Abstract
Delays in bone healing or even the development of a nonunion could be related to the concentrations and/or functions of the bone morphogenetic proteins (BMPs). The RNA expression profile of the BMPs within fracture nonunion tissue is unknown. This preliminary descriptive study was performed to define the RNA profiles of the BMPs, their receptors, and their inhibitors within human fracture nonunion tissue and correlate them to matched healing bone. All patients had hypertrophic nonunions. Tissue samples taken from the nonunion site of 15 patients undergoing surgical treatment for an established nonunion were analyzed. The RNA expression patterns of BMP-2, BMP-4, BMP-5, BMP-6, BMP-7, BMP-8; BMP receptor Types IA, IB, and II; and the BMP inhibitors chordin, Noggin, Drm (Gremlin), and follistatin were determined in the nonunion (fibrous tissue) and healing bone (callus tissue) using quantitative real-time PCR. Comparison between the nonunion and healing bone samples revealed substantially elevated concentrations of BMP-4, Drm/Gremlin, follistatin, and Noggin in nonunion tissue when compared to healing bone. In contrast, BMP-7 concentration was higher in the healing bone. Our data suggest inhibition of BMP-7, by Drm (Gremlin), follistatin, and Noggin and upregulation of BMP-4 may play an integral role in the development of nonunions.
Introduction
The adaptation of fractures to heal is a complex series of events. Accordingly, many proteins are involved in the process of skeletal tissue repair and remodeling. Urist and Strates [21] discovered, more than a half century ago, protein extracts from demineralized bone had the ability to induce bone formation. These proteins were soon to be termed the bone morphogenetic proteins (BMPs) [20]. Subsequent studies demonstrated BMPs are part of the transforming growth factor (TGF)-β superfamily of growth factors. A common feature among the TGF-β superfamily is their seven conserved cysteine regions [5]. More than 20 BMP-related proteins have now been identified and can be classified into multiple subgroups based on their structure and function [1]. They are involved in the development of nearly all organs and tissues, including muscle, cartilage, and bone. In particular, BMPs inhibit muscle differentiation and promote the differentiation of mesenchymal cells into osteoblasts [1]. The mechanisms governing the classical BMP-signaling pathway have been well characterized. They initiate their signaling transduction by binding to serine-threonine kinase receptors, namely BMP receptors Type I and II [4, 7, 24]. There are likewise several types of receptors in each receptor group. Once bound, the activated receptor kinases phosphorylate the transcription factors Smad1, 5, and 8. These Smads are translocated into the nucleus after binding with Smad4 [24]. Once in the nucleus, this complex directs the expression of osteogenic target genes [24].
Regulation of the BMPs is due in part to its inhibitors. Known inhibitors include Noggin, follistatin, Drm/Gremlin, sclerostin, and chordin. They exercise their inhibition at several levels [18, 22]. Chordin and Noggin directly bind to the BMP ligand [2, 17]. Sclerostin may inhibit the Smad cascade by binding to downstream signaling proteins. Investigation via in vitro and in vivo experiments has demonstrated a redundancy in the actions of these inhibitors [14, 17].
The role BMPs or their antagonists may play in the development of fracture nonunions is not well understood. Previous research suggests an interplay between both groups of proteins [10, 11, 13]. Kwong et al. [13] examined the expression of BMP-2, BMP-14, chordin, and Noggin in cartilaginous areas of human fractures that ultimately become nonunions by using immunohistochemical analysis. They reported a reduction in BMP-2 and BMP-14 expression in cartilaginous areas of nonhealing fractures compared to healing fractures. However, there was no difference in the expression of the BMP inhibitors between the two groups of fractures [13]. A similar study by Kloen et al. [11] also studied the localization of BMP-2, BMP-4, BMP-7, and their receptors BMPR-IA, BMPR-IB, and BMPR-II, as well as pSmad1, by immunohistochemical analysis in delayed union and nonunion tissue. They found the seven BMP-signaling components present in 17 of the 21 patients [11].
We designed our study to confirm the suggestion that alterations in the concentrations of BMPs, their receptors, and their inhibitors may be present in fractures that fail to heal. We sought to define the RNA expression levels of most BMPs, the BMP receptors, and BMP antagonists within fracture nonunion tissue and compare this expression profile to healing bone.
