, and written informed consent was signed by all the patients enrolled in this study.
The initial clinical evaluation included the complete history of the patient and a physical examination. We recorded any known cause of ankle trauma and previous failed treatment attempts. Two of us (FV, MC) checked the ankle for instability, malalignment, and range of movement and completed the AOFAS score [35] preoperatively. A standard radiographic examination, including AP and lateral weightbearing views, and MRI of the affected ankle were performed preoperatively.
The scaffolds used in this series for cell support were either a porcine collagen powder (Spongostan® Powder; Johnson & Johnson Medical Ltd, Gargrave, Skipton, UK [54]), which, once mixed with autologous cell concentrate and platelet gel becomes a malleable paste, or a hyaluronic acid membrane (HYAFF®-11; Fidia Advanced Biopolymers [11]) with addition of platelet gel. The secretory granules of platelets, the α granules, contain platelet-derived growth factors AA, BB, and AB; transforming growth factors β1 (TGF-β1) and β2; platelet-derived epidermal growth factor; platelet-derived angiogenesis factor; insulin growth factor 1; and platelet factor 4, which influences bone regeneration [47]. Because of commercial availability, the first 23 patients were treated using the collagen powder, whereas the following 25 patients received the hyaluronic acid membrane.
We performed a laboratory characterization of the implanted biomaterials before the clinical trial to verify the in vitro capability of the biomaterials to support bone marrow-derived cells in terms of cell viability and chondrogenic and osteogenic potential of bone marrow-derived cells.
Cell viability in the biomaterials was determined by the 3-4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich Corp, St Louis, MO)-mitochondrial reduction method using the original protocol of Mosmann as described by Denizot and Lang [17]. Briefly, 1 mL concentrated cells was mixed with the collagen powder or 1 mL was seeded onto a 1- × 1-cm hyaluronic acid membrane scaffold. Samples were incubated at 37°C in a humidified atmosphere of 5% CO2 for 3 hours and then added to alpha-minimal essential medium (α-MEM) (Sigma). Cell viability was monitored after 1, 3, and 7 days from seeding.
To evaluate the chondrogenic and osteogenic potential of bone marrow-derived cells, we isolated mononuclear cells from bone marrow of five male patients (42 ± 12 years old) who underwent surgery for limb nonunion revision and in whom the use of bone marrow to enhance frozen allografts integration was indicated.
A Ficoll-Hypaque density gradient (d = 1.077 g/mL) (Sigma) was used as previously described [57]. Cells were washed twice, resuspended in α-MEM with 15% fetal bovine serum (FBS) (Cambrex Profarmaco, Milan, Italy), counted, and plated at a concentration of 3 × 105 cells/well in a six-well plate. After 24 hours for chondrogenic differentiation, the medium was replaced with complete chondrogenic medium (Dulbecco’s modified Eagle’s medium; Life Technologies, Grand Island, NY) with high glucose supplemented with ITS™ Premix (BD Biosciences, Bedford, MA) (containing 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 5.33 μg/mL linoleic acid, and 1.25 mg/mL bovine serum albumin [BSA]), 10−7 mol/L dexamethasone (Sigma), 37.5 μg/mL ascorbate-2 phosphate (Sigma), and 1 mmol/L sodium pyruvate (Sigma). The medium was with or without the addition of TGF-β1 at 10 ng/mL (R&D Systems, Minneapolis, MN).
For osteogenic differentiation, the initial medium was substituted with an osteogenic medium (α-MEM with 15% FBS containing 75 μg/mL ascorbate-2 phosphate, 0.01 mmol/L β-glycerophosphate (Sigma), or 10−7 mol/L dexamethasone. Cells (for chondrogenesis and osteogenesis) were maintained in culture up to 28 days and the medium was changed twice a week. To evaluate the chondrogenic differentiation, Alcian blue staining was performed. Briefly, cultured cells were washed with phosphate-buffered saline (PBS) and fixed in 10% neutral-buffered formalin for 30 minutes at room temperature. Then, cells were incubated for 3 minutes at room temperature with a 3% acetic acid solution and stained with 1% alcian blue solution (Sigma) for 30 minutes at room temperature. Stained cells were washed extensively in running tap water and rinsed in double deionized water.
To analyze osteogenic differentiation, the calcium deposition was evaluated using alizarin red S staining. Briefly, cultured cells were washed with PBS and fixed in 10% neutral-buffered formalin for 1 hour at room temperature. After washes in double deionized water, cells were dehydrated in an alcohol graded series and stained with 1% alizarin red S solution (Sigma) for 2 minutes. Stained cells were washed extensively with double deionized water to remove the nonspecific precipitation; positive red staining represents calcium deposits on the differentiated cells. Experiments were performed in duplicate wells.
The platelet-rich fibrin gel was produced the day before surgery with the Vivostat System® (Vivolution A/S, 3460 Birkeroed, Denmark). We harvested and processed 120 mL of the patient’s venous blood to provide 6 mL platelet-rich fibrin gel. This product was cryopreserved at –80°C and returned to ambient temperature 30 minutes before the surgical procedure.
The bone marrow was harvested in the same surgical session as the bone marrow-derived cell transplant, from the posterior iliac crest in a sterile regimen, with the patient in a prone decubitus position and under general or spinal anesthesia. After insertion of a marrow needle (size, 11 G × 100 mm) 3 cm deep into the spongy bone, only 5 mL bone marrow was aspirated into a 20-mL plastic syringe internally coated with calcium-heparin solution. Then the needle was rotated 90° and another 5 mL was harvested. Additional harvestings of 5 mL were obtained while rotating and slightly withdrawing the needle in the same location. This procedure was repeated with three to four perforations into the iliac crest, through the same skin opening, until a total of 60 mL bone marrow aspirate was collected. The marrow was aspirated in small fractions from different points to maximize harvesting of the marrow stromal cells and reduce dilution by peripheral blood. The harvested bone marrow was reduced in volume directly in the operating room by removing most of the red cells and plasma. Thus, it was possible to obtain a concentrate of nucleated cells, including stem cells, monocytes, lymphocytes, and other bone marrow resident cells. The bone marrow was concentrated with a cell separator-concentrator (Smart PReP®; Harvest Technologies Corp, Plymouth, MA) by a sterile and disposable dedicated kit (BMAC®; Harvest Technologies Corp) [32]. The 60 mL bone marrow aspirate to be processed, the minimum amount required by the manufacturer, was injected into the posterior portion of the double chamber device. This was placed vertically on the rotor of the centrifuge. A 3-minute centrifugation at 2500 rpm separated the red cells from the other components with the help of a semipermeable membrane; then, during a 2-minute arrest period, the buffy coat separated from the erythrocytes was transferred into the anterior chamber. Finally, a 9-minute phase at 2300 rpm separated the cellular component from the plasma in the anterior chamber; at the end of the entire process, the majority of the red cells remained in the posterior chamber of the container. Once separated from the red cells, the mononuclear cells remained in the anterior chamber in its inferior part covered by the platelet-poor plasma. Finally, they were aspirated from the anterior chamber with a syringe and resuspended in an adequate volume of the platelet-poor plasma to obtain 6 mL cell concentrate.
