Abstract
The importance of the menisci to the well-being of the normal knee is well-documented. However, there is no ideal repair or reconstructive approach for damaged menisci. Gene therapy provides one promising alternative strategy, especially when combined with injectable tissue engineering to achieve minimally invasive clinical application. We asked whether the introduction of human insulin-like growth factor 1 (hIGF-1) gene could improve the repair of full-thickness meniscal defects. We created full-thickness meniscal defects in the “white area” of the anterior horn in 48 goats. Bone marrow stromal cells with the transfection of hIGF-1 gene and injectable calcium alginate gel were mixed together to repair the defects; three control groups included cells without transfection, gel without cells, and defects left empty. After 4, 8, and 16 weeks, the animals were euthanized and the excised defects were examined by macroscopic assessment, histological analysis, electron microscopy, proteoglycan determination, and MRI. Sixteen weeks after surgery the repaired meniscal defects were filled with white tissue similar to that in normal meniscal fibrocartilage. The repair tissue was composed of cells embedded within matrix that filled the spaces of the fibers. The proteoglycan content in the gene-enhanced tissue engineering group was higher than those in the control groups, and less than that in the normal meniscus. The results suggest full-thickness meniscal defects in regions without blood supply can be reconstructed with hIGF-1-enhanced injectable tissue engineering.
Introduction
The meniscus, composed of fibrocartilaginous tissue, plays important roles in the knee [6, 26, 50]. Because degenerative joint disease reportedly develops after meniscectomy [2, 36], how to preserve meniscus function is currently an area of great interest. The periphery of meniscus receives a blood supply and tears in this location are reparable [1, 17, 27]. In contrast, the inner two thirds of meniscus is relatively avascular [27]. The results of meniscal repair in this zone are variable [7, 17, 20, 27, 35], although many techniques have been developed in attempts to promote healing in this region [7, 17, 20, 35, 50]. Results of surgical meniscal repair are not always satisfactory [31] with the failure rate ranging from 0% to 43.5%. Replacement of the meniscus using allograft reportedly has different results with the survival rate ranging from 50% to 82% [57–59] and is limited by immunologic reactions and risks of disease transmission [12, 18, 43].
Growth factors, cell cultures, and scaffolds may be combined to provide tissue-engineered meniscal repair [1, 21, 56, 60]. Alginates are naturally derived polysaccharides that have been used in cell transplantation and tissue engineering [3, 23, 34, 48]. Because solid grafts such as collagen sponges lack the flexibility to fill narrow clefts, one advantage of calcium alginate gel is that the cells-gel mixture can be injected into irregular defects and injuries without the disruption of scaffolds while gaining the advantages of minimally invasive surgery.
Human insulin-like growth factor 1 (hIGF-1) is one of the most critical growth factors in cartilage development and homeostasis [24, 40, 47]. Exposing chondrocytes to hIGF-1 in vitro enhances their metabolism while maintaining the differentiated phenotype [8, 24]. Levels of hIGF-1 in the synovial fluid increase in the patients with osteoarthritis and rheumatoid arthritis (RA) whereas serum hIGF-1 levels decrease in patients with juvenile RA [53]. It could be speculated that elevating the level of hIGF-1 might accelerate the restoration and regeneration of the meniscal tissue. However, any injected protein will be cleared rapidly from the synovial joint; thus, it is difficult to maintain a prolonged exposure to the chondroinductive factor with a one-time delivery of the protein. Cells carrying a transgene for specific proteins may provide a solution to this problem [9, 10, 41]. As an alternative to meniscal fibrochondrocytes for this purpose, autologous bone marrow stromal cells (BMSCs) may be better since they are easy to harvest through a minimally invasive procedure and simple to culture. Moreover, there is no immunologic rejection [21, 29, 44, 61]. Further, in gene-enhanced tissue engineering (GETE), the BMSCs provide an additional advantage of implanting inducible chondroprogenitor cells into the meniscal defects while introducing exogenous cartilage inductive protein. Tissue engineering has been already used to reconstruct the meniscus [28]. In that research, seeding of the scaffolds with autologous chondrocytes enhanced the extent of fibrocartilaginous tissue repair. In another study, a marker gene was introduced into meniscus tissue with a virus vector and the cytokine gene was verified to enhance the synthesis of proteoglycan and collagen in meniscal cells [15].
We therefore asked whether hIGF-1 protein could be secreted by transfected BMSCs to a therapeutic level. We further asked whether hIGF-1 gene transfection in BMSCs combined with ex vivo injectable tissue engineering would enhance the repair of a full-thickness meniscal defect.
Materials and Methods
The hIGF-1 cDNA was amplified from the human hepatocyte cDNA library and then the bicistronic vector pIRES2-EGFP- hIGF-1 was constructed. Bone marrow stromal cells from goats were cultured in vitro and transfected with the hIGF-1 gene by FuGene6. Then the expression of the gene and secretion of the cytokine was determined. Forty-eight adult male Chuandong white goats (body weight, 18–25 kg; age, 1.5–2 years) were randomly divided into four groups: (1) gene-enhanced tissue engineering group (n = 12), which received the hIGF-1 gene-modified BMSCs and calcium alginate gel to repair the meniscal defects; (2) BMSCs group (n = 12), which received the calcium alginate gel seeded with BMSCs; (3) gel group (n = 12), in which only calcium alginate gel was implanted; and (4) a control group (n = 12), in which the meniscal defects were left empty. The full-thickness meniscal defects were mechanically created in a nonvascular area of the menisci penetrating the whole tissue. The different results of repair were evaluated with macroscopic assessment, histological analysis, electron microscopy, proteoglycan determination, and MRI (Fig. 1). The study protocol was approved by the Animal Review Board of the University.
Fig. 1.
A flow diagram of the procedure outlines the steps and the numbers of samples at each step.
