Abstract
Numerous studies have described the use of osteogenic protein-1 (OP-1) in adults, but there are few reports in children. The objectives of this short-term followup cohort study were (1) to examine clinical and radiographic healing of persistent nonunions after OP-1 application in children; and (2) to determine the safety of OP-1 use in this sample. Clinical healing was defined by absence of pain and tenderness at the nonunion site and the ability to fully weight bear on the affected limb. Radiographic healing was determined by bony bridging of the nonunion site in at least one view. Safety was defined as the absence of major adverse events, including allergic reactions, infections, local inflammatory reactions, and heterotopic ossification. OP-1 was used in 19 patients who had an operative procedure for the bridging of persistent nonunions between 1999 and 2007. The mean age was 11.6 years (range, 4.8–20.3 years). Thirteen patients had persistent nonunion after one or more previous surgeries, prior to the initial OP-1 application. A single dose of 3.5 mg of OP-1 mixed with 1 g of Type I bovine collagen was applied to 23 sites of 19 patients. Three patients received additional OP-1 applications. Healing occurred clinically and radiographically in 17 of the 23 sites. Complications included four superficial pin site infections, one deep infection, and two fractures. No major local adverse event related to OP-1 application was noted in our sample. Our findings suggest OP-1 stimulates healing of persistent nonunions without major adverse events in our patient population.
Level of Evidence: Level IV, case series. See the guidelines online for a complete description of levels of evidence.
Introduction
In 1965, Marshall Urist demonstrated that demineralized bone matrix contains certain substances that can induce ectopic new bone formation when implanted in extraskeletal sites in rodents and introduced the term bone morphogenetic proteins (BMPs) to describe these substances [29]. Since then, more than 20 BMPs have been identified. BMPs are members of the transforming growth factor beta (TGF β) super family of extracellular proteins and have numerous effects on the cells of many tissues [30]. Among these, bone morphogenetic protein-2 (BMP-2) and bone morphogenetic protein-7 (BMP-7) possess substantial osteogenic potential [14] and are the only osteoinductive growth factors commercially available. Numerous animal studies [4, 8, 31, 35] demonstrate BMP-2 and BMP-7 stimulate new bone formation in critical-size defects, long-bone nonunions, fracture healing, distraction osteogenesis, spinal fusion, augmentation of autografts and allografts, as well as in many faciomaxillary applications.
In 2001, two large randomized clinical trials demonstrated the safety and efficacy of BMP-2 and BMP-7 for treating acute tibial fractures [16] and tibial nonunions [12], following which the FDA issued regulatory approval for specific indications for the use of the recombinant form of both BMPs. Approval was given for recombinant human bone morphogenetic protein-2 (rhBMP-2, InFuse; Medtronic, Minneapolis, MN) for spinal fusion procedures in skeletally mature patients with degenerative disc disease between L2 and S1, and for the treatment of acute, open fractures of the tibial shaft. Humanitarian Device Exemption was given for the use of recombinant human bone morphogenetic protein-7 (rhBMP-7), also called osteogenic protein-1 (OP-1; Stryker Biotech, Hopkinton, MA) as an alternative to autograft in recalcitrant long-bone nonunions (where use of an autograft is unfeasible and alternative treatments have failed) and as an alternative to autograft in compromised patients requiring revision posterolateral lumbar spinal fusions. Full approval for similar indications has been provided by Health Canada for both BMPs.
Despite the concerns and the high recommended BMP doses, recombinant BMPs continue to be used in humans on a large scale. In addition to the United States, OP-1 has been approved in 28 other countries, including Canada, Australia, and the European Union [27]. Although contraindicated in children, BMPs have been used off-label in some difficult nonunions in children [2, 7, 11, 20, 23]. In these cases, the benefits of using BMPs may outweigh the abovementioned concerns. Very few studies on the use of BMPs in children have been reported. Although the healing rate of these studies varied between 20 and 100%, it is difficult to draw conclusions from these studies because they include small sample sizes ranging from one to five patients [2, 7, 11, 20, 23].