After the bone marrow harvesting phase, we performed ankle arthroscopy with the patient in a supine position and a tourniquet with 280-mm Hg pressure applied at the thigh of the affected limb. Two standard approaches, anteromedial and anterolateral, were made to the ankle. The lesion site was observed and prepared with removal of the detached cartilage fragment and subchondral malacic bone until the healthy bone was reached. With the help of a calibrated probe, we measured the lesion size. Then, the composites to be implanted were prepared. When collagen was used, 1 g powder was mixed with 2 mL bone marrow concentrate and 1 mL platelet-rich fibrin gel to create a malleable paste rich in bone marrow progenitor cells and growth factors. Hyaluronic acid membrane was instead cut in an appropriate size following the area of the lesion and loaded by capillarity with 2 mL bone marrow concentrate and 1 mL platelet-rich fibrin gel. The composites were placed using the same instrumentation as described for the arthroscopic autologous chondrocyte implantation (CITIEFFE SRL, Calderara di Reno, Italy) [22]. The cannula was inserted through the arthroscopic portal closer to the lesion with the help of the dedicated trochar, the distension liquid flux was stopped, and the liquid was removed from the articular environment. The final composite was run through the cannula onto a sliding positioner up to the margin of the lesion, where it was positioned with the help of a probe. We then removed the cannula and accurately fitted the composite into the lesion site using a flattened probe. A layer of platelet-rich fibrin gel then was spread on the implant to provide an additional contribution of growth factors and optimize its stability with the clotting capability of the platelet-rich fibrin gel. We performed multiple sagittal ankle movements under arthroscopic control to verify the stability of the implant. Twenty-two patients underwent associated surgical procedures: osteophytectomy in 17, synovectomy for synovial hypertrophy in two, loose body extraction in two, and calcaneal osteotomy for flatfoot correction in one. The skin was sutured with a single stitch for each portal by an absorbable 3-0 suture.
Active and passive ankle motions were advised the day after surgery. The range of motion was increased gradually according to pain tolerance for the first 3 weeks. Walking with crutches and no weightbearing on the affected ankle was recommended for the first 6 weeks after the surgery. Partial weightbearing increasing to complete weightbearing was permitted from 6 to 8 weeks after surgery, and 4 months after surgery, low-impact sports activity could be resumed. After 10 to 12 months, we allowed running and progressive training for high-impact activities such as tennis and soccer.
We followed patients at 6, 12, 18, and 24 months after surgery. A standard radiographic examination, including AP and lateral weightbearing views, and MRI of the affected ankle were performed at each followup.
At a minimum followup of 12 months (mean, 13 months; range, 12–14 months), five patients who had been treated with collagen paste underwent second-look arthroscopy: in the first three, who were asymptomatic, to evaluate the regenerated tissue at 12 months and in two subsequent symptomatic patients in whom hypertrophic regenerated tissue was evident on MRI. We inspected and evaluated the implant site using the International Cartilage Repair Society visual scoring system [41], including the degree of defect repair, graft integration to adjacent normal articular surface, and gross appearance. Although approaching the ankle tangentially, biopsy specimens as perpendicular to the surface as possible from the central portion of the repaired lesion were obtained for histologic and immunohistologic analyses in the first three patients. The specimens were evaluated independently by two observers (BG, CC).
Biopsy specimens for histologic analysis were fixed in 10% buffered formalin, washed, and decalcified with Formical-2000™ (Decal Chemical Corp, Congers, NY) overnight at room temperature. The samples then were dehydrated in an alcohol graded series and embedded in paraffin. Sections, 5 μm thick, were obtained from the cartilage specimens, and the slides were stored at room temperature. The slides were stained with 0.02% fast green and 0.1% safranin O (Sigma) to identify the presence of accumulated proteoglycans. All the samples were analyzed using a Zeiss Axioscope microscope (Carl Zeiss, Oberkochen, Germany).
For immunohistochemical analyses, we used two monoclonal antibodies on different slides: mouse monoclonal anti-human Type I collagen antibody and mouse monoclonal anti-human Type II collagen antibody (both from Chemicon International, Temecula, CA). Paraffin sections were deparaffinized and rehydrated. Epitope unmasking was performed by treatment with 0.1% hyaluronidase (Sigma) in PBS at 37°C for 5 minutes. After washes, the slides were incubated with 0.04 mol/L Trizma® base saline (TBS) containing 0.1% BSA (Sigma) for 5 minutes at room temperature to prevent nonspecific bindings. The slides were incubated with 2 μg/mL anti-collagen I and 5 μg/mL anti-collagen II diluted in 0.04 mol/L TBS containing 1% BSA and 0.1% Triton X-100™ (Sigma) for 1 hour at room temperature. The slides were washed three times with 0.04 mol/L TBS and incubated with biotinylated secondary antibodies (BioGenex Laboratories Inc, San Ramon, CA) for 20 minutes at room temperature. After three washes with 0.04 mol/L TBS, the samples were incubated with an enzyme-labeled streptavidin reagent (BioGenex) for 20 minutes at room temperature, and after washes, the reactions were developed using fast red substrate (BioGenex). Negative controls were performed by omitting the primary antibody. Slides were counterstained with hematoxylin and eosin and mounted in glycerol gel. All the samples were analyzed using a Zeiss Axioscope microscope.
Continuous data were described as means and standard deviations. Qualitative data are expressed as frequencies and percentages. The Kolmogorov-Smirnov test was used to test the normality of data distribution. The Levene test was used to test the homoscedasticity. Differences between preoperative and various followup data were evaluated with the paired t test for homoscedastic and normally distributed data; otherwise, the nonparametric Wilcoxon-Mann-Whitney test was used. Differences between groups were evaluated with the unpaired t test for homoscedastic and normally distributed data; otherwise, the nonparametric Wilcoxon-Mann-Whitney test was used. The groups also were checked for differences considering the percentage of maximum possible improvement at each followup, calculated as follows: improvement percentage at a set followup = (AOFAS score at the followup−AOFAS preoperative/100−AOFAS preoperative) × 100. Pearson’s correlation was performed to investigate the relationships between continuous variables if they were both normally distributed; otherwise, the Spearman rank correlation test was used. Statistical analysis was performed using SPSS® software (Version 15.0; SPSS Inc, Chicago, IL).
