Abstract
The small GTPase Rho and Rho-associated protein kinase (Rho kinase, ROCK) signal participates in a variety of biological functions including vascular contraction, tumor invasion, and penile erection. Evidence also suggests Rho-ROCK is involved in signaling for mesenchymal cellular differentiation. However, whether it is involved in osteoblastic differentiation is unknown. We therefore asked whether Rho-ROCK signaling participates in recombinant human bone morphogenetic protein (rhBMP-2)-induced osteogenesis both in vitro and in vivo. Continuous delivery of a specific ROCK inhibitor (Y-27632) enhanced ectopic bone formation induced by rhBMP-2 impregnated into an atelocollagen carrier in mice without affecting systemic bone metabolism. Treatment with Y-27632 also enhanced the osteoblastic differentiation of cultured murine neonatal calvarial cells. These effects were associated with increased expression of BMP-4 gene. Expression of a dominant negative mutant of ROCK in ST2 cells promoted osteoblastic differentiation, while a constitutively active mutant of ROCK attenuated osteoblastic differentiation and the ROCK inhibitor reversed this phenotype. Thus, ROCK inhibits osteogenesis, and a ROCK inhibitor in combination with the local delivery of rhBMP/collagen composite may be clinically applicable for stimulating bone formation.
Introduction
Bone morphogenetic protein (BMP), a potent inducer of bone formation both in vitro and in vivo, promotes the differentiation of mesenchymal cells into osteoblasts and is believed responsible for fracture repair or regeneration of bone defects [32, 33]. Implantation of BMP can elicit new bone formation both in orthotopic and heterotopic sites [13, 27], and is used as a bone graft substitute in the clinical setting [8, 24, 30]. Although it is now possible to generate recombinant human BMPs for medical use, more than 1.5 mg/kg of the recombinant protein has been required for bone induction in primates [1, 15], probably due to their reduced capability for tissue regeneration. Therefore, the major challenge still remains in the enhancement of its activity to induce early and optimal bone formation in humans.
The biological activity of BMP is regulated by various growth factors or cytokines through several intracellular signaling pathways, including Smad protein signaling [11, 20]. To explore the mechanism for BMP-induced bone formation, we developed an in vivo experimental model system for the local delivery of recombinant human BMP-2 (rhBMP-2) impregnated in pepsin-digested Type I collagen [29]. With this model we can reproducibly induce ectopic bone and assess bone formation quantitatively in any wild-type or transgenic mice [17, 34]. Our studies suggest granulocyte colony-stimulating factor (G-CSF) [17], basic calponin [34], and TNP-470 [22] (a synthetic anti-angiogenic agent) inhibit BMP-induced bone formation. Using this in vivo system, it should be possible to search for stimulators of BMP-induced bone formation.
The small GTPase Rho and its target Rho-associated protein kinases (Rho kinase, p160ROCK) [9, 18, 19] control cell adhesion [10] and motility [35] through reorganization of the actin cytoskeleton and regulation of actomyosin contractility [3] in a number of cellular processes, including vascular contraction [31], tumor invasion [12], penile erection [2], and apoptosis [4]. Further, several lines of evidence indicated that Rho-ROCK signaling also has been involved in cellular transformation. NIH3T3 cells expressing active Rho or ROCK representing enhanced transformation capability and a specific inhibitor (Y-27632 [31]) for ROCK-inhibited Rho, ROCK, or even Ras-induced cellular transformation [28]. This accumulating evidence suggests Rho-ROCK signaling is also involved as molecular switch for lineage-specific cellular differentiation. However, little is known about the involvement of this signaling for the mesenchymal cellular differentiation including osteogenesis.
To this end, we asked: (1) Does ROCK inhibitor enhance BMP-induced bone formation in vivo? (2) What kind of cells migrate to the BMP/atelocollagen pellets to induce bone formation in vivo? (3) Does ROCK inhibitor itself enhance osteogenic differentiation of primary cultured calvaria cells in vitro? (4) What is the mechanism for ROCK inhibitor-induced osteogenic differentiation? (5) Does ectopic expression of dominant negative ROCK enhance osteoblastic differentiation and does ectopic expression of active ROCK inhibit it?
Materials and Methods
In order to answer our five study questions, we carried out the experiments in the following order. First, we assayed ectopic bone formation using BMP/atelocollagen pellets with or without ROCK inhibitor in mice. Next, we analyzed the phenotype of the cells that migrated to the BMP/atelocollagen ossicles using an in situ hybridization method. We then examined the effects of ROCK inhibitor on osteoblastic differentiation in vitro using primary culture of osteoblast from newborn mice. We then analyzed the mechanism of Rho-ROCK signaling on the expression of BMP transcript (RNA) in mesenchymal ST2 cells in vitro. Finally, we confirmed the inhibitory role of ROCK on the osteogenesis by the introduction and expression of ROCK mutants in ST2 cells.
