Abstract
Legionella bacterium, an intracellular pathogen of mononuclear phagocytes, causes acute fatal pneumonia, especially in patients with impaired cellular immune responses. Until recently, however, the toll-like receptor (TLR) engagement of bacterial proteins derived from Legionella is uncertain. We previously showed that a 19-kDa highly conserved peptidoglycan-associated lipoprotein (PAL) of Legionella pneumophila induced the PAL-specific B cell and T cell responses in mice. In this study, we observed that the rPAL antigen of L. pneumophila, as an effector molecule, activated murine macrophages via TLR2 and produced proinflammatory cytokines such as IL-6 and TNF-α. In both BALB/c and TLR4-deficient C3H/HeJ mice, pretreatment of macrophages with anti-TLR2 mAb showed severely impaired cytokine production in response to the rPAL. In addition, in vitro the rPAL treatment increased the cell surface expression of CD40, CD80, CD86 and MHC I/II molecules. We further showed that the synthetic CpG-oligodeoxynucleotides (CpG ODN) coadministered with the rPAL enhanced IL-12 and IL-6 production and expression of CD40, CD80 and MHC II compared to the rPAL treatment alone. In conclusions, these results indicate that Legionella PAL might activate macrophages via a TLR2-dependent mechanism which thus induce cytokine production and expression of costimulatory and MHC molecules.
Keywords: Legionella pneumophila; Legionnaires' disease; lipoproteins; macrophages, peritoneal; Toll-like receptors
Introduction
Legionella pneumophila, a gram-negative intracellular pathogen, is the etiologic agent of Legionnaires' disease and Pontiac fever. The bacterium is also an important cause of epidemic and sporadic pneumonia worldwide. Transmission to humans occurs through inhalation of aerosols containing L. pneumophila (Steinert et al., 2002). The bacteria reside in the lungs and multiply in the phagosomes of human monocytes (Horwitz et al., 1980) and alveolar macrophages (Nash et al., 1984) by evading phagosome acidification or phagolysosomal fusion (Horwitz, 1983).
Peptidoglycan-associated lipoprotein (PAL) is an outer membrane protein that is commonly present among Legionella species as well as other gram-negative bacteria (Engleberg et al., 1991). A 19 kDa Legionella PAL is of particular interest. Our previous study first demonstrated that rPAL of L. pneumophila, as a vaccine candidate, was a potent activator of PAL-specific B and T cell responses in BALB/c mice immunized with either the rPAL or plasmid DNA PAL (Yoon et al., 2002). Subsequently, the PAL was characterized as a soluble antigen excreted from urine of patients with Legionella pneumonia (Kim et al., 2003).
Members of the toll-like receptor (TLR) family in cells of the innate immune system recognize specific conserved components of microbes, including lipopolysaccharide, peptidoglycan, lipopeptides, lipoteichoic acid and CpG DNA motifs present in bacterial DNA, and initiate the cascade of the inflammatory response, further activating adaptive immunity through the induction of cytokine production and expression of costimulatory molecules (Akira et al., 2001; Barton and Medzhitov, 2002; Dabbagh and Lewis, 2003; Krishnan et al., 2007). Recently there has been broad interest in testing and developing TLR ligands for immune stimulation to enhance antigen-specific responses. Especially, synthetic oligonucleotides containing the CpG motifs (CpG ODN), which are ligands for TLR9, are being developed as a new generation of vaccine adjuvants to enhance immune responses (Krieg and Davis, 2006).
Several studies have reported that TLRs contribute to the host response against L. pneumophila during in vitro and in vivo infection (Girard et al., 2003; Kikuchi et al., 2004; Akamine et al., 2005; Hawn et al., 2006, 2007; Fuse et al., 2007; Newton et al., 2007). However, there have been no studies to elucidate immunostimulatory properties of isolated individual proteins from L. pneumophila in terms of engagement of TLRs and linking to elicit the antigen-specific adaptive immune responses.
The aim of this study was to determine the role of TLRs in the host response to the purified rPAL antigen of L. pneumophila to induce production of proinflammatory cytokines and expression of costimulatory molecules. Furthermore, we examined whether combining treatment of CpG ODN and the rPAL in vitro could enhance the rPAL-induced immune responses.