Results
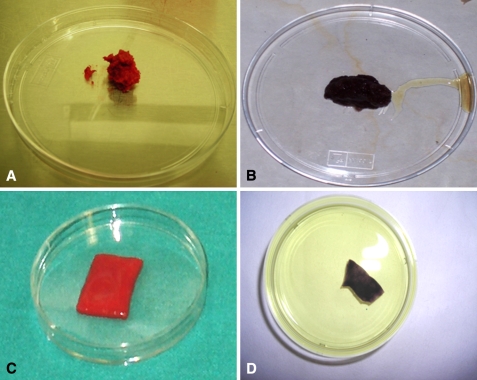
MTT assay performed on the collagen powder and hyaluronic acid membrane seeded with bone marrow-derived cells showed dark staining, giving evidence of a high percentage of viable cells inside the two scaffolds (Fig. 1).
Fig. 1A–D.
The photographs show (A) the collagen powder seeded with concentrated cells, (B) staining of the collagen powder scaffold processed with MTT. The purple is indicative of the cells’ viability; (C) the hyaluronic acid membrane seeded with concentrated cells; and (D) staining of the hyaluronic acid membrane processed with MTT. The purple is indicative of the cells’ viability.
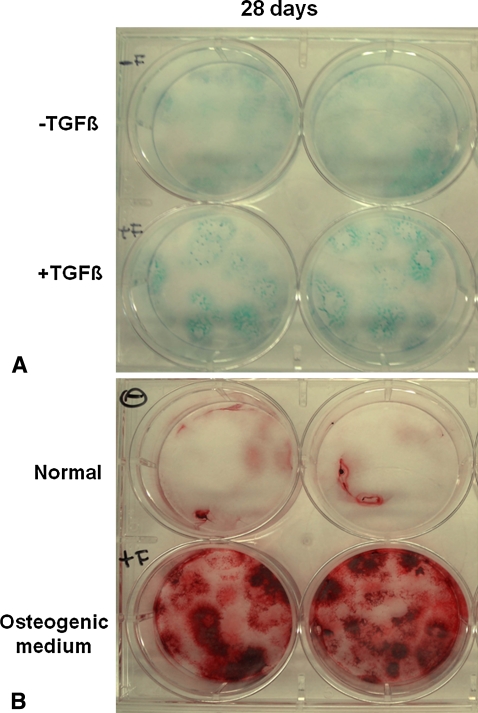
Bone marrow-derived cells were capable of chondrogenic and osteogenic differentiation once grown in suitable media. Particularly, bone marrow-derived cells cultured with TGF-β1 were positive at Day 28 for alcian blue staining, which is specific for glycosaminoglycans (Fig. 2A). Several colonies positive to alizarin red S were found instead in the cells cultured in osteogenic medium at the same experimental time, with calcium precipitates evident inside the colonies (Fig. 2B).
Fig. 2A–B.
The photographs show (A) alcian blue staining of bone mineral-derived cells cultured in the absence (−) or presence (+) of TGF-β1 at 28 days and (B) alizarin red staining of bone mineral-derived cells cultured in normal (−) or osteogenic (+) medium at 28 days. Each evaluation was performed in duplicate.
We had no intraoperative complications. Postoperatively, one patient had a superficial infection of an arthroscopic portal; the infection resolved with oral antibiotic therapy.
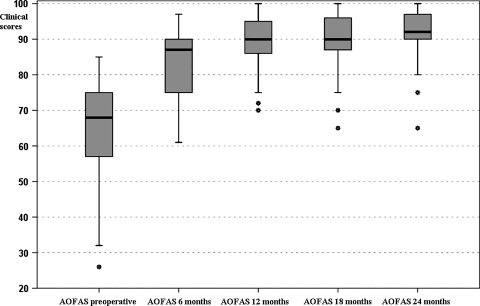
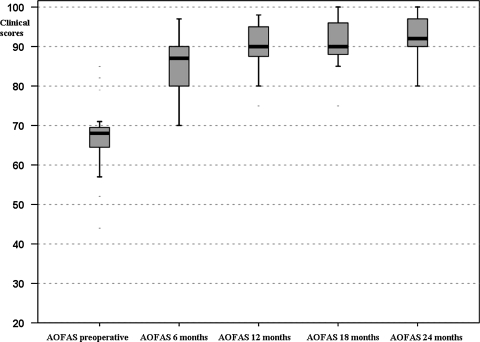
The mean preoperative AOFAS score was 64.4 ± 14.5. The mean AOFAS score improved to 83.3 ± 8.7 at 6 months’ followup, 88.9 ± 8.2 at 12 months, 89.7 ± 8.5 at 18 months, and 91.4 ± 7.7 at 24 months (Table 1; Fig. 3).
Table 1.
Mean AOFAS scores preoperatively and at different followups
| Patient | Age (years) | AOFAS score | ||||
|---|---|---|---|---|---|---|
| Preoperative | 6 months | 12 months | 18 months | 24 months | ||
| 1 | 22 | 75 | 75 | 75 | 75 | 75 |
| 2 | 36 | 38 | 88 | 100 | 100 | 100 |
| 3 | 15 | 71 | 80 | 98 | 100 | 100 |
| 4 | 31 | 68 | 82 | 85 | 88 | 98 |
| 5 | 35 | 68 | 87 | 98 | 98 | 100 |
| 6 | 15 | 82 | 90 | 95 | 95 | 95 |
| 7 | 34 | 85 | 90 | 90 | 90 | 90 |
| 8 | 39 | 64 | 75 | 80 | 82 | 82 |
| 9 | 34 | 36 | 61 | 91 | 89 | 90 |
| 10 | 18 | 85 | 97 | 97 | 97 | 97 |
| 11 | 31 | 68 | 75 | 88 | 88 | 88 |
| 12 | 23 | 74 | 90 | 90 | 90 | 90 |
| 13 | 15 | 75 | 97 | 100 | 100 | 100 |
| 14 | 33 | 70 | 80 | 88 | 90 | 95 |
| 15 | 30 | 41 | 87 | 87 | 87 | 87 |
| 16 | 19 | 78 | 87 | 88 | 90 | 95 |
| 17 | 32 | 51 | 72 | 72 | 65 | 65 |
| 18 | 20 | 64 | 90 | 95 | 95 | 95 |
| 19 | 45 | 69 | 95 | 95 | 95 | 95 |
| 20 | 36 | 65 | 90 | 95 | 97 | 97 |
| 21 | 23 | 57 | 75 | 80 | 80 | 84 |
| 22 | 18 | 69 | 87 | 90 | 92 | 92 |
| 23 | 18 | 69 | 87 | 87 | 88 | 90 |
| 24 | 40 | 67 | 90 | 98 | 100 | 100 |
| 25 | 27 | 68 | 90 | 95 | 100 | 100 |
| 26 | 19 | 74 | 97 | 100 | 100 | 100 |
| 27 | 49 | 64 | 71 | 75 | 75 | 80 |
| 28 | 28 | 57 | 70 | 75 | 75 | 80 |
| 29 | 15 | 68 | 87 | 90 | 90 | 90 |
| 30 | 36 | 68 | 70 | 80 | 85 | 88 |
| 31 | 31 | 35 | 85 | 100 | 100 | 100 |
| 32 | 26 | 44 | 85 | 90 | 90 | 90 |
| 33 | 28 | 78 | 87 | 90 | 90 | 100 |
| 34 | 26 | 79 | 90 | 90 | 90 | 90 |
| 35 | 33 | 55 | 70 | 70 | 70 | 80 |
| 36 | 35 | 54 | 87 | 90 | 92 | 92 |
| 37 | 49 | 64 | 80 | 88 | 90 | 90 |
| 38 | 31 | 79 | 87 | 87 | 87 | 90 |
| 39 | 31 | 26 | 75 | 80 | 80 | 85 |
| 40 | 30 | 52 | 80 | 85 | 87 | 89 |
| 41 | 36 | 32 | 65 | 72 | 75 | 80 |
| 42 | 38 | 61 | 80 | 90 | 92 | 92 |
| 43 | 46 | 78 | 87 | 100 | 100 | 100 |
| 44 | 22 | 66 | 80 | 90 | 90 | 95 |
| 45 | 15 | 82 | 87 | 90 | 95 | 95 |
| 46 | 24 | 80 | 97 | 100 | 100 | 100 |
| 47 | 16 | 64 | 75 | 87 | 90 | 90 |
| 48 | 16 | 75 | 90 | 90 | 90 | 90 |
AOFAS = American Orthopaedic Foot and Ankle Society.