BMP/atelocollagen pellets containing 5 μg of rhBMP-2 (kindly provided by Genetics Institute, Cambridge, MA, through Astellas Pharma Inc., Tokyo, Japan) and 3 mg of atelocollagen (Nitta Gelatin, Osaka, Japan) were implanted into a dorsal subfascial pocket of eight 5-week-old male ICR mice. Six mice were sacrificed at 4 days, six at 1 week, and six at 2 weeks after implantation of the pellets. Recovered ossicles were examined radiographically with a soft xray apparatus (Softex Type SM, Osaka, Japan) and histologically. Calcium content in the ossicles was quantified as follows: the ossicles were defatted with trichloroethylene and methanol (1:1) for 24 hours, dried by evaporation, and then decalcified with 1 mL of 0.6 N HCl for 24 hours. The calcium content of the HCl-supernatants was measured using the calcium C-test (Wako Pure Chemicals, Osaka, Japan). Tissue preparation and in situ hybridization were carried out as previously described [7, 25]. Harvested tissues were fixed in 4% paraformaldehyde, dehydrated in an ethanol series, and embedded in paraffin wax. Six sections 4 μm thick were hybridized with digoxigenin-labeled antisense and sense cRNA probes for mouse BMP-4 [7], mouse osteopontin [25], and mouse a1 chain of procollagen Type I [25]. Hybridization proceeded at 50°C for 16 hours with 0.5 mg/mL of each RNA probe in hybridization buffer (50% deionized formamide, 10% dextran sulfate, 1 × Denhardt’s solution, 4 × SSC, 3 mg/mL of Escherichia coli tRNA), followed by washing and RNase treatment (10 μg/mL) at 37°C for 30 minutes. Hybridized probes were detected using a nucleic acid detection kit (Roche Diagnostic, Japan) according to the manufacturer’s instructions. Sections hybridized with sense probes showed no positive signals.
Tetracycline double-labeling analysis was performed using six undecalcified sections of the tissues. Tetracycline injection (30 mg per kg body weight) was performed at 2 and 4 days (for tibia), or at 3 hours (for ossicles) before sacrifice of the animals. The tibia and the ectopic ossicles were recovered, fixed in 70% methanol, stained in Villanueva bone stain, dehydrated in increasing concentrations of ethanol, defatted in xylene, and embedded in methyl methacrylate. Histomorphometric analyses were performed with a semiautomatic digitizing image analyzer. The system consisted of a light or epifluorescent microscope and a digitizing pad coupled to a computer with histomorphometric software (System Supply Co., Nagano, Japan).
Twenty to thirty primary cultures of murine calvarial cells were obtained from the twenty to thirty neonatal ICR mice 1 day after birth by sequential collagenase/trypsin digestion [34]. The mouse bone marrow-derived stromal cell line ST2 (RCB0224) was obtained from the Riken Cell Bank (Tsukuba, Japan). Neonatal murine calvarial cells were maintained in α-MEM containing 10% FCS, and ST2 cells were maintained in RPMI1640 containing 10% FBS (Invitrogen).
To determine ALP activity, ST2 cells were split, plated at a density of 1 × 104 cells per cm2 and cultured for 24 hours in growth medium on 48 plastic culture dishes. MC3T3-E1 cells were plated at a density of 1 × 105 cells per cm2 and cultured for 24 hours in growth medium. In some experiments, ST2 cells were cultured in α-modified minimum essential medium (a-MEM, Invitrogen) containing 10% FBS on a collagen I-coated culture dish (Celltight C-1, Sumitomo Bakelite Co., Japan). rhBMP-2 and/or Y-27632 were added at various concentrations to the subconfluent cultures in the presence of growth medium. The culture medium was changed daily and the cells were further cultured for the indicated time period. After removing the culture media, cells were washed with PBS and sonicated for 10 seconds at 0°C in 20 mM Tris-HCl, pH 7.5, 0.1% Triton X-100, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride. The ALP activity was determined using p-nitrophenyl-phosphate as a substrate according to the Kind and King’s method (Wako Pure Chemicals).
Primary culture of calvarial cells was plated onto four 12-well plates at the density 2 × 104 cells/cm2 with α-MEM containing 10% FBS. At 18 hours after plating, the culture media were replaced α-MEM containing 5% FBS, 5 mM β-glycerol phosphate (Sigma), and 100 μg/mL ascorbic acid without or with 3, 5, 10, 20, 30 μM Y-27632. Cultured for 16 days, cells were fixed for 30 minutes with 3.7% formaldehyde/PBS at RT. After washing with PBS, nodules were stained with 1% alizarin red S (Sigma) at pH 6.0 [34].
