Abstract
Bone morphogenetic proteins (BMPs) were originally identified as osteoinductive proteins. With cloning of BMP genes, studies of BMPs and their clinical application have advanced. However, with increasing clinical applications, drug delivery systems and production costs have become more important issues. To address these issues, we asked whether E. coli-derived rhBMP-2 (E-BMP-2)-adsorbed porous β-TCP granules could achieve posterolateral lumbar fusion in a rabbit model similar to autogenous bone grafts. Lumbar spinal fusion masses were evaluated by 3-D computed tomography, mechanical testing, and histological analyses 8 weeks after surgery. By these measures E-BMP-2-adsorbed β-TCP granules achieved lumbar spinal fusion in dose-dependent fashion in a rabbit model as well as autogenous bone graft. Our preliminary findings suggest E-BMP-2-adsorbed porous β-TCP could be a novel, effective alternative to autogenous bone grafting for generating new bone and promoting regenerative repair of bone, and potentially utilizable in the clinical setting for treating spinal disorders.
Introduction
Autogenous bone grafting from the iliac bone has commonly been used clinically to promote bone formation, such as in fusion of the unstable spine, repair of large bone defects, and treatment of pseudarthrosis. However, autogenous bone grafting has several disadvantages, including acute and/or chronic pain or dysesthesia, potential risk of wound infection, unsightly scars, and deformity at the donor site [4, 19]. Limited available mass of graft bone is an additional disadvantage, especially in cases of large augmentation and long fusion after correction of scoliosis. To avoid these problems various authors have proposed new materials or agents that can substitute for autogenous bone grafts, such as bioabsorbable polymers, hyaluronic acid, and others [3, 30, 35].
The use of BMPs with their osteoinductive properties [39] has long been considered a promising means of producing such bone graft substitutes. This concept, however, was practically realized after successful cloning of cDNAs of BMPs [42]. Recombinant BMPs (BMP-2 and BMP-7) are currently utilized in combination with bovine collagen carrier in clinical practice to treat skeletal disorders such as open fracture, anterior interbody fusion, and posterolateral lumbar fusion [1, 2, 5–11, 13–17, 20, 22, 23, 25, 26, 31, 34, 41]. However, problems remain with the use of animal-derived collagen, including the risk of generation of antibody or disease transmission and lack of mechanical strength of the carrier collagen. Implants incorporating BMP are also expensive owing to the need for high doses of BMP-2 and would be a barrier to widespread use of such implants. Safe and cost-effective local delivery systems for BMPs avoiding these problems would be important. Better delivery systems and/or ways to reduce the required BMP dose by enhancing BMP action [24, 27, 33, 38] are possible solutions. At the same time, it is important to reduce the cost of production of recombinant human BMP-2 for widespread clinical use of rhBMP-2. The efficiency of production of BMP-2 with E. coli [18, 29] appears superior to that with animal cells, and might provide less expensive BMP-2 for practice use.
We therefore asked whether E. coli-derived rhBMP-2 (E-BMP-2)-adsorbed porous β-TCP granules could achieve posterolateral lumbar fusion in a rabbit model similar to autogenous bone graft as judged by radiographic fusion, mechanical stiffness and histologically evident fusion mass.
Materials and Methods
We used 68 New Zealand white rabbits 18 weeks of age (weight, 2.8–3.2 kg) in this experimental study. Of these, 52 rabbits were equally divided into four groups (13 per group) by dose of E-BMP-2 adsorbed to β-TCP granules (Table 1). Eight rabbits were used as negative controls (implantation of β-TCP granules alone without E-BMP-2) and the remaining eight had only autogenous bone grafting. Posterolateral lumbar spinal fusion was performed with autogenous bone or β-TCP granules adsorbed with five different doses of E-BMP-2. Efficacy of E-BMP-2 for lumbar spinal fusion was evaluated with CT for new bone formation and with fusion scores, the bending load of the fusion required to create 1-mm middle-span deflection, and histological examination with von Kossa staining for mineralization, toluidine blue and TRAP staining for cartilage formation and β-TCP resorption. This protocol including animal care was approved by the Institutional Committee for Animal Care and Experiments of Osaka City University Medical School.
Table 1.