Fig. 3.
Box-and-whisker plots show AOFAS clinical scores preoperatively and at the different followups. Horizontal line = median; box = first to third quartile; error bars = minimum to maximum; dots = outliers.
Of the two patients with preoperative AOFAS scores of 51 and 75, one had a previous ankle fracture treated nonoperatively 7 years before the surgery and the other had previous surgery with microfractures and reported a postoperative infection of the arthroscopic portal.
Forty-five of the 48 patients (94%) participated in a low-impact sports activity at a mean of 4.4 months after the surgery, whereas 37 of the 48 patients (77%) participated in a high-impact sports activity at a mean of 11.3 months.
Patients with the two types of scaffolds showed a similar pattern of improvement at each followup (Table 2; Figs. 4, 5). The percentages of maximum possible improvement also were similar between the groups (Table 3). As the results were not affected by the scaffold used, the successive correlations referred to the total of the patients.
Table 2.
Global clinical results
| Patient group | Time | AOFAS score | p Value* | ||
|---|---|---|---|---|---|
| Mean | SD | Median | |||
| Global | Preoperative | 64.4 | 14.5 | 75.0 | |
| 6 months | 83.3 | 8.7 | 90.0 | 0.0005 | |
| 12 months | 88.9 | 8.2 | 95.0 | 0.0005 | |
| 18 months | 89.7 | 8.5 | 96.5 | 0.0005 | |
| 24 months | 91.4 | 7.9 | 97.5 | 0.0005 | |
| Collagen powder | Preoperative | 62.5 | 18.0 | 78.0 | |
| 6 months | 82.3 | 10.2 | 90.0 | 0.0005 | |
| 12 months | 86.9 | 9.4 | 91.0 | 0.0005 | |
| 18 months | 87.2 | 10.0 | 95.0 | 0.0005 | |
| 24 months | 89.8 | 9.8 | 100.0 | 0.0005 | |
| Hyaluronic acid membrane | Preoperative | 66.2 | 10.5 | 69.5 | |
| 6 months | 84.3 | 7.2 | 90.0 | 0.0005 | |
| 12 months | 90.7 | 6.5 | 96.0 | 0.0005 | |
| 18 months | 92.0 | 6.3 | 97.5 | 0.0005 | |
| 24 months | 92.8 | 5.3 | 97.0 | 0.0005 | |
* Nonparametric Wilcoxon-Mann-Whitney test, followup versus preoperative AOFAS score; AOFAS = American Orthopaedic Foot and Ankle Society; SD = standard deviation.
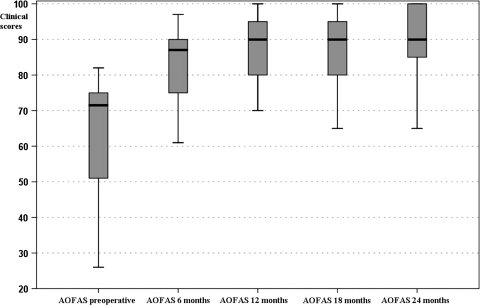
Fig. 4.
Clinical results were analyzed by dividing patients in two groups according to scaffold type used. The box-and-whisker plots show AOFAS clinical scores preoperatively and at the different followups in the group treated with collagen powder scaffolds. Horizontal line = median; box = first to third quartile; error bars = minimum to maximum; dots = outliers.
Fig. 5.
Clinical results were analyzed by dividing patients in two groups according to scaffold type used. Box-and-whisker plots show AOFAS clinical scores preoperatively and at the different followups in the group treated with hyaluronic acid membrane scaffolds. Horizontal line = median; box = first to third quartile; error bars = minimum to maximum; dots = outliers.
Table 3.
Percentages of maximum possible improvement
| Time | Patient group | % of maximum possible improvement | p Value* | ||
|---|---|---|---|---|---|
| Mean | SD | Median | |||
| 6 months | Collagen powder | 52 | 23 | 46 | 0.58 |
| Hyaluronic acid membrane | 53 | 20 | 58 | ||
| 12 months | Collagen powder | 62 | 27 | 63 | 0.18 |
| Hyaluronic acid membrane | 72 | 18 | 69 | ||
| 18 months | Collagen powder | 63 | 27 | 72 | 0.15 |
| Hyaluronic acid membrane | 76 | 17 | 73 | ||
| 24 months | Collagen powder | 72 | 27 | 76 | 0.77 |
| Hyaluronic acid membrane | 78 | 15 | 79 | ||
* Nonparametric Wilcoxon-Mann-Whitney test; SD = standard deviation.
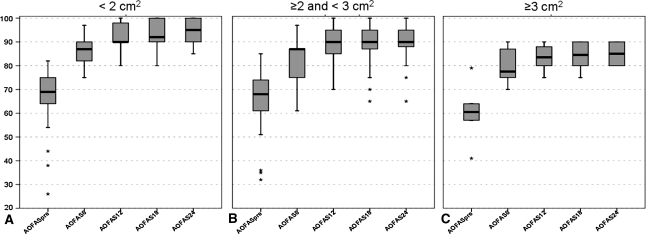
We observed no correlation between the area of the lesion and the preoperative AOFAS score (Table 4), but did observe a relationship between the area of the lesion and the AOFAS score at each followup (Fig. 6). We also observed a relationship between the area of the lesion and the percentage of maximum possible improvement in AOFAS score at each followup. Previous surgeries negatively affected the AOFAS score at 12 and 24 months (Table 5). The percentage of maximum possible improvement in the AOFAS score between the two groups was similar at each followup (Table 5). No relationship was observed between AOFAS scores and depth of the lesion (Table 6).
