Abstract
Wnt signaling is known to be important for diverse embryonic and post-natal cellular events and be regulated by the proteins Dishevelled and Axin. Although Dishevelled is activated by Wnt and involved in signal transduction, it is not clear how Dishevelled-mediated signaling is turned off. We report that guanine nucleotide binding protein beta 2 (Gnb2; Gβ2) bound to Axin and Gβ2 inhibited Wnt mediated reporter activity. The inhibition involved reduction of the level of Dishevelled, and the Gβ2γ2 mediated reduction of Dishevelled was countered by increased expression of Axin. Consistent with these effects in HEK293T cells, injection of Gβ2γ2 into Xenopus embryos inhibited the formation of secondary axes induced either by XWnt8 or Dishevelled, but not by β-catenin. The DEP domain of Dishevelled is necessary for both interaction with Gβ2γ2 and subsequent degradation of Dishevelled via the lysosomal pathway. Signaling induced by Gβ2γ2 is required because a mutant of Gβ2, Gβ2 (W332A) with lower signaling activity, had reduced ability to downregulate the level of Dishevelled. Activation of Wnt signaling by either of two methods, increased Frizzled signaling or transient transfection of Wnt, also led to increased degradation of Dishevelled and the induced Dishevelled loss is dependent on Gβ1 and Gβ2. Other studies with agents that interfere with PLC action and calcium signaling suggested that loss of Dishevelled is mediated through the following pathway: Wnt/Frizzled→Gβγ→PLC→Ca+2/PKC signaling. Together the evidence suggests a novel negative feedback mechanism in which Gβ2γ2 inhibits Wnt signaling by degradation of Dishevelled.
Keywords: dishevelled proteins; feedback, biochemical; Frizzled receptors; heterotrimeric GTP-binding proteins; type C phospholipases; Wnt proteins
Introduction
Wnt signaling plays a pivotal role in diverse biological processes in embryonic development and in adult organisms (Clevers, 2006; Logan and Nusse, 2004; White et al., 2007). Mammals have 19 different Wnt ligands, 10 Frizzled receptors which are similar to serpentine G protein coupled receptors (GPCR) (Schulte and Bryja, 2007), plus other co-receptors such as LRP5/6 (He, 2003; Logan and Nusse, 2004). Binding of Wnt to its receptors activates downstream signaling events, such as the "canonical" pathway, mainly mediated by controlling the level of β-catenin via the ubiquitination/proteasome degradation pathway, or the "non-canonical" pathway which is controlled either via Rac/Rho or Ca+2 signaling (Kohn and Moon, 2005; Veeman et al., 2003). In some specific combinations of Wnt/Frizzled signaling the intracellular protein Dishevelled determines whether signaling proceeds by β-catenin-dependent or independent pathways. It is obvious that tight regulation of the signaling is critical since mis-regulation of Wnt signaling during embryonic development or post-natal life leads to different types of developmental defects, or diseases such as cancer, Alzheimer's disease or osteoporosis and others (http://www.stanford.edu/~rnusse/wntwindow.html).
Axin is a scaffold protein that interacts with a number of proteins to regulate Wnt/β-catenin signaling (Lee et al., 2003; Tolwinski and Wieschaus, 2004; Kikuchi et al., 2006). In the absence of Wnt, Axin is part of a complex with adenomatous polyposis coli (APC) and glycogen synthase kinase 3β (GSK3β) whose action is to phosphorylate cytoplasmic β-catenin and thus target it for ubiquitin- and proteasome-mediated degradation. When, however, Wnt binds to Frizzled and Dishevelled is activated then β-catenin degradation is inhibited, and after accumulating in the cytoplasm translocates to the nucleus where it binds to and activates TCF/LEF transcription factor (Logan and Nusse, 2004; Kikuchi et al., 2006). Although several mechanisms have been proposed how Dishevelled is activated in the presence of Wnt (Li et al., 1999; Chen et al., 2001), it is not clear how the activated Dishevelled signaling is later turned off. One way to turn off activated Dishevelled signaling is by activating the expression of its antagonist naked cuticle which causes down-regulation of Dishevelled (Zeng et al., 2000; Creyghton et al., 2005).
The involvement of trimeric G protein signaling in the regulation of Wnt signaling was suggested by the finding that Frizzled interacts with the Gα subunit of trimeric G proteins in mammalian cells and that G protein signaling directly transduces Frizzled signaling in Drosophila (Katanaev et al., 2005; Liu et al., 2005; Wang et al., 2006). Gα-protein signaling synergistically activates the Wnt/β-catenin pathway in colon cancer cells (Castellone et al., 2005; Yang et al., 2005), and bone formation is promoted by non-canonical Wnt-mediated G-protein signaling (Tu et al., 2007). We have found a role of Gβγ in Wnt signaling through the discovery that in yeast two-hybrid screening Axin binds to Guanine nucleotide binding protein, beta 2 (Gnb2; Gβ2). Here, we provide evidence for a novel negative feedback loop for Wnt signaling in which activated Gβγ signaling can turn off Wnt signaling via degradation of Dishevelled.
Results
Gβ interacts with Axin
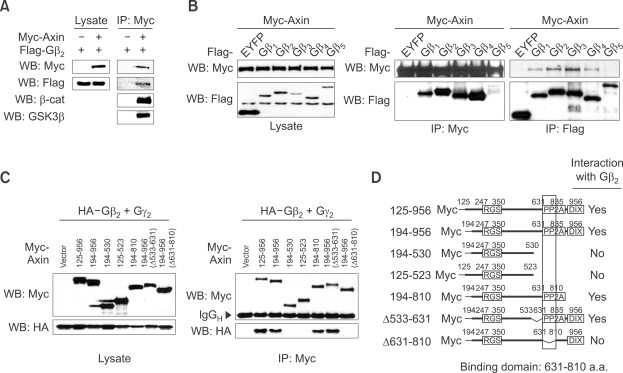
To confirm that the interaction between Axin and Gβ2 occurs in mammalian cells, Myc-tagged Axin and FLAG-tagged Gβ2 were co-transfected in HEK293T cells followed by co-immunoprecipitation (co-IP) experiments (Figure 1A). With the Axin to β-catenin/GSK3β interaction serving as a positive control we found that Axin also interacts with Gβ2. All five isoforms of the Gβ family of heterotrimeric G-protein subunits were tested and found to interact strongly with Axin (Figure 1B) except for Gβ5 which has the least sequence similarity to the other four isoforms Gβ1-4. Because Gβ forms a dimer with Gγ to make a functional unit (Birnbaumer, 2007), we tested whether the dimerization affects binding to Axin. When Gγ2, known to form dimers with Gβ1 or Gβ2 is co-expressed the interaction between Gβ2 and Axin was unaffected (Supplemental Data Figure S1A). Additional co-IP experiments using deletion constructs of Axin determined that amino acids 631-810 of Axin, a sequence which had previously been shown to interact with protein phosphatase 2A (Hsu et al., 1999), were required for interacting with Gβ2 (Figure 1C, 1D and Supplemental Data Figure S1B).