Implant assignment
| Group | Total number | Number harvested | E-BMP-2 (μg/side) | β-TCP (μg/side) | |
|---|---|---|---|---|---|
| At 4 weeks | At 8 weeks | ||||
| Autogenous bone graft | 8 | 0 | 8 | – | – |
| BMP0 | 8 | 0 | 8 | 0 | 500 |
| BMP5 | 13 | 5 | 8 | 5 | 500 |
| BMP15 | 13 | 5 | 8 | 15 | 500 |
| BMP50 | 13 | 5 | 8 | 50 | 500 |
| BMP150 | 13 | 5 | 8 | 150 | 500 |
E-BMP-2 with dimeric molecular structure was produced in human BMP-2 gene-transfected E. coli with monomeric structure and stored in inclusion bodies, which were collected. The molecular structure was unfolded in protein-denaturing agents, and then refolded to form dimeric E-BMP-2 by removal of denaturing agents. Dimeric E-BMP-2 was purified by several steps of chromatography. Details of the procedures for dimerization of cytokines have already been reported [29, 40].
We anesthetized the animals with an intramuscular injection of ketamine (30 mg/kg) and xylazine (10 mg/kg). Flomoxef sodium (60 mg) was administered intramuscularly as a prophylactic antibiotic. Each rabbit underwent a single-level posterolateral intertransverse process fusion at L5–L6. We made a dorsal midline skin incision followed by two paramedian fascial incisions on both sides. The intermuscular plane between the multifidus and longissimus muscles was retracted to expose both transverse processes of L5–L6 and the intertransverse membranes. We used an electric-driven burr (Stryker, Kalamazoo, MI) to decorticate the posterior cortex of the transverse processes, and one of the implant or transplant materials was implanted in both sides (one implant per side). The wounds were then closed with 3–0 nylon sutures and skin staples. After surgery, rabbits had free access to food and water the same as before surgery.
To create the implants, E-BMP-2 freeze-dried powder was dissolved in distilled water to reconstitute before use. We used interconnecting porous β-TCP granules 1 mm to 3 mm in diameter (pore size, 50–350 μm; porosity, 75%) (HOYA Corp, Tokyo, Japan). To prepare one implant to bridge the sides between transverse processes of L5 and L6, 500 mg of β-TCP granules were soaked in 500 μl of distilled water containing one of various dosages of E-BMP-2 (5, 15, 50, or 150 μg) for more than 15 minutes at room temperature. Implants with 500 mg of β-TCP granules soaked with 500 μl of distilled water without E-BMP-2 served for controls. Adsorbance of β-TCP was established by determining the concentration of protein in supernatant after centrifugal separation using Bio-Rad protein assay dye reagent concentrate (Bio-Rad Laboratories, Inc., Hercules, CA). Based on the results of preliminary experiments, it was estimated less than 10% of E-BMP-2 was collected from the supernatant. Autogenous bone chips harvested from the iliac crest (0.8–1 g/side) were also prepared as positive controls.
Four weeks after surgery, five animals from each group were sacrificed by overdose of pentobarbital sodium (50 mg/kg), and the L4–L7 lumbar spines were harvested and processed for further examination. At 8 weeks after surgery, the remaining animals from the experimental and control groups (eight animals per group) were sacrificed and processed in the same fashion.
The lumbar spines were examined for fusion mass condition by anteroposterior plain radiography and CT (GE Yokogawa Medical System, Tokyo, Japan) at 1-mm slice thickness to construct 3-D images every 2 weeks and harvested with dissection of soft tissue at each time point. Fusion was evaluated on sagittal CT view 10 mm lateral from the midline. Three independent observers (SD, TM and HY), who were all spine surgeons, judged the fusions using the following standardized scale: 0 = no bone formation and of β-TCP or transplanted bone absorption between transverse processes, 1 = some bone formation between transverse processes, but β-TCP or transplanted bone absorption was incomplete, 2 = continuous bone bridging between transverse processes and β-TCP or transplanted bone absorption was complete. All three observers agreed on all observations.
Mechanical testing to evaluate the solidity of the L5–L6 fusion site was performed by a three-point flexion-bending test using a materials testing machine (Instron 5882, Instron, Boston, MA). The superior and inferior ends of samples were flattened by trimming, and samples were horizontally held in the apparatus. Three-point bending tests were performed with a 30-mm intersupport distance and a 1 mm/minute head speed. The bending load at 1-mm middle-span deflection was determined from the load-deflection curves.