The objectives of this short-term followup cohort study were to (1) determine clinical and radiographic healing after osteogenic protein-1 (OP-1) application in children presenting with difficult nonunions; and (2) determine the safety of OP-1 use in this sample as verified by the presence or absence of major adverse events, including allergic reactions, infections, local inflammatory reactions, and heterotopic ossification.
Material and Methods
We retrospectively reviewed the charts and radiographs of all 19 patients who underwent surgery with use of OP-1 at our center between 1999 and 2007. There were 13 male and six female patients with a mean age at the time of surgery of 11.6 years (range, 4.8–20.3 years). The most frequent underlying condition was congenital pseudarthrosis of the tibia (CPT) (n = 10). Other indications included proximal femoral focal deficiency (PFFD) with pseudarthrosis of the femoral neck (n = 2), deficient bone formation in a case of congenital short femur treated by distraction osteogenesis (n = 1), osteogenesis imperfecta (OI) with pseudarthrosis of long bones (n = 2), open tibial fracture with bone loss treated by bone transport (n = 1), persistent nonunion of the lateral humeral condyle (n = 1), large unicameral cyst of the femoral neck (n = 1), and bilateral sacral agenesis (n = 1) in which OP-1 was used as an adjunct to spinal instrumentation. Thirteen of the 19 patients had undergone one or more surgeries prior to OP-1 application including nine patients who had bone grafting surgeries previous to OP-1 application (Table 1). Minimum followup was 4 months (average, 26 months; range, 4–56 months). No patients were lost to followup. Informed consent for the use of OP-1 was obtained from the parents and in addition, in patients operated before 2001, we had to obtain special approval from Health Canada for each individual case. However, after 2001, OP-1 was approved by Health Canada for use in recalcitrant long-bone nonunions and a special approval was no longer required. Approval from the Director of Professional Services was obtained to conduct the chart review, in accordance with the guidelines provided from our local Institutional Review Board.
Table 1.
Patient baseline characteristics and surgical details
| Case number | Gender, Age (yr) | Etiology | Bone affected | Indications for OP1 use | Number of previous surgeries | Surgical procedure | OP-1 application (alone/mixed) | Number of application sites | Outcome (healed/not healed) | Time to healing (wk) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1A | M, 14 | CPT | Left tibia | Persistent non-union | 3 | Resection of pseudarthrosis | Alone | 1 | Not healed | – |
| 1B | M, 15 | CPT | Left tibia | Persistent non-union | 4 | Resection of pseudarthrosis | Mixed | 1 | Healed | 11 |
| 2 | M, 10 | PFFD | Right femur | Delayed union of distraction callus | 2 | Femoral lengthening | Mixed | 1 | Not healed | – |
| 3 | M, 11 | CPT | Right tibia | Persistent non-union | 2 | Resection of pseudarthrosis and vascularized bone graft (VBG) | Alone at both VBG sites | 2 | Healed in both sites | 24 and 24 |
| 4 | F, 13 | CPT | Left tibia | Persistent non-union | 4 | Resection of pseudarthrosis | Mixed | 1 | Healed | 18 |
| 5 | M, 8 | CPT | Left tibia | Correction of deformity after healed fracture | 0 | Double osteotomy and bone graft | Mixed | 2 | Healed in both sites | 12 and 12 |
| 6 | M, 12 | CPT | Right tibia | Persistent non-union | 4 | Resection of pseudarthrosis | Mixed | 1 | Healed | 56 |
| 7A | F, 10 | OI - III | Left humerus | Non-union after fracture | 0 | Resection of pseudarthrosis | Mixed | 1 | Not healed | – |
| 7B | F, 10 | OI - III | Left humerus | Persistent non-union | 1 | Resection of pseudarthrosis | Mixed | 1 | Not healed | – |
| 8 | M, 5 | Bone cyst | Right femur | Right femoral proximal metaphysis cyst | 0 | Curetage | Mixed | 1 | Not healed | – |