Table 4.
Relationship between area of lesion, AOFAS score, and percentage of maximum possible improvement in AOFAS score
| Variable | Time | Spearman correlation between lesion area and variable | |
|---|---|---|---|
| p Value | Rho value | ||
| AOFAS score | Preoperative | 0.38 | −0.150 |
| 6 months | 0.008 | −0.376 | |
| 12 months | 0.0006 | −0.477 | |
| 18 months | 0.0004 | −0.493 | |
| 24 months | 0.004 | −0.456 | |
| % of maximum possible improvement | 6 months | 0.008 | −0.380 |
| 12 months | 0.0009 | −0.463 | |
| 18 months | 0.001 | −0.461 | |
| 24 months | 0.0007 | −0.533 | |
AOFAS = American Orthopaedic Foot and Ankle Society.
Fig. 6A–C.
The box-and-whisker plots show the relationship between AOFAS clinical score preoperatively and at the different followups and the area of the lesion by dividing the patients into three groups according to the area of the lesion: (A) < 2 cm2, (B) ≤ 2 and < 3 cm2, and (C) ≥ 3 cm2. Considering 2 cm2 and 3 cm2 as cutoff values, the three groups had different improvement curves. Horizontal line = median; box = first to third quartile; error bars = minimum to maximum; dots = outliers.
Table 5.
Relationship between presence of previous surgeries, AOFAS score, and percentage of maximum possible improvement in AOFAS score
| Variable | Time | p Value* |
|---|---|---|
| AOFAS score | Preoperative | 0.001 |
| 6 months | 0.023 | |
| 12 months | 0.032 | |
| 18 months | 0.030 | |
| 24 months | 0.042 | |
| % of maximum possible improvement | 6 months | 0.987 |
| 12 months | 0.851 | |
| 18 months | 0.912 | |
| 24 months | 0.761 |
* Nonparametric Wilcoxon-Mann-Whitney test, previous surgery versus no surgery for each variable; AOFAS = American Orthopaedic Foot and Ankle Society.
Table 6.
Relationship between depth of lesion, AOFAS score, and percentage of maximum possible improvement in AOFAS score
| Variable | Time | Spearman correlation between lesion depth and variable | |
|---|---|---|---|
| p Value | Rho value | ||
| AOFAS score | Preoperative | 0.17 | −0.200 |
| 6 months | 0.073 | −0.261 | |
| 12 months | 0.666 | −0.064 | |
| 18 months | 0.36 | −0.138 | |
| 24 months | 0.31 | −0.172 | |
| % of maximum possible improvement | 6 months | 0.11 | −0.231 |
| 12 months | 0.544 | −0.090 | |
| 18 months | 0.29 | −0.154 | |
| 24 months | 0.42 | −0.138 | |
AOFAS = American Orthopaedic Foot and Ankle Society.
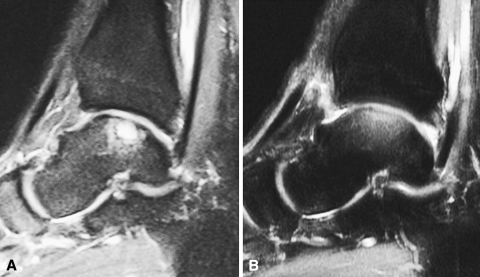
MR images at 24 months’ followup showed newly formed tissue at the lesion site in all patients (Fig. 7). In two patients, MR images at 12 months’ followup showed hypertrophy of the regenerated cartilaginous tissue.
Fig. 7A–B.
(A) A preoperative MR image of a 32-year-old man shows an osteochondral lesion of the talus. (B) At the 2-year followup, the patient’s MR image shows restoration of the cartilage layer and subchondral bone.
The second-look arthroscopies of the first three patients who were treated surgically revealed a continuous, intact regenerated cartilage layer. The other two second-look arthroscopies showed hypertrophic regenerated tissue, confirming the MRI observations. Integration with the healthy cartilage was complete in all patients with a smooth transition zone. The macroscopic aspect of the cartilage was close to normal even in the patients with hypertrophic tissue, once excess cartilage was shaved. The consistency of the regenerated tissue, checked with a probe, appeared similar to the healthy articular cartilaginous tissue. In the three patients operated on first, a biopsy specimen of the transplanted area was obtained, whereas no biopsy was performed in the two patients in whom hypertrophy of the implant was found.
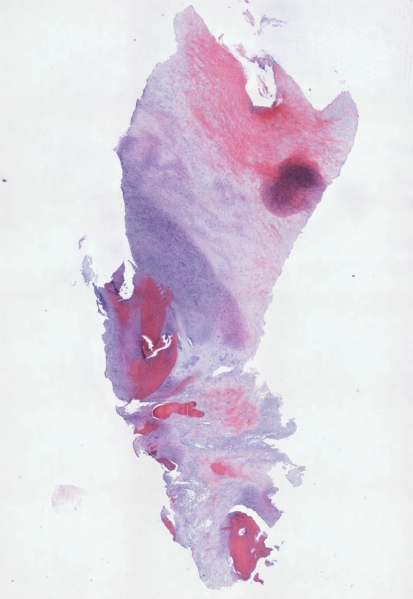
The samples obtained from the biopsies generally showed a structure with various degrees of tissue remodeling. Safranin O staining of the biopsy specimen showed high expression of proteoglycans, whereas the collagen component was less represented (Fig. 8). Collagen Type I immunostaining showed the presence of positive cells, particularly in the middle zone (Fig. 9), whereas collagen Type II showed the presence of some very positive zones throughout the entire thickness of the cartilage (Fig. 10).
Fig. 8.
Histologic observations of a biopsy specimen from a representative patient treated with bone marrow-derived cell transplantation supported on a collagen powder scaffold show repair tissue 12 months after surgery. Safranin O staining shows various degrees of tissue remodeling with many fibrocartilaginous features. Proteoglycans are represented, whereas collagen fibers are limited to a very narrow zone of the superficial layer (Original magnification, ×40).
Fig. 9.
Hematoxylin and eosin staining of a biopsy specimen shows tissue that is highly cellularized and the cells are homogeneously distributed inside the extracellular matrix (Original magnification, ×40).
Fig. 10.