To measure the level of BMP transcript, 39 samples (3 replicates for 13 experiments: zero days; and 1, 2, and 3 days for two Y-27632 treatment and two doses rhBMP-2) of total RNA were prepared from culture cells using Trizol reagent (Life Technologies). RNA was denatured by the treatment with 1.1 M glyoxal, run in a 1.2% agarose gel, and then transferred for 8 hours by capillary action to a Hybond-N+ membrane (Amersham, UK) and UV cross-linked with a cross-linker (1200 watts) (Stratagene). A [α-32P] dCTP-labeled probe was made with Multiprime DNA labeling systems (Amersham). The membrane was hybridized for 2 hours at 65°C with the Rapid-hyb buffer (Amersham) containing the radioactive probe. After hybridization, the filters were washed twice for 10 minutes at room temperature in 0.1% SDS-2 × SSC and twice for 30 minutes at 65°C in 0.1% SDS-0.2 × SSC. Processed filters were analyzed using a FLA-2000 image analyzer (Fujifilm Co, Japan). Full-length cDNA for murine BMP-4 was synthesized by RT-PCR. The reverse transcriptase reaction was carried out using random hexamers and Moloney murine leukemia virus reverse transcriptase (Takara, Otsu, Japan). The PCR reaction profile included 30 cycles of denaturation for 1 minute at 95°C, annealing for 1 minute at 50°C and extension for 1 minute at 72°C using Pfx DNA polymerase (Invitrogen). The PCR product was separated by agarose gel electrophoresis, subcloned into pCR-ScriptSK(+) vector (Stratagene), and sequenced with an ABI PRISM model 377 sequencer (Perkin Elmer). The 1.2 k base pairs of murine full-length BMP-4 cDNA was used for a specific probe for RNA blot. 3′-UTR region of murine cbfa-1 gene was used for RNA blotting as previously reported [7].
cDNAs encoding the catalytic domain of ROCK (amino acids, 1 to 477) and the catalytic domain with a Lys105-> Ala mutation in the ATP-binding region (KD) [9], each tagged at the NH2-terminus with the FLAG epitope, were inserted into the Kpn I and Xba I sites of the pcDNA3 vector (Invitrogen). ST2 cells were transiently transfected with the resulting expression vectors by Lipofectamine Plus reagent (Invitrogen) following the manufacturer’s protocol.
Data were expressed as means ± SEM in all figures. We determined the difference in ossicle wet weight, calcium content, and all bone histomorphometric parameters by the ROCK inhibitor in vivo using Student’s t-test. We determined the difference in ALP activity and osteocalcin production in cultured calvaria cells by the ROCK inhibitor in vitro using Student’s t-test. We determined the difference in BMP-4 transcript and ALP activity by the ROCK inhibitor or expression of ROCK mutants in ST2 cells in vitro using Student’s t-test. All statistical analyses were performed using a JMP8.0 software package (SAS Institute Inc, NC).
Results
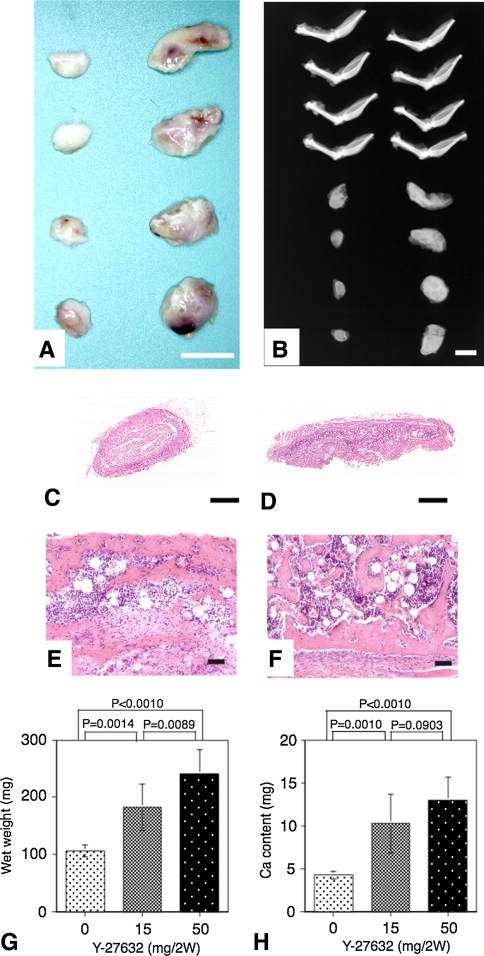
The ossicles induced by the rhBMP-2/collagen composites with Y-27632 treatment for 2 weeks were larger, contained reddish-colored bone marrow and highly dense xray-absorbed calcium when compared with those induced in phosphate buffered saline (PBS)-treated control mice (Fig. 1A–B). Microscopic observations (Fig. 1C–F) revealed the normal bone formation with fully mature bone marrow and no obvious difference apart from bone volume between two groups. The concentrations of the inhibitor in the serum (1 ± 0.3 μM for 15 mg/2w, 3.5 ± 0.7 μM for 50 mg/2w) and the tissue (28.9 ± 12.6 μM for 15 mg/2w, 70.3 ± 18.6 μM for 50 mg/2w) were positively correlated with the bone wet weight (Fig. 1G) or calcium content (Fig. 1H).