The specimens harvested at 8 weeks (n = 3 from each group) after surgery were fixed in 4% paraformaldehyde overnight at 4°C, dehydrated in a graded ethanol series, and embedded in plastic resin. Sections 7 μm in thickness in the region of the intertransverse process were cut in the sagittal plane with a tungsten blade using a rotary microtome (RM2255, Leica Microsystems, Wetzlar, Germany) and stained with hematoxylin and eosin (H-E) after decalcification with 0.5 M EDTA for 30 minutes at room temperature, and stained with toluidine blue and von Kossa and van Gieson staining without decalcification. We examined four sections from each sample taken 10 mm lateral from the midline. Amounts of β-TCP in new bone were observed microscopically; area of residual β-TCP were identified manually using software specialized for bone histomorphometry, OsteoMeasure (Osteometrics Inc, Decatur, GA), and expressed per square micron (μm2). To identify osteoclasts that might have resorbed β-TCP within newly formed bone masses, tartrate-resistant acid phosphatase (TRAP) staining was performed on three sections from specimens taken from each of the five groups of animals sacrificed at 4 weeks after implantation.
We determined differences in the radiographic score, bending load, and histomorphometric measures between the autogenous bone graft group and each of the four E-BMP-2 treatment groups using the Kruskal-Wallis test and the post-hoc Scheffe test. We used StatView-J 5.0 (SAS Institute Inc, CA, USA) for all analyses.
Results
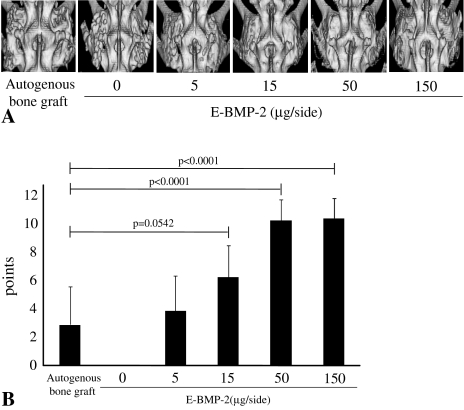
E-BMP-2-adsorbed porous β-TCP granules achieved posterolateral lumbar fusion same as autogenous bone graft evaluated by 3-D CT images (Fig. 1A) and plain radiographs (data not shown). The β-TCP granules appeared replaced by calcified mass with flat surface in E-BMP-2 dose- and time-dependent fashion, though β-TCP granules of the control group without E-BMP-2 continued to be observed throughout the observation period. Moreover, the radiographic scores showed treatment with 15 μg/side of E-BMP-2 achieved spinal fusion similar to autogenous bone graft, and that higher scores were obtained with 50 and 150 μg/side of E-BMP-2 than for the autogenous bone graft control (Fig. 1B).
Fig. 1A–B.
(A) Representative 3D-CT images of posterolateral fusion at 8 weeks after surgery are shown. Fusion masses of groups of BMP15, BMP50 and BMP150 are well remodeled as is the autogenous bone graft group. E-BMP-2 treatment could radiographically achieve lumbar spinal fusion the same as autogenous bone graft. (B) Radiological fusion scores of 3D-CT of BMP50 and BMP150 were higher than autogenous bone graft control group. Values are mean ± standard deviation (SD).
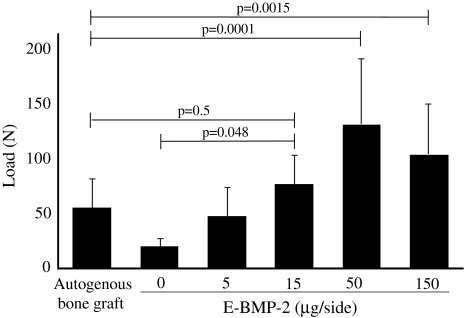
The bending load required to produce 1-mm middle-span deflection of the fusion mass for the BMP15 group was similar to that of the autogenous bone graft control group (Fig. 2). The bending load of the specimens from the BMP50 and BMP150 groups were higher than those in the autogenous bone graft control group (Fig. 2). Specimens from animals with the β-TCP granules retaining E-BMP-2 (50 μg/side or more) had in a larger bending load than that from animals treated with autogenous bone grafts.
Fig. 2.
The bending load at 1-mm middle-span deflection of the fusion mass of the BMP15 group was similar to that of autogenous bone graft control group. More than 15 μg/side of E-BMP-2 treatment could mechanically achieve lumbar spinal fusion as well as autogenous bone graft. The bending loads of the BMP50 and BMP150 were higher than that of the autogenous bone graft control group. Values are mean ± SD.