Figure 1.
Gβ interacts with Axin. (A) Co-immunoprecipitation of myc-Axin and Flag-Gβ2. Myc-tagged Axin and FLAG-tagged Gβ2 were transfected into HEK293T cells. The lysates were first subjected to immunoprecipitation (IP) using anti-Myc antibody, followed by western blotting (WB) using the antibodies indicated on the left side. β-catenin and GSK3β were used for positive controls. (B) Myc-tagged Axin with FLAG-tagged isoforms of Gβ or EYFP were transfected into HEK293T cells. Immunoprecipitation followed by western blotting was performed to test the specificity of interaction between Axin and isoforms of Gβ. (C) Deletion constructs of Axin with HA-tagged Gβ2 and Gγ2 were transfected into HEK293T cells and the expression was confirmed by western blot (left panel). Immunoprecipitation with anti-Myc antibody followed by western blotting was performed to examine specific interaction (right panel). (D) Schematic diagram of deletion constructs and summary of interaction between Axin and Gβ2 was depicted.
Gβ2γ2 inhibits Wnt/β-cat signaling by reducing the level of Dishevelled
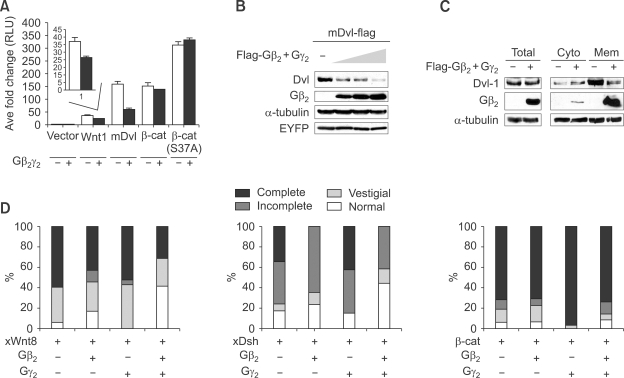
We next examined the functional significance of the Gβγ to Axin interaction through the effect of transient transfection of Gβγ on Wnt mediated TOP-FLASH reporter activity. While it is known that ectopic-expression of Gα activates canonical Wnt signaling (Castellone et al., 2005), we found that co-transfection of Gβ2γ2 inhibited signaling (Supplemental Data Figure 2A). Results from co-transfection experiments designed to individually express either Wnt1, Dishevelled, or β-catenin showed that Gβγ inhibits canonical Wnt signaling at a level upstream of β-catenin and downstream of, or in parallel to, the level of Dishevelled (Figure 2A). We focused on Dishevelled as the target of the inhibition of this since Gβ is known to interact with Dishevelled (Angers et al., 2006). We found that as reporter activity induced by Dishevelled was decreased in a dose-dependent manner by increasing Gβ2γ2 (Supplemental Data Figure S2B) the level of Dishevelled was dramatically reduced; the level of a control protein EYFP was unaffected (Figure 2B). Furthermore although Gβ2 itself (it may form a dimer with endogenous Gγ, but not Gγ2, alone caused a significant reduction, co-transfection of Gβ2γ2 resulted in a greater decrease in the level of Dishevelled than either subunit alone (Supplemental Data Figure S2C).
Figure 2.
Gβ2γ2 inhibits Wnt signaling by reducing the level of Dishevelled (Dvl). (A) Epistasis experiments show Gβγ acts downstream of or in parallel to Dishevelled and upstream of β-catenin. SuperTOPFlash plasmid with empty-vector, Wnt1, mDvl-1, β-catenin or β-catenin S37A was transfected along with either empty-vector or Gβ2γ2, respectively. After transfection (36 h), luciferase assay was performed. The reporter activity induced by transfection of Wnt1 or mDvl-1 was significantly inhibited by co-transfection of Gβ2γ2 (P value < 0.01, n = 3). (B) FLAG-tagged mDvl-1 was transfected alone or with 0.5, 1 and 2 µg of FLAG-tagged Gβ2 and Gγ2 each into HEK293T cells and western blotting was performed to examine the level of mDvl-1. EYFP was used for equal transfection control. (C) To measure the effect of Gβ2γ2 on the level of endogenous Dishevelled-1, HEK293T cells were transfected with Flag tagged Gβ2 and Gγ2 plasmids. After transfection (36 h), cells were lysed and the lysates were fractionated into cytosolic and membrane fractions. The endogenous level of Dishevelled-1 was examined by western blotting. (D) XWnt8 mRNA (10 pg, A, n = 127 embryos), XDsh (500 pg, B, n = 139) or Xβcat (1 ng, C, n = 130) were injected alone or with 1 ng of Gβ2, Gγ2 or both Gβ2 and Gγ2 mRNAs into ventral vegetal blastomeres of 8 cell stage embryos. Axis duplication was scored at tailbud stage (stage35). The percentages of embryos that show varying degrees of axis duplication are presented as bar graphs.
Having established that Gβγ signaling inhibits Wnt signaling via reduction of the level of Dishevelled, we next determined if membrane-associated or cytoplasmic, or both, forms of Dishevelled are targeted. To do this cell lysates were separated into cytoplasm and membrane fractions and analyzed for Dishevelled. After ectopic expression of Gβ2γ2 both endogenous and ectopically expressed Dishevelled present in the membrane fractions were clearly reduced, while the level of Dishevelled in the cytoplasmic fraction was not (Figure 2C and Supplemental Data Figure S2D). Therefore the evidence suggests that Gβ2γ2 inhibits Wnt/β-cat signaling primarily by reducing the level of membrane-associated Dishevelled.