The hIGF-1 cDNA was amplified from the human hepatocyte cDNA library (Clontech Company, Mountain View, CA) using polymerase chain reaction (PCR). Then the PCR products were purified and subcloned into the PMD18-T vectors. Nucleotide sequence analysis was carried out by the chain termination method. The correct plasmids were selected out and cloned into the pIRES2-EGFP plasmids (Clontech Company). To construct the eukaryotic expression vectors, the PMD18-T-hIGF-1 and pIRES2-EGFP were digested by Xho I and EcoR I and then the corresponding segments were retrieved and inserted into the multiple cloning sites of the pIRES2-EGFP vectors.
Bone marrow aspirates were obtained aseptically from the iliac crests of the same 48 healthy goats mentioned above. The aspirates were plated on 35-mm dishes (12 dishes per animal) and cultured as a monolayer at 37°C in 5% carbon dioxide. The cultured BMSCs were maintained in a mixture of Dulbecco’s modified Eagle medium containing 10% fetal bovine serum for 5 days. Then the cells were transfected with plasmid pIRES2-EGFP-hIGF-1 at a ratio of 3:1 using FuGene6 Transfect Reagent (Roche Company, Basel, Switzerland) following the manufacturer’s instructions. To increase the efficiency, the cells were treated with hyaluronidase at a density of 4 U/mL for 12 hours before and in the transfection procedure [31]. The EGFP expression was observed under an inverted fluorescent microscope in a dark room and stimulated with 488-nm ultraviolet light. The EGFP-positive cells were counted in consecutive fields of vision to calculate the transfect efficiency. The concentrations of hIGF-1 in supernatant were determined using the enzyme-linked immunosorbent assay kit and protocol (Diagnostics Laboratory Systems, Webster, TX). The cells and scaffold complex were created by vortexing an aliquot of the cell suspension with a sodium alginate solution to yield a cellular density of 3 × 107 cells/mL in a 1.2% (weight/volume) sodium alginate solution and were stored in an incubator temporarily for implantation.
Each animal was anesthetized with an intravenous injection of xylazine at a dose of 0.2 mg/kg. Arthrotomies were performed on both knees under sterile conditions. The medial menisci were exposed and full-thickness defects were created with a 3-mm-diameter cutting cylinder at the anterior horn of the menisci in an avascular area penetrating the whole tissue. The cells-calcium alginate gel was formed by injecting the cells-sodium alginate solution and 102 mM calcium chloride simultaneously into the defects. After 10 minutes of polymerization, the incision was closed in layers and bandaged.
The knees were not immobilized after the operation and the animals were allowed free cage activities. All of the animals had free access to food and water. Four, 8, and 16 weeks after surgery, eight knees from each of the four groups were harvested and were grossly evaluated to assess the signs of infection, osteoarthritic changes, and the repair outcomes.
Limbs of Week 16 were selected for MRI (two limbs in each group). MRI scans were made on a high-resolution 1.5-Tesla scanner (Magnetom Vision; Siemens, Iselin, NJ). The signal and appearance of the defects were reported by a radiologist (WJX) experienced in MRI of the knees, who was blinded to the groups. The menisci were fixed in 4% paraformaldehyde for 12 hours and then embedded in paraffin. Sagittal 4.5-μm sections of the specimens were cut at the repair sites and stained with hematoxylin and eosin, toluidine blue, and Masson’s trichrome. Three sections from each sample were deparaffinized by xylene, passed through decreasing concentrations of ethanol, then the samples were incubated with a primary rabbit anti-Type I collagen IgG (Chemicon, Temecula, CA) diluted at a ratio of 1:500 at 4°C overnight and exposed to a 1:1000 dilution biotin-conjugated secondary IgG for 1 hour. Sections were incubated with avidin-biotin-peroxidase reagent and exposed to diaminobenzidine. Control tissue for the primary antibodies was bovine skin. As the control tissue for the secondary IgG, sections were processed as stated previously with the omission of the primary antibodies. The repair tissue (two samples from each group) was fixed in a 2% glutaric dealdehyde solution for 4 hours and was post-fixed in 1% osmium tetroxide for 2 hours. The tissue was then dehydrated in graded concentrations of ethanol and embedded in Epon 812. Ultrathin (40–60 nm) sections were cut and stained with uranyl acetate and lead citrate. Transmission electron microscope examination was carried out on an electron microscope (Hitachi 7100-B, Tokyo, Japan). The tissues were dried by a critical point drier (Quorum K850, Kent, UK, sputtered with BAL-TEC ion, and observed on a scanning electron microscope (Quanta-200; Philip, Zaterdag, Holland). Both the normal meniscal tissue from additional healthy goats and the repair tissue of GETE and BMSCs groups (six samples from each group) at 16 weeks were prepared for glycosaminoglycan assay. Fifty milligrams of the tissue were digested in lysate solution, freeze-dried, and dissolved in 1 mL phosphate-buffered saline solution. Fifty-microliter samples were added into 750-μL dimethylmethylene blue solution and the optical density of each sample was determined at 525 nm. Mixed isomer shark chondroitin sulfate was used to construct the standard curve. The quality of repair was assessed by three histopathologists (XMX, YZW, CYL) by grading the histological sections of the middle of repair areas from each group using a modified cartilage repair scoring scale. Ten sections from five samples from each group, 16 weeks after repair, were examined by an observer blinded to the groups (YZW) using histomorphometric software (CMIAS pathologic image analysis system; BASU Co, Beijing, China) to measure the percentage of repair tissue (pores within the tissue not included), the number of fibrochondrocytes in per unit area of repair tissue, the percentage of fibrocartilage-like tissue in per unit area with Masson’s trichrome stain, and the intensity of the collagen Type I stain. Some data were not determined because no fibrochondrocytes, cartilage-like tissue, or positive coloration of Type I collagen stain were found in the gel and control groups.
