Abstract
The adaptor protein, LAD/TSAd, plays essential roles in T cell activation. To further understand the functions of this protein, we performed yeast two-hybrid screening using TSAd as bait and identified 67 kDa laminin binding protein (LBP) as the interacting partner. Subsequently, TSAd-LBP interaction was confirmed in D1.1 T cell line. Upon costimulation by T cell receptor (TCR) plus laminin crosslinking or TCR plus integrin α6 crosslinking, LBP was coimmunoprecipitated with TSAd. Moreover, TCR plus laminin costimulation-dependent T cell migration was enhanced in D1.1 T cells overexpressing TSAd but was disrupted in D1.1 cells overexpressing dominant negative form of TSAd or TSAd shRNA. These data show that, upon TCR plus integrin costimulation, TSAd associates with LBP and mediates T lymphocyte migration.
Keywords: cell movement; focal adhesion protein-tyrosine kinases; integrins; laminin binding protein 67-kDa, rat; Sh2d2a protein, mouse
Introduction
T cell trafficking is a critical element of cell-mediated immune responses. Combinations of various stimuli, such as chemokines, engagement of antigen receptors and binding of integrins to their ligands, trigger the migration of T cells within lymphatic systems and tissues. In particular, integrin-mediated signaling events are important in ECM-mediated migration of T cells. Upon binding to their ligands, integrins initiate cascades that activate multiple kinases, including focal adhesion kinase (FAK) and Src (Guan, 1997). Once activated, these kinases phosphorylate and activate various substrates mediating the migration of T cells. For example, Crk-associated substrate (Cas) lymphocyte-type (Cas-L) is tyrosine-phosphorylated upon engagement of T cell receptor (TCR) and β1 integrin and mediates T cell migration (Ohashi et al., 1999, van Seventer et al., 2001). Moreover, the SLAP-130/FYB adapter protein is tyrosine-phosphorylated upon α4 or β1 integrin stimulation, and enhances cell migration (Hunter et al., 2000).
We previously identified the murine adaptor protein, LAD (p56lck-interacting adaptor protein), as a binding partner of p56lck (Choi et al., 1999). The LAD protein was separately identified as Rlk/Txk- and Itk-binding adapter protein (RIBP) and MEKK2-binding protein (Rajagopal et al., 1999, Sun et al., 2001). The human counterpart of mLAD was identified as the TSAd (T cell-specific adapter protein) (Spurkland et al., 1998). To avoid confusion, we will refer this adaptor protein as TSAd. TSAd is an adaptor protein mainly expressed in T cells, which contains several protein-protein interaction domains, including a SH2 domain, a proline-rich SH3 binding motif and several phosphotyrosine sites. Upon T cell activation, TSAd is tyrosine-phosphorylated, associates with p56lck and is redistributed from the cytoplasm to the plasma membrane and plays essential roles in p56lck-mediated T cell activation (Choi et al., 1999). Consistent with this finding, proliferation of TSAd-deficient T cells in response to TCR-mediated activation was significantly impaired. Furthermore, TSAd-deficient T cells were defective in the generation of IL-2 and IFN-γ, but not IL-4 (Rajagopal et al., 1999). In addition to a function in T cell activation, TSAd was shown to associate with G protein β subunit and mediate chemokine-dependent T cell migration (Park et al., 2007). In response to chemokine, TSAd associates with the tyrosine kinase p56lck and ZAP-70 and is essential for the activation of ZAP-70 (Park et al., 2007). In addition, TSAd is a regulator of T cell death and TSAd-deficient mice show impaired cell-death and lupus-like autoimmunity (Rajagopal et al., 1999).
In this report, to search for the additional functions of TSAd in T cells, we performed yeast two-hybrid screening and identified 67kDa non-integrin laminin binding protein (LBP) as the binding partner. LBP cDNA encodes a polypeptide of 295 amino acids with a calculated molecular mass of 32 kDa, which is post-translationally modified to the 45 kDa and 67 kDa forms (Rao et al., 1989, Menard et al., 1997). Post-translational processing may involve acylation and homodimerization (Buto et al., 1998, Nelson et al., 2008). LBP binds laminin with high affinity. The LBP-binding motif in laminin (-YIGSR-) is distinct from the integrin-binding motif containing the RGD sequence (Clement et al., 1990). LBP associates with the integrin α6 chain for surface expression as well as high-affinity binding to laminin (Ardini et al., 1997; Buto et al., 1998). In T cell lineage, the protein is expressed on normal, activated mature T cells (both CD4+ and CD8+) primarily of the memory subtype, representing ~10-15% of the total human peripheral blood T cell population (Canfield and Khakoo, 1999). LBP-expressing T lymphocytes additionally express the integrin α6 and β1 chains which form the laminin receptor, VLA-6. In conjunction with VLA-6, LBP binds laminin with high affinity and mediates adherence of T cells to the extracellular matrix containing laminin (Canfield and Khakoo, 1999).