To confirm that Gβγ can regulate Wnt signaling in an in vivo system, we used Xenopus embryos because Wnt, Dishevelled and β-catenin induce a secondary embryonic axis if they are injected into the vegetal side of early blastomeres. We expected that Gβγ would inhibit the formation of secondary axes induced by the injection of XWnt8, and XDsh but not β-catenin mRNA. The results show that Gβ2 and Gβ2γ2, but not Gγ2 alone, significantly reduced secondary axis formation after injection of XWnt8; the percentage of normal embryos was increased from 6 to 41 (Figure 2D, left panel). Similarly, secondary axes induced by the injection of XDsh mRNA were considerably reduced by the co-injection of Gβ2 mRNA alone (but not by Gγ2 mRNA), and reduced even more when Gβ2 and Gγ2 mRNAs were co-injected; the percentage of normal embryos increased from 17 to 44 (Figure 2D, middle panel). In addition, the percentage of embryos that showed complete axis duplication was reduced from 34 to 0 when Gβ2γ2 mRNA was co-injected with XDsh mRNA. As expected from the lack of an effect on β-catenin-mediated signaling in cell culture the increased secondary axis formation induced by the injection of β-catenin mRNA was unaffected by the co-injection of Gβ2, Gγ2 and Gβ2γ2 (Figure 2D). Taken together these results from cell culture and Xenopus development suggest that Gβγ inhibits the Wnt/β-cat pathway in vivo by creating a signal for the loss of Dishevelled.
Interaction between Gβ and the DEP domain and membrane localization of Dishevelled is necessary for down-regulation of Dishevelled
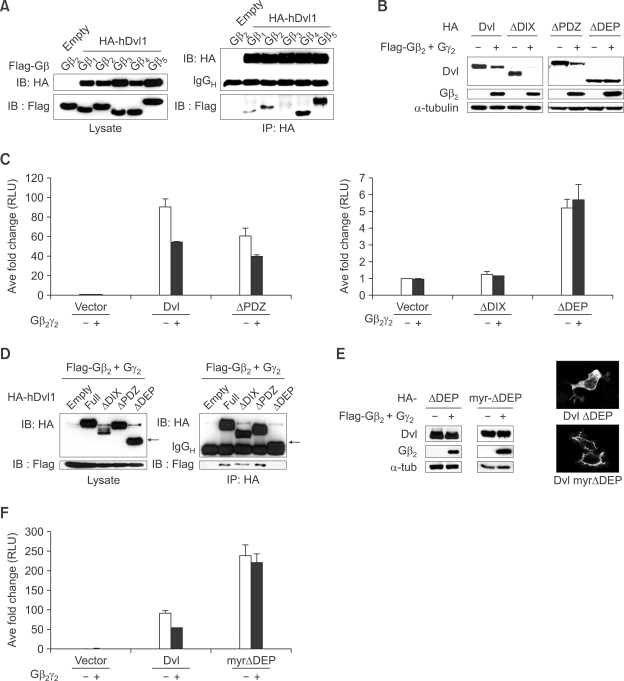
We determined if Dishevelled can interact with Gβ family members by co-IP experiments and found that all Gβ proteins except for Gβ3 interact with Dishevelled (Figure 3A). Dishevelled has three conserved domains; DIX, PDZ and DEP (Wallingford and Habas, 2005). To identify which domain of Dishevelled is involved in the degradation induced by Gβγ, we used deletion constructs (Supplemental Data Figure S3) which were co-transfected with Gβ2γ2. Only the DEP domain including C-terminal domain was found to be necessary for Gβ2γ2-mediated degradation of Dishevelled (Figure 3B). It is known that DIX domain is necessary for the induction of the level of β-catenin (Kishida et al., 1999). Consistent with this published result ectopic expression of DIX domain-deleted Dishevelled (ΔDIX-Dishevelled) neither induce β-catenin/Tcf mediated reporter activity nor respond to Gβγ (Figure 3C), while the level of ΔDIX-Dishevelled was reduced by Gβγ (Figure 3B). As expected luciferase-reporter activity induced by DEP domain-deleted Dishevelled (ΔDEP-Dishevelled) was not inhibited by the co-expression of Gβ2γ2 (Figure 3C). We reasoned that ΔDEP-Dishevelled could not be down-regulated by Gβ2γ2 since it could not bind to Gβ2. To test this hypothesis we performed co-IP experiments and found that as expected ΔDEP-Dishevelled does not bind to Gβ2 (Figure 3D). These results suggest that interaction of Gβ with the DEP domain of Dishevelled is necessary for down-regulation of Dishevelled by Gβγ. Since it is known that the DEP domain is necessary for the membrane localization of Dishevelled when Frizzled is over-expressed (Axelrod et al., 1998), and the membrane fraction of Dishevelled is targeted for loss mediated by Gβ2γ2 (Figure 2C and Supplemental Data Figure 2D), the ΔDEP-Dishevelled protein may be resistant to down-regulation partly due to its lack of membrane localization. To test this hypothesis, a myristoylation/palmitoylation modification sequence (Simons et al., 2005) was added to ΔDEP-Dishevelled (myr-ΔDEP-Dishevelled) to target localization to the membrane and the effect of Gβ2γ2 assessed. Although the myr-ΔDEP-Dishevelled protein localized to the membrane, its level was not reduced by Gβ2γ2 (Figure 3E). Additionally, the robust luciferase-reporter activity induced by myr-γDEP-Dishevelled was not blocked by Gβ2γ2 (Figure 3F). These data suggest that membrane localization of Dishevelled alone may not be enough to induce degradation, although it may aid degradation of Dishevelled (See Figure 5B). It is of course also possible that the forced localization of Dishevelled in the membrane does not sufficiently mimic the position in the membrane or interaction with membrane proteins after normal Wnt/Frizzled signaling. The results of these experiments suggest that the interaction between Gβ and the DEP domain and membrane localization of Dishevelled is necessary for down-regulation of Dishevelled.