| 9 | M, 14 | Bone transport | Right tibia | Non-union after bone transport | 3 | Resection of pseudarthrosis | Alone | 1 | Healed | 28 |
| 10 | M, 20 | CPT | Left tibia | Persistent non-union | 2 | Resection of pseudarthrosis and osteotomy | Alone | 1 | Not healed | – |
| 11A | F, 15 | CPT | Left tibia | Non-union | 0 | Resection of pseudarthrosis | Mixed | 1 | Not healed | – |
| 11B | F, 16 | CPT | Left tibia | Persistent non-union | 1 | Resection of pseudarthrosis | Mixed | 1 | Not healed | – |
| 11C | F, 17 | CPT | Left tibia | Persistent non-union | 2 | Resection of pseudarthrosis | Mixed | 1 | Healed | 31 |
| 12 | F, 6 | PFFD | Right femur | Persistent non-union of femoral neck osteotomy | 3 | Resection of pseudarthrosis | Mixed | 1 | Not healed | – |
| 13 | M, 11 | PFFD | Left femur | Persistent non-union of femoral neck osteotomy | 2 | Resection of pseudarthrosis | Mixed | 1 | Healed | 60 |
| 14 | M, 11 | CPT | Right tibia | Tibial deformity | 0 | Osteotomy | Alone | 1 | Healed | 8 |
| 15 | M, 4 | CPT | Right tibia | Tibial deformity | 1 | Resection of pseudarthrosis | Alone | 1 | Healed | 13 |
| 16 | M, 11 | OI - IV | Right tibia | Non-union of previous osteotomy | 3 | Resection of pseudarthrosis | Mixed | 1 | Healed | 14 |
| 17 | F, 7 | Sacrum agenesis | Sacrum | Non-union | 1 | Bone graft | Mixed | 2 | Healed in 1 site | 8 |
| 18 | F, 9 | Persistent non-union of lateral condyle | Right humerus | Non-union | 3 | Resection of pseudarthrosis | Mixed | 1 | Healed | 12 |
| 19 | M, 8 | CPT | Left tibia | Tibial deformity | 0 | Double osteotomy | Alone | 2 | Healed in both sites | 16 and 22 |
CPT = congenital pseudarthrosis of the tibia; PFFD = proximal femoral focal deficiency; OI = osteogenesis imperfecta.
All operations were performed by the two senior authors (FF and RH). In most cases, the nonvascular tissues at the nonunion site were excised until bleeding bone was exposed. Irrigation of the wound and very good hemostasis were conducted prior to OP-1 application. The OP-1 implant was supplied by Stryker Biotech (Hopkinton, Mass.). Each sterile package contained 3.5 mg of the OP-1 mixed with 1 g of Type I bovine collagen (the total reconstituted volume was approximately 4 mm per unit). Not more than one vial of OP-1 was used in each of the 19 cases. In the majority of cases (16 of 23 application sites), OP-1 was applied in conjunction with an autogenous iliac crest bone graft (mixed). The anatomical sites where OP-1 was applied included 12 tibias, four femurs, two humeri, and one sacrum. Four patients had an OP-1 application to two different anatomical sites during the same surgery (three patients with CPT had OP-1 applications to both proximal and distal tibia; one patient with sacral agenesis had a bilateral application) bringing the total number of sites that received OP-1 to 23. Repeat OP-1 application was performed in three patients because of a persistent nonunion; one patient had two additional applications and two patients had one additional application each.
Most of these patients are regularly followed clinically and radiographically until skeletal maturity, specifically children with CPT, PFFD and OI. Two pediatric orthopedic surgeons (BD, RH) evaluated patients for clinical evidence of healing, including the absence of pain and tenderness at the nonunion site and the ability to fully weight bear on the affected limb with an orthosis. The same two raters (BD, RH) independently evaluated all radiographs for healing and reached a consensus. For radiographic evidence of healing, we followed the criterion of Friedlaender et al. [12], where radiographic healing was determined by the presence of bone bridging at the site of the nonunion in at least one view. Similar to the study of Friedlaender et al. [12], we did not use more rigorous radiological criteria. A nonunion was considered healed if it fulfilled both clinical and radiographic criteria.