Materials and Methods
We prospectively enrolled 15 patients (11 male and four female) with established fracture nonunions of at least 6 months’ duration between August 2007 and March 2008. A nonunion was defined as the absence of osseous healing at 6 months after the initial injury. The patient cohort had a mean age of 46 years (range, 32–80 years; standard deviation, 14 years). The anatomic sites involved were two femoral shafts, two subtrochanteric femurs, two tibial shafts, one tibial plateau, one distal tibia, two fibular shafts, three midshaft clavicles, one proximal humerus, and one ulnar shaft. Ten of the 15 patients developed a nonunion in a weightbearing bone. No specimens were taken from the axial skeleton. The average time interval between the time of index fracture and the time of nonunion surgery was 16 months (range, 0.5–6 years). All patients presented with pain, deformity, and decreased function of the involved extremity. Twelve of the fifteen (80%) subjects presented with a failed osteosynthesis from previous surgery. Three of the fifteen (20%) patients had previous use of a bone stimulator. All the nonunions were of the hypertrophic type. We excluded patients with malignancy, pregnancy, age of less than 18 years, and the use of corticosteroids or hormones. We obtained prior IRB approval and all subjects gave their informed consent before their inclusion in the study.
At the time of nonunion repair surgery, we collected tissue samples from two distinct sites in each patient: fibrous tissue from the nonunion site and healing bone from the surrounding region (range, 1–2 samples per site); we used tissue consistency, color, and toughness to distinguish healing bone from the surrounding scar. Tissue samples taken were collected utilizing a standard protocol (no electrocautery was used). All surgical specimens were retrieved by one surgeon. One half of the collected tissue was placed in an RNA-stabilizing solution (Qiagen, Crawley, West Sussex, UK) while the other half was placed in phosphate-buffered saline (PBS) with proteinase inhibitors. All samples were stored in liquid nitrogen. The fracture nonunion tissue and healing bone samples were first analyzed for RNA expression levels of most human BMPs (BMP-2, -4, -5, -6, -7, and -8), three BMP receptors (BMP receptor Types IA, IB, and II), and four BMP inhibitor genes (Noggin, sclerostin, follistatin, Drm/Gremlin), and significant results were confirmed on a protein level (Table 1).
Table 1.
Bone morphogenic protein/receptor/inhibitor and tissue type
| Protein | Tissue type | Tissue type |
|---|---|---|
| BMP-2 | Nonunion | Healing bone |
| BMP-4 | Nonunion | Healing bone |
| BMP-5 | Nonunion | Healing bone |
| BMP-6 | Nonunion | Healing bone |
| BMP-7 | Nonunion | Healing bone |
| BMP-8 | Nonunion | Healing bone |
| BMP Receptor 1A | Nonunion | Healing bone |
| BMP Receptor 1B | Nonunion | Healing bone |
| BMP Receptor 2 | Nonunion | Healing bone |
| DRM (Gremlin) | Nonunion | Healing bone |
| Chordin | Nonunion | Healing bone |
| Follistatin | Nonunion | Healing bone |
| Noggin | Nonunion | Healing bone |
For RNA extraction, tissue was weighed and homogenized to powder in reagent (TRI Reagent®; Molecular Research Center, Inc, Cincinnati, OH) (1 mL/0.1 g tissue) using mortar and pestle with liquid nitrogen. One hundred microliters of BCP reagent per 1 mL TRI Reagent® was added. Particulates were pelleted at 12,000 g for 15 minutes at 4°C and the aqueous layer recovered. The aqueous layer was placed into a fresh tube. Equal volumes of 70% ethanol were added and mixed. Samples were applied to spin columns (RNeasy® Mini Kit; Qiagen), centrifuged at full speed for 15 seconds, and the flow-through discarded. Columns were then washed and eluted according to the manufacturer’s instructions. RNA samples were quantified and qualified (260/280 optical density ratio > 1.6) using a spectrophotometer and stored at −80°C.
Complementary DNA was synthesized from 1 μg of total RNA using reverse transcriptase (ImProm-II™ Reverse Transcription System; Promega, Madison, WI) and random hexamers in a total volume of 20 μL according to the manufacturer’s instructions. Complementary DNA was stored at −20°C.