Figure 3.
DEP domain of Dishevelled (Dvl) is required for degradation of Dishevelled by Gβγ. (A) Dishevelled interacts with all isoforms of Gβ except for Gβ3. HA-tagged human Dv-1 was co-transfected with all isoforms of Gβ into HEK293T cells. Immunoprecipitation followed by western blotting was performed. (B) The level of DEP domain deleted Dishevelled was not reduced by Gβ2γ2. Various deletion constructs of Dishevelled were co-transfected with Gβ2γ2 into HEK293T cells and western blotting was performed. (C) The data from luciferase assay confirm the result obtained in (B). Luciferase assay was performed using superTOPFlash and pTK-renilla luciferase after co-transfection of various deletion constructs of Dishevelled and Gβ2γ2. (D) DEP domain of Dishevelled is required for interaction with Gβ2γ2. Various deletion constructs of Dishevelled with Gβ2γ2 were co-transfected into HEK293T cells and immunoprecipitation followed by western blotting was performed. Arrow indicates ΔDEP-Dishevelled. (E) Membrane localization of ΔDEP-Dishevelled via myristoylation/ palmitoylation signal is not sufficient for the down-regulation of Dishevelled by Gβ2γ2. ΔDEP-Dishevelled and myr-ΔDEP, which has a myristoylation/ palmitoylation signal sequence to the N-terminal of ΔDEP-Dishevelled, were transfected alone or with Gβ2γ2 into HEK293T cells. After fractionation, western blotting was performed using the membrane fraction (left panel). Indirect immunofluorescence analysis with anti-HA antibody shows myr-ΔDEP-Dishevelled is highly localized in plasma membrane (right panel). (F) Luciferase assay using superTOPFlash was conducted to confirm the result obtained in (E).
Figure 5.
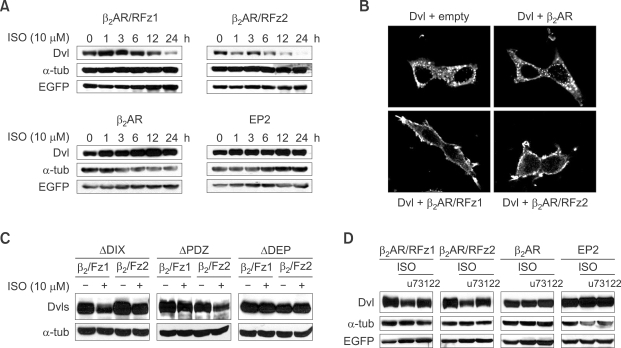
Activation of Fz leads to down-regulation of Dishevelled (Dvl) via PLC signaling. (A) Activation of Frizzled leads to down-regulation of Dishevelled. Dishevelled was co-transfected with β2-adrenergic/rat Fz1 (β2AR/RFz1), β2-adrenergic/rat Fz2 chimeric receptor (β2AR/RFz2), Gs-coupled receptor β2-adrenergic receptor (β2AR) or EP2 into HEK293T cells. Isoproterenol was added at 10 µM at the times indicated in the figure. The level of Dishevelled was measured by western blot analysis. EGFP was used for equal transfection control. (B) Activation of chimeric Frizzled recruits Dishevelled to plasma membrane. Dishevelled was co-transfected with receptors described in figure into HEK293T cells and the cellular localization of Dishevelled was detected via indirect immunofluorescence. (C) DEP domain is necessary for the downregulation of Dishevelled via activation of β2AR/RFz1 and β2AR/RFz2. β2AR/RFz1 and β2AR/RFz2 plasmids were co-transfected with Dishevelled constructs (Supplemental Data Figure S3) as indicated in figure and the cells were treated with isoproterenol (10 µM for 24 h before harvest) followed by western blot analysis. (D) Down-regulation of Dishevelled by Frizzled activation is blocked by treatment with a PLC inhibitor. Dishevelled was co-transfected with each receptor described in figure into HEK293T cells, which were treated with isoproterenol at 10 µM for 24 h before harvest (36 h). U73122, a PLC inhibitor, was added at 10 µM for 24 h before harvest. Western analysis was performed with antibodies indicated in figure.
Dishevelled degradation by Gβ2γ2 is mediated via the lysosomal degradation pathway and Ca+2/PKC signaling is involved in that process
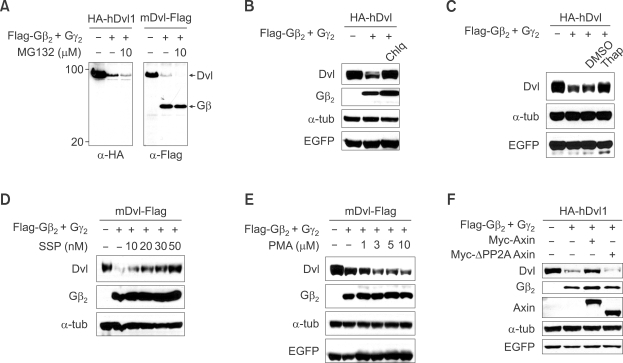
Dishevelled has been shown to be degraded using either the proteasomal or lysosomal pathway (Miyazaki et al., 2004; Creyghton et al., 2005; Simons et al., 2005; Angers et al., 2006; Zhang et al., 2006). Which pathway is involved in the Gβγ-mediated reduction of Dishevelled was tested using pathway-specific inhibitors. Treatment with MG132, a proteasome-pathway inhibitor, did not block Gβγ mediated reduction of Dishevelled (Figure 4A) nor were any cleavage products of N-terminal and C-terminal epitope-tagged Dishevelled observed (Figure 4A). Additional proteasome pathway calpain inhibitors ALLN or ALLM also did not block the degradation of Dishevelled (data not shown). Dishevelled degradation mediated by Gβ2γ2, however, was blocked by the lysosomal-pathway degradation inhibitor chloroquine (Figure 4B) suggesting that Gβγ-mediated reduction of Dishevelled occurs in lysosomes.
Figure 4.