Results
Legionella PAL activates TLR 2-mediated signaling in BALB/c and C3H/HeJ macrophages
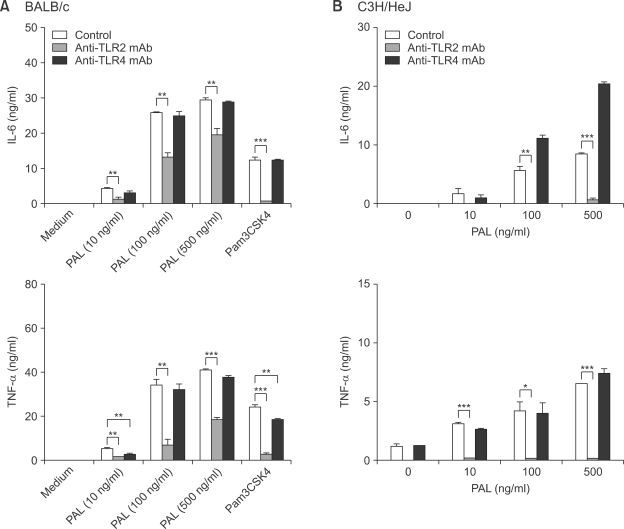
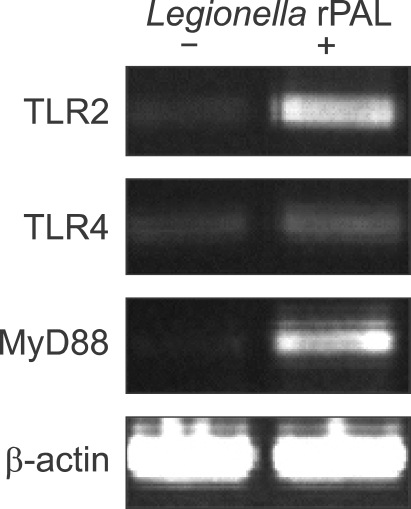
To explore the involvement of TLR2 or TLR4 in Legionella PAL-induced cell activation, peritoneal macrophages from BALB/c mice were incubated with or without anti-TLR2 and -TLR4 mAb respectively, and then stimulated with various concentrations of the rPAL for 18 h (Figure 1). In the absence of TLR blocking antibodies, the production of IL-6 and TNF-α was increased by the rPAL in a dose-dependent fashion. In experiments with pretreatment of TLR antibodies, the rPAL-induced IL-6 production was significantly inhibited by the anti-TLR2 mAb, but it was unaffected by the anti-TLR4 mAb (Figure 1A). Similarly, the rPAL-induced TNF-α production was significantly inhibited by the anti-TLR2 mAb, but no significant change was observed between untreated and anti-TLR4 mAb treated macrophages (Figure 1B). We used in parallel Pam3CSK4 as a positive control of TLR2 agonist for the TLR2-dependant stimulation. These findings suggest that Legionella PAL might induce cytokine production by interaction with TLR2. We also verified TLR2-dependant stimulation by rPAL in macrophages from LPS-hyporesponsive, TLR4-mutant C3H/HeJ mice, as a control system for TLR4 signaling (Braedel-Ruoff et al., 2005). Similar to BALB/c macrophages, C3H/HeJ macrophages significantly increased IL-6 and TNF-α levels in response to rPAL treatment, which was greatly inhibited by TLR2 blocking antibody. There was no inhibition of the cytokine production in cells treated with anti-TLR4 mAb. We further investigated TLR-mediated signaling by the rPAL of L. pneumophila at the gene expression level. The mRNA expression of TLR2, TLR4 and MyD88 were analyzed in BALB/c macrophages with or without rPAL treatment by RT-PCR. Compared to the untreated controls, TLR2 and MyD88 mRNA expression was prominent with rPAL treatment (Figure 2). In contrast, the TLR4 mRNA expression was very weak. These findings indicate that Legionella PAL-mediated signaling might modulate TLR2 and possibly, MyD88 expression.
Figure 1.
Production of IL-6 and TNF-α in BALB/c mouse macrophages after stimulation with rPAL. Peritoneal macrophages from BALB/c (A) or C3H/HeJ (B) mice were pretreated with or without 10 µg/ml of anti-TLR2 or -TLR4 mAb. After 1h cells were stimulated for 18 h with various concentrations (10, 100 and 500 ng/ml) of rPAL. The amounts of IL-6 and TNF-α in culture supernatants were determined by using ELISA. As a positive control for TLR2-dependant stimulation, TLR2 agonist, Pam3CSK4 (100 ng/ml) was used. Data are expressed as mean ± SD and represent three separate experiments (*P < 0.05, **P < 0.01, ***P < 0.001).