Fig. 1A–H.
ROCK inhibitor stimulated the ectopic bone formation in vivo induced by BMP. (A) Macroscopic pictures of ectopic ossicles 2 weeks after implantation of BMP/atelocollagen composites containing 5 μg of rhBMP-2, in control (left) and Y-27632-treated (right) mice are shown. Scale bar, 10 mm. (B) Radiographs of the ossicles and the bones of the lower extremities in control (left) and Y-27632-treated (right) mice are shown. Scale bar, 10 mm. Cross-sections of the ossicles in (C) control and (D) Y-27632-treated mice are shown (Stain, hematoxylin and eosin; original magnification ×10; scale bar, 1 mm). Calcified trabeculae and maturated bone marrow can be seen in both (E) control and (F) Y-27632-treated mice (Stain, hematoxylin and eosin; original magnification ×10; scale bar, 100 μm). (G) Wet weight of each ossicle is present (mean ± SEM, n = 4). (H) Calcium content of each ossicle is present (mean ± SEM, n = 4) (in the absence of Y-27632).
The treatment of ROCK inhibitor did not affect (p > 0.1) the three osteoblastic and three osteoclastic parameters in the tibia (Table 1). In the ossicles, one bone-formation parameter (BV/TV) increased (p = 0.027) and two bone absorption parameters (Oc.S/BS and N.Oc/BS) decreased (p = 0.019 and 0.011, respectively) by the treatment with ROCK inhibitor. Taken together, the treatment of ROCK inhibitor per se did not affect the general bone metabolism in the mice, and the stimulatory effects of the inhibitor on the osteogenesis were restricted to the BMP-2/collagen composite.
Table 1.
Histomorphometric analysis of tibia and ossicles in the mice treated with ROCK inhibitor (Y-27632) (values expressed as mean ± SD)
| Tibia | |||
|---|---|---|---|
| Variable | Control | Y-27632 | p Value |
| BV/TV (%) | 12.48 ± 1.37 | 13.01 ± 1.47 | 0.301 |
| Ob.S/BS (%) | 8.81 ± 2.33 | 8.18 ± 1.40 | 0.673 |
| BFR/BS (mm3/mm2/year) | 0.0741 ± 0.025 | 0.0709 ± 0.0243 | 0.860 |
| ES/BS (%) | 32.8 ± 4.35 | 34.4 ± 3.22 | 0.569 |
| Oc.S/BS (%) | 7.99 ± 2.36 | 8.20 ± 1.84 | 0.863 |
| N.Oc/BS (number/mm) | 2.16 ± 0.54 | 2.16 ± 0.55 | 0.988 |
| Ossicles | |||
|---|---|---|---|
| Variable | Control | Y-27632 | p Value |
| BV/TV (%) | 9.77 ± 4.55 | 26.15 ± 6.39 | 0.027* |
| Ob.S/BS (%) | 5.86 ± 3.32 | 9.72 ± 4.41 | 0.141 |
| BFR/BS (mm3/mm2/year) | 0.418 ± 0.062 | 0.480 ± 0.111 | 0.397 |
| ES/BS (%) | 93.1 ± 3.82 | 85.5 ± 7.21 | 0.57 |
| Oc.S/BS (%) | 35.5 ± 3.74 | 28.1 ± 4.79 | 0.019* |
| N.Oc/BS (number/mm) | 10.08 ± 1.17 | 6.78 ± 2.12 | 0.011* |
BV/TV = bone volume/total volume; Ob.S/BS = osteoblast surface/bone surface; and BFR/BS = bone formation rate/bone surface are osteoblastic parameters. ES/BS = erosion surface/bone surface; Oc.S/BS = osteoclast surface/bone surface; and N.Oc/BS = number of osteoclast/bone surface are osteoclastic parameters.
* p < 0.05.