Differences between the total histological scores of the sections from various 16 weeks samples were determined by one-way analysis of variance. The differences of the filling percentage of repair tissue among the GETE, BMSCs, and gel group were compared using analysis of variance. Newman-Keuls multiple comparison analysis was performed to identify differences. Student’s t test was used to determine whether the GETE group was better than the BMSCs group in terms of fibrochondrocyte number, the percentage of fibrocartilage-like tissue, and the absorbance. The data were expressed as mean ± standard deviation (SD) of separate experiments.
Results
Four hours after transfection, green fluorescence expressed in part of the BMSCs with high intensity and gradually increased to peak at 48 hours and then decreased. The transfection efficiency was 22% ± 2.4%. The hIGF-1 concentration in the supernatants of untransfected cells was 1.667 ± 0.3 ng/mL and increased (p = 0.0001) to 3.28 ng/mL 4 hours after transfection. The peak concentration was 34.75 ng/mL at 48 hours and then decreased to 16.39 ng/mL at Day 10.
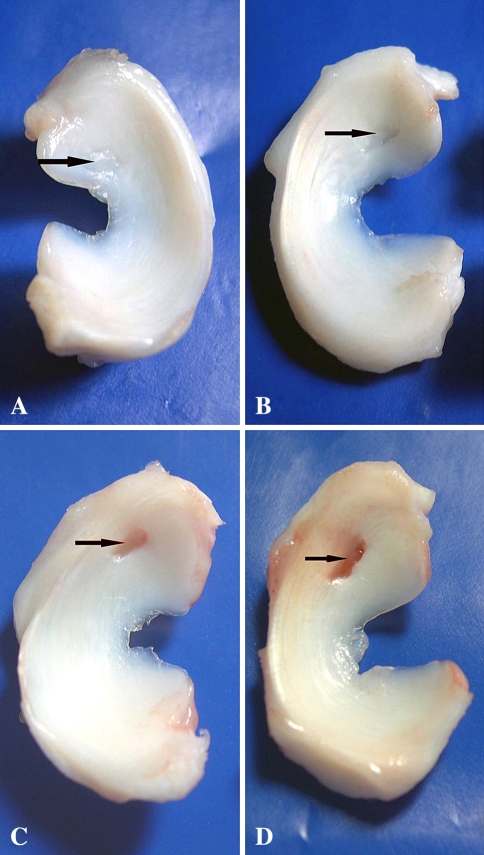
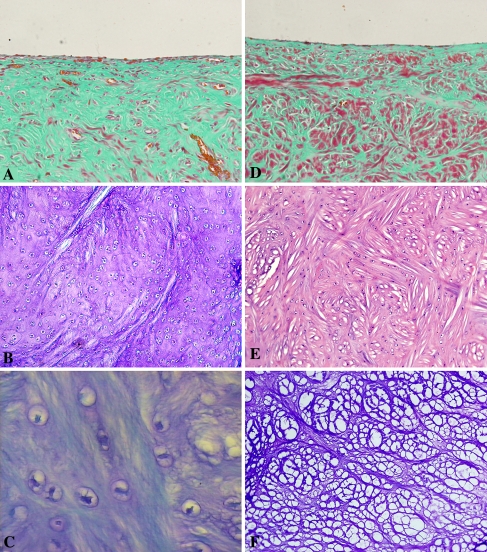
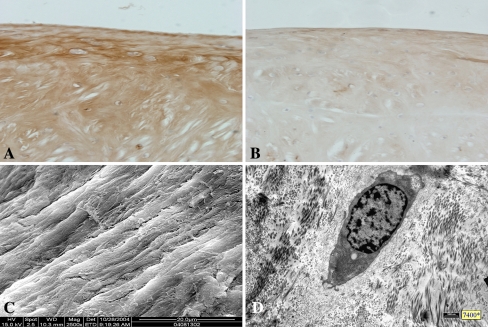
The defects in GETE group were filled completely with white and tenacious tissue, and the boundaries were difficult to discern (Fig. 2A), while margins of the defects in the BMSCs group were recognizable and lacked continuity between defects and host tissue (Fig. 2B). Repair tissue in the gel group was tenacious and gray with clear margins (Fig. 2C). The defects in the control group were filled with little thin and soft fibrous tissue with clear margins (Fig. 2D). In the GETE group, large numbers of cells were distributed within gel fibers (Fig. 3A–B). The repair tissue stained with metachromatic matrix intensely and evenly (Fig. 3C). Cells distributed diffusely within the network structure formed by gel fibers in the BMSCs group (Fig. 3D). Cells aligned with the fibers and some space among the fibers was filled with extracellular matrix (Fig. 3E). In the gel group, an empty network formed by gel fibers filled the deeper part of the defects without cell deposition (Fig. 3F). However, cartilaginous tissue in BMSCs group was less than that in GETE group (Fig. 4A–B). The structure of adjacent fibrocartilage was loose and full of multiple fissures. Scanning electron microscope revealed regular fibers arranged in superficial repair tissue of the GETE and BMSCs groups. The surface was smooth with tiny fissures among some fibers (Fig. 4C). Transmission electron microscope confirmed the existence of round and oval chondrocyte-like cells in differently oriented fibers (Fig. 4D).
Fig. 2A–D.
Macroscopic evaluation of the repair tissue (16 weeks) is shown. (A) In the GETE group, the defects were filled completely with white and rough tissue. The boundary between the host and repair tissue was difficult to discern. (B) In the BMSCs group, the material in the defect was white and almost filled the defects completely with discernible margins. (C) In the gel group, the bottom of the defects was covered with partial gel-like tissue incompletely with discernible edges. (D) The defects were filled with thin and soft fibrous connective tissue in the control group. GETE = gene-enhanced tissue engineering; BMSCs = bone marrow stromal cells.
Fig. 3A–F.