Figure 2.
Effect of Legionella PAL on TLR2, TLR4 and MyD88 mRNA expression. Peritoneal macrophages from BALB/c mice were treated with or without 1 µg/ml of rPAL for 18 h. Total RNA was then extracted from the macrophages and subjected to RT-PCR, as described in Methods section. The β-actin gene expression was examined as a positive control.
Coadministration of CpG ODN with Legionella PAL synergistically enhances cytokine production in murine macrophages
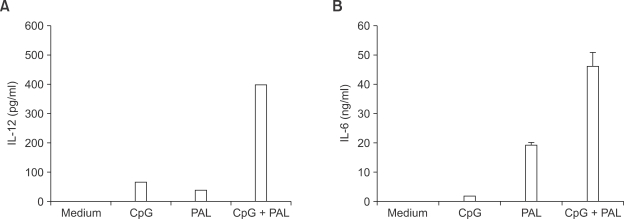
We next examined whether cytokine production by Legionella PAL antigen might be further enhanced by coadministration with CpG ODN. IL-12 production was observed in macrophages when treated with CpG ODN, the rPAL or both (Figure 3A). Interestingly, combined treatment of CpG ODN and the rPAL, increased IL-12 production by up to sevenfold, compared to the rPAL alone. As expected, CpG ODN alone induced minimal release of IL-6 (Figure 3B). However, the levels of IL-6 production were significantly increased by coadministration of CpG ODN and the rPAL. These results indicate that the combination of CpG ODN and Legionella PAL might synergistically activate macrophages to produce cytokines.
Figure 3.
Co-stimulation of Legionella PAL with CpG ODN induces significantly increased production of IL-12 and IL-6. BALB/c mice macrophages were incubated for 18 h with culture medium, CpG ODN (3 µg/ml), rPAL (100 ng/ml), and the combination of CpG ODN and rPAL. The cell supernatants were collected and analyzed by ELISA for IL-12p40 (A) and IL-6 (B) levels. Results are expressed as means of triplicate wells ± SD.
Legionella PAL increases expression of cell surface molecules in murine macrophages
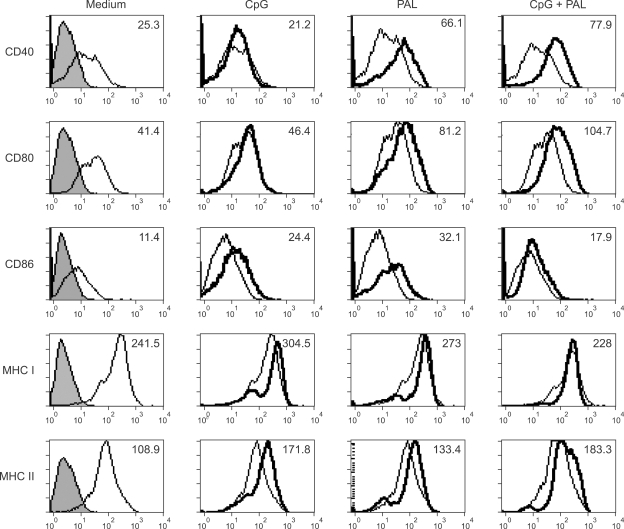
To elucidate whether the rPAL or the rPAL plus CpG ODN can induce expression of cell surface molecules in macrophages, in terms of activation of the adaptive immune responses, peritoneal macrophages were analyzed by flow cytometry after 24 h incubation with the rPAL, CpG ODN, or CPG ODN plus the rPAL (Figure 4). The rPAL-treated macrophages demonstrated increased expression of the B7 family (CD80 and CD86), MHC II and in particular, CD40 molecules compared to the untreated control. However, coadministration of CpG ODN and the rPAL was associated with little increase in expression of MHC II, CD40 and CD80 and decrease in expression of CD86 and MHC I, compared to the rPAL or CpG ODN alone. Collectively, these results indicate that macrophage activated with Legionella PAL upregulates expression of costimulatory molecules, and especially, upregulation of CD40 molecule may participate in T or B cell activation as a predominant costimulatory signal. In addition, CpG ODN could modulate the PAL-specific immune responses.
Figure 4.