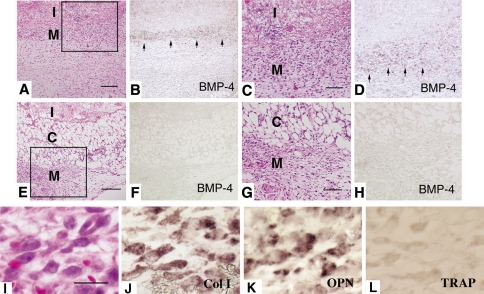
On Day 4 postimplantation, numerous mesenchymal cells were recruited adjacent to BMP/collagen implants in Y-27632-treated mice (Fig. 2A and C). Surrounding mesenchymal cells were separated from BMP/collagen pellet by loose connective tissue in PBS-treated mice (Fig. 2E and G). In situ hybridization analysis revealed the transcripts for BMP-4 (Fig. 2B and D) were localized in mesenchymal cells close to the BMP/collagen implant in Y-27632-treated mice. However, the signals for BMP-4 transcripts were marginal in PBS-treated mice (Fig. 2F and H). The migrating cells were also positive for Type I collagen and osteopontin, and negative for tartrate-resistant acid phosphatase (TRAP) (Fig. 2I–L).
Fig. 2A–L.
Localization of BMP-4 transcripts in tissue sections on Day 4 after implantation. (A and B), (C and D), (E and F), (G and H) are adjacent sections. C and G are higher magnifications of square area of A and B, respectively. (Scale bar: A and E; 50 μm. C and G; 25 μm, I: implant, C: loose connective tissue, M: mesenchymal cell layer) (A–H.) Hematoxylin and eosin staining and in situ hybridization for antisense cRNA probe for BMP-4. (A–D) Y-27632-treated mice or (E–H) PBS-treated mice (original magnifications ×100). (I–L) Higher magnification of migrating cells towards rhBMP-2 pellet as shown by: (I) hematoxylin and eosin staining; in situ hybridization using antisense cRNA for (J) Type I collagen and (K) osteopontin; and (L) TRAP, stained with tartrate resistant acid phosphatase (TRAP). (Scale bar, 10 μm and original magnifications ×200).
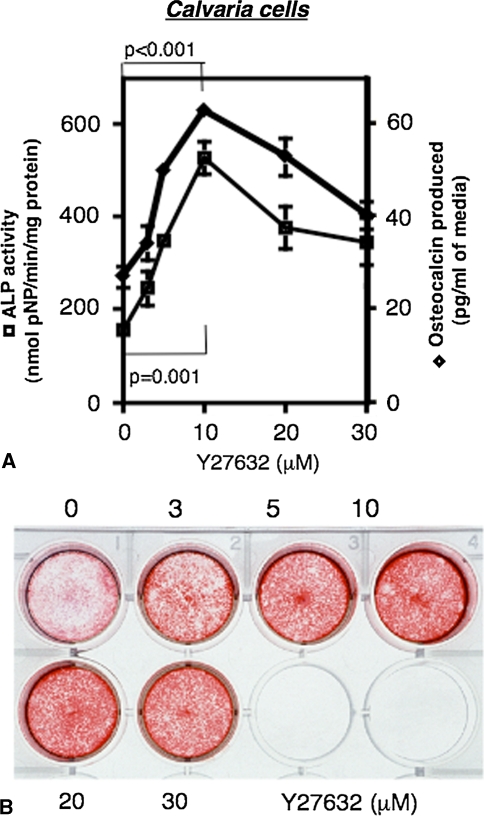
In murine calvarial cultures, the ROCK inhibitor not only stimulated ALP activity and osteocalcin production in a dose-dependent manner, but also enhanced nodule formation (Fig. 3A–B).
Fig. 3A–B.
Stimulated ALP activity, osteocalcin production and nodule formation by ROCK inhibitor in primary culture of neonatal murine calvarial cells. (A) Stimulated ALP activity (■) and osteocalcin production (◆) by ROCK inhibitor in calvarial cells for 4 days (mean ± SEM, n = 6) (in the absence of Y-27632). (B) Nodule formation of calvarial cells for 16 days in the presence of given concentrations of Y-27632.