Determining the safety of OP-1 use in this sample consisted of evaluating the presence of any adverse event occurring following OP-1 application. Since OP-1 application is not a standard treatment procedure, extra caution was taken to identify all adverse events, including peri- and postoperative complications, presence of local inflammatory reactions, heterotopic ossification, infection, refractures, and the consequences of applying OP-1 more than once (repetitive use).
Results
Healing occurred in 17 of the 23 sites at the last followup and was judged to have healed between 8 and 60 weeks. Healing was not achieved in six patients (six sites) at followup times ranging between 18 to 52 months (Table 2). Case 11 successfully healed (Fig. 1A–F). Case 2 underwent an iliac crest bone graft to the femur despite OP-1 application due to persistent nonunion (Fig. 2A–C). Healing rates of the present study were compared to that of five other pediatric studies that used OP-1 (Table 3).
Table 2.
Outcome of patients in whom healing was not achieved after OP-1 application
| Case number | Etiology | Outcome |
|---|---|---|
| 2 | Congenital short femur, femoral lengthening resulting in poor regenerate. | Healed after iliac crest bone grafting. |
| 7 | Osteogenesis imperfecta. Non-union of the distal humerus. | No further surgery. Asymptomatic with brace. |
| 8 | Cyst in the proximal femur. | Lost to follow-up. |
| 10 | Congenital pseudarthrosis of the tibia (CPT). | Refused further surgery. Healed clinically but not radiologically. |
| 12 | Proximal femoral focal deficiency (PFFD). Pseudarthrosis of the femoral neck. | Waiting for further surgery. |
| 17 | Sacral agenesis. | Asymptomatic. No further surgery planned for the time being. |
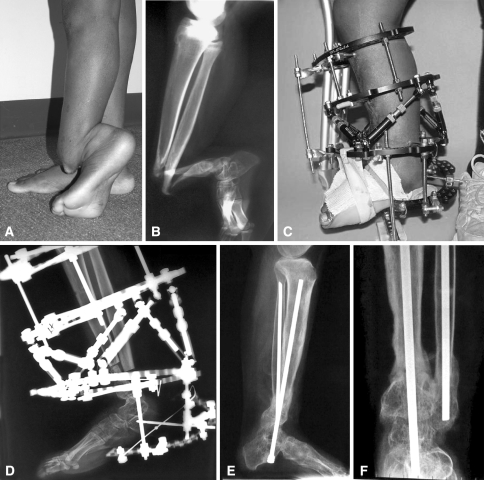
Fig. 1A–F.
(A) This 15-year-old patient with congenital pseudarthrosis of the tibia (CPT) had severe angulation of her left tibia, (B) illustrated here in a preoperative radiograph. (C) Postoperative realignment is shown with a circular fixator after excision of the pseudarthrosis site, OP-1 application mixed with iliac crest bone grafting and gradual correction; (D) also shown radiographically. (E) Lateral and (F) anteroposterior view radiographs 31 weeks after the initial surgery show healing of the pseudarthrosis site.
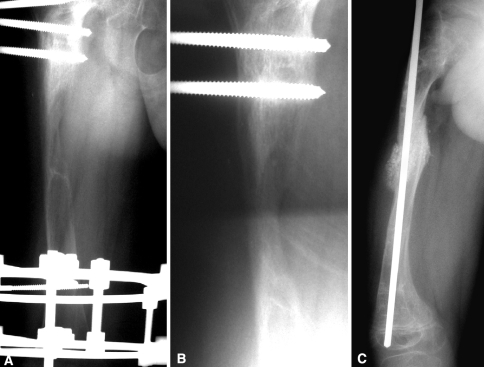
Fig. 2A–C.
These radiographs illustrate (A) poor regenerate bone of a congenital short femur with an Ilizarov fixator 7 months after lengthening. (B) The bone was still not healed 1 month post-OP-1 application in the middle of the regenerate through a mini incision. (C) Bone healing was achieved following iliac crest bone grafting.
Table 3.