Dishevelled (Dvl) degradation by Gβ2γ2 is mediated via the lysosomal degradation pathway and Ca+2/PKC signaling is involved in that process. (A-C) HA-tagged human Dishevelled-1 or Flag tagged mouse Dishevelled-1 plasmid was transfected alone or with Gβ2γ2 into HEK293T cells and the cells were treated with MG132 (10 µM for 4 h before harvest, (A)), chloroquine (50 µM for 16 h before harvest, (B)) and thapsigargin (5 µM for 1h and cells were incubated for 24 h more without drug before harvest, (C)) and the level of Dishevelled was measured by western analysis. (D and E) Flag tagged mouse Dishevelled-1 plasmid was co-transfected with Gβ2γ2 and EGFP into HEK293T cells and the cells were treated with different concentrations of PKC antagonist (16h before harvest, (D)) or agonist (16 h before harvest, (E)). Western blot analysis was then performed with antibodies indicated in the figure. (F) Axin blocks degradation of Dishevelled by Gβ2γ2. Dishevelled was transfected alone (lane 1) or with Gβ2γ2 (lanes 2-4) into HEK293T cells along with Myc-tagged Axin (lane 3) or ΔPP2A Axin (lane 4) and western blotting was performed with antibodies indicated in the figure. EGFP was used for equal transfection control.
The identification of the signal pathway downstream of Gβγ which leads to lysosomal degradation of Dishevelled is also important. After confirming that activation of Gβγ signaling causes translocation of PKCα from the cytoplasm to the plasma membrane (Sheldahl et al., 1999) (Supplemental Data Figure S4A) we used a mutant form Gβ2 (W332A) which is able to bind to Gγ but has reduced signaling activity (Ford et al., 1998) in order to determine whether signaling activity of Gβγ is needed. Gβ2 (W332A) had a much lower ability than the wild type to reduce Dishevelled abundance (Supplemental Data Figure S4B) and it had reduced ability to inhibit Wnt-mediated luciferase-reporter activity (Supplemental data Figure S4C). These data suggest that activation of downstream signaling by Gβγ plays an important role in the downregulation of Dishevelled. Gβ2 (W332A)'s lower ability (Supplemental Data Figure S4B and S4C) was not a result of reduced interaction with Dishevelled because it can interact with Dishevelled as well or even better than wild type Gβ2 in co-IP experiments (Supplemental Data Figure S4D).
Having shown that activation of downstream signaling by Gβγ is required for the degradation of Dishevelled we examined the role of Ca+2/PKC signaling (Sheldahl et al., 1999) by using inhibitors or activators of this pathway. Thapsigargin, which inhibits cytoplasmic calcium signaling by diverting Ca+2 released from the ER from the cytoplasm to outside of the cell (Westfall et al., 2003) clearly blocked Gβγ-mediated loss of Dishevelled (Figure 4C). Staurosporine, a PKC inhibitor, blocked whereas PMA, a PKC activator, increased the loss of Dishevelled by Gβγ (Figure 4D and 4E). Although the many isoforms of PKC precluded easy identification of the specific isoforms responsible for the effect, these preliminary data suggest that Ca+2/PKC signaling is involved in the Gβγ mediated loss of Dishevelled.
Axin blocks Gβγ-mediated loss of Dishevelled
Our initial finding that Axin binds Gβ2 (Figure 1) led to the hypothesis that interaction with Gβγ might control the loss of Dishevelled but left the role of Axin unexplained. As previously mentioned co-transfection of Axin blocked Gβγ-mediated loss of Dishevelled, but an ΔPP2A-Axin construct which lacks the interaction domain with protein phosphatase 2A (PP2A) and does not interact with Gβγ (Supplemental Data Figure S1B), did not inhibit loss of Dishevelled by Gβγ (Figure 4F). It is conceivable that Axin sequesters Gβγ from interaction with Dishevelled thereby blocking loss of Dishevelled mediated by Gβγ. The possibility that Axin blocks downregulation of Dishevelled by Gβγ through competitive inhibition of interaction between Gβγ and Dishevelled or interference of Gβγ signaling is discussed later in a summary model (Figure 7).
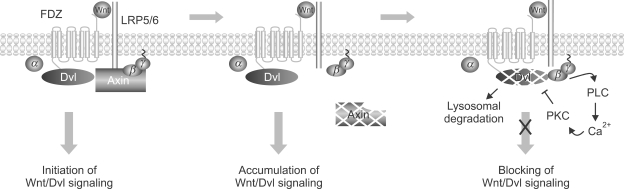
Figure 7.
Model for a negative feedback mechanism of Wnt signal transduction by Gβγ signaling. Left panel: In the presence of Wnt, Dishevelled (Dvl) and Axin (for canonical Wnt signaling) are translocated to membrane-bound Frizzled and LRP, respectively, and initiate downstream signaling. Released Gα subunits from Gβγ may then synergistically enhance canonical Wnt signaling. During this time if LRP-bound Axin binds the Gβγ subunit and blocks the downstream signaling of Gβγ then Dishevelled will not be degraded and signaling will continue. Middle panel: Upon further Wnt signaling LRP-bound Axin is degraded which leads to release of Gβγ subunits from Axin. Right panel: The released Gβγ subunits interact with Dishevelled, which lead to its destruction via a signaling pathway including PLC, Ca+2 and PKC. The activation of the Gβγ signaling cascade leads to degradation of Dishevelled in lysosomes and thereby turns off Wnt signaling.
