Abstract
Bone injuries have a systemic influence on the remodeling of bone. This effect has not been examined concerning its extent and duration. We measured the systemic effect of distraction osteogenesis on the remodeling of bones of the axial skeleton by means of the mineral apposition rate and bone formation rate in an animal experiment. Distraction osteogenesis was performed on the tibiae of 24 mature Yucatan minipigs. After a 4-day latency period, the tibiae were distracted 2 mm/day for 10 days. The ensuing consolidation phase lasted 10 days. Three fluorescent labeling substances were applied intravenously: calcein green at the second postoperative day, tetracycline 1 day after the end of the distraction phase, and xylene orange 2 days before sacrifice. We prepared ground sections from the ninth right ribs. The mineral apposition rate and bone formation rate were measured histomorphometrically on labeled osteons. The median mineral apposition rate during distraction was 2.39 μm/day (2.12–2.62 μm/day), which was higher than the rate during consolidation (median, 1.62 μm/day; 1.54–1.84 μm/day). The median bone formation rate confirmed this result and was 840.51 μm2/day (744.20–1148.26 μm2/day) during distraction and 384.25 μm2/day (330.84–467.71 μm2/day) during consolidation. Thus, a short period of distraction osteogenesis appears to have an anabolic effect on the mineral apposition rate of remote cortical bone.
Introduction
Bone injuries have a stimulating effect on the growth of distant, unaffected bones. The reasons for this are systemic hormonal changes [3] and locally released growth factors that enter the circulation [10, 14, 32]. The local and systemic changes after skeletal injury are well-described [24, 25]. The healing of an injured bone or bone marrow is accompanied by a systemically stimulating effect on the production of bone at distant intact localizations of the skeleton [24]. This has been named the systemic acceleratory phenomenon (SAP) [24, 25]. Bone repair after skeletal injury follows a sequence of local and systemic mechanisms of regulation [33]. The initial assumption that hormonal changes caused the systemic stimulating effect [9, 21, 22] was largely rejected when subsequent studies showed locally released growth factors that enter the circulation were primarily responsible for the SAP [4, 5, 12, 31]. Involved mediators are bone morphogenetic proteins, insulin-like growth factor, transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF), fibroblast growth factor, prostaglandins, and hormones, such as parathormone, growth hormone, and glucocorticoids [5, 35]. The injury of the bone marrow and the resulting release of osteogenic substances primarily lead to this stimulation [10]. It has been shown that osteoblastic cells will be recruited to aid the healing of a distant bone defect [30]. Any systemic influence has been described as small [11] or absent [37] in cases of solely cortical lesions. SAP appears primarily to arise from cancellous bone [24] and leads to bone growth at sites of the skeleton remote from the injury [1, 3, 15, 19, 20]. Elevated cell numbers, increased cell activity, or both may be the cause of this stimulation [18, 28].
Initiation of the systemic effects quickly follows an injury [11, 37]. However, the magnitude and duration of the influence on bone remodeling distant to the injured site has not been fully determined due to the heterogeneity of the examined material with varying species, diverse fractures types and accompanying injuries (eg, those to soft tissue) (Table 1). While most fracture repair is based on endochondral ossification, repair during distraction osteogenesis is primarily based on intramembranous ossification [2, 34]. The mechanical stimulus on the cells through distraction contributes largely to the release of mediators. The mechanical strain stimulates proliferation rate, as well as mitogen production and mitotic cell activity. Therefore, systemically effective factors such as TGF-β [24] and PDGF [26] are released, which increase osteoblast activity and influence the formation of callus [6–8, 27]. Thus, distraction osteogenesis allows exploration of the influence of mechanical stimulus on the systemic stimulation as compared to rigidly fixed bone injuries [8, 36].
Table 1.
MAR values from studies on intact bone without any skeletal injury
| Study | Species | Bone | Bone type | MAR (μm/day) |
|---|---|---|---|---|
| Frost [13] | Human | Various | Trabecular | 1.10 |
| Piert et al. [29] | Minipig | Iliac crest | Trabecular | 1.84 |
| Mosekilde et al. [23] | Sinclair minipig | Vertebra | Trabecular | 1.47 |
| Funk et al. (current study) | Yucatan minipig | Rib | Cortical | 1.55 |
MAR = mineral apposition rate.
