Abstract
The neuronal calcium sensor (NCS) proteins regulate signal transduction processes and are highly conserved from yeast to humans. We report complete NMR chemical shift assignments of the NCS homolog from fission yeast (Schizosaccharomyces pombe), referred to in this study as Ncs1p. (BMRB no. 16446).
Keywords: NCS, Ncs1p, Fission yeast, Calcium, EF-hand, NMR, S. pombe
Biological context
Neuronal calcium sensor (NCS) proteins belong to a sub-branch of the calmodulin superfamily that regulate a variety of physiological target proteins in the brain and retina (Ames et al. 1996; Braunewell and Gundelfinger 1999; Burgoyne et al. 2004). The best characterized NCS protein is recoverin that serves as a Ca2+ sensor in retinal rod and cone cells where it controls the desensitization of rhodopsin (Dizhoor et al. 1991; Erickson et al. 1998; Kawamura 1993). The NCS family also includes neuronal Ca2+ sensors such as neurocalcin (Hidaka and Okazaki 1993), hippocalcin (Kobayashi et al. 1993), and frequenin (Pongs et al. 1993), as well as yeast homologs, S. cerevisiae Frq1 (Hendricks et al. 1999) and S. pombe Ncs1p (Hamasaki-Katagiri et al. 2004). All members of the NCS family have around 200 amino acid residues, contain N-terminal myristoylation, and possess four EF-hands.
Three-dimensional structures are now known for many NCS proteins, including recoverin (Ames et al. 1997; Flaherty et al. 1993), frequenin (Bourne et al. 2001), Frq1 (Strahl et al. 2007), neurocalcin (Vijay-Kumar and Kumar 1999), and GCAPs (Ames et al. 1999; Stephen et al. 2007). The Ca2+-bound NCS proteins share a common fold with four EF-hands arranged in a tandem array and an exposed N-terminus. The structure of Ca2+-free recoverin contains a covalently attached myristoyl group buried inside the protein hydrophobic core (Tanaka et al. 1995). Binding of Ca2+ to recoverin leads to extrusion of its myristoyl group, termed the calcium-myristoyl switch, that enables recoverin to bind to membrane targets only at high Ca2+ levels (Dizhoor et al. 1993; Zozulya and Stryer 1992). By contrast, frequenin (NCS-1) and yeast Frq1 contain exposed myristoyl groups in their Ca2+-free state and therefore lack a Ca2+ myristoyl switch (Ames et al. 2000; O’Callaghan and Burgoyne 2004). Also, the recent x-ray structure of Ca2+-bound GCAP1 (Stephen et al. 2007) showed the myristoyl group to be sequestered in a unique environment flanked by N- and C-terminal helices, very different from the myristate binding pocket seen in Ca2+-free recoverin. The atomic-resolution structures of other myristoylated NCS proteins are needed to better define the range and different types of NCS protein-myristate interactions. We report here NMR resonance assignments of myristoylated S. pombe Ncs1p in the Ca2+-free state as a first step toward elucidating the protein structure and environment around the N-terminal myristoyl group.
Methods and experiments
Expression and purification of Ncs1p
Recombinant myristoylated neuronal calcium sensor-1 (Ncs1p) was uniformly 15N- or 15N/13C-labeled by co-expressing Ncs1p and yeast N-myristoyl CoA-transferase in E. coli strain, BL21(DE3) grown on M9 medium supplemented with 15N-NH4Cl and/or 13C6-glucose. Recombinant protein expression was induced by exogenously adding myristic acid (10 mg/l) and 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) to cells grown overnight at 25°C. Typically, a 1-L culture yields about 30 mg of myristoylated protein. Detailed procedures for purifying myristoylated Ncs1p are described elsewhere (Hamasaki-Katagiri et al. 2004).
NMR spectroscopy
Samples of recombinant Ca2+-free myristoylated Ncs1p (0.5–0.7 mM) were prepared in 90%/10% H2O/D2O or 100% D2O with 5 mM Tris-d11 (pH 7.4), 4 mM DTT-d11 and 0.3 mM EDTA-d12. NMR experiments were conducted using Bruker Advance 600 or 800 MHz spectrometer equipped with a triple resonance cryogenic probe. All experiments were performed at 310 K. Backbone and side-chain chemical shift assignments were obtained using 15N-HSQC, HNCO, HNCACB, CBCACONH, HBHACONH and 15N-HSQC-TOCSY (mixing time of 60 ms) spectra (Ikura et al. 1990). Methyl group side-chain resonances were assigned using 13C-CT-HSQC and 13C-HCCH-TOCSY (Kay et al. 1993). For aromatic side-chain chemical shift assignments, HBCBCGCDHD, HBCBCGCDCEHE, 13C-CT-HSQC-TOCSY spectra (Yamazaki et al. 1993) along with 13C-HSQC-NOESY, recorded with a mixing time of 120 ms, were used. NMR data were processed using NMRPipe (Delaglio et al. 1995) software package and analyzed using SPARKY.