Effect of PAL or CpG ODN on the surface expression of costimulatory and MHC molecules. Peritoneal macrophages (1 × 106 cells/ml) were cultured with media alone, CpG ODN (10 µg/ml), rPAL (10 µg/ml) or CpG ODN plus rPAL, and analyzed for expression of CD40, CD80, CD86, MHC I and MHC II by flow cytometry, as detailed in the Methods section. Gray filled histograms: isotype matched controls, Thin-line histograms: untreated cells, Thick-line histograms: CpG, rPAL or CpG+rPAL treated cells. Mean Fluorescence Intensity (MFI) was given on the top right upper corner of each panel.
Discussion
Several studies have identified the role of TLR2 in innate immune responses against L. pneumophila infection, although other TLRS might also contribute to innate immunity against this organism (Akamine et al., 2005; Archer and Roy, 2006; Hawn et al., 2006; Fuse et al., 2007). TLR2 plays a pivotal role in the bacterial recognition and host resistance against the intracellular growth of L. pneumophila (Akamine et al., 2005; Fuse et al., 2007). TLR2 deficient mice attenuated IL-12p40 production (Akamine et al., 2005), and showed higher mortality to L. pneumophila infection compared to wild-type and TLR4-deficient mice (Hawn et al., 2006; Fuse et al., 2007). The purified LPS of L. pneumophila has been shown to be involved in TLR2-mediated macrophage activation (Girard et al., 2003; Braedel-Ruoff et al., 2005; Fuse et al., 2007), however, other bacterial components of L. pneumophila have not yet been studied.
In the present study, we have demonstrated that the 19 kDa, purified rPAL of L. pneumophila, one of cell-wall associated lipoproteins, could significantly induce production of proinflammatory cytokines such as IL-6 and TNF-α in macrophages from BALB/c or TLR4 deficient C3H/HeJ mice. The responses were significantly inhibited by TLR2 blocking antibody, but not affected by TLR4 blocking antibody, indicating that Legionella PAL appears to be a potent TLR2 agonist. This finding is consistent with the general notion that TLR2 recognizes various bacterial lipoproteins (Brightbill et al., 1999; Hirschfeld et al., 1999; Lien et al., 1999; Takeuchi et al., 1999).
Recently, Fuse et al. (2007) reported TLR2 as a recognition molecule of L. pneumophila LPS for induction of macrophage inflammatory protein-2 (MIP-2). They demonstrated that MIP-2 production was reduced in lungs from TLR2-deficient mice infection model and bone-marrow-derived macrophages in response to L. pneumophila LPS. One interesting observation of their study is that higher concentrations of LPS are able to overcome the lack of TLR2, suggesting the involvement of other factors. Therefore, it might be interesting to examine whether Legionella PAL as a TLR2 ligand involve in pulmonary inflammatory responses or MIP-2 production in murine infection models.
Legionella bacterium replicates in alveolar macrophages, but the disease results from a complex series of interactions with host (Cianciotto, 2001). Although Legionella species-common PAL antigen has been characterized as the most abundant protein of the soluble fractions (Kim et al., 2003), its role in pathogenesis is unknown. In the present study, in vitro rPAL-stimulated macrophages induced effectively IL-6 and TNF-α production in dose-dependent fashion, mediated by TLR2 signaling. This suggests that the PAL-induced cytokine mobilization can be either protective or detrimental, depending on the extent of the cytokine released during the innate immune responses. Newton et al. (2000) observed that survival of BALB/c mice from sublethal challenge of L. pneumophila was associated with elevated acute phase cytokines (TNF-α, IL-1β and IL-6), and susceptible IL-4 deficient mice showed higher levels of the cytokines. In addition, the PAL of gram-negative bacteria, as a naturally occurring TLR2 agonist, is shed by bacteria into circulation of sepsis animal and induces cytokine production by macrophages (Hellman et al., 2002; Liang et al., 2005). All these findings support that TLR2-mediated macrophage activation by Legionella PAL might play in part an important role in pathogenesis during the acute stage of Legionella infection.
In the present study, we have also shown that TLR2 as well as MyD88 mRNA expression was prominent in the rPAL-treated macrophages compared to the untreated controls. This indicates that TLR2-mediated signaling by the rPAL might be involved in initiating the MyD88 dependent response, although further studies using TLR2-deficient or MyD88-deficient mice are needed to confirm this. It is not clear whether additional MyD88-dependent, TLR2-independent pathways is crucial for full protection against L. pneumophila (Archer and Roy, 2006; Hawn et al., 2006). Moreover, the potential ability of the rPAL to activate other TLRS remains to be determined in future experiments.