Specific oligonucleotide primers for most of the human BMP genes (BMP-2, -4, -5, -6, -7, and -8), three BMP receptor genes (BMP receptor Types IA, IB, and II), and four BMP inhibitor genes (Noggin, sclerostin, follistatin, Drm/Gremlin) were bought from Integrated DNA Technologies, Inc (Coralville, IA). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin (mRNA) genes were used as an endogenous control to normalize for differences in the amount of total RNA in each sample. The oligonucleotide sequences were taken from the scientific literature [12, 23]. Quantitative real-time PCR was performed using the ABI Prism® 7700 Sequence Detection System (PE Applied Biosystems, Inc, Foster City, CA) according to the manufacturer’s protocol. Each reaction was performed in 25 μL and contained the following: the equivalent 1 ng of cDNA, the SYBR® Green PCR Master Mix (PE Applied Biosystems), and 100 nmol/L each of the forward and reverse primer. Conditions for the PCR were 2 minutes at 55°C, 10 minutes at 95°C, and then 40 cycles, each consisting of 15 seconds at 95°C and 1 minute at 60°C. The ABI Prism® 7700 measured the cycle-cycle changes in fluorescence in each sample and generated a kinetic profile of DNA amplification over the 40-cycle PCR. The cycle number (termed the cycle threshold [Ct]) at which amplification entered the exponential phase was determined and this number was used as an indicator of the amount of target RNA in each tissue. The quantitative real-time PCR reactions were performed in triplicate.
Gene expression levels relative to that of GAPDH were calculated from the Ct values (calculated as GAPDH mRNA Ct − gene Ct), using an assumption of maximal amplification efficiency in each reaction.
Protein was extracted from seven patient tissue samples (both nonunion and fracture callus) using T-PER® extraction buffer (Pierce Biotech, Rockford, IL) and homogenized to powder under mortar and pestle with liquid nitrogen. The protein was spun down and the supernatant quantified using a spectrophotometer. Approximately 20 μg protein was loaded onto a 15% SDS-PAGE gel and electrophoresed in Tris-glycine buffer (0.025 mol/L Tris base, 0.192 mol/L glycine, 0.1% sodium dodecyl sulfate) at 30 mA per gel for 1 hour. The protein was then transferred onto a nitrocellulose membrane (0.45-mm pore size) with a transblot apparatus (Bio-Rad Laboratories, Inc, Hercules, CA) at 250 mA for 1 hour. For the Western blot assay, the membranes were blocked in 10% nonfat milk (nonfat milk in 0.02 mol/L Tris base, 0.385 mol/L NaCl, and 0.1% Tween® 20, pH 7.5 [TBS-T]) at 4°C overnight. The following day, the membranes were incubated for 1 hour with the indicated primary antibody (BMP-2, BMP-4, and BMP-7 [Santa Cruz Biotechnology, Inc, Santa Cruz, CA]) with dilutions according to the manufacturer at room temperature. Membranes were rinsed three times with TBS-T. Finally, blots were probed with goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology) for 30 minutes, rinsed three times with TBS-T, and developed with enhanced luminescence reagents followed by exposure of film for 5 to 10 minutes (ECL™ Western Blot Detection System; Amersham Pharmacia Biotech, Inc, Piscataway, NJ).
Due to limited amounts of tissue specimen, seven of the 15 patients’ samples underwent immunohistochemistry staining. These samples were stored in liquid nitrogen without RNA-stabilizing solution. At the time for immunohistochemistry, the tissue was subsequently fixed in 10% neutral-buffered formalin for 48 hours. The samples were washed and then dehydrated through a graded ethanol treatment. After dehydration, each sample was embedded in paraffin and sectioned to a thickness of 5 to 6 μm. If needed, surface decalcification was performed before sectioning using DeCal Solution (Polysciences, Inc, Warrington, PA) for 1 day. Hematoxylin and eosin staining was performed to allow for comparative histologic analysis. Polyclonal antibodies for BMP-2 and BMP-7 (Santa Cruz Biotechnology) were used for immunostaining of ten sections from each of the seven samples. Immunohistochemical processing was performed with use of the avidin-biotin-peroxidase complex method. The Vectastain® Elite ABC Rabbit IgG Kit (Vector Laboratories, Burlingame, CA) was used for both the BMP-2 and BMP-7 antibodies. Preliminary studies were performed to determine the appropriate concentration (1:100) for each antibody. The samples were prepared for the immunohistochemical reaction by first removing the paraffin from the sections and rehydrating the tissue samples. Endogenous peroxidase activity was blocked with methanol containing 0.3% hydrogen peroxide for 30 minutes at room temperature, followed by washing with PBS solution. The samples were subjected to antigen retrieval by placing them in citrate buffer and heating them to boiling in a microwave, after which they were allowed to stand for 30 minutes at room temperature. Nonspecific binding was inhibited with protein block and 0.5% bovine serum albumin for 30 minutes at room temperature in a humid chamber. Immunostaining was then carried out by applying the appropriate concentration of antibodies to each sample and incubating overnight at 4°C. For negative controls, normal rabbit IgG in PBS solution was used in place of the primary antibodies. The following day, the samples were washed in PBS solution and were incubated with the biotinylated secondary antibody for 1 hour at room temperature. The avidin-biotin-peroxidase complex (Vectastain® kit) was then added for 30 minutes at room temperature. The color reaction was developed with use of 3, 3′-diaminobenzidine tetrachloride (DAB) (Sigma-Aldrich Corp, St Louis, MO). Counterstaining was performed with use of hematoxylin or methyl green, and a coverslip was applied with Permount® (Fisher Chemical Co, Fairlawn, NJ).