The histological analysis for the sections of 16 weeks is shown. (A) In the GETE group, the surface was smooth and the cartilaginous specific coloration existed between fibers of the gel (Stain, Masson’s trichrome; original magnification, × 100). (B) A large amount of cells was intensively distributed within gel fibers uniformly (Stain, alcian blue; original magnification, × 200) and the matrix was evenly stained (Stain, alcian blue; original magnification, × 400) (C). (D) The net-like fibers in the BMSCs group were relatively thick and the cartilaginous matrix was less than the GETE group (Stain, Masson’s trichrome; original magnification, × 100). (E) Many homogeneous cells distributed within the net (Stain, hematoxylin and eosin; original magnification, × 100). (F) Empty network formed by gel fibers existed in the gel group without cell deposition (Stain, alcian blue; original magnification, × 100). GETE = gene-enhanced tissue engineering; BMSCs = bone marrow stromal cells.
Fig. 4A–D.
(A) Type I collagen stain for the repair tissue of 16 weeks. Deep-stained brown granules deposited in the matrix of GETE group (Stain, type I collagen immunohistochemistry; original magnification, × 200), and the coloration was deeper than the BMSCs group (Stain, Type I collagen immunohistochemistry; original magnification, × 200) (B). (C) Scanning electron microscopic observation for the repair tissue in GETE group of 16 weeks demonstrated regular fibers in similar diameters arranged superficially with matrix filled between the fibers and covered the surface of some fibers. (D) Transmission electron microscope analysis of samples from 16 weeks confirmed round and oval chondrocyte-like cells in differently oriented fibers with multiple cell processes and abundant rough endoplasmic reticulum and ribosomes. GETE = gene-enhanced tissue engineering; BMSCs = bone marrow stromal cells.
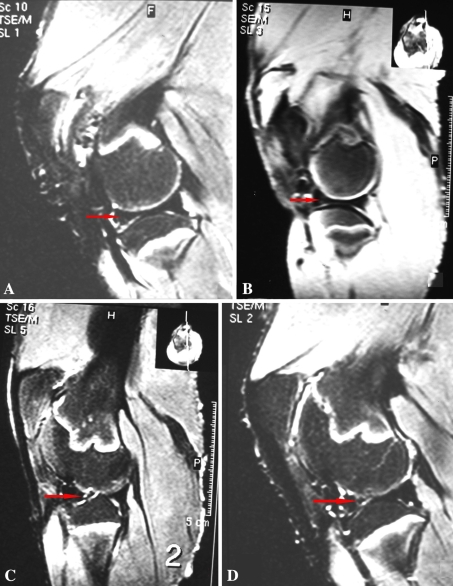
On MRI, the surfaces of the anterior horn in GETE group appeared smooth and continuous with small local high-signal regions (Fig. 5A). In the BMSCs group, a relatively smooth surface was seen in the defects with local tiny, superficial irregularities (Fig. 5B). Partial defects were filled with high-signal material in the gel group (Fig. 5C) and local high-signal existed in the anterior horn of the control menisci (Fig. 5D). Glycosaminoglycan content in normal meniscus was 14.69 ± 0.28 mg/g. In the GETE group, it decreased to 14.20 ± 0.12 mg/g in repair tissue (p < 0.01), but was more than the BMSCs group (13.72 ± 0.18 mg/g) (p < 0.01). The repair tissue in the GETE group contained a fibrocartilage-like matrix that stained greater (p < 0.01) than that in the BMSCs group (Table 1). More filling percentage of repair tissue (p < 0.01) was observed in the GETE group when compared with the percentage in the BMSCs group or gel group. Quantitative histological assessments followed the scale described by Schreiber et al. [49] and were modified by the authors. Some items more suitable for reflecting the biological repair of the meniscus were added including bonding to the host tissue, overall healthy fibrochondrocyte number, and the matrix porosity (Table 2). The intraobserver estimations have 8.6% variability and interobserver estimations have 12.2% variability. The histologic scores in the GETE group were higher (p < 0.01) those in the other groups and the differences (p < 0.01) among BMSCs, gel, and control groups. When compared with the BMSCs group, larger fibrochondrocyte numbers (p < 0.01) and higher percentage of cartilage-like tissue (p < 0.01) were observed in the GETE group.
Fig. 5A–D.
(A) Sagittal turbo T2-weighted MRI images of 16 weeks samples demonstrated the surface of the anterior horn of the menisci in the GETE group appeared smooth and continuous with tiny locally high signal regions. (B) In the BMSCs group, the surface was relatively smooth with local tiny superficial irregularities. Partial defect was filled by high signal material in the gel group (C) and stripped high signal existed in the anterior horn of the control meniscus (D). GETE = gene-enhanced tissue engineering; BMSCs = bone marrow stromal cells.
Table 1.
Results of histomorphometric and stereologic analyses
| Variable | GETE | BMSCs | Gel | Control | Normal | p Value |
|---|---|---|---|---|---|---|
| Absorbance of Type I collagen stain | 0.78 ± 0.03 | 0.57 ± 0.08 | ND | ND | 0.89 ± 0.04 | < 0.01 |
| Filling percent of repair tissue | 0.95 ± 0.04 | 0.86 ± 0.08 | 0.62 ± 0.11 | 0.12 ± 0.03 | 1.00 | < 0.01 |
| Histologic scores of the repair tissue | 27.83 ± 1.08 | 22.33 ± 1.17 | 12.42 ± 1.96 | 8.08 ± 2.62 | 31.00 | < 0.01 |
| Fibrochondrocytes number of per unit area (× 104/mm2) | 2.44 ± 0.36 | 1.48 ± 0.28 | ND | ND | 0.82 ± 0.17 | < 0.01 |
| Percent of cartilage-like tissue | 0.92 ± 0.05 | 0.74 ± 0.09 | ND | ND | 1.00 | < 0.01 |
GETE = gene-enhanced tissue engineering; BMSCs = bone marrow stromal cells; ND = not determined.
Table 2.