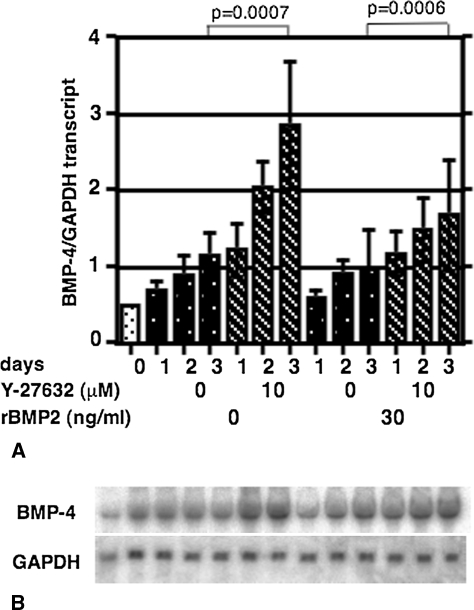
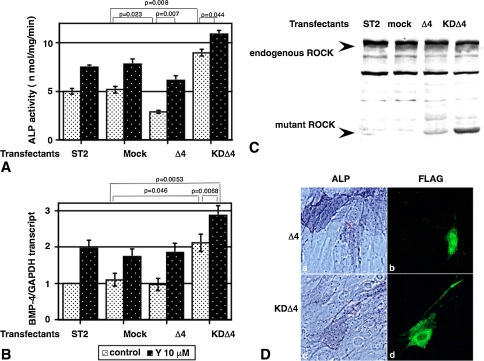
Ten μM of Y-27632 enhanced (two- to threefold compared to the control; p = 0.0007) the expression of BMP-4 mRNA level in ST2 cells (Fig. 4A, hatched bars). This effect appeared specific because adding rhBMP-2 did not alter the BMP-4 mRNA level in ST2 cells (Fig. 4B). Expression of the active ROCK mutant (Δ4) in cultured ST2 cells (Fig. 5C) decreased the ALP activity (Fig. 5A, bar 5) in the absence of ROCK inhibitor. The addition of the ROCK inhibitor partly reversed these inhibitory actions (Fig. 5A, bar 6). Conversely the expression of a dominant negative ROCK construct (KDΔ4, Fig. 5C) enhanced ALP activity (Fig. 5A, bar 7) and BMP-4 expression (Fig. 5B, bar 7), and the addition of the ROCK inhibitor stimulated ALP activity and BMP-4 expression (Fig. 5A–B, bar 8). Furthermore, the ST2 cells expressing dominant negative ROCK represented ALP-positive staining (Fig. 5D, lower panel). Active ROCK mutant expressing ST2 cells never showed ALP positive staining (Fig. 5D, upper panels).
Fig. 4A–B.
Effects of ROCK inhibitors and BMP-2 on the expression of BMP-4 in vitro. (A) The level of transcript was normalized with control GAPDH transcript and shown as mean ± SEM of three determinations. Ten μM of Y-27632 enhanced (two- to threefold compared to the control) the expression of BMP-4 mRNA level in ST2 cells. (B) Representative blots of BMP-4 and GAPDH are shown.
Fig. 5A–D.
Effects of expression of active (Δ4) or dominant negative ROCK (KDΔ4) mutants on the osteoblastic differentiation in ST2 cells in vitro. (A and B) ST2 cells were transiently transfected with vectors alone (mock) or with vectors encoding Δ4 or KDΔ4. ALP activity (A) and the level of BMP-4 transcript (B) were determined in the absence (open bars) or presence (filled bars) of ROCK inhibitor at 72 hours after the transfection. The level of transcript was normalized with control GAPDH transcript. Both ALP activity and the level of BMP-4 were shown as mean ± SEM of six determinations. (C) Immunoblot analysis of the same cell lysates with antibodies to ROCK [23] after SDS-7% PAGE. Arrows, positions of endogenous ROCK and recombinant ROCK mutants. (D) ALP staining (right panels, original magnification ×200) and anti-FLAG Abs immunostaining (left panels, original magnification ×200) of ST2 cells transfectants.
Discussion
The small GTPase Rho and Rho-associated protein kinase (Rho kinase, ROCK) signal participates in a variety of biological functions including vascular contraction, tumor invasion, and penile erection. However, whether it is involved in osteoblastic differentiation is unknown. In order to examine the biological role of ROCK in osteogenesis, we asked the following five questions: (1) Does ROCK inhibitor enhance BMP-induced bone formation in vivo? (2) What kinds of cells migrate to the BMP/atelocollagen pellets to induce bone formation in vivo? (3) Does ROCK inhibitor itself enhance osteogenic differentiation of primary cultured calvaria cells in vitro? (4) What is the mechanism for ROCK inhibitor induced osteogenic differentiation? (5) Does ectopic expression of dominant negative ROCK enhance osteoblastic differentiation and does ectopic expression of active ROCK inhibit it?
We acknowledge the following limitations. Since our study has been carried out using a mouse ectopic bone formation system in vivo (mice) and in vitro, the data may not reflect that in human osteogenesis. First, we assume the signaling pathways in vitro are similar to those in vivo, although the in vivo biological environment may have additional or alternate pathways. Indeed, systemic delivery of ROCK inhibitor by osmotic pump only stimulated the ectopic BMP-induced bone formation in mice (Fig. 1 and Table 1), but did not affect the endogenous bone metabolism in tibia (Table 1). Second, given the limited experimental conditions, we consider this a pilot study. Further studies will be required to confirm these observations across a wide range of conditions and models.