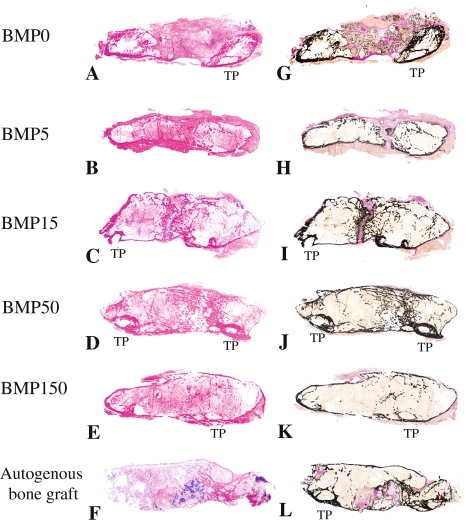
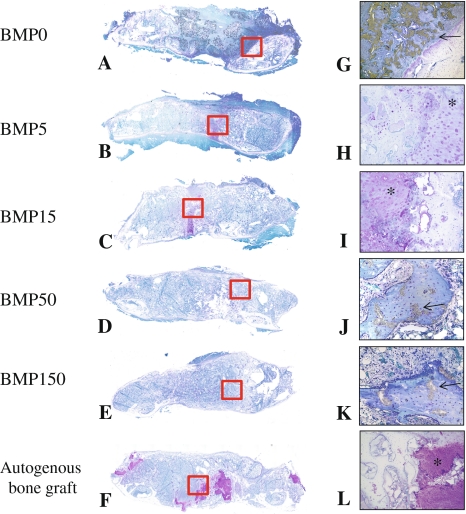
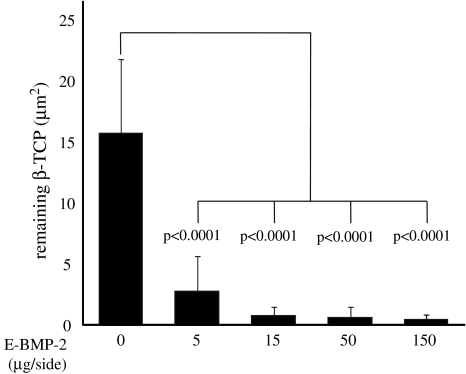
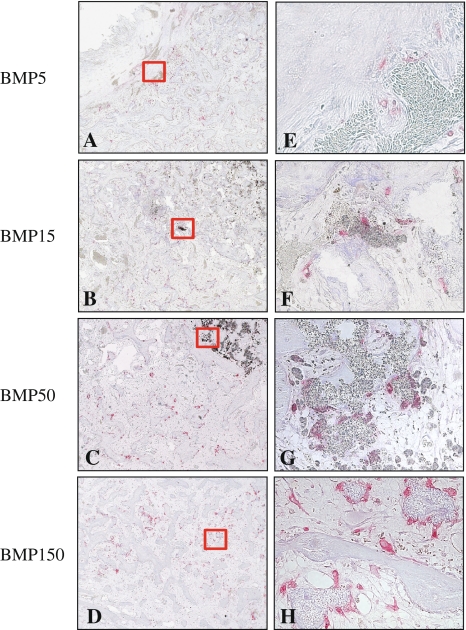
Histological examination revealed E-BMP-2 adsorbed β-TCP granules achieved fusion between transverse processes as well as autogenous bone graft. Eight weeks after operation, the bone masses with the peripheral cortical bone bridging the transverse processes achieved with more than 50 μg/side of E-BMP-2 (Fig. 3D, E, J and G) were similar to those achieved with autogenous bone graft (Fig. 3F and L) although the groups treated with less than 15 μg/side of E-BMP-2 (Fig. 3A–C, G–I) could not be achieved complete bone bridging. Toluidine blue-stained specimens from the groups of BMP5, BMP15, and the autogenous bone grafting control group (Fig. 4B and H, C and I, F and L, respectively) revealed metachromatic cartilage formation in the middle of the new bony mass bridging the transverse processes though there is no cartilage residue in the bone mass between transverse processes of the groups treated with more than 50 μg/side of E-BMP-2 (Fig. 4D and E). Higher-magnification views of bridging bone masses showed remaining β-TCP granules in these groups (Fig. 4G–K). In the controls without E-BMP-2, only fibrous tissue and remaining β-TCP without new bone were seen (Fig. 4A and G). The amount of β-TCP remaining within new bone mass was reduced (p < 0.0001) in all BMP groups compared with the control group treated without E-BMP-2 (Fig. 5). Furthermore, within newly formed bone in the E-BMP-2-treated groups (Fig. 6A–D), osteoclasts (TRAP-positive giant cells) were predominantly seen on the surfaces of the β-TCP in a dose dependent manner (Fig. 6E–H), suggesting more rapid resorption of β-TCP within the BMP-induced new bone mass and remodeling of the fusion masses.