Assignments and data deposition
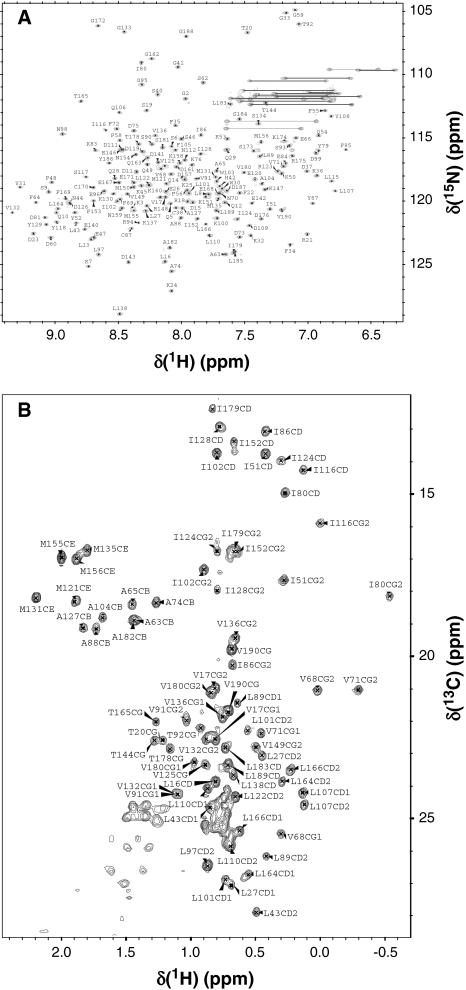
Figure 1 presents HSQC spectra of myristoylated Ca2+-free Ncs1p at pH 7.4 to illustrate representative backbone and side chain resonance assignments. NMR assignments were based on 3D heteronuclear NMR experiments performed on 13C/15N-labeled Ncs1p (residues 2–190). The protein sample in this study consists of 189 native residues with a myristoyl group covalently attached at the N-terminus (Gly 2). All non-proline residues exhibited strong backbone amide resonances with uniform intensities, indicative of a well-defined three-dimensional protein structure. More than 95% of the backbone resonances (1HN, 15N, 13Cα, 13Cβ, and 13CO) and ~82% of aliphatic side chain resonances were assigned. The chemical shift assignments (1H, 15N, 13C) of Ca2+-free Ncs1p have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) under accession number 16446.
Fig. 1.
Two-dimensional 15N-HSQC a and 13C-CT-HSQC b NMR spectra of Ca2+-free Ncs1p recorded at 800 MHz proton frequency. Amide resonances from residues in the downfield region (N77, G78, F82, N113, G114, and indole amide protons of W30 and W103) are not shown in a. Side chain amide resonances of Asn and Gln are connected with solid lines. The assignment of backbone amide and side-chain methyl groups are indicated in a and b, respectively. Unresolved methyl groups of valine and leucine are designated as CG and CD, respectively
The chemical shift index of each amino acid residue reveals a protein secondary structure in Ncs1p that closely resembles the canonical secondary structure and topology seen in other NCS proteins. Ncs1p contains 10 α-helices and two antiparallel β-sheets (α1: 9–17; α2: 25–35; β1: 42–44; α3: 44–55; α4: 61–72; β2: 79–81; α5: 82–90; α6: 101–108; β3: 115–117; α7: 118–131; α8: 145–154; β4: 163–165; α9: 166–174; α10: 179–186). The NMR assignments reported here for Ca2+-free Ncs1p are overall similar to those reported previously for Ca2+-free recoverin (BMRB 4030). Similar chemical shifts are seen for conserved residues in the N-terminal region that might interact with the myristoyl group, suggesting that Ncs1p contains a myristoyl group in a sequestered environment similar to that seen previously in Ca2+-free recoverin (Tanaka et al. 1995).