Healing outcome and adverse events in pediatric OP-1 studies
| Authors, year | Sample size | Healing rate (%) | Mean healing time (weeks) | Adverse events |
|---|---|---|---|---|
| Dohin et al. (current study) | 19 | 74 | 22 | One patient experienced a severe pin site infection, four patients had superficial pin site infections, one patient with CPT sustained an undisplaced fracture of the tibia while having the fixator still in place and another patient with CPT had a refracture after healing. |
| Kujala et al. 2008 [20] | 1 | 100 | 32 | Refracture and deep infection of the pseudarthrosis site four months after initial healing, requiring one more operation. |
| Burkart and Rommens 2008 [7] | 1 | 100 | 24 | No adverse event reported. |
| Anticevic et al. 2006 [2] | 1 | 100 | 4 | Not reported. |
| Fabeck et al. 2006 [11] | 1 | 100 | 5 | Not reported. |
| Lee et al. 2006 [23] | 5 | 20 | 24 | One patient had sterile serous drainage from the wound, which was treated with antibiotic. |
There were no allergic or anaphylactic reactions. One patient (Case 11) developed a severe deep infection that necessitated several debridements under general anesthesia. This patient had two successive applications of OP-1 until the pseudarthrosis site healed. At the 31-week followup, the patient was walking with full weight bearing without support and had complete radiographic healing (Fig. 1). Four patients had superficial pin site infections that were successfully treated with intravenous antibiotics. One patient with CPT (Case 4) had a refracture of the tibia 13 months after successful initial OP-1 application. Additional surgery included iliac crest bone graft and stabilization with an Ilizarov frame. The frame was removed 4 months after this latter surgery as healing was achieved. Another patient (Case 11) sustained an undisplaced fracture of the proximal tibia while having the fixator in place which resolved with nonoperative treatment. There were no patients with local inflammatory reactions or heterotopic ossification. No adverse event was related to additional applications of OP-1 or application of OP-1 at more than one site.
Discussion
OP-1 possesses osteogenic potential contributing to the healing of persistent nonunions. Numerous studies have described the use of OP-1 in adults, but there are few reports in children. Although the healing rate of the pediatric studies varied between 20 and 100%, conclusions cannot be drawn because these studies included very small sample sizes. The objectives of this study were to examine clinical and radiographic healing after OP-1 application in children presenting with persistent nonunions, and to determine the safety of OP-1 use in this sample as verified by the presence or absence of major adverse events.
There are several limitations to this study. First, this study consists of a small series of patients with different pathologies affecting various anatomical sites. Second, we had no control group and OP-1 was always applied in conjunction with other surgical techniques of stabilization and/or cancellous bone grafts. Thus, it is difficult to define the exact role of OP-1 in the healing process, especially as a substantial number of patients who had CPT were relatively close to skeletal maturity and therefore maybe healed because of the bone graft, the revised internal fixation, or due to a decreased risk of fracture with increasing age. Third, the followup was not long enough in most cases to get to the point of refracture, specifically when considering cases of CPT in which a higher refracture rate is expected. Fourth, factors such as age, gender, diagnosis, site of nonunion, previous surgeries, method of OP-1 application, and number of OP-1 application sites may have an impact on healing. However, the small sample size and heterogeneous etiologies precluded such analyses. Fifth, we did not rigorously assess the growth plates, therefore we are unable to conclude whether OP-1 had any effect on the growth plates. Sixth, we did not measure BMP antibodies in our patients. In two studies, circulating antibodies against Type 1 collagen and anti-OP-1 [12] or anti-BMP-2 [16] antibodies were detected in a small proportion of patients treated with BMPs, however studies [13, 16] have concluded that there was insufficient information to establish a causal relationship between these antibodies and absence of ossification.