Here, we have found that TSAd associates with LBP in response to TCR + integrin coengagement and mediates laminin-dependent FAK phosphorylation and cell migration.
Results
Identification of LBP as a binding partner of TSAd
To identify the specific binding partners, we performed yeast two-hybrid screening with TSAd as bait. Following the screening of 2 × 106 independent colonies of a murine T cell lymphoma cDNA library, approximately 40 strong positive clones were isolated. Partial nucleotide sequencing of these cDNA fragments revealed that nine clones encoded a portion of the same protein, specifically, 67 kDa non-integrin LBP.
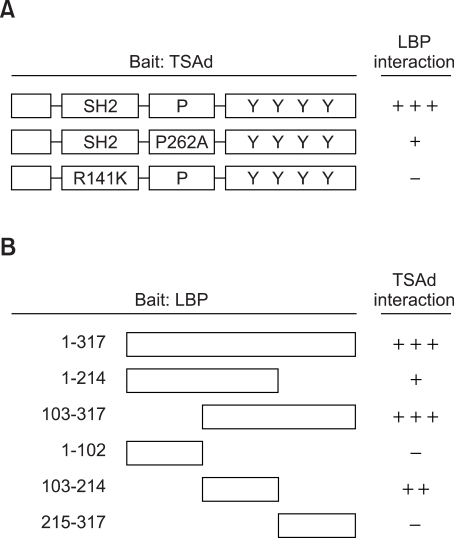
To delineate the domains involved in binding, deletion or point mutants of TSAd shown in Figure 1 were employed for a yeast two-hybrid assay. Among the TSAd mutants tested, point mutants of the proline-rich motif (P262A) and the SH2 domain (R141K) displayed reduced and no binding to LBP, respectively (Figure 1A). These results indicate that the region encompassing SH2 and proline-rich motif is involved in binding to LBP. However, it is unlikely that SH2/phosphotyrosine or proline-rich motif/SH3 domain interactions are involved in binding, since yeast has no reported tyrosine kinase activity and the SH3 domain is not present in LBP. In addition, out of the deletion mutants of LBP tested, the mutant encoding amino acids 103-214 of human LBP displayed binding to TSAd (Figure 1B).
Figure 1.
Mapping by yeast two-hybrid assay of the LBP binding sites in TSAd (A) and the TSAd binding sites in LBP. Binding affinity was scored as +++ (deep blue), ++ (intermediate blue), + (weak blue) or - (white) upon X-Gal staining. SH2; Src homology domain, P; proline-rich motif, Y; tyrosine phosphorylation sites.
TSAd binds to LBP upon engagement of TCR and integrin
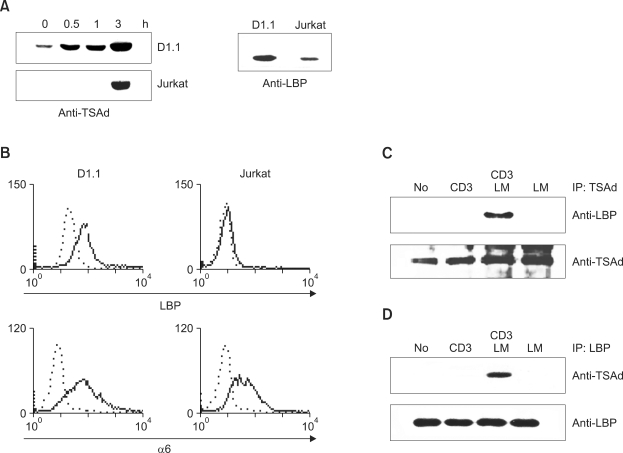
As the expression of both TSAd and LBP is known to be induced by TCR activation, we employed the D1.1 T cell line, the Jurkat subclone with activated phenotype for further characterization of TSAd-LBP interactions (Canfield and Khakoo, 1999, Choi et al., 1999). To determine the suitability of D1.1 cells for our study, TSAd and LBP levels were analyzed. As shown in Figure 2A. left panel, TSAd levels were induced in both D1.1 and parental Jurkat T cells following CD3 stimulation and significantly higher in D1.1 cells compared to Jurkat T cells. For the analysis of LBP expression, anti-LBP antibodies were generated by immunizing mice and rabbits with GST fusion proteins encompassing amino acids 215-294 of LBP. Both antibodies detected the 45 kDa form of LBP in Western blotting of (Figure 2A, right panel and data not shown) and the level of LBP in D1.1 cells was found approximately 3-fold higher, compared to Jurkat T cells.