As our previous study has shown that Legionella PAL induces strong PAL-specific antibody and cellular responses in BALB/c mice immunized with pcDNA3-PAL or the rPAL (Yoon et al., 2002), we are especially interested in developing strategies to enhance Legionella PAL-induced adaptive immunity by stimulating TLR-mediated innate immune responses. In our present study, coadministration of CpG ODN, as a TLR9 agonist, with the rPAL induced synergistic production of inflammatory cytokines (IL-12p40 and IL-6) compared to the rPAL alone. CpG ODN is known to directly stimulate B cells and DCs, thereby enhancing the production of Th1-biased cytokines (IFN-γ and IL-12) as well as proinflammatory cytokines (IL-1, IL-6, IL-18 and TNF) (Krieg and Davis, 2006). Coadministration of CpG ODN with the rPAL antigen might favorably shift the rPAL-induced Th2 biased response to Th1 responses (Chu et al., 1997; Yoon et al., 2002). Especially, marked increase of IL-12 production would be beneficial in controlling early dissemination of L. pneumophila (Tateda et al., 2001a, 2001b). Together with these data, cooperated signaling via TLR2 and TLR9 stimulation might be used as one of new strategies to promote Th1 responses for vaccine development against various infection diseases.
Rogers and colleagues (2007) reported that exposure of bone marrow derived DCs to L. pneumophila increased expression of TLR2 and TLR4 and enhanced expression of costimulatory (CD40 and CD86) and MHC molecules. In the present study, we have shown that macrophages treated with the rPAL induced increased expression of CD40, CD80, CD86 and MHC I/II, and in particular, the combined treatment of CpG ODN and the rPAL had prominently increased the expression of CD40, which might lead to a greater chance of interaction between CD40 and CD40L and macrophage activation for IL-12 production (Grewal et al., 1997; Mathur et al., 2006). Therefore, these data support that Legionella PAL antigen is fully competent for stimulation of immune responses by expression of costimulatory and MHC molecules as well as cytokine production, and the addition of CpG ODN might expect to induce the synergistic effect via activation of TLR2- and TLR9-mediated signaling pathways.
In summary, our results clearly demonstrated the involvement of TLR2 in the recognition of Legionella PAL by murine peritoneal macrophages. Furthermore, we suggest that Legionella PAL as a vaccine antigen may activate the innate immunity at least through the TLR2-mediated signaling pathway, and then link to the development of the PAL-specific adaptive immunity. Furthermore, the selective targeting of bacterial cell components to TLR stimulation would appear to be a great assurance for vaccine development against infectious diseases.
Methods
Production and purification of a recombinant lipoprotein
The full protein-coding region of the pal gene of 19 kDa lipoprotein (Engleberg et al., 1991) was PCR-amplified from a DNA template derived from L. pneumophila serogroup 1 strain with a pair of primers: the XbaI containing sense primer 5'-TCTAGATTGTGGAATGAAAGCCGGATCGT-3' and SalI containing anti-sense primer 5'-GTCCACCCATGAGGCGAAAGGAAGCATC-3'. The amplified DNA was digested with XbaI and SalI, and ligated into an XbaI/SalI-cut pMAL-c2X vector (New England Biolabs Inc., Ipswich, MA). The plasmid construct was used to transform Escherichia coli BL21(DE3) pLysS (Novagen, Madison, WI) for the production of the recombinant PAL protein fused to a 43 kDa maltose-binding protein (MBP). The pal gene with in frame cloning in the plasmid construct was identified by sequence analysis. The MBP-PAL fusion protein was purified using the pMAL protein fusion and purification system (New England Biolabs Inc.) according to the manufacturer's instructions. The 63 kDa purified fusion protein was cleaved with factor Xa at 23℃ for 24 h and then applied to DEAE-sepharose resin. In this step, a free rPAL (19 kDa) was separately collected from the flow-through fraction. The protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). The protein was identified by SDS-PAGE and immunoblotting with rabbit anti-rPAL IgG (Kim et al., 2003) (data not shown). To eliminate contaminating LPS from the rPAL preparation, all reagents were prepared with endotoxin-free solutions. The rPAL used in the experiments was tested for the LPS concentration by the Limulus amebocyte lysate test (BioWhitttaker, Walkersville, MD) and contained less than 20 pg of endotoxin per ml.