Meniscal repair histologic scoring scale
| Characteristics of the tissue repair | Points |
|---|---|
| A. Nature of the predominant tissue | |
| Typical fibrocartilage | 5 |
| Greater than 50% fibrocartilage | 4 |
| 50% fibrocartilage/50% fibrous tissue or scaffold | 3 |
| Greater than 50% fibrous tissue | 2 |
| Fibrous tissue | 0 |
| B. Toluidine blue staining of matrix | |
| Normal staining | 3 |
| Moderate | 2 |
| Slight | 1 |
| None | 0 |
| C. Surface | |
| Smooth and intact surface | 3 |
| Superficial lamination | 2 |
| Slight disruption | 1 |
| Severe disruption | 0 |
| D. Integrity | |
| Normal structure | 2 |
| Slight disruption | 1 |
| Severe disruption | 0 |
| E. Thickness | |
| 75%–100% of adjacent tissue | 2 |
| 50%–74% of adjacent tissue | 1 |
| 0%–49% of adjacent tissue | 0 |
| F. Bonding to host tissue | |
| Bonded | 2 |
| Partially bonded | 1 |
| Not bonded | 0 |
| G. Repair tissue in the defect | |
| Normal cell morphology and matrix staining | 3 |
| Cell clustering and normal matrix staining | 2 |
| Cell degeneration and decreased matrix staining | 1 |
| Severe cell degeneration, poor or no matrix staining | 0 |
| H. The adjacent host tissue | |
| Normal cell morphology and matrix staining | 3 |
| Cell clustering and normal matrix staining | 2 |
| Decreased matrix staining | 1 |
| Poor or no matrix staining | 0 |
| I. Overall healthy cartilage cell number | |
| Similar with normal fibrocartilage | 3 |
| Slightly less than normal fibrocartilage | 2 |
| Much less than normal fibrocartilage | 1 |
| Almost without cartilage cells | 0 |
| J. The matrix porosity | |
| Thick matrix without pores | 5 |
| Thick matrix with some pores | 4 |
| Semithick matrix with many pores | 3 |
| Porous matrix | 2 |
| Fibrous matrix | 0 |
| Maximum possible score | 31 |
Discussion
Growth factor gene therapy and tissue engineering are emerging fields that have promising application in muscle and skeletal repair [1, 10, 21, 22, 25, 37, 49, 51]. In theory, cell-mediated gene transfer combines the supply of a chondrogenic cell population with the production of appropriate growth factors directly to the lesions. We therefore asked whether (1) hIGF-1 protein could be secreted by the transfected BMSCs with a therapeutic level, and (2) hIGF-1 gene transfection could improve the results of the BMSCs-based ex vivo injectable tissue engineering to repair a full-thickness meniscal defect.
One major limitation of our study is the limited number of goats, which was not large enough to provide definitive conclusions, but rather preliminary ones. Second, long-term studies are required to evaluate the fate of transplanted cells in terms of their viability and dedifferentiation as well as the durability of the regenerated tissue to reduce the risk of osteoarthritis. Third, only Type I collagen was assessed in this study. Other extracellular matrix molecules existing in fibrocartilaginous tissues should be taken into account. For example, Type II collagen, which is also important for tissue function, should be examined in future studies. In this preliminary research, the repair results were examined from several aspects, including histomorphometry, electron microscope, glycosaminoglycan assessment, and image analysis. The findings suggested some positive aspects of this original ex vivo injectable gene-enhanced tissue engineering in repairing a full-thickness model of meniscal defect.
Few studies have focused on the application of growth factors to meniscal tissue or cells [4, 15, 21]. In research related to gene-modified bovine meniscal cells, adenovirus-mediated hepatocyte growth factor gene therapy enhanced blood vessel formation in the subcutaneous pouch of athymic nude mice for 8 weeks [21]. We found medium from transfected BMSCs we tested contained a hIGF-1 level as high as 34.75 ng/mL. Previous studies suggest hIGF-1 at 50 ng/mL optimally stimulates matrix elaboration and the development of a cartilage-specific matrix structure, whereas as low as 10 ng/mL is sufficient to produce stimulation of the proliferative and metabolic actions of cultured chondrocytes [30, 42, 52].
Tissue engineering has been used to regenerate meniscal tissue in the rat. Scaffolds derived from normal menisci of rats seeded with bone marrow-derived stromal cells can form meniscal tissues within 4 weeks [62]. In this study, the volume of the repair tissue in the gel group decreased gradually. This is probably because of the insufficient intensity that could not bear stress. In groups with cell transplantation, the secreted matrix occupied the residual space after degradation. The configuration and texture of the meniscus of the goat are similar to that of the human being. Function and bearing of the fibrocartilaginous structure in this animal enabled it to serve as the model for meniscal repair and transplant in the literature [5, 11, 38, 39, 46].
Gene transfer provides a promising alternative strategy for cytokine application [10, 25, 37, 41]. However, the results of in vitro experiments suggested direct injection of adenoviral vectors into the meniscus transduced only a few cells. In some cases, this strategy provides no new cells to repopulate the damaged areas [14]. Ex vivo gene-enhanced tissue engineering has been applied to the repair of cartilage. Gene encoding hIGF-1 was transfected by the adenovirus to enhance cartilage healing in an equine femoropatellar joint model. A single 15-mm cartilage defect received AdIGF-1-modified chondrocytes under arthroscopy [13]. The gross and histological appearances of IGF-1-modified repair tissue were improved over control defects. The entire procedure of the GETE method has not been used in meniscal defects so far. In this preliminary study, the repaired menisci in the GETE groups showed few fissures and consisted of cells embedded in a matrix containing Type I collagen. This indicated that the new differentiated cells had synthesized appropriate matrix components. The majority of the GETE was used as the virus vector. Gene expression and transduction efficiency are higher and can last longer in virus gene therapy than the nonviral method. With retrovirus, the efficiency can exceed 92.3% in rabbit chondrocytes and 74.7% in human chondrocytes. In vivo expression within osteochondral defects lasts more than 4 weeks [55]. With adeno-associated virus, the efficiency was 81.6% in lapine meniscal cells in vitro. When the virus was injected into the meniscal tears in a lapine model, transgene expression continued in cells adjacent to the tear for at least 20 days [32]. Theoretically, integrating exogenous gene into the genome of the undifferentiated mesenchymal cells by virus confronts the risk of insertional mutagenesis. Cationic liposomes and other lipid-based systems have the advantage of being easy and safe to prepare and they do not restrict the size of the DNA delivered [19, 54]. Madry and Trippel [33] examined lipid-mediated gene transfer methods of transfection for chondrocytes. FuGENE 6 transfection produced maximum levels of transgene expression (7.8%–41%) compared with other commercially available liposomes. After transplantation genetically modified chondrocytes kept producing beta-galactosidase for 2 weeks [33]. In our detection in vitro, the transfection efficiency by using liposome was up to 22% with a peak cytokine concentration of 34.75 ng/mL, which was comparable to the efficiency reported.