Image analysis of the immunohistochemistry specimens was performed by IP Lab 4.0 software (BD Biosciences, San Jose, CA). Under a magnification of ×100, the number of cells staining positively for each antibody was assessed by observing a total of five fields of at least 10 cells each. We performed the image analysis for the two tissue types, bone and fibrous tissue. Again no attempt was made to quantify the intensity of staining. The cells were evaluated simply for the presence or absence of staining. This was performed by two independent observers (KI, LK) who were blinded to the tissue type and to the antibody tested.
BMP-2, BMP-4, and BMP-7 underwent further testing by SDS-PAGE/Western immunoblotting. The Western immunoblotting was evaluated by the presence or absence of bands in relation to the β-actin control. No attempt was made to quantify the intensity of staining. This was performed by two independent observers (KI, LK) who were blinded to the tissue type and to the antibody tested.
Data analysis for the quantitative real-time PCR, to compare the nonunion tissue and fracture callus tissue RNA profiles, was performed by a paired Student’s t test with equal variance. We determined differences in each protein tested between the nonunion tissue and healing bone. Statistical analyses were carried out on Stata® software (StataCorp LP, College Station, TX).
Results
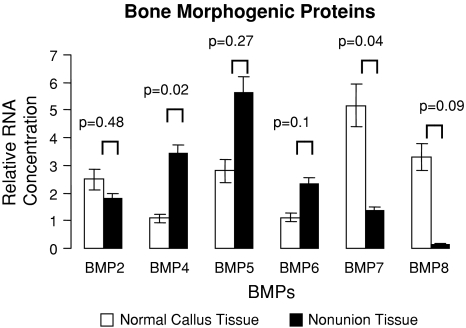
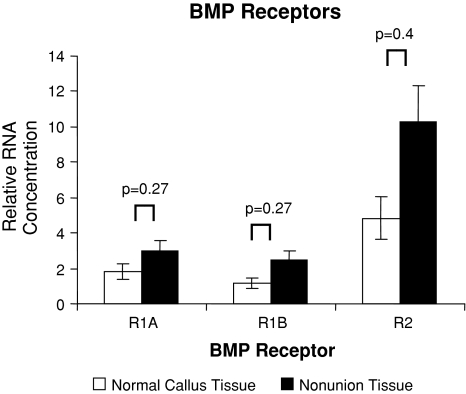
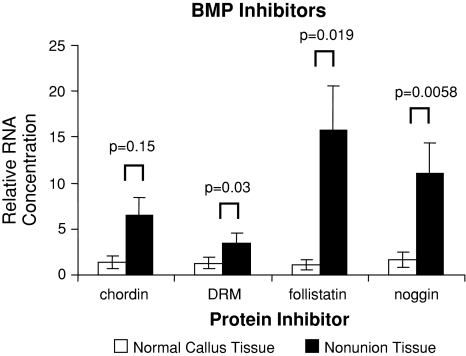
The mRNA expression for most of the known human BMPs, their receptors, and their inhibitors were observed in both nonunion and matched fracture callus tissue. All 13 genes investigated were at the level of assay detection. BMP gene expression in healing bone displayed several upregulated genes between the two tissues (Fig. 1). However, we consistently found an increased level of BMP antagonist genes in nonunion tissue when compared to fracture callus tissue (Fig. 2). The BMP receptors were expressed but did not demonstrate any significant differences (Fig. 3). BMP-4 was upregulated (p = 0.02) in nonunion tissue when compared to the fracture callus tissue. The compared RNA levels of the BMP antagonists Drm/Gremlin (p = 0.02), follistatin (p = 0.019), and Noggin (p = 0.058) were upregulated in the nonunion tissues. BMP-7 was increased (p = 0.035) in the fracture callus tissue.
Fig. 1.