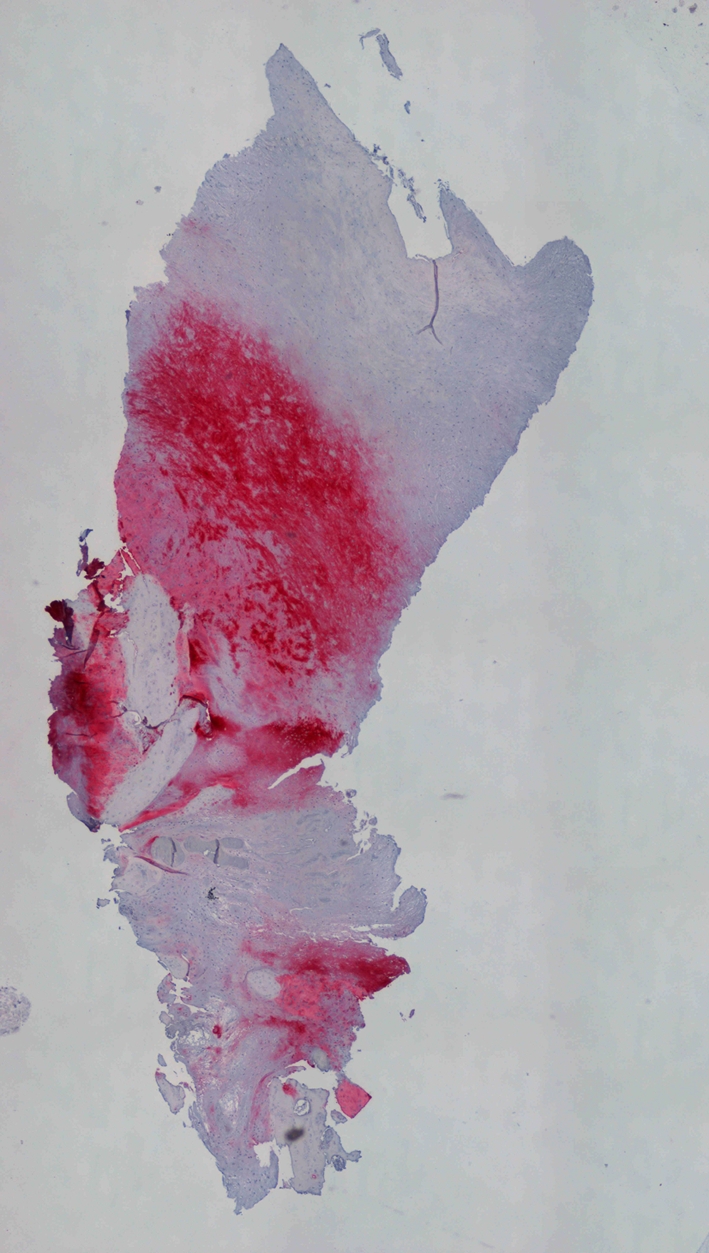
Immunohistochemical evaluation of collagen Type II, using a new fuchsin stain, showed that the tissue is positive to this specific marker particularly in the medial and deep zone (Original magnification, ×40).
Discussion
Autologous chondrocyte implantation is widely considered state of the art in cartilage regeneration, having shown its ability to regenerate reparative tissue with physical characteristics similar to healthy cartilage [51, 52]. Furthermore, the use of a tissue-engineered biomaterial composed of autologous chondrocytes grown on a scaffold entirely made of HYAFF-11® enhanced applicability of the technique, permitting a completely arthroscopic procedure in the knee and ankle [22, 42, 43]. Nevertheless, the need for two surgical operations and the high costs resulting from cell expansion are major drawbacks of the technique, which may never be overcome. Furthermore, chondrocytes are differentiated cells, which are able to regenerate only a cartilaginous structure. The rationale of this study is based on the hypothesis that bone marrow-derived cells may be used for osteochondral lesions of the talus repair, overcoming all the drawbacks raised from autologous chondrocyte implantation. To support this hypothesis, we conducted a preclinical laboratory study to verify the in vitro chondrogenic and osteogenic potential of bone marrow-derived cells and the in vitro capability of the biomaterials used in the study to support bone marrow-derived cells. We then conducted a prospective clinical study to investigate the validity of the one-step arthroscopic technique, based on bone marrow-derived cells, in osteochondral lesions of the talus repair, in terms of AOFAS score; the influence of scaffold type, lesion area, previous surgeries, and lesion depth on AOFAS score; and the ability to regenerate osteochondral tissue, using MRI, second-look arthroscopies, and histologic analysis.
Although the lack of a control group is a limitation of the study, nevertheless, there is general agreement in the literature that, in the type of lesion considered in this study (Type II lesions), surgical treatment is mandatory [25, 33]. Furthermore, the clinical and histologic results of these series are consistent with those of previously published series of patients with similar lesions treated by arthroscopic autologous chondrocyte implantation, considered the gold standard treatment for this type of lesion [22, 23, 25, 26]. The use of two different scaffolds for bone marrow-derived cell support, owing to commercial availability of the materials during the study, is also a weakness of the study. Nevertheless, the results obtained with the two scaffolds showed a similar pattern of improvement (Figs. 4, 5; Table 2), indicating both scaffolds are equivalent to support bone marrow-derived cells for transplantation. The limited number of followup biopsy specimens also may be considered a limitation of the study. Nevertheless, biopsy morbidity in an asymptomatic patient is to be considered and evaluation by alternative methods such as qualitative MRI T2 mapping is in progress [60].
The in vitro experiment reported in this study confirmed bone marrow-derived cells are able to differentiate into chondral and osseous lineages, thus supporting the hypothesis that in vivo bone marrow-derived cells may be able to fill the whole thickness of a defect and secrete some trophic molecules, which contribute to regeneration of damaged tissue [15], the final result being cartilage on the top and bone on the bottom. Transplantation of the entire bone marrow cellular pool by transferring the entire regenerative potential present in the bone marrow to the lesion site permits the cells to be processed directly in the operating room without the need for a laboratory phase and thus allows the technique to be performed in one step. Moreover, the use of a concentrator device produces bone marrow-derived cells with similar or greater functional activity, similar colony-forming capacity, and a superior migratory activity compared with the more traditional product obtained using Ficoll isolation [31].
Bone marrow-derived cell transplantation resulted in AOFAS scores similar to those reported for other widely used techniques (Table 7).
Table 7.
Comparison of techniques for treatment of osteochondral lesions and published results
| Study | Lesion type or size | Evaluation score | Surgical procedure | Number of patients | Followup (months)* | Preoperative score | Followup score |
|---|---|---|---|---|---|---|---|
| Gobbi et al. [27] (2008) | Ferkel Classification Types IIB, III, and IV | AOFAS | Chondroplasty OATS Microfracture | 33 | 53 (24–119)† | Chondroplasty: 36.8* OATS: 31.1* Microfracture: 33.8* | Chondroplasty: 82.7* OATS: 85.4* Microfracture: 83.8* |
| Nam et al. [48] (2009) | 273 mm2* | AOFAS | ACI open ACI + bone graft | 11 | 38 (24–60)† | 47.4 ± 17.4‡ | 84.3 ± 8.1‡ |
| Baums et al. [5] (2006) | 2.3 cm2 (1–6.25)† | AOFAS | ACI open | 12 | 63 (44–48)† | 43,5 (37–57)† | 88.4 (66–100)† |
| Giannini et al. [22] (2008) | 1.6 cm2 (0.5–2.5)† | AOFAS | Arthroscopic ACI | 46 | 36* | 57.2 ± 14.3‡ | 89.5 ± 13.4‡ |
| Current study | 2.07 ± 0.48 cm2‡ | AOFAS | BMDC transplantation | 48 | 29 (24–35)† | 64.4 ± 14.5‡ | 91.4 ± 7.7‡ |
Values are expressed as * means; †means, with ranges in parentheses; or ‡means ± standard deviations; AOFAS = American Orthopaedic Foot and Ankle Society; OATS = osteoarticular transfer system; ACI = autologous chondrocyte implantation; BMDC = bone marrow-derived cell.