We postulated the systemic stimulation, as reflected by the mineral apposition rate (MAR) and the bone formation rate (BFR), during the distraction phase is increased compared to the consolidation phase.
Materials and Methods
Cortical rib bone specimens of 24 minipigs that underwent distraction osteogenesis were analyzed histomorphometrically to compare MAR and BFR during distraction and the following consolidation phase. Therefore, MAR and BFR were measured once during distraction and repeated in the same animal group during consolidation by applying sequential fluorochrome labeling. We performed distraction osteogenesis on the left tibiae of 24 mature female Yucatan minipigs with a mean age of 77 weeks (range, 32–116 weeks). Under sterile conditions and general anesthesia, a 1.5-cm piece of the fibula was excised using a lateral approach. A premounted half-ring fixator was fixed to the tibia, which was then osteotomized sparing the periosteum through an anteromedial approach at midshaft. After a postoperative latency period of 4 days, the tibiae were distracted 1 mm every 12 hours for 10 days. Following this was a consolidation period that lasted 10 days.
For fluorescent polychrome labeling, three different substances were introduced intravenously through a jugular-implanted port system at defined points in time during the experiment: calcein green (Sigma Aldrich Chemie GmbH, Steinheim, Germany) at a dose of 15 mg/kg body weight (BW) on Day 2 postoperatively; tetracycline hydrochloride (Supramycin® pro infusione; Grünenthal GmbH, Aachen, Germany) at 25 mg/kg BW on Day 16; and xylene orange (Sigma Aldrich) at 90 mg/kg BW on Day 23.
On Day 25, the animals were sacrificed by injecting 40 mL Thiopental (Byk Gulden, Altana Pharma Deutschland GmbH, Konstanz, Germany) for deep sedation intravenously and, after cessation of breathing, producing cardiac arrest with 40 mL 14.9% potassium chloride. The ninth right rib was explanted and fixed in neutral isotonic formalin solution by applying the principle of immersion fixation. The rib was chosen as the specimen because it is the only nonweightbearing bone in quadrupeds suitable for the examination of cortical changes in tubular bones.
Using a cutting grinding system (Exakt-Trennschleifsystem-Makro; Exakt Apparatebau GmbH, Norderstedt, Germany), three 3-mm-thick sections were cut out of each rib. The specimens were taken dorsally 2 cm from the costovertebral joint, from the middle of each rib, and ventrally 2 cm from the bone-cartilage border. While sectioning, we ensured the cutting band was orthogonal to the tangent of the axis of the rib.
The bone specimens were processed under the exclusion of light and infiltrated with a synthetic fluid on a methylmethacrylate base (Technovit 7200 VLC®; Heraeus Kulzer, Weinheim, Germany). The specimens were further treated using the sandwich technique and finally ground to a thickness of about 80 μm using a microgrinding system (Phoenix 3000; Fa. Jean Wirtz GmbH & Co KG, Düsseldorf, Germany).
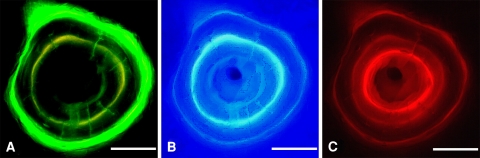
We digitized the ground sections using a direct light microscope, a digital camera, and the image analysis program KS 400 3.0 (Kontron Elektronik GmbH, Eching, Germany). Therefore, the three fluorochromes were stimulated with the respective spectrum of light, which was possible through the interposition of suitable filter systems consisting of an excitation filter, a dichromatic division mirror, and a blocking filter (filter combinations: I 3 for calcein, D for tetracycline, N 2.1 for xylene orange; Leica, Bensheim, Germany). A macro especially manufactured for this experiment was applied for the collection of data. All intact completely or nearly completely depicted osteons with a threefold marking were examined histomorphometrically. Therefore, the circle-like structures were read into the computer individually using the camera and microscope at a magnification of 40 × 10 × 0.55 (Fig. 1A–C). Due to the different glowing intensities of the fluorochromes, the exposure time of the camera was varied for each of them. The digitized images were contrast enhanced and combined into one image for each osteon to optimally depict all three labels (Fig. 2). The MAR in μm/day and BFR in μm2/day were then determined for the time periods between the applications of calcein and tetracycline (distraction phase), tetracycline and xylene orange (consolidation phase), and calcein and xylene orange (total period of observation).