We found continuous delivery of a specific ROCK inhibitor (Y-27632) enhanced ectopic bone formation induced by rhBMP-2 impregnated into an atelocollagen carrier in mice without affecting systemic bone metabolism. Treatment with Y-27632 also enhanced the osteoblastic differentiation of cultured murine neonatal calvarial and ST2 cells. These effects were associated with increased expression of BMP-4 gene. Expression of a dominant negative mutant of ROCK in ST2 cells promoted osteoblastic differentiation, while a constitutively active mutant of ROCK attenuated osteoblastic differentiation and the ROCK inhibitor reversed this phenotype. Thus, ROCK apparently plays a negative role in osteogenesis. Mundy et al. [23] reported the stimulation of bone formation in rodents by a statin (inhibitor for HMG-CoA reductase) from the screening of more than 30,000 compounds derived from natural products. The effect of the statin may lower the cellular cholesterol level, which is required for the geranylgeranylation of Rho to show full activity, as the authors postulated. If this is the case, a ROCK inhibitor might be a more promising and specific stimulator for the promoting of osteogenesis, since it exists downstream in the signal transduction cascade. Consistent with the results, Ohnaka et al. [26] reported that pitavastatin, a newly developed statin, enhanced BMP-2 and osteocalcin expression in Rho-associated kinase dependent manner in primary cultured human osteoblasts. They also reported that hydroxyfasudil, a specific inhibitor for ROCK, increased BMP-2 and osteocalcin production. Here, we further confirmed these results by the expression of active and negative ROCK protein in the cells and also presented the enhanced osteogenesis in vivo animal model.
Employing this in vivo animal model using recombinant human BMP-2 containing Type I collagen deposit, we have already screened a number of compounds, including clinically approved antiosteoporotic drugs, such as ipriflavon, vitamin D analogue, or estrogen analogue. However, none of the compounds demonstrated the positive effect for osteogenesis in vivo. Only estrogen showed high-density bone without changing the ossicle size. We also recently reported the inhibitors for MEK (MAP kinase kinase)-enhanced osteogenesis (ALP activity, osteocalcin secretion, and nodule formation) in the culture cells [6]. However, we could not check the effect of MEK inhibitor on the in vivo BMP-induced osteogenesis because of the limiting availability of the compound. So far the ROCK inhibitor presented here is the only compound in which we found the positive effect for osteogenesis in vivo.
We also found the expression of cbfa-1, a downstream key regulator for osteogenesis [5, 14], was not increased by the ROCK inhibitor and/or rhBMP-2 treatment. Cbfa-1 is a crucial regulator for osteoblastic differentiation; however, the cbfa-1 gene expression during the osteoblastic differentiation is reportedly crucial in some cases and also cell-type specific [5, 14]. Consistent with our results, most recently Mizuno and Kuboki [21] reported that the cbfa-1 gene expression was independent for the osteoblastic differentiation lineage using rat bone marrow stromal cells cultured with Type I collagen. Other posttranslational mechanisms, such as phosphorylation or complex formation of the cbfa-1 protein, should be considered for the activity of this transcriptional factor. In addition, the ROCK inhibitor did not alter the phosphorylation of smad 1/5 [16], a direct downstream target of BMP-2/4 signaling (data not shown).
Enhancement of the healing process for nonunion fractures, reconstruction of local bone defects resulting from surgical resection of bone tumors, and acceleration of an interbody fusion for degenerative spinal disorders are major problems in orthopaedic and craniofacial surgery. Also diseases involving systemic bone loss, such as osteoporosis are major global public health problems. The molecules or genes responsible for this negative control mechanism of osteoblastic differentiation have not yet been identified. The identification of Rho-associated kinase as a negative regulator of bone formation suggests that the ROCK inhibitors such as Y-27632, in conjunction with a local delivery of rhBMPs/collagen, may have therapeutic utility in the clinical setting.
Acknowledgments
We thank Drs. M. Uehata and T. Murozono (Mitsubishi Tanabe Pharma Inc.) for the supply and the concentration measurement of Y-27632 by HPLC.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangement, etc) that might pose a conflict of interest in connection with the submitted article. This work is supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan, as well as by research grants from the Yamanouchi Foundation for Research on Metabolic Disease.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan.