Fig. 3A–L.
Representative longitudinal histology sections of fusion mass of each group 8 weeks after surgery are shown. (A) BMP0; (B) BMP5; (C) BMP15; (D) BMP50; (E) BMP150; (F) autogenous bone graft (Stain, hematoxylin and eosin; original magnification, ×1). (G) BMP0; (H) BMP5; (I) BMP15; (J) BMP50; (K) BMP150; (L) autogenous bone graft (Stain, von Kossa and van Gieson; original magnification, ×1). Newly formed bone mass between transverse processes of BMP50 and 150 showed completely continuous cortical bone as well as autogenous bone graft control group. More than 50 μg/side of E-BMP-2 treatment could histologically achieve lumbar spinal fusion as well as autogenous bone graft.
Fig. 4A–L.
Representative longitudinal histology sections of fusion mass of each group at 8 weeks after surgery are shown. (A) BMP0; (B) BMP5; (C) BMP15; (D) BMP50; (E) BMP150; (F) autogenous bone graft (Stain, Toluidine blue; original magnification, A–F: ×1). (G) BMP0; (H) BMP5; (I) BMP15; (J) BMP50; (K) BMP150; (L) autogenous bone graft (Metachromatic; original magnification ×100 for G and L, original magnification ×200 for H, I, J and K). Positive cartilage remnant in the fusion mass is not present in only section of groups of BMP50 and BMP150. More than 50 μg/side of E-BMP-2 treatment could histologically achieve lumbar spinal fusion as well as autogenous bone graft. Arrows indicate residual β-TCP.
Fig. 5.
Amounts of residual β-TCP were quantitatively assessed using bone histomorphometry software and expressed per square micron. Resorption of β-TCP was higher in the groups treated with E-BMP-2 than in the group treated without E-BMP-2. E-BMP-2 treatment could histologically achieve remodeling of newly formed fusion mass the same as autogenous bone graft. Values are mean ± SD.
Fig. 6A–H.
Representative longitudinal histology sections of fusion mass of each group at 4 weeks after surgery are shown. (A and E) BMP5; (B and F) BMP15: (C and G) BMP50; (D and H) BMP150. (Stain, TRAP; original magnification, A–D: x100, E–H: ×400). Right panels show higher magnifications of each left panel. E-BMP-2 treatment could histologically achieve remodeling of newly formed fusion mass as same as autogenous bone graft. TRAP-positive giant cells, that are osteoclasts, are abundant in the BMP50 and BMP150 groups.
Discussion
BMPs were originally identified as osteoinductive proteins by Urist more than 40 years ago. Since cloning of BMP genes, studies of BMPs and their clinical application have advanced. However, with progression of clinical application, issues related to drug delivery systems and production costs have arisen. β-TCP granules are widely used in clinical situations and E. coli-derived rhBMP-2 (E-BMP-2) can be produced at less cost compared with mammalian cell-derived BMP-2. Therefore we asked whether E. coli-derived rhBMP-2 (E-BMP-2) adsorbed on porous β-TCP granules could achieve posterolateral lumbar fusion in a rabbit model similar to that of autogenous bone graft.
We note several limitations. First, we used a rabbit posterolateral spinal fusion model. For clinical application, studies must be performed in much larger animals including sheep or nonhuman primates since the efficacy of cytokines and BMPs vary in differing species. Second, we followed the process of spinal fusion for only 8 weeks. Although this time period was determined by longitudinal followup of 3D-CT, different results might have been obtained at later stages. Third, our sample size in each group was not sufficient to yield substantial effects and the study is likely underpowered; we therefore consider the study preliminary. On the other hand, we previously found E-BMP-2 has osteoinductive activity equivalent to that currently produced in BMP-2 gene-transfected animal cells (Chinese hamster ovary cells) [45]. However, it is essential to evaluate the safety of E-BMP-2 for clinical use. In several previous studies using E-BMP-2 [18, 29, 45], its safety was not sufficiently examined. Evaluation of the safety of E-BMP-2 is required in preclinical studies.