Figure 2.
TSAd associates with LBP in response to TCR + laminin (LM) stimulation. (A) D1.1 and Jurkat cells were stimulated with anti-CD3 for the indicated times, and cell lysates were subjected to Western blotting with anti-mTSAd Ab or anti-LBP Ab. (B) FACS histograms of D1.1 cells (left columns) and Jurkat cells (right columns) depicting surface expression of LBP and integrin α6. In each panel, staining with the indicated Ab is depicted by a solid line in comparison with an isotype control (broken line). (C) D1.1 cells were transfected with the expression plasmids encoding GFP-tagged TSAd and stimulated with anti-CD3 Ab, anti-CD3 Ab + laminin or laminin. Subsequently, GFP-tagged TSAd was immunoprecipitated with anti-TSAd Ab, and immunoprecipitates were subjected to western blotting with anti-LBP Ab and anti-TSAd Ab. (D) D1.1 cells were prepared as described in (C). Endogenous LBP was immunoprecipitated with anti-LBP Ab, and the immunoprecipitates were analyzed by Western blotting with anti-TSAd or anti-LBP Ab.
The elevated expression of TSAd and LBP in D1.1 cells is consistent with the theory that D1.1 cells have activated T cell phenotypes. Previous reports show that surface expression of LBP is observed only in activated T cells, and that interactions with the integrin α6 chain are required for this process (Ardini et al., 1997; Canfield and Khakoo, 1999). We confirmed these previous findings in D1.1 cells. On FACS analysis, surface expression of LBP and integrin α6 was detected only in D1.1, but not Jurkat T cells (Figure 2B). These data show that D1.1 cells are suitable for analyzing TSAd-LBP interactions.
Binding between TSAd and LBP was investigated by coimmunoprecipitation studies in D1.1 cells. Since the size of hTSAd (52 kDa) is almost equal to that of the immunoglobulin heavy chain, confirmation of the amount of immunoprecipitated TSAd by Western blotting was sometimes difficult, due to overlap with the heavy chain band. Therefore, we used D1.1 cells transfected with expression plasmid encoding GFP-tagged TSAd (TSAd-GFP) with increased size. Subsequently, D1.1 cells were stimulated with anti-CD3 Ab, anti-CD3 Ab + laminin or laminin. Next, cell lysates were subjected to immunoprecipitation with anti-TSAd Ab and the immunoprecipitates were analyzed by Western blotting with anti-LBP Ab. As shown in Figure 2C, LBP was detected in anti-TSAd immunoprecipates upon CD3 + laminin stimulation, but not after CD3 or laminin stimulation only. Conversely, as shown in Figure 2D, TSAd was readily detected in LBP immunoprecipitates, following CD3 + laminin, but not CD3 or laminin, stimulation of D1.1 cells. These results indicate that in T cells, TSAd binds to LBP only during simultaneous stimulation by both TCR and laminin.
TSAd binds LBP upon stimulation of TCR + integrin α subunit
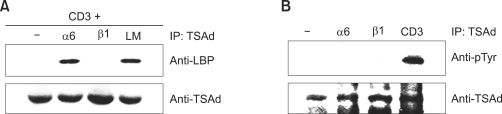
Since α6β1 is the major laminin-binding integrin, we next examined whether α6 or β1 stimulation induce the association of TSAd with LBP. Upon engagement of CD3 + α6 integrin, but not CD3 + β1 integrin, TSAd was detected in anti-LBP immunoprecipitates (Figure 3A). We thus propose that TSAd acts as an adaptor protein in integrin α6-mediated signaling through association with LBP in activated T cells.
Figure 3.