Fig. 1A–C.
The three fluorescent labels were digitalized with their respective filter combinations: (A) calcein green, (B) tetracycline, and (C) xylene orange. Scale bar = 50 μm.
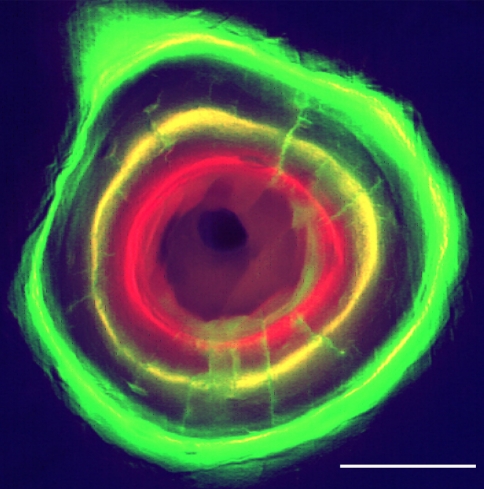
Fig. 2.
The three images of an osteon were combined into one and contrast enhanced. Scale bar = 50 μm.
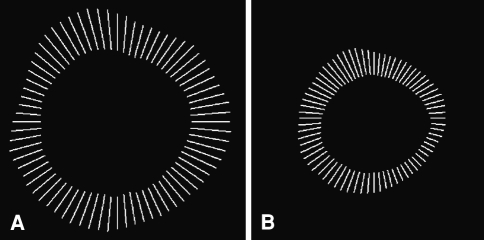
The individual rings of labeled osteons were segmented using a grey scale and if necessary manually closed. Manual processing was necessary when the segmented lines were not continuous. For this reason, only completely or nearly completely closed circlelike structures could be evaluated. If gaps larger than 10% of the circumference were found, manual processing would have been too arbitrary, and thus, the osteon was excluded from measurement. The closed structures constituted approximately 87% of the total number of observed threefold labeled rings. We were able to evaluate 266 osteons. Through filling of the enclosed areas within the rings, three circle-like areas were created from each osteon (Fig. 3A–B). The areas between the fluorochrome labels were measured and the values divided by the number of days of distraction and consolidation, respectively, to yield the BFR during both phases. On the basis of the largest area, the center of gravity of the Haversian system was determined. From this, a corona of 72 rays was generated. The mean was calculated per osteon from the distances of the rays between the fluorochromic bands divided by the time interval between the applications (Fig. 4A–B). This value corresponded to the MAR in μm/day for the respective interval between marker substance applications.
Fig. 3A–B.
The areas between the labeled rings of the osteon show the mineral apposition during (A) the distraction phase and (B) the consolidation phase.
Fig. 4A–B.
The length of the rays between the fluorescent labels indicates the mineral apposition in micrometers of (A) the distraction phase and (B) the consolidation phase.
Due to the small number of animals, the Wilcoxon sign-rank test for connected paired samples was applied to show differences between distraction and consolidation phase. Displayed are medians and 25–75 percentiles. Statistical evaluation of data was performed using SPSS® 9.0 software (SPSS Inc, Chicago, IL).
Results
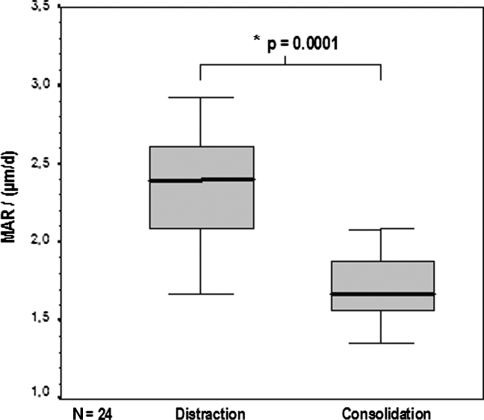
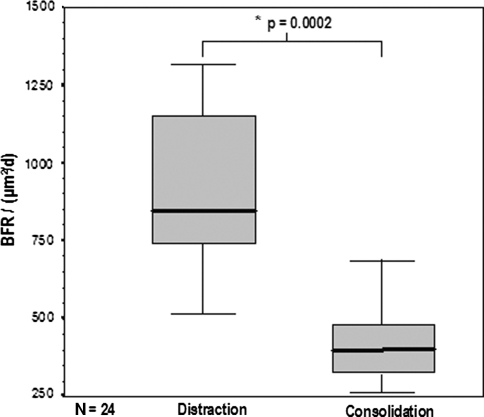
The median MAR during the distraction phase was higher (p = 0.0001) than that for the consolidation phase: 2.39 μm/day (2.12–2.62 μm/day) versus 1.62 μm/day (1.54–1.84 μm/day), respectively. The median BFR during distraction was also higher (p = 0.0002) than that for consolidation: 840.51 μm2/day (744.20–1148.26 μm2/day) versus 384.25 μm2/day (330.84–467.71 μm2/day). Thus, the MAR was 47.5% higher during the distraction phase than during the consolidation phase (Fig. 5), and the BFR 45.7% higher (Fig. 6).