Quantitative real-time PCR revealed comparative expression of BMP genes isolated from normal/callus tissue and nonunion tissue with oligospecific BMP primers, normalized to GAPDH. Error bars = standard error.
Fig. 2.
Quantitative real-time PCR revealed comparative expression of BMP receptor genes isolated from normal/callus tissue and nonunion tissue with oligospecific BMP receptor primers, normalized to GAPDH. Error bars = standard error.
Fig. 3.
Quantitative real-time PCR revealed comparative expression of BMP inhibitor genes isolated from normal/callus tissue and nonunion tissue with oligospecific BMP inhibitor primers, normalized to GAPDH. Error bars = standard error.
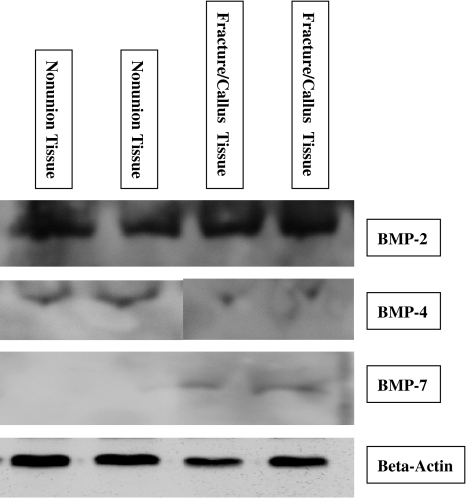
The Western immunoblots were read by two independent observers. Consistent with the mRNA expression pattern, BMP-2 was present among both types of tissue samples (Fig. 4). BMP-4 was detected in nonunion samples but decreased in healing bone samples (Fig. 4). BMP-7 was detected in the healing bone but was absent in the nonunion samples (Fig. 4).
Fig. 4.
Tissue lysates of nonunion tissue and normal/callus tissue from different but matched patients were subjected to SDS-PAGE and detected by Western blot analysis with antibodies specific for BMP-2, BMP-4, BMP-7, and β-actin.
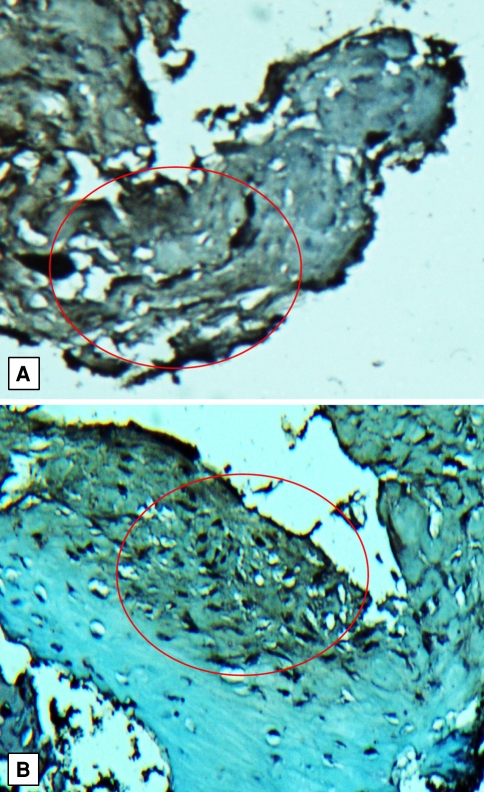
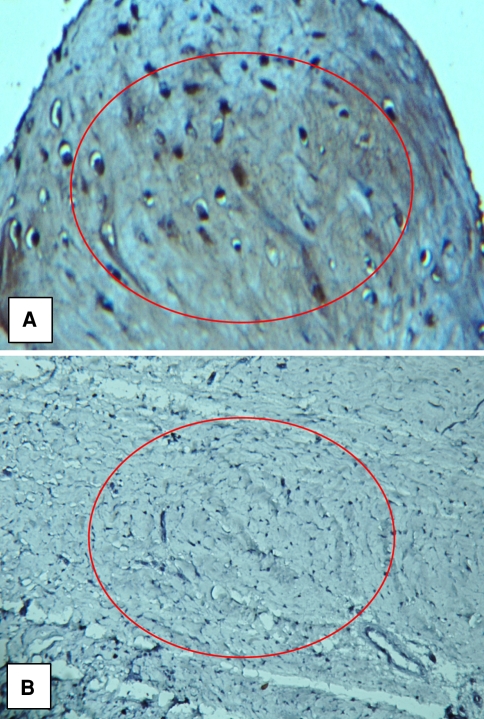
Immunostaining for BMP-2 and BMP-7 confirmed our findings with quantitative real-time PCR and Western blot analysis (Figs. 5, 6). Immunostaining for BMP-7 was absent in the nonunion specimens and the test sections were indistinguishable from the negative control section. Immunostaining for BMP-7 was present in the fracture callus specimens. Sections immunostained for BMP-2 exhibited variable positivity depending on the area of the section examined. Positive immunostaining was restricted consistently to the fibrous tissue of the nonunion tissue. No positive staining was visible in 10% to 20% of the area of the sections, but in approximately 70% of the section area the staining was diffusely positive. In the remainder of the section area, the staining was present in discrete, variably sized foci within the tissue substance and strongly positive.