We observed clinical and radiographic healing in 17 of 23 sites, thus giving an overall healing rate of 74%. This compares favorably with other reported studies in the literature, which report a success rate varying between 20% and 100%. In the pediatric literature, we identified five reports of OP-1 use, four which concluded with successful healing after several previous failed surgeries [2, 7, 11, 20] (Table 3). Similarly, healing rates following OP-1 use in adults range between 75% and 89% [12, 24]. It remains unclear as to why union did not occur in all patients and why the clinical response to BMPs in humans is not as robust as reported in animal studies [10, 22]. Several possibilities could explain the unpredictable results of BMPs in humans: the presence of BMP antagonists [9, 21, 32], the stimulatory effect BMPs have on osteoclasts and bone resorption [15], the presence of anti-BMP antibodies [12, 13, 16], the very short half-life of BMPs [30], the absence of an adequate delivery system for BMPs [28], and the lack of sufficient numbers of responding cells at the site of application in the host [10]. It is possible that the addition of a single BMP (BMP-2 or BMP-7) to the defective bony site may not be enough to fully promote osteogenesis, and that a cocktail of growth factors with simultaneous or sequential activity may be required [10]. Furthermore, the poor healing environment that is associated with these difficult and challenging conditions, such as congenital pseudarthrosis of the tibia [20], may defy any type of bone graft substitute.
The local adverse events experienced by our patients were few and consistent with other reports in the pediatric literature [2, 7, 11, 20, 23]. Other studies using BMPs in an adult population also report no or very few local adverse events related to BMP-7 application for long-bone nonunion [24] or for the healing of tibial fractures [12, 26]. None of our patients experienced a local inflammatory reaction, nor did they develop ectopic ossification at the site of OP-1 application. While this local complication is very rare with the use of OP-1 in long bones, two recent case studies have reported ectopic ossification in the humerus after OP-1 or rhBMP-2 application [3, 34]. The incidence of infection was also very low in our series and included one patient who developed a deep infection and four patients with superficial infections; however these infections are not believed to be directly related to OP-1.
Both OP-1 and rhBMP-2 are contraindicated in patients who may be allergic to any of the materials contained in the devices, patients who have an infection near the area of the surgical incision, and during pregnancy as BMPs can cross the placenta and the long-term effects on the fetus remain unknown [28]. These should be used with caution in women of child-bearing age. Patients who have a tumor removed from the area of the implantation site or currently have a tumor in that area should not be administered BMPs. Concerns regarding the use of BMPs in children include the unknown effects BMPs may have on the growth plate; therefore the use of BMPs in skeletally immature patients is not usually recommended. In addition, the clinical consequences of anti-BMP antibody formation and of the repetitive use of OP-1 require further investigation. Other side effects of BMPs may include ectopic ossification and local inflammatory reactions; however, two studies suggest that when BMPs are used in the current therapeutic dosages there is a negligible risk of excessive bone formation at the implantation site [12, 16].
The long-term effects of BMPs, specifically regarding carcinogenesis, remain largely unknown. Although BMPs are expressed in many tumors [25], no cases of malignancy have been reported as a direct result of BMP application. The effect of local application of BMPs in cases of existing malignancies remains controversial; a beneficial effect of BMP-7 or BMP signaling in inhibiting cancer cells has been reported [5, 6, 17, 33], while other studies [18, 19] have reported a negative effect. Several authors have indicated that OP-1 does not seem to have a carcinogenic effect [25, 28, 30]. As data on safety of BMPs in cancer patients remains inconclusive [1], we do not recommend using BMPs in children with cancer.
Despite the limitations, our data suggest OP-1 is associated with few complications in the short term and results in healing of most complicated fractures in children. We recommend using OP-1 in children with persistent nonunion who have not responded to standard methods of treatment and as a first line of treatment for cases of CPT. Before recommending the use of OP-1 in children with bone cysts, a randomized clinical trial comparing the efficacy of OP-1 with steroid injection (standard care) needs to be completed. We should not forget that BMPs are only signals and that without correction of alignment, rigid fixation, and a healthy viable environment, they will not work. Future studies on OP-1 use in children including a larger sample size, longer followup period to get to the point of refracture in cases of CPT, and more comprehensive adverse event analysis (including immunological studies) are warranted to investigate its long-term safety in this population.