TSAd associates with LBP in response to TCR + integrin α6 stimulation. (A) D1.1 cells were transfected with expression plasmids encoding TSAd-GFP and stimulated with anti-CD3 + integrin α6, integrin β1 or laminin (LM) for 2 h. Cell lysates were analyzed by immunoprecipitation with anti-TSAd Ab and subsequent western blotting with anti-LBP Ab and anti-TSAd Ab. (B) D1.1 cells processed as described in (A) were stimulated by anti-integrin α6, integrin β1 or anti-CD3 Ab for 30 min. From cell lysates, TSAd-GFP was immunoprecipitated and immunoprecipitates were subjected to immunoboltting with anti-pTyr (4G10) Ab or anti-TSAd Ab.
We next examined the tyrosine phosphorylation status of TSAd upon TCR or integrin stimulation. Western analysis of TSAd immunoprecipitates showed that stimulation of CD3, but not integrin α6 or β1, led to tyrosine phosphorylation of TSAd (Figure 3B). In addition, we investigated the possibility of whether LBP was tyrosine phosphorylated in D1.1 cells. No evidence of LBP phosphorylation was observed upon integrin or TCR stimulation (data not shown).
TSAd colocalizes with LBP on the cell membrane upon CD3 + laminin stimulation
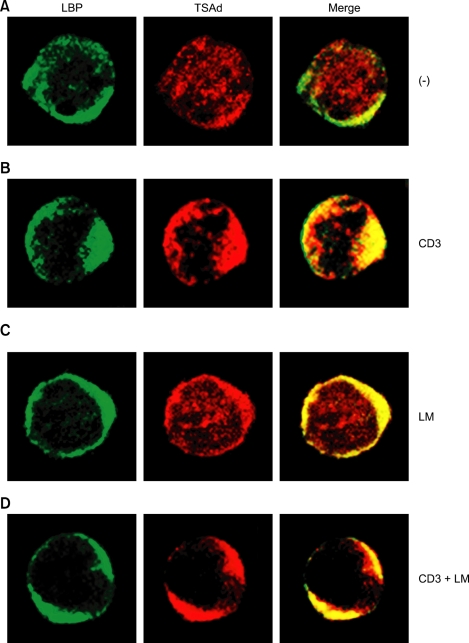
Having determined that TSAd associates with LBP upon TCR + integrin stimulation, we were interested in examining the specific sites of localization. In non-stimulated D1.1 cells (Figure 4A), most LBP localized in the plasma membrane, whereas TSAd was observed throughout the cell. Co-localization of LBP and TSAd was minimal in this state. When stimulated with anti-CD3 Ab, significant amount of TSAd and LBP was translocated to the capping sites on the cell membrane (Figure 4B). Upon stimulation with laminin (Figure 4C), TSAd and LBP partially colocalized in the cell membrane, although considerable amount of TSAd was still found throughout the cell. In cells stimulated with anti-CD3 Ab + laminin for 1h (Figure 4D), LBP and TSAd completely co-localized at capping sites on the membrane. Thus it seems that, in resting cells, LBP and TSAd are located in different compartments, LBP on the membrane and TSAd in the cytosol, and TCR + integrin stimulation is required for the translocation of TSAd to membrane and colocalization of TSAd with LBP. These results are consistent with the results observed in the coimmunoprecipitation assay (Figure 2C, 2D and 3A) in that the combined actions of both TCR and integrin signaling are required for binding of TSAd to LBP.
Figure 4.
Upon CD3 + laminin (LM) stimulation, TSAd colocalizes with LBP on cell membrane. D1.1 cells were unstimulated (A), stimulated with anti-CD3 Ab (B), laminin (C), or anti-CD3 Ab + laminin (D) and were stained using rabbit anti-TSAd Ab and mouse anti-LBP Ab. Subsequently, cells were exposed to TRITC-conjugated anti-rabbit secondary Ab and FITC-conjugated anti-mouse secondary Ab.