Fig. 5A–B.
Immunohistochemistry staining of BMP-2 in (A) callus tissue and (B) nonunion tissue is shown. Paraffin-embedded callus or nonunion tissue was dehydrated, stained with DAB (brown staining) specific to BMP-2 antibody, and counterstained with hematoxylin. Red circle indicates areas of BMP-2 staining (original magnification, ×132).
Fig. 6A–B.
Immunohistochemistry staining of BMP-7 in (A) callus tissue and (B) nonunion tissue is shown. Paraffin-embedded callus or nonunion tissue was dehydrated, stained with DAB (brown staining) specific to BMP-7 antibody, and counterstained with hematoxylin. Red circle indicates areas of BMP-7 staining and absence of staining (original magnification, ×200).
Discussion
Fracture nonunion tissue and healing bone may demonstrate various gene profiles. The BMPs are a group of growth factors well known for their osteogenic potential [17]. The ability for a fracture to heal maybe dependent on the concentration and/or function of the BMPs. Our purpose was to confirm the suggestion that alterations in the concentrations of BMPs, their receptors, and their inhibitors may be present in fractures that fail to heal.
This was a preliminary descriptive study comparing the mRNA profiles of nonunion tissue and healing bone which has some limitations. First, we did not obtain healed bone specimens from matched controls. This would have been the ideal control. We acknowledge that the “healed bone” area may well not represent “normal” bone since this comes from a fracture that did not ultimately heal completely. Second is the small sample size. An increase in sample size would produce stronger data interpretation and provide enough data for a formal study. Third is an inability to enroll a uniform patient population. A homogeneous population, in regards to anatomic location, patient age, and fracture age, would lead to more reliable results. Fourth, all of the specimens were of the hypertrophic nonunion nature. We did not study atrophic and oligotrophic nonunions. These might yield varying results due to the different local microenvironments. Fifth, there is a heterogeneity in the fractures examined (the anatomic sites). Weight bearing bones have different mechanical environments which can lead to different results. Finally, we did not take into account the early time points of a developing nonunion tissue. All specimens were taken after the diagnosis of an established fracture nonunion was made (> 6 months). Early gene expression during the development of a fracture nonunion could display correlating trends with respect to the BMP/BMP antagonist concentrations and be markedly different from later time points. We observed no differences between the ages of the nonunion, although previous animal studies have found differences [15].
The RNA expression profile of the 13 different BMPs, their receptors, and their antagonists were altered between the nonunion tissue and the healing bone tissue. We found healing bone to express an upregulation of BMP-7 and nonunion tissue to have an increased expression of BMP-4, Drm (Gremlin), follistatin, and Noggin. BMP-7 is an integral part of the transformation of mesenchymal cells into bone and cartilage. This occurs by the induction of osteoblast differentiation by the transcription of numerous osteogenic genes [24]. It is inhibited by Noggin and chordin. The osteogenic capability of BMP-7 (or osteogenic protein 1) has led to its use as a clinical surgical supplement [6]. Our data are similar to those in a previous study on atrophic nonunions produced in rats [15]. They found the gene expression of BMP-7 was substantially lower in nonunions compared to standard healing fractures at several time points. Both our data and the cited results can support the protein’s clinical role in the treatment of bone nonunions [6]. We found BMP-4 RNA was upregulated in the nonunion tissue. That finding was confirmed at the protein level by our protein analyses. BMP-4 is believed to be involved in bone and cartilage development [3]. It also has a role in human embryonic development, where BMP-4 is a critical signaling molecule required for the early differentiation of the embryo and establishing the dorsal-ventral axis [3]. A recent investigation, however, reported the loss of BMP-4 had no deleterious consequences in the embryologic development of the appendicular skeleton [19]. Decreased amounts of BMP-4 are also implicated in fibrodysplasia ossificans progressive [9]. Accordingly, BMP-4’s diminished role in both skeletal development and in fibrodysplasia ossificans progressiva may demonstrate similar inhibitory roles found within fracture nonunions. BMP-4 shares a similar structure and function to BMP-2. Although BMP-2 is upregulated in the callus tissue, it was not substantially upregulated in this study. Our protein analyses also suggest BMP-2 is present in both nonunion and fracture callus specimens.