References
- 1.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;6:721–727. [DOI] [PMC free article] [PubMed]
- 2.Chitaley K, Wingard CJ, Webb RC, Branam H, Stopper VS, Lewis RW, Mills TM. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nature Med. 2001;7:119–122. [DOI] [PubMed]
- 3.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. [DOI] [PMC free article] [PubMed]
- 4.Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK 1. Nature Cell Biol. 2001;3:339–345. [DOI] [PubMed]
- 5.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa-1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. [DOI] [PubMed]
- 6.Higuchi C, Myoui A, Hashimoto N, Kuriyama K, Yoshioka K, Yoshikawa H, Itoh K. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of extracellular matrix. J Bone Miner Res. 2002;17:1785–1794. [DOI] [PubMed]
- 7.Hirota S, Takaoka K, Hashimoto J, Nakase T, Takemura T, Morii E, Fukuyama A, Morihana K, Kitamura Y, Nomura S. Expression of mRNA of murine bone-related proteins in ectopic bone induced by murine bone morphogenetic protein-4. Cell Tissue Res. 1994;277:27–32. [DOI] [PubMed]
- 8.Hoshino M, Namikawa T, Kato M, Terai H, Taguchi S, Takaoka K. Repair of bone defects in revision hip arthroplasty by implantation of a new bone-inducing material comprised of recombinant human BMP-2, Beta-TCP powder, and a biodegradable polymer: an experimental study in dogs. J Orthop Res. 2007;25:1042–1051. [DOI] [PubMed]
- 9.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed]
- 10.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. [DOI] [PubMed]
- 11.Itoh F, Asao H, Sugamura K, Heldin CH, Dijke PT, Itoh S. Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. EMBO J. 2001;20:4132–4142. [DOI] [PMC free article] [PubMed]
- 12.Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nature Med. 1999;5:221–225. [DOI] [PubMed]
- 13.Kaito T, Myoui A, Takaoka K, Saito N, Nishikawa M, Tamai N, Ohgushi H, Yoshikawa H. Potentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA-PEG/hydroxyapatite composite. Biomaterials, 2005;26:73–79. [DOI] [PubMed]
- 14.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. [DOI] [PubMed]
- 15.Krause F, Younger A, Weber M. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. J Bone Joint Surg Am. 2008;90:1168–1169. [PubMed]
- 16.Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signaling pathways converge on the TGF-β family mediator Smad1. Nature. 1997;389:618–622. [DOI] [PubMed]
- 17.Kuwabara H, Wada T, Oda T, Yoshikawa H, Sawada N, Kokai Y, Ishii S. Overexpression of the granulocyte colony-stimulating factor gene impairs bone morphogenetic protein responsiveness in mice. Lab Invest. 2001;81:1133–1141. [DOI] [PubMed]
- 18.Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051–29054. [DOI] [PubMed]
- 19.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for the small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed]
- 20.Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001;187:265–276. [DOI] [PubMed]
- 21.Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J Biochem. 2001;129:133–138. [DOI] [PubMed]
- 22.Mori S, Yoshikawa H, Hashimoto J, Ueda T, Funai H, Kato M, Takaoka K. Antiangiogenic agent (TNP-470) inhibition of ectopic bone formation induced by bone morphogenetic protein-2. Bone. 1998;22:99–105. [DOI] [PubMed]
- 23.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. [DOI] [PubMed]
- 24.Murakami N, Saito N, Horiuchi H, Okada T, Nozaki K, Takaoka K. Repair of segmental defects in rabbit humeri with titanium fiber mesh cylinders containing recombinant human bone morphogenetic protein-2 (rhBMP-2) and a synthetic polymer. J Biomed Mater Res. 2002;62:169–174. [DOI] [PubMed]
- 25.Nakase T, Nomura S, Yoshikawa H, Hashimoto J, Hirota S, Kitamura Y, Oikawa S, Ono K, Takaoka K. Transient and localized expression of bone morphogenetic protein 4 messenger RNA during fracture healing. J Bone Miner Res. 1994;9:651–659. [DOI] [PubMed]
- 26.Ohnaka K, Shimoda S, Nawata H, Shimokawa H, Kaibuchi K, Iwamoto Y, Takayanagi R. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun. 2001;287:337–342. [DOI] [PubMed]
- 27.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252. [DOI] [PubMed]
- 28.Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. [DOI] [PMC free article] [PubMed]
- 29.Takaoka K, Koezuka M, Nakahara H. Telopeptide-depleted bovine skin collagen as a carrier for bone morphogenetic protein. J Orthop Res. 1991;9:902–907. [DOI] [PubMed]
- 30.Tamai N, Myoui A, Hirao M, Kaito T, Ochi T, Tanaka J, Takaoka K, Yoshikawa H. A new biotechnology for articular cartilage repair: subchondral implantation of a composite of interconnected porous hydroxyapatite, synthetic polymer (PLA-PEG), and bone morphogenetic protein-2 (rhBMP-2). Osteoarthritis Cartilage. 2005;13:405–417. [DOI] [PubMed]
- 31.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. [DOI] [PubMed]
- 32.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. [DOI] [PubMed]
- 33.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. [DOI] [PubMed]
- 34.Yoshikawa H, Taniguchi S, Yamamura H, Mori S, Sugimoto M, Miyado K, Nakamura K, Nakao K, Katsuki M, Shibata N, Takahashi K. Mice lacking smooth muscle calponin display increased bone formation that is associated with enhancement of bone morphogenetic protein responses. Genes Cells. 1998;3:685–695. [DOI] [PubMed]
- 35.Yoshioka K, Matsumura F, Akedo H, Itoh K. Small GTP-binding protein Rho stimulates the actomyosin system, leading to invasion of tumor cells. J Biol Chem. 1998;273:5146–5154. [DOI] [PubMed]