The main parameter that influenced the functional score was the area of the lesion. The presence of previous surgery negatively influenced the score preoperatively and postoperatively, whereas no differences were seen in percentage of clinical improvement compared with patients with no previous surgeries.
Histologic and immunohistologic results obtained confirmed the presence of new cartilaginous tissues with various degrees of tissue remodeling toward hyaline cartilage, consistent with the histologic results of autologous chondrocyte implantation where the newly formed tissue also shows various degrees of tissue remodeling [22]. Furthermore, other studies regarding histologic evaluation of the reparative tissue obtained after autologous chondrocyte implantation report less glycosaminoglycan concentration and discrepancies with healthy cartilage in histologic and immunohistochemical appearances, resulting in tissues that may need a longer time for maturation [46]. Possibly, histologic evaluation at a longer followup would be needed to evaluate whether this tissue would evolve toward more hyaline characteristics.
Although longer followup is needed to confirm the validity of the repair overtime, the arthroscopic one-step technique represents an advance in osteochondral regeneration, achieving high clinical scores with the formation of repair tissue and without the major disadvantages of previous techniques.
Acknowledgments
We thank Carola Cavallo, PhD, for bioptic evaluation and preclinical analysis; Annarita Cenacchi, MD, and Annalisa Gabriele, MD, Immunohematology and Transfusional Service, Istituto Ortopedico Rizzoli, for bone marrow harvesting and concentration procedures; and Keith Smith for English revision.
Footnotes
One or more of the authors (SG, BG) received funding from the Fondazione Monte dei Paschi di Siena.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Aigner J, Tegeler J, Hutzler P, Campoccia D, Pavesio A, Hammer C, Kastenbauer E, Naumann A. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J Biomed Mater Res. 1998;42:172–181. [DOI] [PubMed]
- 2.Altman RD, Kates J, Chun LE, Dear DD, Eyre D. Preliminary observations of chondral abrasions in a canine model. Ann Rheum Dis. 1992;51:1056–1062. [DOI] [PMC free article] [PubMed]
- 3.Angermann P, Jensen P. Osteochondritis dissecans of the talus: long-term results of surgical treatment. Foot Ankle. 1989;10:161–163. [DOI] [PubMed]
- 4.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. [DOI] [PubMed]
- 5.Baums MH, Heidrich G, Schultz W, Steckel H, Kahl E, Klinger HM. Autologous chondrocyte implantation of the ankle: a 2- to 5-year follow-up. J Bone Joint Surg Am. 2006;88:303–308. [DOI] [PubMed]
- 6.Beris AE, Lykissas MG, Papageorgiou CD, Georgoulis AD. Advances in articular cartilage repair. Injury. 2005;36(suppl 4):S14–S23. [DOI] [PubMed]
- 7.Bosien WR, Staples OS, Russel SW. Residual disability following acute ankle sprains. J Bone Joint Surg Am. 1955;37:1237–1243. [PubMed]
- 8.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. [DOI] [PubMed]
- 9.Brittberg M. Autologous chondrocyte transplantation. Clin Orthop Relat Res. 1999;367(suppl):S147–S155. [DOI] [PubMed]
- 10.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. [DOI] [PubMed]
- 11.Brun P, Abatangelo G, Radice M, Zacchi V, Guidolin D, Daga Gordini D, Cortivo R. Chondrocyte aggregation and reorganization into three-dimensional scaffolds. J Biomed Mater Res. 1999;46:337–346. [DOI] [PubMed]
- 12.Buckwalter JA, Lohmander S. Operative treatment of osteoarthrosis: current concepts review. J Bone Joint Surg Am. 1994;76:1405–1418. [DOI] [PubMed]
- 13.Buckwalter JA, Mow VC, Ratcliffe A. Restoration of injured or degenerated articular cartilage. J Am Acad Orthop Surg. 1994;2:192–201. [DOI] [PubMed]
- 14.Canale ST, Belding RH. Osteochondral lesions of the talus. J Bone Joint Surg Am. 1980;62:97–102. [PubMed]
- 15.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. [DOI] [PubMed]
- 16.Capone C, Frigerio S, Fumagalli S, Gelati M, Principato MC, Storini C, Montinaro M, Kraftsik R, De Curtis M, Parati E, De Simoni MG. Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS ONE. 2007;2:e373. [DOI] [PMC free article] [PubMed]
- 17.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modification to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;22:271–277. [DOI] [PubMed]
- 18.Dominici M, Pritchard C, Garlits JE, Hofmann TJ, Persons DA, Horwitz EM. Hematopoietic cells and osteoblasts are derived from a common marrow progenitor after bone marrow transplantation. PNAS. 2004;101:11761–11766. [DOI] [PMC free article] [PubMed]
- 19.Ferruzzi A, Buda R, Faldini C, Vannini F, Di Caprio F, Luciani D, Giannini S. Autologous chondrocyte implantation in the knee joint: open compared with arthroscopic technique. Comparison at a minimum follow-up of five years. J Bone Joint Surg Am. 2008;90(suppl 4):90–101. [DOI] [PubMed]
- 20.Franchini M, Dupplicato P, Ferro I, De Gironcoli M, Aldegheri R. Efficacy of platelet gel in reconstructive bone surgery. Orthopedics. 2005;28:161–163. [DOI] [PubMed]
- 21.Galois L, Freyria AM, Herbage D, Mainard D. Cartilage tissue engineering: state-of-the-art and future approaches. Pathol Biol Paris. 2005;53:590–598. [DOI] [PubMed]
- 22.Giannini S, Buda R, Di Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36:873–880. [DOI] [PubMed]
- 23.Giannini S, Buda R, Faldini C, Vannini F, Bevoni R, Grandi G, Grigolo B, Berti L. Surgical treatment of osteochondral lesions of the talus (OLT) in young and active patients: guidelines for treatment and evolution of the technique. J Bone Joint Surg Am. 2005;87(suppl 2):28–41. [DOI] [PubMed]
- 24.Giannini S, Buda R, Grigolo B, Vannini F. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001;22:513–517. [DOI] [PubMed]
- 25.Giannini S, Buda R, Grigolo B, Vannini F, De Franceschi L, Facchini A. The detached osteochondral fragment as a source of cells for autologous chondrocyte implantation (ACI) in the ankle joint. Osteoarthritis Cartilage. 2005;13:601–607. [DOI] [PubMed]
- 26.Giannini S, Vannini F. Operative treatment of osteochondral lesions of the talar dome: current concepts review. Foot Ankle Int. 2004;25:168–175. [DOI] [PubMed]