Preparation of peritoneal macrophage cells
Six-week-old female BALB/c and C3H/HeJ mice were purchased from Dae Han Bio Link Inc. (Seoul, Korea). Mouse peritoneal macrophages were isolated by peritoneal lavage with ice-cold sterile physiologic saline 5 days after intraperitoneal injection of 3 ml of 3% (w/v) sterile brewer thioglycollate (Becton Dickinson, Franklin Lakes, NJ). The cells were washed, resuspended in RPMI 1640 containing 10% FBS (Gibco, Grand island, NY), 1% penicillin-streptomycin (Gibco) and plated in 96-well flat bottomed plates (Nunclon Surface, Roskilde, Denmark) at a concentration of 1 × 105 cells per well. Following incubation in a humidified 5% CO2 incubator for 24 h at 37℃, the cells were washed three times with culture medium before the specific treatment.
Cytokine ELISA
For blockade of the TLRs, macrophages (1 × 105 cells) were preincubated with 10 µg/ml of anti-TLR2 mAb (clone T2.5, eBioscience, CA) or anti-TLR4 mAb (clone MTS 510, eBioscience) for 1 h. Macrophages were pulsed with rPAL (10-500 ng/ml), Pam3CSK4 (100 ng/ml), CpG ODN (3 µg/ml) or CpG ODN (3 µg/ml) plus rPAL (100 ng/ml) for 18 h at 37℃ in a humidified 5% CO2 environment. The cell supernatants were collected and stored at -20℃. IL-12p40 (BioSource, Camarillo, CA), IL-6 (BioSource) and TNF-α (Serotec, Oxford, UK) levels were determined by ELISA kits according to the manufacturers' instructions. The optical density was measured at 450 nm by a VERSAmax reader (Molecular Devices Co., Sunnyvale, CA). Pam3CSK4, a synthetic lipopeptide was purchased from Invivogen (San Diego, CA). CpG ODN was purchased from Bioneer Co. (Daejeon, Korea). The sequences of the CpG ODN used were 5'-TCCATGACGTTCCTGACGTT-3' (CpG DNA, ODN1826).
RT-PCR
The peritoneal macrophages (1 × 106 cells) were stimulated with 1 µg/ml of the rPAL for 18 h at 37℃. Total RNA was isolated from macrophage cultures using Trizol reagent (Invitrogen, Camarillo, CA) according to the manufacturer's protocol. To synthesize the first strand cDNA, reverse transcription of total RNA was performed with avian myeloblastosis virus RTase (Promega, Madison, WI). For the PCR amplification, the sample was heated at 94℃ for 5 min, and then subjected to 30 amplification cycles of 1 min at 94℃, 30 s at 60℃, 1 min at 72℃, and 5 min at 72℃. PCR products were visualized with ethidium bromide in 1.5% agarose gels. The specific primer sets used were as follows: 5'-TTCCTACAGTGAGCAGGATTCCCAT-3' and anti-sense: 5'-CTTTTCGATGGAATCGATGATGTGT-3' for TLR2, sense: 5'-TAGCCATTGCTGCCAACATCATCCA-3' and anti-sense: 5'-TGCCAGAGCGGCTGCTCAGAAACT-3' for TLR4, sense: 5'-GGTCCATTGCCAGCGAGCTAATTG-3' and anti-sense: 5'-AATCAGTCGCTTCTGTTGGACACCT-3' for MyD88, and sense: 5'-AAGACCTCTATGCCAACACAGTGCT-3' and anti-sense: 5'-CCACACAGAGTACTTGCGCTCAGG-3' for β-actin.
Flow cytometry
The peritoneal macrophages (1 × 106 cells/ml) were incubated with CpG ODN (10 µg/ml), rPAL (10 µg/ml) or the combination of CpG ODN and rPAL for 24 h. The cells were harvested and washed with ice-cold PBS containing 2% FBS and stained with FITC- or PE-conjugated mAbs against CD40 (clone 3/23) (Santa cruze, Biotec. Inc.), CD80 (B7-1, clone 16-10A1) (eBioscience), CD86 (B7-2, clone GL-1) (eBioscience), MHC I (clone 34-1-2S) (eBioscience) and MHC II (clone M5/114.15.2) (eBioscience). In all experiment the cells were first treated with aggregated 1 µg/ml 2.4G2 (anti-FcrRII and -III: BD Phar-Mingen, San Diego, CA) to block nonspecific IgG binding. Cells for MHC I and II molecules were simultaneously stained with the indicated FITC- or PE-conjugated mAb for 30 min at 4℃. After immunolabeling, cells were washed in PBS with FBS, fixed in 1% paraformaldehyde. Ten thousand events were acquired on FACScan flow cytometer (Becton Dickinson, San Jose, CA). FACS data were analyzed using CellQuest software (Becton Dickinson).