Noggin, chordin, Drm/Gremlin, and sclerostatin are extracellular inhibitors of BMPs. Our observations suggest gene expression of these BMP antagonists (with the exception of chordin) are upregulated in nonunion tissue. Since the BMP antagonists have the ability to block certain osteogenic BMPs, it makes sense for them to be upregulated in the nonunion tissue. These proteins thus have an inverse correlation to the levels of BMPs found in nonunion tissue. These data differ from those in the study by Niikura et al. [15] on atrophic nonunions. Their results displayed a downregulation of gene expression of these extracellular BMP antagonists except for chordin in the nonunion tissue. Time and species can account for these differences. Niikura et al. [15] only measured time points up to 28 days of nonunion time in rat femur model. It is difficult to compare time courses from different species, but our study in human nonunion specimens displayed an average age of 16 months [15]. The induction of BMP inhibitors may result from an accumulation over time, where the increased amounts are needed to take physiologic effect.
Previous immunohistochemical studies have demonstrated BMPs and their signaling components are present in both the fibrous tissue of nonunions and the callus tissue of healing bone [11]. Bone fails to heal despite the presence of BMPs, as demonstrated by their immunohistochemical assays [10]. The differentiation and fate of osteoblasts are controlled by various extracellular signals, such as Noggin and the BMPs [8]. This is especially true for BMP-2 and BMP-7. We found an increased expression of BMP-7 in healing bone and upregulated BMP-4, Drm (Gremlin), follistatin, and Noggin in human nonunion tissue. These results are variable when compared to related studies in the literature (Table 2) [10, 12, 13, 16]. Our analyses suggest these proteins may have an impact in the development of a fracture nonunion.
Table 2.
Comparison of the current BMP expression literature
| Authors | Proteins studied | Specimens; outcome measures | Results |
|---|---|---|---|
| Kloen et al. 2002 [11] | BMP-2, BMP-4, BMP-7, BMPR-IA, BMPR-IB, BMPR-II, pSmad1 | 4 delayed union, 17 nonunion human tissue; Immunohistochemistry | All proteins studied detected in most specimens 17/21 |
| Kwong et al. 2009 [13] | BMP-2, BMP-14, chordin, Noggin | 7 nonhealing human fractures; Immunohistochemistry | decrease in BMP-2, BMP-14, no change in chordin, Noggin |
| Onishi et al. 1998 [16] | BMP-2, BMP-4, BMP-7 BMP Receptor 2 |
Rat Fracture Model; Immunohistochemistry | All proteins are present during various stages of bone healing |
| Niikura et al. 2006 [15] | Microarray including BMP-2, BMP-3, BMP-4, BMP-6, BMP-7, GDF-5, GDF-7, Noggin, drm, screlostin, BAMBI, chordin | Rat Nonunion Model; Genechip microarray and real-time quantitative RT-PCR | several BMPs and inhibitors are temporally induced and down regulated |
| Fajardo et al. 2009 [current study] | BMP-2, BMP-4, BMP-5, BMP-6,BMP-7, BMP-8 BMP Receptors 1A, 1B, 2 chordin, Drm, Noggin, follistatin |
15 human hypertrophic nonunions and 15 healing bone specimens; Real-Time PCR, Western Blot, IHC | BMP-7 upregulated in healing bone; BMP-8, Noggin, chordin, follistatin upregulated in nonunion tissue |
Acknowledgments
We thank Kiril Ilalov, Li Kong, and Michael Walsh for their help in this research endeavor.