TSAd is required for integrin-mediated T cell migration
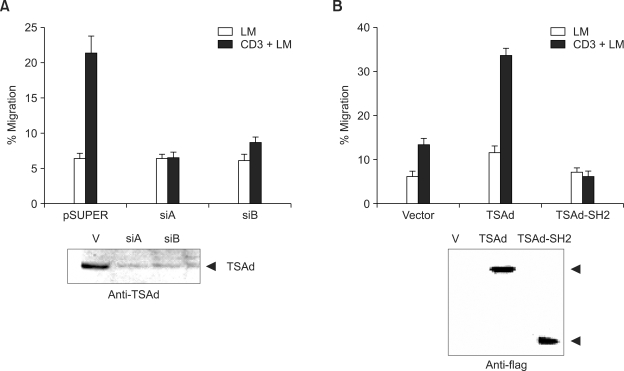
Having found the TSAd-LBP interaction upon laminin stimulation, we were interested in testing the possible role of TSAd in laminin-dependent T cell migration. D1.1 cells were transfected with pSUPER, or two TSAd shRNA-expressing constructs; siA or siB for the migration assay. At 12 h after transfection, cells were transferred and incubated for 6 h in the upper chamber of transwell inserts with filters coated with laminin or anti-CD3 Ab + laminin. Subsequently, filters were removed and the numbers of migrating cells were counted. As shown in Figure 5A, anti-CD3 Ab + laminin-induced T cell migration observed in pSUPER-transfected cells was almost completely disrupted in cells transfected with siA or siB, implying that TSAd is required for TCR and integrin-mediated T cell migration. To ensure that shRNA was active, control lysates were prepared from the same cells and the level of TSAd was determined by Western blotting with anti-mTSAd antibodies (Figure 5A, lower panel).
Figure 5.
TSAd mediates T cell migration on laminin (LM). (A) D1.1 cells were transfected with pSUPER or pSUPER plasmids expressing two types of hTSAd-shRNA (siA or siB). Migration properties of cells were analyzed by incubation for 6 h in the upper chamber of transwell inserts with filters coated with laminin or anti-CD3 Ab + laminin. Results are expressed as the percentage of fully migrated cells on the lower side of the filters. Cell numbers were counted in four separate microscope fields. The bars represent the mean ± SD of triplicate samples. Data are representative of five independent experiments (Upper panel). As control for the level of TSAd, the same cells were analyzed by Western blotting with anti-TSAd Ab (lower panel). (B) D1.1 cells were transfected with expression plasmids encoding FLAG-tagged TSAd or the SH2 domain of TSAd (TSAd-SH2). Subsequently, migration properties of cells were assayed as described in (A) (upper panel). Levels of transfected genes were analyzed by Western blotting with anti-FLAG Ab (lower panel).
To further assess the role of TSAd in integrin-mediated T cell migration, we tested the migration of D1.1 cells stably expressing either the TSAd protein or the SH2 domain of TSAd. The SH2 domain of TSAd was previously characterized as dominant-negative by its ability to impair T cell receptor-mediated activation of the IL-2 promoter (Choi et al., 1999). As shown in Figure 5B, D1.1 cells expressing wild-type TSAd displayed a marked increase in migration, whereas cells expressing the TSAd-SH2 domain displayed impaired migration on anti-CD3 Ab + laminin-coated filters. Analysis of lysates from the same cells showed that TSAd or TSAd-SH2 are properly expressed with the expected sizes (Figure 5B, lower panel). These findings, combined with results shown in Figure 2 and 3 indicate that, upon TCR and integrin α6 stimulation, TSAd associates with LBP and mediates T cell migration through ECM.
TSAd is required for integrin-mediated FAK phosphorylation
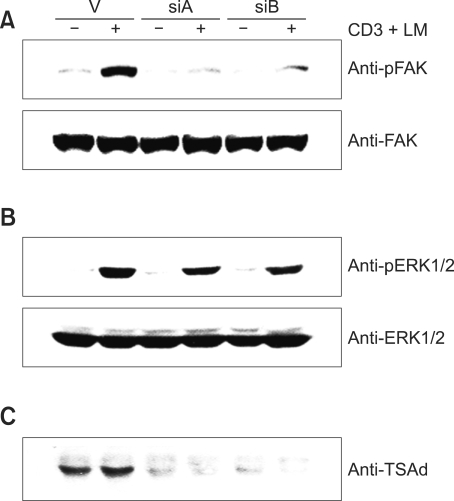
Focal adhesion kinase (FAK) is implicated in the regulation of integrin-mediated spreading and cell migration. To investigate the molecular mechanisms of the action of TSAd in T cell migration, we examined the activation status of FAK by measuring the tyrosine phosphorylation of FAK. To assay the phosphorylation status, an antibody specifically recognizing the autophosphorylation site, phospho-Tyr397 of FAK, was employed for Western analysis. As shown in Figure 6A, FAK was tyrosine-phosphorylated following CD3 + laminin stimulation of D1.1 cells transfected with control vector, but not in cells expressing TSAd shRNA, indicating that TSAd is required for TCR + integrin-mediated tyrosine phosphorylation of FAK. In addition, we tested the possibility that TSAd coimmunoprecipitates with FAK upon anti-CD3 Ab + laminin stimulation. No evidence for binding was observed (data not shown). Therefore, it seems that TSAd regulates FAK indirectly. We additionally examined the possibility of TSAd involvement in the activation of ERK1/2, based on the report that stimulation of α6 integrin facilitates Erk2 activation (Wei et al., 1998). Upon CD3 + laminin stimulation, ERK1/2 was phosphorylated approximately to the same levels in pSUPER, siA and siB-transfected D1.1 cells (Figure 6B). From these data, we conclude that TSAd is involved in integrin-mediated FAK phosphorylation, but not ERK activation.