Fig. 5.
The boxplot diagram depicts the higher (p = 0.0001) MAR during the distraction phase than during the consolidation phase. Solid bar in box = median value; box = 27–75 percentile; error bars = minimum/maximum value.
Fig. 6.
The boxplot diagram depicts the higher (p = 0.0002) BFR during the distraction phase than during the consolidation phase. Solid bar in box = median value; box = 27–75 percentile; error bars = minimum/maximum value.
Discussion
Bone injuries have a systemic influence on bone remodeling. In this animal experiment, we examined the systemic influence of distraction osteogenesis on cortical bone through histomorphometric analysis of MAR and BFR.
We acknowledge several limitations. First, we did not compare the effect of different types of injuries but rather illustrated the principal of remote effects during short-term distraction osteogenesis as a special form of bone injury. Second, only the two described parameters were measurable in the same animals consecutively, which provided a steady study setting, as all other parameters of possible bias stayed unchanged. We believed other histomorphometric or immunohistochemical parameters for bone turnover were not suitable for comparing consecutive measurements in the same animal. Third, blood chemistry specimens for consecutive measurements of systemic secretion of growth factors were lost. Although this is a limitation, the standardized and automated method of analyzing the bone formation parameters with fluorescent labeling in a steady animal model made it possible to show the short-term effect of distraction osteogenesis as a special type of bone injury for the first time in cortical bone.
In our study, a 10-day tibial distraction produced a measurable systemic anabolic effect on bone remodeling when compared to the values measured during the following consolidation phase in the same animals (Table 1). The results of this experiment confirm previously performed studies examining the systemic influence of local skeletal injury [3, 4, 8, 10, 12, 16, 24, 25, 32] (Table 2). Our results demonstrate the quick onset of systemic stimulation of bone metabolism. The magnitude seems comparable to the systemic effect of other bone injuries, although measurement parameters and methods varied as well as the examined species (Table 2). The duration of the stimulation appears closely related to the duration of the stimulus of distraction osteogenesis. Otherwise we would not have found differences between the distraction phase and the consolidation phase which directly followed. To clarify magnitude and duration of the systemic acceleratory phenomenon, further studies should compare the effects of different types of bone injuries in a single experimental model.
Table 2.
Effects of different bone injuries on bone remodeling as evaluated in various studies
| Study | Species | Parameter of measurement | Bone | Type of injury | Time point of measurement | Result |
|---|---|---|---|---|---|---|
| Bab et al. [3] | Rat | MAR, mineralization lag time, osteoid, osteoblasts, cartilage thickness | Mandibular condyles | Tibial bone marrow ablation | 3–18 days | Increase in bone and cartilage formation |
| Gazit et al. [14] | Rat | Histomorphometry (surface area) | Mandibular condyles | Tibial bone marrow ablation | 10 days | Healing marrow is responsible for osteogenic response |
| Einhorn et al. [10] | Rat | MAR | Both tibiae | Femoral cortical drilling + nailing + fracture | Various | Cortical injury did not produce increase, nailing did increase MAR for about 3 weeks, additional fracture did not alter effect |
| Hansen-Algenstaedt et al. [16] | Beagle | VEGF | Muscle samples | Distraction osteogenesis | 25 days | Increased expression during distraction |
| Buckley et al. [8] | Avian | DNA synthesis, cell division | Calvarial osteoblast-like cell culture | Cyclic strain | 72 hours | Increase and orientation of cells |
| Foldes et al. [12] | Human | Osteocalcin, alkaline phosphatase | Iliac crest | Bone marrow aspiration | 1 day to 5 weeks | Increase in serum |
| Müller et al. [24] | Rat | X-ray density, dry weight, ash weight, Ca2+ content, mineralizing surface, MAR, BFR | Femora, tibiae, vertebrae | Drill hole | 7 days | Increase in trabecular bone, not in cortical bone |
| Müller et al. [25] | Rat | X-ray bone density | Femora, tibiae, vertebrae | Drill hole + immobilization | 7 days | Decrease in systemic bone density |
| Funk et al. (current study) | Minipig | MAR, BFR | Rib cortex | Distraction osteogenesis | 10 days | Increase in cortical bone |
MAR = mineral apposition rate; BFR = bone formation rate; VEGF = vascular endothelial growth factor.