Statistical analysis
All data were expressed as means ± SD. Data analysis was performed using the Sigma stat software (Systat Software Inc., San Jose, CA). Differences between group means were tested for statistical significance by one-way ANOVA and Duncan method post-hoc comparison. A P value of < 0.05 was considered statistically significant.
Acknowledgments
We thank M.D. Woon Ki Paik for his critical reading of this manuscript. This work was partly funded by the Korea Research Foundation grant (KRF-2005-015-E00139) and by Korea University.
Abbreviations
- CpG ODN
synthetic CpG-oligodeoxynucleotides
- rPAL
recombinant peptidoglycan-associated lipoprotein
- TLRs
toll-like receptors
References
- 1.Akamine M, Higa F, Arakaki N, Kawakami K, Takeda K, Akira S, Saito A. Differential roles of Toll-like receptors 2 and 4 in in vitro responses of macrophages to Legionella pneumophila. Infect Immun. 2005;73:352–361. doi: 10.1128/IAI.73.1.352-361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 3.Archer KA, Roy CR. MyD88-dependent responses involving toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires' disease. Infect Immun. 2006;74:3325–3333. doi: 10.1128/IAI.02049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton GM, Medzhitov R. Control of adaptive immune responses by Toll-like receptors. Curr Opin Immunol. 2002;14:380–383. doi: 10.1016/s0952-7915(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 5.Braedel-Ruoff S, Faigle M, Hilf N, Neumeister B, Schild H. Legionella pneumophila mediated activation of dendritic cells involves CD14 and TLR2. J Endotoxin Res. 2005;11:89–96. doi: 10.1179/096805105X35189. [DOI] [PubMed] [Google Scholar]
- 6.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 7.Cianciotto NP. Pathogenicity of Legionella pneumophila. Int J Med Microbiol. 2001;291:331–343. doi: 10.1078/1438-4221-00139. [DOI] [PubMed] [Google Scholar]
- 8.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dabbagh K, Lewis DB. Toll-like receptors and T-helper-1/T-helper-2 responses. Curr Opin Infect Dis. 2003;16:199–204. doi: 10.1097/00001432-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Engleberg NC, Howe DC, Rogers JE, Arroyo J, Eisenstein BI. Characterization of a Legionella pneumophila gene encoding a lipoprotein antigen. Mol Microbiol. 1991;5:2021–2029. doi: 10.1111/j.1365-2958.1991.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 11.Fuse ET, Tateda K, Kikuchi Y, Matsumoto T, Gondaira F, Azuma A, Kudoh S, Standiford TJ, Yamaguchi K. Role of Toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model. J Med Microbiol. 2007;56:305–312. doi: 10.1099/jmm.0.46913-0. [DOI] [PubMed] [Google Scholar]
- 12.Girard R, Pedron T, Uematsu S, Balloy V, Chignard M, Akira S, Chaby R. Lipopolysaccharides from Legionella and Rhizobium stimulate mouse bone marrow granulocytes via Toll-like receptor 2. J Cell Sci. 2003;116:293–302. doi: 10.1242/jcs.00212. [DOI] [PubMed] [Google Scholar]
- 13.Grewal IS, Borrow P, Pamer EG, Oldstone MB, Flavell RA. The CD40-CD154 system in anti-infective host defense. Curr Opin Immunol. 1997;9:491–497. doi: 10.1016/s0952-7915(97)80100-8. [DOI] [PubMed] [Google Scholar]
- 14.Hawn TR, Smith KD, Aderem A, Skerrett SJ. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J Infect Dis. 2006;193:1693–1702. doi: 10.1086/504525. [DOI] [PubMed] [Google Scholar]
- 15.Hawn TR, Berrington WR, Smith IA, Uematsu S, Akira S, Aderem A, Smith KD, Skerrett SJ. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J Immunol. 2007;179:6981–6987. doi: 10.4049/jimmunol.179.10.6981. [DOI] [PubMed] [Google Scholar]
- 16.Hellman J, Roberts JD, Jr, Tehan MM, Allaire JE, Warren HS. Bacterial peptidoglycan-associated lipoprotein is released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J Biol Chem. 2002;277:14274–14280. doi: 10.1074/jbc.M109696200. [DOI] [PubMed] [Google Scholar]