- 27.Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006;22:1085–1092. Erratum in: Arthroscopy. 2008;24:A16. [DOI] [PubMed]
- 28.Hangody L, Feczkó P, Bartha L, Bodó G, Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res. 2001;391(suppl):S328–S336. [DOI] [PubMed]
- 29.Hangody L, Kish G, Karpati Z, Szerb I, Eberhardt R. Treatment of osteochondritis dissecans of the talus: use of the mosaicplasty technique: a preliminary report. Foot Ankle Int. 1997;18:628–634. [DOI] [PubMed]
- 30.Hangody L, Kish G, Modis L, Szerb I, Gaspar L, Dioszegi Z, Kendik Z. Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven years results in 36 patients. Foot Ankle Int. 2001;22:552–558. [DOI] [PubMed]
- 31.Hermann PC, Huber SL, Herrler T, von Hesler C, Andrassy J, Kevy SV, Jacobson MS, Heeschen C. Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant. 2008;16:1059–1069. [DOI] [PubMed]
- 32.Hernigou P, Baujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. [DOI] [PubMed]
- 33.Hunziker EB. Articular cartilage repair: basic science and clinical progress: a review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. [DOI] [PubMed]
- 34.Kacena MA, Gundberg CM, Horowitz MC. A reciprocal regulatory interaction between megacaryocytes, bone cells and hematopoietic stem cells. Bone. 2006;39:978–984. [DOI] [PubMed]
- 35.Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349–353. [DOI] [PubMed]
- 36.Kreuz PC, Steinwachs M, Erggelet C, Lahm A, Henle P, Niemeyer P. Mosaicplasty with autogenous talar autograft for osteochondral lesions of the talus after failed primary arthroscopic management: a prospective study with a 4-year follow-up. Am J Sports Med. 2006;34:55–63. [DOI] [PubMed]
- 37.Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1999;81:1229–1235. [DOI] [PubMed]
- 38.Lepore AC, Han SS, Tiler-Polsz CJ, Cai J, Rao MS, Fischer I. Transplantation into the adult CNS. Neuron Glia Biol. 2004;1:113–126. [DOI] [PMC free article] [PubMed]
- 39.Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signalling. J Bone Miner Res. 2006;21:626–636. [DOI] [PubMed]
- 40.Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C, Bertoja AZ, Giardino R, Fornasari PM, Mercuri M, Picci P. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095–3100. [DOI] [PubMed]
- 41.Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S, Stauffer E; International Cartilage Repair Society. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85:45–57. [DOI] [PubMed]
- 42.Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, Kon E, Pederzini L, Rosa D, Sacchetti GL, Stefani G, Zanasi S. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;435:96–105. [DOI] [PubMed]
- 43.Marcacci M, Kon E, Zaffagnini S, Filardo G, Delcogliano M, Neri MP, Iacono F, Hollander AP. Arthroscopic second generation autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2007;15:610–619. [DOI] [PubMed]
- 44.Mazzucco L, Medici D, Serra M, Panizza R, Rivara G, Orecchia S, Libener R, Cattana E, Levis A, Betta PG, Borzini P. The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion. 2004;44:1013–1018. [DOI] [PubMed]
- 45.Moran ME, Kim HK, Salter RB. Biological resurfacing of full-thickness defects in patellar articular cartilage of the rabbit: investigation of autogenous periosteal grafts subjected to continuous passive motion. J Bone Joint Surg Br. 1992;74:659–667. [DOI] [PubMed]
- 46.Moriya T, Wada Y, Watanabe A, Sasho T, Nakagawa K, Mainil-Varlet P, Moriya H. Evaluation of reparative cartilage after autologous chondrocyte implantation for osteochondritis dissecans: histology, biochemistry, and MR imaging. J Orthop Sci. 2007;12:265–273. [DOI] [PubMed]
- 47.Nair MB, Varma HK, John A. Platelet-rich plasma and fibrin glue-coated bioactive ceramics enhance growth and differentiation of goat bone marrow-derived stem cells. Tissue Eng. 2009;15:1–13. [DOI] [PubMed]
- 48.Nam EK, Ferkel RD, Applegate GR. Autologous chondrocyte implantation of the ankle: a 2- to 5-year follow-up. Am J Sports Med. 2009;37:274–284. [DOI] [PubMed]
- 49.O’Driscoll S. The healing and regeneration of articular cartilage: current concepts review. J Bone Joint Surg Am. 1998;80:1795–1812. [PubMed]
- 50.Olmsted-Davis EA, Gugala Z, Camargo F, Gannon FH, Jackson K, Kienstra KA, Shine HD, Lindsey RW, Hirschi KK, Goodell MA, Brenner MK, Davis AR. Primitive adult hematopoietic stem cells can function as osteoblast precursors. PNAS. 2003;100:15877–15882. [DOI] [PMC free article] [PubMed]
- 51.Oreffo RO, Cooper C, Mason C, Clements M. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1:169–178. [DOI] [PubMed]
- 52.Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2–12. [DOI] [PubMed]
- 53.Pritsch M, Horoshovsky H, Farine I. Arthroscopic treatment of the osteochondral lesion of the talus. J Bone Joint Surg Am. 1986;68:862–865. [PubMed]
- 54.Ragusa R, Faggian G, Rungatscher A, Cugola D, Marcon A, Mazzucco A. Use of gelatin powder added to rifamycin versus bone wax in sternal wound hemostasis after cardiac surgery. Interact Cardiovasc Thorac Surg. 2007;6:52–55. [DOI] [PubMed]
- 55.Sanchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18:93–103. [PubMed]
- 56.Schenck R, Goodnight JM. Osteochondritis dissecans: current concepts review. J Bone Joint Surg Am. 1996;78:439–456. [PubMed]
- 57.Schutze N, Noth U, Schneidereit J, Hendrich C, Jakob F. Differential expression of CCN-family members in primary human bone marrow-derived mesenchymal stem cells during osteogenic, chondrogenic and adipogenic differentiation. Cell Commun Signal. 2005;3:5. [DOI] [PMC free article] [PubMed]
- 58.Sledge S. Microfracture techniques in the treatment of osteochondral injuries. Clin Sports Med. 2001;20:365–377. [DOI] [PubMed]
- 59.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. [DOI] [PubMed]
- 60.White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241:407–414. [DOI] [PubMed]
- 61.Yamashita F, Sakakida K, Suzu F, Takai S. The transplantation of an autogenic osteochondral fragment for osteochondritis dissecans of the knee. Clin Orthop Relat Res. 1985;201:43–50. [PubMed]