One problem with wide clinical use of BMPs is their high cost of production, since they are originally derived from mammalian CHO cells [42]. To address this issue, Sebald et al. devised a novel method to produce rhBMP-2 derived from E. coli and convert BMP monomers to biologically active dimers (E-BMP-2) [18, 29]. E-BMP-2 has osteoinductive activity the same as CHO-derived rhBMP-2 both in vivo and in vitro [45]. However, these reports included only results for E-BMP-2-induced ectopic bone formation. Our question in this study was whether E-BMP-2-adsorbed β-TCP can achieve lumbar spinal fusion in a rabbit model the same as autogenous bone graft. Our findings suggest E-BMP-2-adsorbed β-TCP granules can effectively achieve posterolateral spinal fusion in a rabbit model the same as autogenous bone graft. On the other hand, from the original phase of preclinical and clinical application of BMPs, they have been locally delivered with Type I collagen sponges [5, 6, 10]. Although it is now possible to generate large amounts of recombinant human BMPs for medical use, the major challenge remains in the development of optimal local delivery systems for these proteins. To address this issue, we have investigated a biodegradable polymer as a carrier for BMPs yielding slow release and increasing the effects of BMPs [30], while Seeherman et al. have investigated the efficacy of calcium phosphate paste as a carrier for rhBMP-2 for the treatment of osteotomy [35]. We have generated an osteoinductive composite consisting of rhBMP-2, β-TCP powder, and biodegradable polymer for the treatment of various skeletal disorder models [12, 26, 36]. However, the results of these investigations are not yet clinically applicable because of the use of agents not approved for clinical use, such as biodegradable polymers. To address this problem, we used β-TCP, which is widely applied clinically, as a delivery system for BMP. As a result the necessary amount of BMP-2 to obtain lumbar spinal fusion is higher than that reported in our previous study [26]. We found lumbar spinal fusion occurred with the use of β-TCP and extended release of E-BMP-2 as previously described [32, 43]. Large quantities of BMPs facilitated osteoclast formation and resultant resorption and remodeling of newly formed bone in a dose-dependent manner (Figs. 5, 6), as previously reported [36]. This excessive osteoclast differentiation and bone resorption is an indirect effect of osteoblast differentiation and RANKL (receptor activator of NFκB ligand) expression induced by BMP stimulation [28, 37, 46] and a direct effect of BMP on osteoclastogenesis [44]. As previously described [21], these observations also indicate that excessive amounts of BMP might fail to yield required new bone formation, and that it is important to use optimal doses of BMP in clinical situations such as spinal fusion. E-BMP-2-adsorbed β-TCP granules may thus be useful as new materials for osteogenesis, especially for spinal fusion, and successfully address issues related to the clinical application of BMPs noted above.
Our preliminary findings suggest E-BMP-2-adsorbed porous β-TCP could be an effective alternative to autogenous bone grafting for generation of new bone and promotion of regenerative repair of bone, and potentially utilizable in the clinical setting for the treatment of spinal disorders.
Acknowledgments
We thank Drs. A. Suzuki, K. Yano, T. Matsumoto, H. Yasuda, K. Sugama, H. Irie, and A. Yamada, W. Fukushima, M Fukui, and also Ms. A. Inagaki, Ms. K. Hata, Ms. K. Kamei, and Ms. Y. Hanamoto for technical and statistical assistance. E-BMP-2 was produced and kindly provided by Dr. W. Sebald (Würzburg, Germany) and donated to us through Osteopharma Inc. (Osaka, Japan). The β-TCP granules were donated by HOYA Corp. (Tokyo, Japan).
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Project Grants 16109009 and 1679085 to KT, and 19791018 to YI).