Acknowledgments
We thank Jeff Walton for technical support and help with NMR experiments. Work supported by NIH grants (EY012347) to J.B.A and (RR11973) to the UC Davis NMR facility.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Ames JB, Tanaka T, Stryer L, Ikura M. Portrait of a myristoyl switch protein. Curr Opin Struct Biol. 1996;6:432–438. doi: 10.1016/S0959-440X(96)80106-0. [DOI] [PubMed] [Google Scholar]
- Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. doi: 10.1038/38310. [DOI] [PubMed] [Google Scholar]
- Ames JB, Dizhoor AM, Ikura M, Palczewski K, Stryer L. Three-dimensional structure of guanylyl cyclase activating protein-2, a calcium-sensitive modulator of photoreceptor guanylyl cyclases. J Biol Chem. 1999;274:19329–19337. doi: 10.1074/jbc.274.27.19329. [DOI] [PubMed] [Google Scholar]
- Ames JB, Hendricks KB, Strahl T, Huttner IG, Hamasaki N, Thorner J. Structure and calcium-binding properties of Frq1, a novel calcium sensor in the yeast Saccharomyces cerevisiae. Biochemistry. 2000;39:12149–12161. doi: 10.1021/bi0012890. [DOI] [PubMed] [Google Scholar]
- Bourne Y, Dannenberg J, Pollmann VV, Marchot P, Pongs O. Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor1) J Biol Chem. 2001;276:11949–11955. doi: 10.1074/jbc.M009373200. [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Gundelfinger ED. Intracellular neuronal calcium sensor proteins: a family of EF-hand calcium-binding proteins in search of a function. Cell Tissue Res. 1999;295:1–12. doi: 10.1007/s004410051207. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, O’Callaghan DW, Hasdemir B, Haynes LP, Tepikin AV. Neuronal Ca2+-sensor proteins: multitalented regulators of neuronal function. Trends Neurosci. 2004;27:203–209. doi: 10.1016/j.tins.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeiffer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Rrolley D, Walsh KA, Philipov PP, Hurley JB, Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991;251:915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Chen CK, Olshevskaya E, Sinelnikova VV, Phillipov P, Hurley JB. Role of the acylated amino terminus of recoverin in Ca(2+)-dependent membrane interaction. Science. 1993;259:829–832. doi: 10.1126/science.8430337. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Lagnado L, Zozulya S, Neubert TA, Stryer L, Baylor DA. The effect of recombinant recoverin on the photoresponse of truncated rod photoreceptors. Proc Natl Acad Sci USA. 1998;95:6474–6479. doi: 10.1073/pnas.95.11.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KM, Zozulya S, Stryer L, McKay DB. Three-dimensional structure of recoverin, a calcium sensor in vision. Cell. 1993;75:709–716. doi: 10.1016/0092-8674(93)90491-8. [DOI] [PubMed] [Google Scholar]
- Hamasaki-Katagiri N, Molchanova T, Takeda K, Ames JB. Fission yeast homolog of neuronal calcium sensor-1 (Ncs1p) regulates sporulation and confers calcium tolerance. J Biol Chem. 2004;279:12744–12754. doi: 10.1074/jbc.M311895200. [DOI] [PubMed] [Google Scholar]
- Hendricks KB, Wang BQ, Schnieders EA, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Okazaki K. Neurocalcin family: a novel calcium-binding protein abundant in bovine central nervous system. Neurosci Res. 1993;16:73–77. doi: 10.1016/0168-0102(93)90074-Z. [DOI] [PubMed] [Google Scholar]
- Ikura M, Kay LE, Bax A. A novel approach for sequential assignment of 1H, 13C, and 15 N spectra of proteins: heteronuclear triple-resonanc three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry. 1990;29:4659–4667. doi: 10.1021/bi00471a022. [DOI] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993;362:855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Kay LE, Xu GY, Singer AU, Muhandiram DR, Forman-Kay JD. A gradient-enhanced HCCH-TOCSY experiment for recording side-chain 1H and 13C correlations in H2O samples of proteins. J Magn Reson. 1993;101:33–337. [Google Scholar]
- Kobayashi M, Takamatsu K, Saitoh S, Noguchi T. Myristoylation of hippocalcin is linked to its calcium-dependent membrane association properties. J Biol Chem. 1993;268:18898–18904. [PubMed] [Google Scholar]
- O’Callaghan DW, Burgoyne RD. Identification of residues that determine the absence of a Ca(2+)/myristoyl switch in neuronal calcium sensor-1. J Biol Chem. 2004;279:14347–14354. doi: 10.1074/jbc.M310152200. [DOI] [PubMed] [Google Scholar]
- Pongs O, Lindemeier J, Zhu XR, Theil T, Engelkamp D, Krah-Jentgens I, Lambrecht HG, Kock KW, Schwerner J, Rivosecchi R, Mallart A, Galceran J, Canal I, Barbas JA, Ferrus A. Frequenin-a novel calcium-binding protein that modulates synaptic efficacy. Neuron. 1993;11:15–28. doi: 10.1016/0896-6273(93)90267-U. [DOI] [PubMed] [Google Scholar]
- Stephen R, Bereta G, Golczak M, Palczewski K, Sousa MC. Stabilizing function for myristoyl group revealed by the crystal structure of a neuronal calcium sensor, guanylate cyclase-activating protein 1. Structure. 2007;15:1392–1402. doi: 10.1016/j.str.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl T, Huttner IG, Lusin JD, Osawa M, King D, Thorner J, Ames JB. Structural insights into activation of phosphatidylinositol 4-kinase (Pik1) by yeast frequenin (Frq1) J Biol Chem. 2007;282:30949–30959. doi: 10.1074/jbc.M705499200. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ames JB, Harvey TS, Stryer L, Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature. 1995;376:444–447. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar S, Kumar VD. Crystal structure of recombinant bovine neurocalcin. Nat Struct Biol. 1999;6:80–88. doi: 10.1038/4956. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Forman-Kay JD, Kay LE. Two-dimensional NMR experiments for correlating 13C and 1H/chemical shifts of aromatic residues in 13C-labeled proteins via scalar couplings. J Am Chem Soc. 1993;115:11054–11055. doi: 10.1021/ja00076a099. [DOI] [Google Scholar]
- Zozulya S, Stryer L. Calcium-myristoyl protein switch. Proc Natl Acad Sci USA. 1992;89:11569–11573. doi: 10.1073/pnas.89.23.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]