- 17.Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 18.Horwitz MA, Silverstein SC. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz MA. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MJ, Sohn JW, Park DW, Park SC, Chun BC. Characterization of a lipoprotein common to Legionella species as a urinary broad-spectrum antigen for diagnosis of Legionnaires' disease. J Clin Microbiol. 2003;41:2974–2979. doi: 10.1128/JCM.41.7.2974-2979.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuchi T, Kobayashi T, Gomi K, Suzuki T, Tokue Y, Watanabe A, Nukiwa T. Dendritic cells pulsed with live and dead Legionella pneumophila elicit distinct immune responses. J Immunol. 2004;172:1727–1734. doi: 10.4049/jimmunol.172.3.1727. [DOI] [PubMed] [Google Scholar]
- 22.Krieg AM, Davis HL. CpG ODN as a Th1 immune enhancer for prophylactic and therapeutic vaccines. In: Hackett CJ, Harn DA Jr, editors. Vaccine Adjuvants. New Jersey, USA: Humana Press Inc.; 2006. pp. 87–110. [Google Scholar]
- 23.Krishnan J, Selvarajoo K, Tsuchiya M, Lee G, Choi S. Toll-like receptor signal transduction. Exp Mol Med. 2007;39:421–438. doi: 10.1038/emm.2007.47. [DOI] [PubMed] [Google Scholar]
- 24.Liang MD, Bagchi A, Warren HS, Tehan MM, Trigilio JA, Beasley-Topliffe LK, Tesini BL, Lazzaroni JC, Fenton MJ, Hellman J. Bacterial peptidoglycan-associated lipoprotein: a naturally occurring toll-like receptor 2 agonist that is shed into serum and has synergy with lipopolysaccharide. J Infect Dis. 2005;191:939–948. doi: 10.1086/427815. [DOI] [PubMed] [Google Scholar]
- 25.Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, Carroll JD, Espevik T, Ingalls RR, Radolf JD, Golenbock DT. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 26.Mathur RK, Awasthi A, Saha B. The conundrum of CD40 function: host protection or disease promotion? Trends Parasitol. 2006;22:117–122. doi: 10.1016/j.pt.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Nash TW, Libby DM, Horwitz MA. Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J Clin Invest. 1984;74:771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton CA, Perkins I, Widen RH, Friedman H, Klein TW. Role of Toll-like receptor 9 in Legionella pneumophila-induced interleukin-12p40 production in bone marrow-derived dendritic cells and macrophages from permissive and nonpermissive mice. Infect Immun. 2007;75:146–151. doi: 10.1128/IAI.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton C, McHugh S, Widen R, Nakachi N, Klein T, Friedman H. Induction of interleukin-4 (IL-4) by legionella pneumophila infection in BALB/c mice and regulation of tumor necrosis factor alpha, IL-6, and IL-1beta. Infect Immun. 2000;68:5234–5240. doi: 10.1128/iai.68.9.5234-5240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers J, Hakki A, Perkins I, Newton C, Widen R, Burdash N, Klein T, Friedman H. Legionella pneumophila infection up-regulates dendritic cell Toll-like receptor 2 (TLR2)/TLR4 expression and key maturation markers. Infect Immun. 2007;75:3205–3208. doi: 10.1128/IAI.01950-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinert M, Hentschel U, Hacker J. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol Rev. 2002;26:149–162. doi: 10.1111/j.1574-6976.2002.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Ogawa T, Takada H, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 33.Tateda K, Moore TA, Deng JC, Newstead MW, Zeng X, Matsukawa A, Swanson MS, Yamaguchi K, Standiford TJ. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol. 2001a;166:3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 34.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun. 2001b;69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon WS, Park SH, Park YK, Park SC, Sin JI, Kim MJ. Comparison of responses elicited by immunization with a Legionella species common lipoprotein delivered as naked DNA or recombinant protein. DNA Cell Biol. 2002;21:99–107. doi: 10.1089/104454902753604970. [DOI] [PubMed] [Google Scholar]