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Akamaru T, Suh D, Boden SD, Kim HS, Minamide A, Louis-Ugbo J. Simple carrier matrix modifications can enhance delivery of recombinant human bone morphogenetic protein-2 for posterolateral spine fusion. Spine. 2003;28:429–434. [DOI] [PubMed]
- 2.Allen RT, Lee YP, Stimson E, Garfin SR. Bone morphogenetic protein-2 (BMP-2) in the treatment of pyogenic vertebral osteomyelitis. Spine. 2007;32:2996–3006. [DOI] [PubMed]
- 3.Arosarena OA, Collins WL. Bone regeneration in the rat mandible with bone morphogenetic protein-2: a comparison of two carriers. Otolaryngol Head Neck Surg. 2005;132:592–597. [DOI] [PubMed]
- 4.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. [DOI] [PubMed]
- 5.Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662–2673. [DOI] [PubMed]
- 6.Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine. 1995;20:412–420. [DOI] [PubMed]
- 7.David SM, Gruber HE, Meyer RA, Jr., Murakami T, Tabor OB, Howard BA, Wozney JM, Hanley EN, Jr. Lumbar spinal fusion using recombinant human bone morphogenetic protein in the canine. A comparison of three dosages and two carriers. Spine. 1999;24:1973–1979. [DOI] [PubMed]
- 8.Dimar JR, Glassman SD, Burkus KJ, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine. 2006;31:2534–2539; discussion 2540. [DOI] [PubMed]
- 9.Glassman SD, Dimar JR, 3rd, Burkus K, Hardacker JW, Pryor PW, Boden SD, Carreon LY. The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine. 2007;32:1693–1698. [DOI] [PubMed]
- 10.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84:2123–2134. [DOI] [PubMed]
- 11.Grauer JN, Patel TC, Erulkar JS, Troiano NW, Panjabi MM, Friedlaender GE. 2000 Young Investigator Research Award winner. Evaluation of OP-1 as a graft substitute for intertransverse process lumbar fusion. Spine. 2001;26:127–133. [DOI] [PubMed]
- 12.Hoshino M, Namikawa T, Kato M, Terai H, Taguchi S, Takaoka K. Repair of bone defects in revision hip arthroplasty by implantation of a new bone-inducing material comprised of recombinant human BMP-2, Beta-TCP powder, and a biodegradable polymer: an experimental study in dogs. J Orthop Res. 2007;25:1042–1051. [DOI] [PubMed]
- 13.Hsu WK, Wang JC. The use of bone morphogenetic protein in spine fusion. Spine J. 2008;8:419–425. [DOI] [PubMed]
- 14.Itoh H, Ebara S, Kamimura M, Tateiwa Y, Kinoshita T, Yuzawa Y, Takaoka K. Experimental spinal fusion with use of recombinant human bone morphogenetic protein 2. Spine. 1999;24:1402–1405. [DOI] [PubMed]
- 15.Johnsson R, Stromqvist B, Aspenberg P. Randomized radiostereometric study comparing osteogenic protein-1 (BMP-7) and autograft bone in human noninstrumented posterolateral lumbar fusion: 2002 Volvo Award in clinical studies. Spine. 2002;27:2654–2661. [DOI] [PubMed]
- 16.Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32:2885–2890. [DOI] [PubMed]
- 17.Konishi S, Nakamura H, Seki M, Nagayama R, Yamano Y. Hydroxyapatite granule graft combined with recombinant human bone morphogenic protein-2 for solid lumbar fusion. J Spinal Disord Tech. 2002;15:237–244. [DOI] [PubMed]
- 18.Kubler NR, Reuther JF, Faller G, Kirchner T, Ruppert R, Sebald W. Inductive properties of recombinant human BMP-2 produced in a bacterial expression system. Int J Oral Maxillofac Surg. 1998;27:305–309. [DOI] [PubMed]
- 19.Kurz LT, Garfin SR, Booth RE, Jr. Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324–1331. [DOI] [PubMed]
- 20.Lewandrowski KU, Nanson C, Calderon R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: a report of five cases. Spine J. 2007;7:609–614. [DOI] [PubMed]
- 21.Martin GJ, Jr., Boden SD, Marone MA, Marone MA, Moskovitz PA. Posterolateral intertransverse process spinal arthrodesis with rhBMP-2 in a nonhuman primate: important lessons learned regarding dose, carrier, and safety. J Spinal Disord. 1999;12:179–186. [PubMed]
- 22.Minamide A, Kawakami M, Hashizume H, Sakata R, Tamaki T. Evaluation of carriers of bone morphogenetic protein for spinal fusion. Spine. 2001;26:933–939. [DOI] [PubMed]
- 23.Minamide A, Kawakami M, Hashizume H, Sakata R, Yoshida M, Tamaki T. Experimental study of carriers of bone morphogenetic protein used for spinal fusion. J Orthop Sci. 2004;9:142–151. [DOI] [PubMed]