Causes for the systemic stimulatory effect are believed to be mainly due to the release of local growth factors and to a lesser extent to hormonal changes occurring in the course of bony injury and the subsequent repair [5, 10, 22]. The injury of the bone marrow is regarded as the trigger of this process [14]. Vice-versa, it has been shown that distant osteoblastic cells can be recruited to a bone healing site via circulation, the stimulus for their relocation again being the release of growth factors [30].
Despite the opinion that the SAP occurs in cancellous but not in cortical bone [24], our study shows a substantial systemic influence on cortical bone applying polychrome fluorescent labeling. The previous assumption that systemic effects may be detected within the fast-reacting cancellous bone but not within cortical bone might be explained by choice of methods of examination. We used a relatively easy method to confirm increased MAR and BFR within cortical bone.
In contrast to other investigations, which usually describe the acceleratory phenomenon examining long tubular bones of the weightbearing lower extremities or hind and fore extremities in quadrupeds, our study shows the effect within the nonweightbearing ribs.
We do not know the duration of SAP and our experiment did not allow such an assessment. However, it may be assumed the stimulating effect subsides shortly after the end of the distraction since our study still showed a difference even between the short periods of distraction and consolidation. The accelerated bone metabolism supposedly does not outlast the stimulation for long since most of the involved growth factors have a very short half-life, and as described by Holbein et al. [17], only the active but not the bound factor is responsible for the effect. Einhorn et al. [10] report a duration of the anabolic effect of skeletal injury of about 30 days in rats. They inflicted a single injury on the rats and not a 10-day period of distraction as in our study. Our data with a short period of injury demonstrated a difference between the MAR in the distraction phase and in the following consolidation phase, which indicates the brevity of the systemic effect of callus distraction on bone metabolism.
Distraction osteogenesis represents a special impairment of the bone marrow; hence, it makes sense its effect on the systemic bone remodeling differs from other types of skeletal injury [17, 36]. The comparison of serum specimens from patients with distraction osteogenesis and patients with rigidly stabilized proximal tibial osteotomy revealed callotasis but not osteotomy enhanced the proliferative activity [36]. Although we analyzed only bone histomorphometric parameters, our data suggest, in accordance with other investigations, the mechanical stimulus of callus distraction leads to an SAP via the release of various mediators [14]. Additionally, only during distraction was a rise in active TGF-β observed, a mediator whose release is probably regulated through mechanical strain [24, 26]. Cyclic stretching of osteoblastic cell cultures stimulated the cell proliferation and increased the production of TGF-β in another study [27].
Many investigations concerning changes in bone tissue use the untreated contralateral side as control. According to our data and those of other studies, one should bear in mind the systemic effects of a local skeletal injury and their possible influence on the outcome. The investigation of the different extents of the influence of various types of injury on bone remodeling, however, will need to be the subject of further studies. The stretching of muscle and nerve tissue, skin, and blood vessels may also have a systemic effect on the bone metabolism [25].
Distraction osteogenesis has a systemic anabolic effect on cortical bone of nonweightbearing ribs. The cascade of growth factor and hormone release and distribution remain to be elucidated, as do the possible mechanical influences on their release. Understanding the mechanisms would give further insight into the duration and dimension of the effect in different types of injuries.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Charité–University Medicine Berlin.