Injecting transfected cells without a carrier causes wide distribution of the cells instead of filling into the defects, which may lead to quick degradation and limited number of cells for reconstruction. Direct delivery of genes into the living fibrochondrocytes alone will not provide the sharp increase in the number of intralesional chondrogenic or meniscal cells, which are necessary to fill the defects. In one study, adult human BMSCs were transduced with adeno-associated virus containing transforming growth factor beta-1 (TGF-β1) [45]. Then the cells were implanted into the osteochondral defects of athymic rats. Improved cartilage repair was observed after 12 weeks. The TGF-β1 gene-modified BMSCs with polylactide scaffolds have been used in other research to repair cartilage defects in rabbits with similar findings [16]. Although short-term gene delivery may limit the repair results to some degree, temporary gene expression is still ideal for some tissue engineering, in which the process of new tissue formation needs to stop after the repair has been completed. A few BMSCs were transfected using the current method in this research. Cells trapped within the scaffold expressed the transgene and served as a local source of hIGF-1 synthesis. The glycosaminoglycan assay and histological analysis in this issue suggested there was more extracellular matrix synthesized in the GETE group but the level was still lower than that in normal tissue, indicating the limited but effective stimulatory effect of the hIGF-1 protein secreted by auto-BMSCs. Although fibrochondrocytes in the repair tissue of the GETE and BMSCs groups were more prevalent than in normal tissue, a conclusion could not be made that the repair results were better than normal.
This preliminary study demonstrated the efficacy of liposomal transfection of BMSCs with the hIGF-1 gene to deliver biologically effective concentrations of hIGF-1 and suggested the value of liposome-mediated ex vivo gene therapy for improving meniscus healing.
Acknowledgments
We thank Dr Xiaokui Hou and Dr Yingzhen Wang for assistance with the manuscript. We also thank Dr Guanzhen Yang and Dr Xiangfu Wu for their technical assistance on molecular technology and Dr Chengyu Lv, Dr Xiaoming Xing, and Dr Wenjian Xu for their help with the histologic and imageologic analysis. We thank Hao Xu for his assistance on statistical analysis.
Footnotes
Two of the authors (HZ, PL) received funding from the National Natural Science Foundation of China (No 30801167).
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Department of Joint Surgery, The Affiliated Hospital of Tsingtao University, Tsingtao, China.
References
- 1.Adams SB Jr, Randolph MA, Gill TJ. Tissue engineering for meniscus repair. J Knee Surg. 2005;18:25–30. [DOI] [PubMed]
- 2.Arnoczky SP, Warren RF, Spivak JM. Meniscal repair using an exogeneous fibrin clot. An experimental study in dogs. J Bone Joint Surg Am. 1988;70:1209–1217. [PubMed]
- 3.Becker TA, Kipke DR, Brandon T. Calcium alginate gel: a biocompatible and mechanically stable polymer for endovascular embolization. J Biomed Mater Res. 2001;54:76–86. [DOI] [PubMed]
- 4.Bhargava MM, Hidaka C, Hannafin JA, Doty S, Warren RF. Effects of hepatocyte growth factor and platelet-derived growth factor on the repair of meniscal defects in vitro. In Vitro Cell Dev Biol Anim. 2005;41:305–310. [DOI] [PubMed]
- 5.Bradley MP, Fadale PD, Hulstyn MJ, Muirhead WR, Lifrak JT. Porcine small intestine submucosa for repair of goat meniscal defects. Orthopedics. 2007;30:650–656. [DOI] [PubMed]
- 6.Brindle T, Nyland J, Johnson DL. The meniscus: review of basic principles with application to surgery and rehabilitation. J Athl Train. 2001;36:160–169. [PMC free article] [PubMed]
- 7.DeHaven KE, Sebastianelli WJ. Open meniscus repair. Indications, technique, and results. Clin Sports Med. 1990;9:577–587. [PubMed]
- 8.Dupont J, Holzenberger M. Biology of insulin-like growth factors in development. Birth Defects Res C Embryo Today. 2003;69:257–271. [DOI] [PubMed]
- 9.Evans CH, Ghivizzani SC, Smith P, Shuler FD, Mi Z, Robbins PD. Using gene therapy to protect and restore cartilage. Clin Orthop Relat Res. 2000;379(1):S214–S219. [DOI] [PubMed]
- 10.Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC. Osteoarthritis gene therapy. Gene Ther. 2004;11:379–389. [DOI] [PubMed]
- 11.Fabbriciani C, Lucania L, Milano G, Schiavone Panni A, Evangelisti M. Meniscal allografts: cryopreservation vs deep-frozen technique. An experimental study in goats. Knee Surg Sports Traumatol Arthrosc. 1997;5:124–134. [DOI] [PubMed]
- 12.Garrett JC, Steensen RN. Meniscal transplantation in the human knee: a preliminary report. Arthroscopy. 1991;7:57–62. [DOI] [PubMed]