- 24.Nakagawa K, Imai Y, Ohta Y, Takaoka K. Prostaglandin E2 EP4 agonist (ONO-4819) accelerates BMP-induced osteoblastic differentiation. Bone. 2007;41:543–548. [DOI] [PubMed]
- 25.Namikawa T, Terai H, Hoshino M, Kato M, Toyoda H, Yano K, Nakamura H, Takaoka K. Enhancing effects of a prostaglandin EP4 receptor agonist on recombinant human bone morphogenetic protein-2 mediated spine fusion in a rabbit model. Spine. 2007;32:2294–2299. [DOI] [PubMed]
- 26.Namikawa T, Terai H, Suzuki E, Hoshino M, Toyoda H, Nakamura H, Miyamoto S, Takahashi N, Ninomiya T, Takaoka K. Experimental spinal fusion with recombinant human bone morphogenetic protein-2 delivered by a synthetic polymer and beta-tricalcium phosphate in a rabbit model. Spine. 2005;30:1717–1722. [DOI] [PubMed]
- 27.Ohta Y, Nakagawa K, Imai Y, Katagiri T, Koike T, Takaoka K. Cyclic AMP enhances Smad-mediated BMP signaling through PKA-CREB pathway. J Bone Miner Metab. 2008;26:478–484. [DOI] [PubMed]
- 28.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. [DOI] [PMC free article] [PubMed]
- 29.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237:295–302. [DOI] [PubMed]
- 30.Saito N, Okada T, Horiuchi H, Murakami N, Takahashi J, Nawata M, Ota H, Nozaki K, Takaoka K. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat Biotechnol. 2001;19:332–335. [DOI] [PubMed]
- 31.Sandhu HS, Kanim LE, Kabo JM, Toth JM, Zeegen EN, Liu D, Delamarter RB, Dawson EG. Effective doses of recombinant human bone morphogenetic protein-2 in experimental spinal fusion. Spine. 1996;21:2115–2122. [DOI] [PubMed]
- 32.Santoni BG, Pluhar GE, Motta T, Wheeler DL. Hollow calcium phosphate microcarriers for bone regeneration: in vitro osteoproduction and ex vivo mechanical assessment. Biomed Mater Eng. 2007;17:277–289. [PubMed]
- 33.Sasaoka R, Terai H, Toyoda H, Imai Y, Sugama R, Takaoka K. A prostanoid receptor EP4 agonist enhances ectopic bone formation induced by recombinant human bone morphogenetic protein-2. Biochem Biophys Res Commun. 2004;318:704–709. [DOI] [PubMed]
- 34.Schimandle JH, Boden SD, Hutton WC. Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine. 1995;20:1326–1337. [PubMed]
- 35.Seeherman HJ, Bouxsein M, Kim H, Li R, Li XJ, Aiolova M, Wozney JM. Recombinant human bone morphogenetic protein-2 delivered in an injectable calcium phosphate paste accelerates osteotomy-site healing in a nonhuman primate model. J Bone Joint Surg Am. 2004;86:1961–1972. [DOI] [PubMed]
- 36.Taguchi S, Namikawa T, Ieguchi M, Takaoka K. Reconstruction of bone defects using rhBMP-2-coated devitalized bone. Clin Orthop Relat Res. 2007;461:162–169. [DOI] [PubMed]
- 37.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. [DOI] [PubMed]
- 38.Toyoda H, Terai H, Sasaoka R, Oda K, Takaoka K. Augmentation of bone morphogenetic protein-induced bone mass by local delivery of a prostaglandin E EP4 receptor agonist. Bone. 2005;37:555–562. [DOI] [PubMed]
- 39.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. [DOI] [PubMed]
- 40.Weigel U, Meyer M, Sebald W. Mutant proteins of human interleukin 2. Renaturation yield, proliferative activity and receptor binding. Eur J Biochem. 1989;180:295–300. [DOI] [PubMed]
- 41.Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2007;8:1011–1018. [DOI] [PubMed]
- 42.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. [DOI] [PubMed]
- 43.Wu CH, Hara K, Ozawa H. Enhanced osteoinduction by intramuscular grafting of BMP-beta-TCP compound pellets into murine models. Arch Histol Cytol. 1992;55:97–112. [DOI] [PubMed]
- 44.Yamamoto Y, Udagawa N, Matsuura S, Nakamichi Y, Horiuchi H, Hosoya A, Nakamura M, Ozawa H, Takaoka K, Penninger JM, Noguchi T, Takahashi N. Osteoblasts provide a suitable microenvironment for the action of receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2006;147:3366–3374. [DOI] [PubMed]
- 45.Yano K, Hoshino M, Ohta Y, Manaka T, Naka Y, Imai Y, Sebald W, Takaoka K. Osteoinductive capacity and heat stability of recombinant human bone morphogenetic protein-2 produced by Escherichia coli and dimerized by biochemical processing. J Bone Miner Metab. 2009;27:355–363. [DOI] [PubMed]
- 46.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. [DOI] [PubMed]