Acknowledgments
We thank Denis Alves, Guylaine Bédard, and Marc Lepik from the Medical Illustration Department of the Shriners Hospital for Children for assistance with the images.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Shriners Hospital for Children, Montreal, Canada.
References
- 1.American Academy of Orthopaedic Surgeons. BMPs and cancer: is the risk real? Available at: http://www.aaos.org/news/aaosnow/may08/research7.asp. Accessed August 25, 2008.
- 2.Anticevic D, Jelic M, Vukicevic S. Treatment of a congenital pseudarthrosis of the tibia by osteogenic protein-1 (bone morphogenetic protein-7): a case report. J Pediatr Orthop B. 2006;15:220–221. [DOI] [PubMed]
- 3.Axelrad TW, Steen B, Lowenberg DW, Creevy WR, Einhorn TA. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J Bone Joint Surg Br. 2008;90:1617–1622. [DOI] [PubMed]
- 4.Bax B, Wozney J, Ashhurst D. Bone morphogenetic protein-2 increases the rate of callus formation after fracture of the rabbit tibia. Calcif Tissue Int. 1999;65:83–89. [DOI] [PubMed]
- 5.Buijs JT, Henriquez NV, van Overveld PGM, van der Horst G, Que I, Schwaninger R, Rentsch C, ten Dijke P, Cleton-Jansen A-M, Driouch K, Lidereau R, Bachelier R, Vukicevic S, Clezardin P, Papapoulos SE, Cecchini MG, Lowik CWGM, van der Pluijm G. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007;67:8742–8751. [DOI] [PubMed]
- 6.Buijs JT, Rentsch CA, van der Horst G, van Overveld PGM, Wetterwald A, Schwaninger R, Henriquez NV, ten Dijke P, Borovecki F, Markwalder R, Thalmann GN, Papapoulos SE, Pelger RCM, Vukicevic S, Cecchini MG, Lowik CWGM, van der Pluijm G. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am J Pathol. 2007;171:1047–1057. [DOI] [PMC free article] [PubMed]
- 7.Burkhart KJ, Rommens PM. Intramedullary application of bone morphogenetic protein in the management of a major bone defect after an Ilizarov procedure. J Bone Joint Surg Br. 2008;90:806–809. [DOI] [PubMed]
- 8.den Boer FC, Bramer JAM, Blokhuis TJ, Van Soest EJ, Jenner JMGT, Patka P, Bakker FC, Burger EH, Haarman HJTM. Effect of recombinant human osteogenic protein-1 on the healing of a freshly closed diaphyseal fracture. Bone. 2002;31:158–164. [DOI] [PubMed]
- 9.Dimitriou R, Tsiridis E, Carr I, Simpson H, Giannoudis PV. The role of inhibitory molecules in fracture healing. Injury. 2006;37S:S20–S29. [DOI] [PubMed]
- 10.Einhorn TA. Clinical applications of recombianat human BMPs: early experience and future development. J Bone Joint Surg Am. 2003;85:82–88. [DOI] [PubMed]
- 11.Fabeck L, Ghafil D, Gerroudj M, Baillon R, Delincé P. Bone morphogenetic protein 7 in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 2006;88:116–118. [DOI] [PubMed]
- 12.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001; 83:S151–S158. [PMC free article] [PubMed]
- 13.Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br. 1999;81:710–718. [DOI] [PubMed]
- 14.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36:S20–S27. [DOI] [PubMed]
- 15.Giannoudis PV, Kanakaris NK, Einhorn TA. Interaction of bone morphogenetic proteins with cells of the osteoclast lineage: review of the existing evidence. Osteoporos Int. 2007;18:1565–1581. [DOI] [PubMed]
- 16.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84:2123–2134. [DOI] [PubMed]