- 13.Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007;89:672–685. [DOI] [PubMed]
- 14.Goto H, Shuler FD, Lamsam C. Transfer of LacZ marker gene to the meniscus. J Bone Joint Surg Am. 1999;81:918–925. [DOI] [PubMed]
- 15.Goto H, Shuler FD, Nivibizi C, Fu FH, Robbins PD, Evans CH. Gene therapy for meniscal injury: enhanced synthesis of proteoglycan and collagen by meniscal cells transduced with a TGFbeta(1)gene. Osteoarthritis Cartilage. 2000;8:266–271. [DOI] [PubMed]
- 16.Guo X, Zhang Q, Yang S, Shao Z, Yuan Q, Pan Z, Tang S, Liu K, Quan D. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor beta1 gene. Biomed Mater. 2006;1:206–215. [DOI] [PubMed]
- 17.Hanks GA, Gause TM, Sebastianelli WJ, O’Donnell CS, Kalenak A. Repair of peripheral meniscal tears: open versus arthroscopic technique. Arthroscopy. 1991;7:72–77. [DOI] [PubMed]
- 18.Heckmann TP, Barber-Westin SD, Noyes FR. Meniscal repair and transplantation: indications, techniques, rehabilitation, and clinical outcome. J Orthop Sports Phys Ther. 2006;36:795–814. [DOI] [PubMed]
- 19.Helgren I, Drvota V, Pieper R, Enoksson S, Blomberg P, Islam KB. Highly efficient cell-mediated gene transfer using non-viral vectors and FuGene6: in vitro and in vivo studies. Cell Mol Life Sci. 2000;57:1326–1333. [DOI] [PMC free article] [PubMed]
- 20.Henning CE, Lynch MA, Yearout KM, Vequist SW, Stallbaumer RJ, Decker KA. Arthroscopic meniscal repair using an exogenous fibrin clot. Clin Orthop Relat Res. 1990;252:64–72. [PubMed]
- 21.Hidaka C, Ibarra C, Hannafin JA, Torzilli PA, Quitoriano M, Jen SS. Formation of vascularized meniscal tissue by combining gene therapy with tissue engineering. Tissue Eng. 2002;8:93–105. [DOI] [PubMed]
- 22.Hoben GM, Athanasiou KA. Meniscal repair with fibrocartilage engineering. Sports Med Arthrosc. 2006;14:129–137. [DOI] [PubMed]
- 23.Holte O, Tonnesen HH, Karlsen J. Measurement of diffusion through calcium alginate gel matrices. Pharmazie. 2006;61:30–34. [PubMed]
- 24.Itoh K, Suzuki S, Kuroda T. Effects of local administration of insulin-like growth factor-I on mandibular condylar growth in rats. J Med Dent Sci. 2003;50:79–85. [PubMed]
- 25.Ivkovic A, Pascher A, Hudetz D, Jelic M, Haspl M, Windhager R, Pecina M. Current concepts in gene therapy of the musculoskeletal system. Acta Chir Orthop Traumatol Cech. 2006;73:115–122. [PubMed]
- 26.Johnson TC, Evans JA, Gilley JA, Delee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16:254–261. [DOI] [PubMed]
- 27.Kollias SL, Fox JM. Meniscal repair. Where do we go from here? Clin Sports Med. 1996;15:621–630. [PubMed]
- 28.Kon E, Chiari C, Marcacci M, Delcogliano M, Salter DM, Martin I, Ambrosio L, Fini M, Tschon M, Tognana E, Plasenzotti R, Nehrer S. Tissue engineering for total meniscal substitution: animal study in sheep model. Tissue Eng Part A. 2008;14:1067–1080. [DOI] [PubMed]
- 29.Leo AJ, Grande DA. Mesenchymal stem cells in tissue engineering. Cells Tissues Organs. 2006;183:112–122. [DOI] [PubMed]
- 30.Loeser RF, Shanker G. Autocrine stimulation by insulin-like growth factor 1 and insulin-like growth factor 2 mediates chondrocyte survival in vitro. Arthritis Rheum. 2000;43:1552–1559. [DOI] [PubMed]
- 31.Lozano J, Ma CB, Cannon WD. All-inside meniscus repair: a systematic review. Clin Orthop Relat Res. 2007;455:134–141. [DOI] [PubMed]
- 32.Madry H, Cucchiarini M, Kaul G, Kohn D, Terwilliger EF, Trippel SB. Menisci are efficiently transduced by recombinant adeno-associated virus vectors in vitro and in vivo. Am J Sports Med. 2004;32:1860–1865. [DOI] [PubMed]
- 33.Madry H, Trippel SB. Efficient lipid-mediated gene transfer to articular chondrocytes. Gene Ther. 2000;7:286–291. [DOI] [PubMed]
- 34.Mahler S, Desille M, Fremond B, Chesne C, Guillouzo A, Campion JP. Hypothermic storage and cryopreservation of hepatocytes: the protective effect of alginate gel against cell damages. Cell Transplant. 2003;12:579–592. [DOI] [PubMed]
- 35.McCarty EC, Marx RG, DeHaven KE. Meniscus repair: considerations in treatment and update of clinical results. Clin Orthop Relat Res. 2002;402:122–134. [DOI] [PubMed]
- 36.McDermott ID, Amis AA. The consequences of meniscectomy. J Bone Joint Surg Br. 2006;88:1549–1556. [DOI] [PubMed]
- 37.Mi Z, Ghivizzani SC, Lechman ER, Jaffurs D, Glorioso JC, Evans CH, Robbins PD. Adenovirus-mediated gene transfer of insulin-like growth factor 1 stimulates proteoglycan synthesis in rabbit joints. Arthritis Rheum. 2000;43:2563–2570. [DOI] [PubMed]
- 38.Miller MD, Kline AJ, Jepsen KG. “All-inside” meniscal repair devices: an experimental study in the goat model. Am J Sports Med. 2004;32:858–862. [DOI] [PubMed]