References
- 1.Andersson SM, Nilsson BE. Changes in bone mineral content following tibial shaft fractures. Clin Orthop Relat Res. 1979;144:226–229. [PubMed]
- 2.Aronson J, Good B, Stewart C, Harrison B, Harp J. Preliminary studies of mineralization during distraction osteogenesis. Clin Orthop Relat Res. 1990;250:43–49. [PubMed]
- 3.Bab I, Gazit D, Massarawa A, Sela J. Removal of tibial marrow induces increased formation of bone and cartilage in rat mandibular condyle. Calcif Tissue Int. 1985;37:551–555. [DOI] [PubMed]
- 4.Bab I, Gazit D, Muhlrad A, Shteyer A. Regenerating bone marrow produces a potent growth-promoting activity to osteogenic cells. Endocrinology. 1988;123:345–352. [DOI] [PubMed]
- 5.Bab IA, Einhorn TA. Polypeptide factors regulating osteogenesis and bone marrow repair. J Cell Biochem. 1994;55:358–365. [DOI] [PubMed]
- 6.Brighton CT, Sennett BJ, Farmer JC, Iannotti JP, Hansen CA, Williams JL, Williamson J. The inositol phosphate pathway as a mediator in the proliferative response of rat calvarial bone cells to cyclic biaxial mechanical strain. J Orthop Res. 1992;10:385–393. [DOI] [PubMed]
- 7.Brighton CT, Strafford B, Gross SB, Leatherwood DF, Williams JL, Pollack SR. The proliferative and synthetic response of isolated calvarial bone cells of rats to cyclic biaxial mechanical strain. J Bone Joint Surg Am. 1991;73:320–331. [PubMed]
- 8.Buckley MJ, Banes AJ, Levin LG, Sumpio BE, Sato M, Jordan R, Gilbert J, Link GW, Tran Son Tay R. Osteoblasts increase their rate of division and align in response to cyclic, mechanical tension in vitro. Bone Miner. 1988;4:225–236. [PubMed]
- 9.Dekel S, Lenthall G, Francis MJ. Release of prostaglandins from bone and muscle after tibial fracture: an experimental study in rabbits. J Bone Joint Surg Br. 1981;63:185–189. [DOI] [PubMed]
- 10.Einhorn TA, Simon G, Devlin VJ, Warman J, Sidhu SP, Vigorita VJ. The osteogenic response to distant skeletal injury. J Bone Joint Surg Am. 1990;72:1374–1378. [PubMed]
- 11.Enneking WF. The repair of complete fractures of rat tibias. Anat Record. 1948;101:515–537. [DOI] [PubMed]
- 12.Foldes J, Naparstek E, Statter M, Menczel J, Bab I. Osteogenic response to marrow aspiration: increased serum osteocalcin and alkaline phosphatase in human bone marrow donors. J Bone Miner Res. 1989;4:643–646. [DOI] [PubMed]
- 13.Frost H. Measurement of human bone formation by means of tetracycline labelling. Can J Biochem Physiol. 1963;41:31–42. [PubMed]
- 14.Gazit D, Karmish M, Holzman L, Bab I. Regenerating marrow induces systemic increase in osteo- and chondrogenesis. Endocrinology. 1990;126:2607–2613. [DOI] [PubMed]
- 15.Günzel I, Müller WA. Bone mineral metabolism in mice after fracture of tibiae, double labelling with 47CA and 224RA. Biophysik. 1973;10:267–272. [DOI] [PubMed]
- 16.Hansen-Algenstaedt N, Algenstaedt P, Bottcher A, Joscheck C, Schwarzloh B, Schaefer C, Muller I, Koike C, Ruther W, Fink B. Bilaterally increased VEGF-levels in muscles during experimental unilateral callus distraction. J Orthop Res. 2003;21:805–812. [DOI] [PubMed]
- 17.Holbein O, Neidlinger-Wilke C, Suger G, Kinzl L, Claes L. Ilizarov callus distraction produces systemic bone cell mitogens. J Orthop Res. 1995;13:629–638. [DOI] [PubMed]
- 18.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;238:249–281. [PubMed]
- 19.Kowalewski K, Yong S. Bone and urinary hydroxyproline in normal and hypothyroid rats with a long bone fracture. Acta Endocrinol. 1967;56:547–553. [DOI] [PubMed]