Activation of Frizzled signaling induces loss of Dishevelled
Because Frizzled is known to be a G-protein coupled receptor (Schulte and Bryja, 2007) and would be expected to increase the level of dissociated Gβγ from Gα in a more physiological manner, we examined whether activation of Frizzled signaling could induce loss of Dishevelled. To activate Frizzled signaling HEK293T cells were transfected with β2AR/RFz1 and β2AR/RFz2, which are chimeric receptors that are used to activate the canonical and non-canonical Wnt/Frizzled signaling pathways, respectively, when isoproterenol (Iso) is added (Liu et al., 2001; Ahumada et al., 2002). As expected after cells with β2AR/RFz1 and β2AR/RFz2 were treated with isoproterenol (Iso) Dishevelled levels were reduced (Figure 5A) similarly to that found when Gβγ was increased by transfection. Control GPCRs, which are not related with Wnt/Frizzled signaling, like β2AR that can, or EP2 that cannot be activated by the treatment of Iso were both found to be unable to cause loss of Dishevelled (Figure 5A). Why the chimeric receptors β2AR/RFz1 and β2AR/RFz2, but not β2AR, leads to loss of Dishevelled while both types can activate a Gβγ signal upon Iso treatment could be a result of a difference in ability to induce membrane translocation of Dishevelled. We therefore examined Dishevelled localization after co-expressing these same chimeric or non-chimeric receptors. Consistent with this hypothesis, β2AR/RFz1 and β2AR/RFz2 can induce translocation of Dishevelled to the plasma membrane, but β2AR could not (Figure 5B). Thus the activation of Gβ signaling alone from a receptor is normally insufficient to allow Dishevelled level to be reduced and other signaling events initiated by β2AR/RFz1 and β2AR/RFz2 are necessary. Although ectopic expression of Gβγ alone led to the downregulation of Dishevelled (Figure 2 and Supplemental Data Figure S2), it is possible that overexpression bypasses the requirement for Frizzled for membrane localization of Dishevelled in that situation. Which domain of Dishevelled is necessary for the β2AR/RFz1 and β2AR/RFz2 mediated loss of Dishevelled was examined next. Of the Dishevelled deletion constructs used (Supplemental Data Figure S3) only the ΔDEP-Dishevelled was not degraded after the activation of β2AR/RFz1 or β2AR/RFz2 (Figure. 5C). This result is consistent with the previous results (Figure 3) that showed that the DEP domain plays a critical role in the Gβγ mediated degradation of Dishevelled.
We next turned our attention to the signaling events occurring after the Frizzled receptor and especially the possible role of phospolipase C (PLC) signaling, which is known to be activated by Gβγ (Ahumada et al., 2002). Addition of a specific PLC inhibitor, u73122 (Lockhart and McNicol, 1999) to cells expressing β2AR/RFz1 or β2AR/RFz2 and induced with Iso prevented the loss of Dishevelled (Figure 5D). Because PLC involvement occurs after Gβγ and before Ca+2/PKC the pathway involved in the downregulation of Dishevelled is expected to follow this order (Wnt/Frizzled→Gβγ→PLC→Ca+2/PKC signaling) (Figure 4C-E and 5D).
Gβγ signaling is necessary for the Wnt-mediated loss of Dishevelled
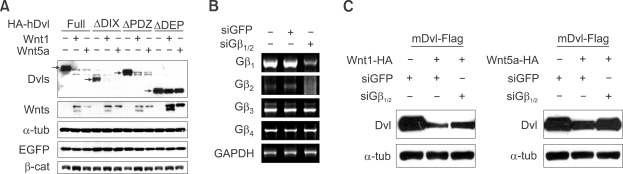
Interestingly, co-transfection of Wnt1 or Wnt5A with Dishevelled can reduce the level of Dishevelled (but not a control protein EGFP) just as with activation of Frizzled receptors by Iso (Figure 6A). As expected all deletion versions of Dishevelled except ΔDEP-Dishevelled were degraded by the co-expression of Wnt1 or Wnt5a. For these experiments we confirmed that the Wnt1 signaling pathway is intact and functional as indicated by an increased level of β-catenin upon co-transfection of Wnt1 (Figure 6A). To further confirm that Gβγ signaling is necessary for the Wnt-mediated loss of Dishevelled, siRNA that can knockdown both Gβ1 and Gβ2 (Krumins and Gilman, 2006) was added to HEK293T cells. The cells with that had been given siRNA had lowered Gβ1 and Gβ2, but not other control, mRNAs (Figure 6B) and had the expected diminished block in the loss of Dishevelled induced by either Wnt1 or Wnt5a (Figure 6C). The lack of complete blocking may be a result of incomplete downregulation of Gβ1 and Gβ2 or functional redundancies of other Gβ proteins whose mRNA levels were not reduced with these siRNAs. It is of course also possible that Wnt signaling causes downregulation of Dishevelled by pathways in addition to those involving Gβγ signaling. Nevertheless our data support the view that endogenous Gβγ signaling plays a critical role in loss of Dishevelled caused by Wnt.
Figure 6.
Expression of Wnts reduces the level of Dishevelled (Dvl) and the degradation is blocked by knock-down of Gβ1 and Gβ2. (A) Dishevelled is down-regulated by Wnt expression and DEP domain of Dishevelled is required for this down-regulation. Full length and deletion constructs of Dishevelled were co-transfected with mouse Wnt1 or Wnt5a into HEK293T cells and western blot analysis was performed. EGFP was used equal transfection control and the level of β-catenin was measured to show that Wnt1 signaling is properly conducted. (B) HEK293T cells were transfected with siRNA for GFP or Gβ1/Gβ2, and the level of isoforms of Gβ1-4 was measured by RT-PCR analysis using specific primers targeting each Gβ isoform at 36 h after transfection. Gβ5 was not shown since its expression seems to be very low. (C) Wnt induced down-regulation of Dishevelled is blocked by knock-down of Gβ1 and Gβ2. Dishevelled was co-transfected with Wnt1 or Wnt5a into HEK293T cells. siRNAs for GFP or Gβ1/2 was transfected as depicted in figure. Western analysis was performed to measure the level of Dishevelled.
Discussion
These data on the role of Gβγ signaling in Wnt mediated loss of Dishevelled can be incorporated into the following model based on work by others and our current results (Figure 7). In the presence of Wnt, Dishevelled and Axin (for canonical Wnt signaling) are translocated to membrane-bound Frizzled and LRP, respectively, and initiate downstream signaling (Mao et al., 2001; Tamai et al., 2004). Released Gα subunits from Gβγ may then synergistically enhance canonical Wnt signaling (Castellone et al., 2005). During this time if LRP-bound Axin binds the Gβγ subunit and blocks the downstream signaling of Gβγ (as we show in Figure 1 and 4F) then Dishevelled will not be degraded and signaling will continue. Upon further Wnt signaling LRP-bound Axin is degraded (Willert et al., 1999; Mao et al., 2001) and now Gβγ subunits interact with Dishevelled to lead to its destruction via a signaling pathway including PLC, Ca+2 and PKC (Figure 3-5). The activation of the Gβγ signaling cascade leads to degradation of Dishevelled in lysosomes (Figure 3) and thereby turns off Wnt signaling. This model suggests that the downregulation of Dishevelled by Gβγ signaling is a novel negative feedback mechanism for regulation of Wnt signaling and provides some ideas for further investigation regarding the role of Gβγ signaling in Wnt signaling.