- 39.Miller MD, Ritchie JR, Gomez BA, Royster RM, DeLee JC. Meniscal repair. An experimental study in the goat. Am J Sports Med. 1995;23:124–128. [DOI] [PubMed]
- 40.Neidel J, Schulze M, Sova L. Insulin-like growth factor I accelerates recovery of articular cartilage proteoglycan synthesis in culture after inhibition by interleukin 1. Arch Orthop Trauma Surg. 1994;114:43–48. [DOI] [PubMed]
- 41.Nixon AJ, Haupt JL, Frisbie DD, Morisset SS, McIlwraith CW, Robbins PD, Evans CH, Ghivizzani S. Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene Ther. 2005;12:177–186. [DOI] [PubMed]
- 42.Nixon AJ, Saxer RA, Brower-Toland BD. Exogenous insulin-like growth factor-I stimulates an autoinductive IGF-1 autocrine/paracrine response in chondrocytes. J Orthop Res. 2001;19:26–32. [DOI] [PubMed]
- 43.Noyes FR, Barber-Westin SD, Rankin M. Meniscal transplantation in symptomatic patients less than fifty years old. J Bone Joint Surg Am. 2004;86:1392–1404. [DOI] [PubMed]
- 44.Oreffo RO, Cooper C, Mason C, Clements M. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1:169–178. [DOI] [PubMed]
- 45.Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X, Chu CR. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14:804–813. [DOI] [PubMed]
- 46.Ritchie JR, Miller MD, Bents RT, Smith DK. Meniscal repair in the goat model. The use of healing adjuncts on central tears and the role of magnetic resonance arthrography in repair evaluation. Am J Sports Med. 1998;26:278–284. [DOI] [PubMed]
- 47.Rogachefsky RA, Dean DD, Howell DS, Altman RD. Treatment of canine osteoarthritis with insulin-like growth factor-1 (IGF-1) and sodium pentosan polysulfate. Osteoarthritis Cartilage. 1993;1:105–114. [DOI] [PubMed]
- 48.Sakai S, Masuhara H, Yamada Y, Ono T, Ijima H, Kawakami K. Transition of mechanical property of porous alginate scaffold with cells during culture period. J Biosci Bioeng. 2005;100:127–129. [DOI] [PubMed]
- 49.Schreiber RE, Ilten-Kirby BM, Dunkelman NS, Symons KT, Rekettye LM, Willoughby J, Ratcliffe A. Repair of osteochondral defects with allogeneic tissue engineered cartilage implants. Clin Orthop Relat Res. 1999;367(Suppl):S382–395. [DOI] [PubMed]
- 50.Seedholm BB, Hargreaves DJ. Transmission of load in the knee joint with special reference to the role of the menisci. Part II. Experimental result, discussion and conclusion. Eng Med. 1979;8:220–229. [DOI]
- 51.Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J Bone Joint Surg Am. 1997;79:1770–1777. [DOI] [PubMed]
- 52.Takahashi T, Ogasawara T, Kishimoto J, Liu G, Asato H, Nakatsuka T, Uchinuma E, Nakamura K, Kawaguchi H, Chung UI, Takato T, Hoshi K. Synergistic effects of FGF-2 with insulin or IGF-1 on the proliferation of human auricular chondrocytes. Cell Transplant. 2005;14:683–693. [DOI] [PubMed]
- 53.Tavera C, Abribat T, Reboul P, Dore S, Brazeau P, Pelletier JP. IGF and IGF-binding protein system in the synovial fluid of osteoarthritic and rheumatoid arthritic patients. Osteoarthritis Cartilage. 1996;4:263–274. [DOI] [PubMed]
- 54.Tomita T, Hashimoto H, Tomita N, Morishita R, Lee SB, Hayashida K. In vivo direct gene transfer into articular cartilage by intraarticular injection mediated by HVJ (Sendai virus) and liposomes. Arthritis Rheum. 1997;40:901–906. [DOI] [PubMed]
- 55.Ueblacker P, Wagner B, Vogt S, Salzmann G, Wexel G, Krüger A, Plank C, Brill T, Specht K, Hennig T, Schillinger U, Imhoff AB, Martinek V, Gansbacher B. In vivo analysis of retroviral gene transfer to chondrocytes within collagen scaffolds for the treatment of osteochondral defects. Biomaterials. 2007;28:4480–4487. [DOI] [PubMed]
- 56.Vacanti CA. The history of tissue engineering. J Cell Mol Med. 2006;10:569–576. [DOI] [PMC free article] [PubMed]
- 57.Van Arkel ER, de Boer HH. Survival analysis of human meniscal transplantations. J Bone Joint Surg Br. 2002;84:227–231. [DOI] [PubMed]
- 58.Verdonk PC, Demurie A, Almgvist KF, Veys EM, Verbruggen G, Verdonk R. Transplantation of viable meniscal allograft. Survivorship analysis and clinical outcome of one hundred cases. J Bone Joint Surg Am. 2005;87:715–724. [DOI] [PubMed]
- 59.Verdonk PC, Verstraete KL, Almgvist KF, De Cuyper K, Veys EM, Verbruggen G, Verdonk R. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14:694–706. [DOI] [PubMed]
- 60.Vinatier C, Guicheux J, Daculsi G, Layrolle P, Weiss P. Cartilage and bone tissue engineering using hydrogels. Biomed Mater Eng. 2006;16(Suppl):S107–S113. [PubMed]
- 61.Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–429. [DOI] [PubMed]
- 62.Yamasaki T, Deie M, Shinomiya R, Yasunaga Y, Yanada S, Ochi M. Transplantation of meniscus regenerated by tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. Artif Organs. 2008;32:519–524. [DOI] [PubMed]