- 20.Lowe J, Bab I, Stein H, Sela J. Primary calcification in remodeling Haversian systems following tibial fracture in rats. Clin Orthop Relat Res. 1983;176:291–297. [PubMed]
- 21.Meller Y, Kestenbaum RS, Mozes M, Mozes G, Yagil R, Shany S. Mineral and endocrine metabolism during fracture healing in dogs. Clin Orthop Relat Res. 1984;187:289–295. [PubMed]
- 22.Meller Y, Shainkin-Kestenbaum R, Shany S, Zuilli I, Yankowitz N, Giat J, Konforti A, Torok G. Parathyroid hormone, calcitonin, and vitamin D metabolites during normal fracture healing in humans: a preliminary report. Clin Orthop Relat Res. 1984;183:238–245. [PubMed]
- 23.Mosekilde L, Weisbrode S, Safron J, Stills H, Jankowsky M, Ebert D, Danielsen C, Sogaard C, Franks A, Stevens M, Paddock C, Boyce R. Calcium-restricted ovariectomized Sinclair S-1 minipigs: an animal model of osteopenia and trabecular plate perforation. Bone. 1993;14:379–382. [DOI] [PubMed]
- 24.Müller M, Schilling T, Minne HW, Ziegler R. A systemic acceleratory phenomenon (SAP) accompanies the regional acceleratory phenomenon (RAP) during healing of a bone defect in the rat. J Bone Miner Res. 1991;6:401–410. [DOI] [PubMed]
- 25.Müller M, Schilling T, Minne HW, Ziegler R. Does immobilization influence the systemic acceleratory phenomenon that accompanies local bone repair? J Bone Miner Res. 1992;7:S425–S427. [DOI] [PubMed]
- 26.Mundy GR. Factors which stimulate bone growth in vivo. Growth Reg. 1993;3:124–128. [PubMed]
- 27.Neidlinger-Wilke C, Wilke HJ, Claes L. Cyclic stretching of human osteoblasts affects proliferation and metabolism: a new experimental model and its application. J Orthop Res. 1994;12:70–78. [DOI] [PubMed]
- 28.Parfitt AM, Mundy GR, Roodman GD, Hughes DE, Boyce BF. A new model for the regulation of bone resorption with particular reference to the effects of bisphosphonates. J Bone Miner Res. 1996;11:150–159. [DOI] [PubMed]
- 29.Piert M, Zittel T, Becker G, Jahn M, Stahlschmidt A, Maier G, Machulla HJ, Bares R. Assessment of porcine bone metabolism by dynamic [18F]fluoride ion PET: correlation with bone histomorphometry. J Nucl Med. 2001;42:1091–1100. [PubMed]
- 30.Shirley D, Marsh D, Jordan G, McQuaid S, Li G. Systemic recruitment of osteoblastic cells in fracture healing. J Orthop Res. 2005;23:1013–1021. [DOI] [PubMed]
- 31.Simmons DJ. Fracture healing perspectives. Clin Orthop Relat Res. 1985;200:100–113. [PubMed]
- 32.Sumner DR, Turner TM, Urban RM, Leven RM, Hawkins M, Nichols EH, McPherson JM, Galante JO. Locally delivered rhTGF-ß2 enhances bone ingrowth and bone regeneration at local and remote sites of skeletal injury. J Orthop Res. 2001;19:85–94. [DOI] [PubMed]
- 33.Suva LJ, Seedor JL, Endo N, Quartuccio HA, Thompson DD, Bab I, Rodan GA. Pattern of gene expression following rat tibial marrow ablation. J Bone Miner Res. 1993;8:379–388. [DOI] [PubMed]
- 34.Tajana GF, Morandi M, Zembo MM. The structure and development of osteogenetic repair tissue according to Ilizarov technique in man: characterization of extracellular matrix. Orthopedics. 1989;12:515–523. [DOI] [PubMed]
- 35.Urist MR, Silverman BF, Büring K, Dubuc FL, Rosenberg JM. The bone induction principle. Clin Orthop Relat Res. 1967;53:243–283. [DOI] [PubMed]
- 36.Weiss S, Baumgart R, Jochum M, Strasburger CJ, Bidlingmaier M. Systemic regulation of distraction osteogenesis: a cascade of biochemical factors. J Bone Miner Res. 2002;17:1280–1289. [DOI] [PubMed]
- 37.White AA 3rd, Panjabi MM, Southwick WO. The four biomechanical stages of fracture repair. J Bone Joint Surg Am. 1977;59:188–192. [PubMed]