- 17.Hallahan AR, Pritchard JI, Chandraratna RAS, Ellenbogen RG, Geyer JR, Overland RP, Strand AD, Tapscott SJ, Olson JM. BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med. 2003;9:1033–1038. [DOI] [PubMed]
- 18.Hsu M-Y, Rovinsky S, Penmatcha S, Herlyn M, Muirhead D. Bone morphogenetic proteins in melanoma: angel or devil? Canc Metastasis Rev. 2005;24:251–263. [DOI] [PubMed]
- 19.Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K, Imamura T. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27:6322–6333. [DOI] [PubMed]
- 20.Kujala S, Vähäsarja V, Serlo W, Jalovaara P. Treatment of congenital pseudarthrosis of the tibia with native bovine BMP Acta Orthop Belg. 2008;74:132–136. [PubMed]
- 21.Kwong FN, Hoyland JA, Freemont AJ, Evans CH. Altered relative expression of BMPs and BMP inhibitors in cartilaginous areas of human fractures progressing towards nonunion. J Orthop Res. 2009;27:752–757. [DOI] [PMC free article] [PubMed]
- 22.Lane JM. BMPs: why are they not in everyday use? J Bone Joint Surg Am. 2001;83:S161–S162. [PubMed]
- 23.Lee FY, Sinicropi SM, Lee FS, Vitale MG, Roye DJ, Choi IH. Treatment of congenital pseudarthrosis of the tibia with recombinant human bone morphogenetic protein-7 (rhBMP-7). A report of five cases. J Bone Joint Surg Am. 2006;88:627–633. [DOI] [PubMed]
- 24.McKee MD. Recombinant human bone morphogenic protein-7: applications for clinical trauma. J Orthop Trauma. 2005;19:S26–S28. [DOI] [PubMed]
- 25.Obert L, Deschaseaux F, Garbuio P. Critical analysis and efficacy of BMPs in long bones non-union. Injury. 2005;36:S38–S42. [DOI] [PubMed]
- 26.Ristiniemi J, Flinkkila T, Hyvonen P, Lakovaara M, Pakarinen H, Jalovaara P. RhBMP-7 accelerates the healing in distal tibial fractures treated by external fixation. J Bone Joint Surg Br. 2007;89:265–272. [DOI] [PubMed]
- 27.Stryker. OP-1 Implant for Fracture Repair. Available at: http://www.stryker.com/en-us/products/Orthobiologicals/Osteoinductive/OP-1/index.htm. Accessed August 25, 2008.
- 28.Termaat M, Den Boer FC, Bakker FC, Patka P, Haarman HJTM. Current concepts review. Bone Morphogenetic Proteins. Development and clinical efficacy in the treatment of fractures and bone defects. J Bone Joint Surg Am. 2005;87:1367–1378. [DOI] [PubMed]
- 29.Urist M. Bone: formation by autoinduction. Science. 1965;150:893–899. [DOI] [PubMed]
- 30.Vaibhav B, Nilesh P, Vikram S, Anshul C. Bone morphogenetic protein and its application in trauma cases: a current concept update. Injury. 2007;38:1227–1235. [DOI] [PubMed]
- 31.Watanabe K, Tsuchiya H, Sakurakichi K, Tomita K. Bone transport using hydroxapatite loaded with bone morphogenetic protein in rabbits. J Bone Joint Surg Br. 2007;89:1122–1129. [DOI] [PubMed]
- 32.Westerhuis RJ, van Bezooijen RL, Kloen P. Use of bone morphogenetic proteins in traumatology. Injury. 2005;36:1405–1412. [DOI] [PubMed]
- 33.Wu WKK, Sung JJ, Wu YC, Li ZJ, Yu L, Cho CH. Bone morphogenetic protein signalling is required for the anti-mitogenic effect of the proteasome inhibitor MG-132 on colon cancer cells. Br J Pharmacol. 2008;154:632–638. [DOI] [PMC free article] [PubMed]
- 34.Wysocki RW, Cohen MS. Ectopic ossification of the triceps muscle after application of bone morphogenetic protein-7 to the distal humerus for recalcitrant nonunion: a case report. J Hand Surg Am. 2007;32:647–650. [DOI] [PubMed]
- 35.Yasko A, Lane J, Fellinger E, Rosen V, Wozney J, Wang E. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992;74:659–670. [PubMed]