Footnotes
One of the authors (KE) has received research support from Synthes, Inc, West Chester, PA; Stryker Orthopaedics, Mahway, NJ; and Biomet Inc, Warsaw, IN; and is a consultant for Exactech, Inc, Gainesville, FL.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Abe E. Function of BMPs and BMP antagonists in adult bone. Ann N Y Acad Sci. 2006;1068:41–53. [DOI] [PubMed]
- 2.Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O’Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas, SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–673. [DOI] [PubMed]
- 3.Behesti H, Holt JK, Sowden JC. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev Biol. 2006;6:62. [DOI] [PMC free article] [PubMed]
- 4.Brownlow HC, Reed A, Simpson AH. Growth factor expression during the development of atrophic non-union. Injury. 2001;32:519–524. [DOI] [PubMed]
- 5.Cheng H. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003;85:1544–1552. [DOI] [PubMed]
- 6.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83 Suppl 1(Pt 2):S151–S158. [PMC free article] [PubMed]
- 7.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. [DOI] [PMC free article] [PubMed]
- 8.Hendy GN, Kaji H, Sowa H, Lebrun JJ, Canaff L. Menin and TGF-beta superfamily member signaling via the Smad pathway in pituitary, parathyroid and osteoblast. Horm Metab Res. 2005;37:375–379. [DOI] [PubMed]
- 9.Kaplan FS, Fiori J, LS DLP, Ahn, J, Billings PC, Shore EM. Dysregulation of the BMP-4 signaling pathway in fibrodysplasia ossificans progressiva. Ann N Y Acad Sci. 2006;1068:54–65. [DOI] [PubMed]
- 10.Kloen P, Di Paola M, Borens O, Richmond J, Perino G, Helfet DL, Goumans MJ. BMP signaling components are expressed in human fracture callus. Bone. 2003;33:362–371. [DOI] [PubMed]
- 11.Kloen P, Doty SB, Gordon E, Rubel IF, Goumans MJ, Helfet DL. Expression and activation of the BMP-signaling components in human fracture nonunions. J Bone Joint Surg Am. 2002;84:1909–1918. [DOI] [PubMed]
- 12.Kochanowska I, Chaberek S, Wojtowiczi A, Marczynski B, Wlodarski K, Dytko M, Ostrowski K. Expression of genes for bone morphogenetic proteins BMP-2, BMP-4 and BMP-6 in various parts of the human skeleton. BMC Musculoskelet Disord. 2007;8:128. [DOI] [PMC free article] [PubMed]
- 13.Kwong FN, Hoyland JA, Freemont AJ, Evans CH. Altered relative expression of BMPs and BMP inhibitors in cartilaginous areas of human fractures progressing towards nonunion. J Orthop Res. 2009;27:752–757. [DOI] [PMC free article] [PubMed]
- 14.Leboy PS. Regulating bone growth and development with bone morphogenetic proteins. Ann N Y Acad Sci. 2006;1068:14–18. [DOI] [PubMed]
- 15.Niikura T, Hak DJ, Reddi AH. Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. J Orthop Res. 2006;24:1463–1471. [DOI] [PubMed]
- 16.Onishi T, Ishidou Y, Nagamine T, Yone K, Imamura,T, Kato M, Sampath TK, Sakou T. Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone. 1998;22:605–612. [DOI] [PubMed]
- 17.Rosen V. BMP and BMP inhibitors in bone. Ann N Y Acad Sci. 2006;1068:19–25. [DOI] [PubMed]
- 18.Tsialogiannis E, Polyzois I, Oak Tang Q, Pavlou G, Tsiridis E, Heliotis M, Tsiridis E. Targeting bone morphogenetic protein antagonists: in vitro and in vivo evidence of their role in bone metabolism. Expert Opin Ther Targets. 2009;13:123–137. [DOI] [PubMed]
- 19.Tsuji K, Cox K, Bandyopadhyay A, Harfe BD, Tabin CJ, Rosen V. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am. 2008;90 Suppl 1:14–18. [DOI] [PubMed]
- 20.Urist MR. A morphogenetic matrix for differentiation of bone tissue. Calcif Tissue Res. 1970;Suppl:98–101. [DOI] [PubMed]
- 21.Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971;50:1392–1406. [DOI] [PubMed]
- 22.Winkler D, Yu C, Geoghegan JC, Ojala EW, Skonier KE, Shpektor D, Sutherland MK, Latham JA. Noggin and sclerostin bone morphogenetic protein antagonists form a mutually inhibitory complex. J Biol Chem. 2004;279:36293–36298. [DOI] [PubMed]
- 23.Wordinger RJ, Agarwal R, Talati M, Fuller J, Lambert W, Clark AF. Expression of bone morphogenetic proteins (BMP), BMP receptors, and BMP associated proteins in human trabecular meshwork and optic nerve head cells and tissues. Mol Vis. 2002;8:241–250. [PubMed]
- 24.Wu X, Shi W, Cao X. Multiplicity of BMP signaling in skeletal development. Ann N Y Acad Sci. 2007;1116:29–49. [DOI] [PubMed]