We showed that Axin does not interact with Gβ5 (Figure 1B), whereas Dishevelled does not interact with Gβ3 (Figure 3A) and the interaction between Dishevelled and Gβ2 is necessary for the down-regulation of Dishevelled by Gβ2. Therefore we expected that Gβ3 has weaker effect while Gβ5 shows stronger effect than Gβ2 on the down-regulation of Dishevelled. Figure 3A and repeated experiments (data not shown) reproducibly show that Gβ2 has the strongest effect while Gβ3 and Gβ5 exhibit very weak effect. The result with Gβ3 is consistent with our proposed model, while the result with Gβ5 is not easily explainable. It may be possible that the interaction between Axin and Gβ plays other unknown roles, such as recruitment of Dishevelled/Axin complex to Gβγ, in the regulation of Dishevelled.
Although the mechanism is not clear, Dishevelled can, depending on the nature of the upstream Wnt/Frizzled signaling, selectively determine whether canonical or non-canonical signaling is used (Schulte and Bryja, 2007). Here we found that β2AR/RFz2, which is known to activate non-canonical Wnt signaling (Ahumada et al., 2002), or Wnt5a led to loss of Dishevelled (Figure 5 and 6). Therefore it is conceivable that Gβγ signaling can control both canonical and non-canonical Wnt signaling by determining the amount of Dishevelled at the membrane. Although it is not known whether Axin is translocated to the membrane in non-canonical Wnt signaling, it is possible that Axin plays the same role in reduction of Dishevelled to turn off non-canonical Wnt signaling. Other unknown molecules may have similar roles to control Gβγ signaling for the downregulation of Dishevelled.
We can now add Gβγ to the list of proteins including NEDL1 (Miyazaki et al., 2004), Naked cuticle (Creyghton et al., 2005), inversin (Simons et al., 2005), KLHL12 (Angers et al., 2006), and dapper (Zhang et al., 2006) that are responsible for the regulation of Dishevelled level in cells. Naked cuticle in particular is similar to Gβγ's role in that both establish a negative feedback loop to shut down Wnt signaling (Creyghton et al., 2005). That there are so many proteins involved suggests that the precise regulation of Dishevelled level is crucial to many processes. Mis-regulation of Dishevelled is known to be involved in nephronophthis type II (Simons et al., 2005), an autosomal cystic kidney disease, and non small cell lung cancer (NSCLC) (Uematsu et al., 2003). Whether Gβγ is playing a role in these diseases will be important to determine.
Methods
Plasmids
All isoforms of Gβ cDNAs were obtained by RT-PCR using total RNA from whole mouse embryos and cloned in pCMV4-Flag and pCS2-HA backbone vectors. HA-tagged hDishevelled constructs were obtained from Dr. Kikuchi (Hiroshima University, Japan). Details about myc-tagged Axin and Flag-tagged mDishevelled constructs are described elsewhere (Fagotto et al., 1999). SuperTopFlash reporter was obtained from Dr. Moon (University of Washington). β2AR/RFz1 and β2AR/RFz2 were obtained from Dr. Malbon (State University of New York at Stony Brook). β2AR and EP2 were obtained from Dr. Gutkind (National Institute of Health).
Tissue culture and transfection
HEK293T cells were grown in DMEM supplemented with 10% FBS in a 37℃ humidified incubator containing 5% CO2. Transient transfections of plasmids were performed via the calcium phosphate method.
Co-immunoprecipitation and western analysis
For the co-immunoprecipitation, HEK293T cells (5 × 106) were lysed in RIPA buffer (25 mM Tris-HCl at pH 8.0, 150 mM NaCl, 10% glycerol, 1% Igepal CA-630, 0.25% deoxycholic acid, 2 mM EDTA, 1 mM NaF, 50 mM glycerophosphate). Lysates were cleared by centrifugation and immunoprecipitation was performed using monoclonal anti Myc or anti HA (sc-7392; Santa Cruz Biotechnology, Santa Cruz, CA) or anti Flag (F3165; Sigma) antibodies and protein A/G plus agarose. Western blot experiments were performed as previously described (Fagotto et al., 1999).
Membrane-cytosolic fractionation
Cells were washed in PBS and scraped in lysis buffer containing 10 mM Tris-HCL (pH7.4), 140 mM NaCl, 5 mM EDTA, 2 mM DTT, 0.5 mM PMSF and 1 mg/ml leupeptin. Cells were lysed by strokes in a chilled homogenizer at 4℃. The lysates were centrifuged at 1,000 × g for 10 min to remove unbroken cells and nuclei. The cleared lysates were subject to ultracentrifugation at 100,000 × g for fractionation. The pellet was dissolved using RIPA buffer (containing 0.05% SDS).
Luciferase reporter assay
Cells seeded in 12-well plates were co-transfected with 500 ng superTOP-FLASH, 50 ng pTK-Renilla (Promega) and effector plasmids. The final amount of DNA in all transfections was brought to a total of 1,000-1,500 ng with empty vectors. Assays were performed in accordance with the dual luciferase assay protocols (Promega) using the luminometer. The Renilla activity was used to normalize TOP-FLASH activity.
Axis duplication assay using Xenopus embryo
10 pg of Wnt8, Dsh and β-catenin mRNAs were co-injected with 1 ng of Gβ2 and Gγ2 to ventral-vegetal one blastomere of 8 cell-stage embryos. Axis duplication was monitored at tailbud stage (stage 37), and embryos were classified as complete axis duplication, incomplete axis duplication, vestigial or normal. The phenotypes for the classification are followings: complete axis duplication, duplicated trunk and complete secondary head structures including duplicated cement gland and pair of eyes; incomplete axis duplication, duplicated trunk only or duplicated trunk and incomplete head structures; vestigial, no duplicated trunk but slight dorsalization.
Usage of siRNA
The sequences of siRNA for Gβ1 and Gβ2 were adapted from Krumins and Gilman (2006). The sequences of siRNAs; siGβ1/Gβ2, 5'-UACGACGACUUCAACUGCATT-3'/5'-UGCAGUUGAAGUCGUCGUATT-3'; siGFP, 5'-GUUCAGCGUGUCCGGCGAGTT-3 '/5'-CUCGCCGGACACGCUGAACTT-3'. 200 nM siRNAs were transfected into HEK293T using calcium phosphate.
Supplemental data
Supplemental Data include four figures and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-41-10-02.pdf.
Acknowledgements
We appreciate Dr. Frank Costantini and Mr. Eric Schulze for critical reading and editing manuscript. This work was supported by the grant from Korea Research Foundation (KRF-C00534) and the sabbatical research grant from University of Seoul to E. Jho. JK Han was supported from Advanced Basic Research Laboratory Program of KRF (R14-2002-012-01001-0). H. Jung, H. Kim and S. Lee were supported by the Brain Korea 21 program.
Abbreviations
- APC
adenomatous polyposis coli
- GPCR
G protein coupled receptors
- GSK3β
glycogen synthase kinase 3β
- Gβ2
guanine nucleotide binding protein β2
- NSCLC
non small cell lung cancer
- PP2A
protein phosphatase type 2A
- Iso
isoproterenol
- β2AR
β2 adrenergic receptor
Supplementary Material
Supplemental Data
References
- 1.Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science. 2002;298:2006–2010. doi: 10.1126/science.1073776. [DOI] [PubMed] [Google Scholar]
- 2.Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, Moon RT. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat Cell Biol. 2006;8:348–357. doi: 10.1038/ncb1381. [DOI] [PubMed] [Google Scholar]
- 3.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnbaumer L. Expansion of signal transduction by G proteins. The second 15 years or so: from 3 to 16 alpha subunits plus betagamma dimers. Biochim Biophys Acta. 2007;1768:772–793. doi: 10.1016/j.bbamem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Hu LA, Semenov MV, Yanagawa S, Kikuchi A, Lefkowitz RJ, Miller WE. beta-Arrestin1 modulates lymphoid enhancer factor transcriptional activity through interaction with phosphorylated dishevelled proteins. Proc Natl Acad Sci USA. 2001;98:14889–14894. doi: 10.1073/pnas.211572798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Creyghton MP, Roel G, Eichhorn PJ, Hijmans EM, Maurer I, Destree O, Bernards R. PR72, a novel regulator of Wnt signaling required for Naked cuticle function. Genes Dev. 2005;19:376–386. doi: 10.1101/gad.328905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, Miller RJ, Jan LY, Lefkowitz RJ, Hamm HE. Molecular basis for interactions of G protein betagamma subunits with effectors. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 11.He X. A Wnt-Wnt situation. Dev Cell. 2003;4:791–797. doi: 10.1016/s1534-5807(03)00165-5. [DOI] [PubMed] [Google Scholar]
- 12.Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439–3445. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- 13.Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi A, Kishida S, Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med. 2006;38:1–10. doi: 10.1038/emm.2006.1. [DOI] [PubMed] [Google Scholar]
- 15.Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Krumins AM, Gilman AG. Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. J Biol Chem. 2006;281:10250–10262. doi: 10.1074/jbc.M511551200. [DOI] [PubMed] [Google Scholar]
- 18.Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Yuan H, Weaver CD, Mao J, Farr GH, 3rd, Sussman DJ, Jonkers J, Kimelman D, Wu D. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 1999;18:4233–4240. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, Moon RT, Malbon CC. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 22.Lockhart LK, McNicol A. The phospholipase C inhibitor U73122 inhibits phorbol ester-induced platelet activation. J Pharmacol Exp Ther. 1999;289:721–728. [PubMed] [Google Scholar]
- 23.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 24.Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki K, Fujita T, Ozaki T, Kato C, Kurose Y, Sakamoto M, Kato S, Goto T, Itoyama Y, Aoki M, Nakagawara A. NEDL1, a novel ubiquitin-protein isopeptide ligase for dishevelled-1, targets mutant superoxide dismutase-1. J Biol Chem. 2004;279:11327–11335. doi: 10.1074/jbc.M312389200. [DOI] [PubMed] [Google Scholar]
- 26.Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- 28.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 30.Tolwinski NS, Wieschaus E. Rethinking WNT signaling. Trends Genet. 2004;20:177–181. doi: 10.1016/j.tig.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uematsu K, He B, You L, Xu Z, McCormick F, Jablons DM. Activation of the Wnt pathway in non small cell lung cancer: evidence of dishevelled overexpression. Oncogene. 2003;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 33.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 34.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 35.Wang HY, Liu T, Malbon CC. Structure-function analysis of Frizzleds. Cell Signal. 2006;18:934–941. doi: 10.1016/j.cellsig.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Westfall TA, Hjertos B, Slusarski DC. Requirement for intracellular calcium modulation in zebrafish dorsal-ventral patterning. Dev Biol. 2003;259:380–391. doi: 10.1016/s0012-1606(03)00209-4. [DOI] [PubMed] [Google Scholar]
- 37.White BD, Nguyen NK, Moon RT. Wnt signaling: it gets more humorous with age. Curr Biol. 2007;17:R923–R925. doi: 10.1016/j.cub.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 38.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang M, Zhong WW, Srivastava N, Slavin A, Yang J, Hoey T, An S. G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the β-catenin pathway. Proc Natl Acad Sci USA. 2005;102:6027–6032. doi: 10.1073/pnas.0501535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng W, Wharton KA, Jr, Mack JA, Wang K, Gadbaw M, Suyama K, Klein PS, Scott MP. naked cuticle encodes an inducible antagonist of Wnt signalling. Nature. 2000;403:789–795. doi: 10.1038/35001615. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Gao X, Wen J, Ning Y, Chen YG. Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J Biol Chem. 2006;281:8607–8612. doi: 10.1074/jbc.M600274200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data