Figure 6.
TSAd is required for anti-CD3 Ab + laminin (LM)-induced FAK phosphorylation. (A) D1.1 cells were transfected with pSUPER or plasmids expressing two types of hTSAd-shRNA (siA or siB) and incubated for 30 min on plates coated with anti-CD3 Ab + laminin. Cell lysates were subjected to Western blotting with anti-p-FAK or anti-FAK Ab. (B) TSAd is not involved in CD3 + laminin-stimulated ERK1/2 phosphorylation. The same cell lysates were subjected to Western blotting with anti-p-ERK1/2 or anti-ERK1/2 antibody. (C) As controls for the level of TSAd, the same cells were analyzed by Western blotting with anti-TSAd Ab.
Discussion
Here, we present a novel function of TSAd and LBP in regulating the motility of T cells. Combined with the previous findings on TSAd and LBP function, the findings in this report suggest the following model for the action mechanism of TSAd in TCR and integrin-dependent migration. Upon TCR stimulation, TSAd is phosphorylated by p56lck. Subsequent engagement of integrin α6β1 and LBP with the laminin-containing extracellular matrix induces the association of TSAd with LBP. Once recruited to the integrin complex via association with LBP, TSAd may recruit other signaling molecules, leading to the phosphorylation of FAK and T cell migration through the extracellular matrix.
Migration of T cells is crucial for the regulation of immune responses, especially inflammation, since lymphocytes must reach pathogens sequestered in inflammatory sites. The requirement of TSAd in laminin-mediated migration suggests the possibility that the protein is involved in inflammation. This theory is consistent with the finding that the expression of TSAd (Figure 2A and ref [Choi et al., 1999]) and surface LBP (Figure 2B and ref [Canfield and Khakoo, 1999]) are inducible by T cell activation. During T cell activation or differentiation to effector T cells, TSAd expression is induced by TCR stimulation and subsequently, TSAd form the complex with integrin α6β1 and LBP and may facilitate the migration of T cells through local inflamed tissue.
TSAd is essential in the integrin α6-mediated signaling pathway and may therefore be responsible for the previously reported functions of integrin α6β1. A integrins family includes diverse integral membrane glycoproteins that function as non-covalently associated αβ heterodimers. Several members of the integrin family, including α1β1, α2β1, α3β1, α6β1, α7β1 and α6β4 heterodimers, serve as laminin receptors on a variety of cell types (Belkin and Stepp, 2000). Particularly, mature effector T cells require the α6β1 integrin for their functions. Effector/memory T cells express several fold more α6β1 integrin than naïve cells, and interact more efficiently with laminin (Shimizu et al., 1990). Upregulation of α6β1 by IL-12 and α interferon promotes the migration of human type 1 helper cells (Colantonio et al., 1999). In the view of these established functions of integrin α6β1, we suggest that TSAd may operate in the migration of effector/memory T cells rather than naïve cells.
In the yeast two-hybrid assay, TSAd-LBP interaction was detected even in the absence of TCR + laminin stimulation which was shown to be essential for the interaction in human T cell line. It seems that, as shown in Figure 4, TCR + laminin stimulation induces the colocalization of TSAd and LBP, which is required for the subsequent association of TSAd and LBP. In yeast two-hybrid assay, overexpressed TSAd and LBP were enforced to colocalize in the nuclear compartment by the presence of nuclear localization signal in both bait and prey, which is presumably sufficient to allow the association of TSAd with LBP.
In conclusion, our study establishes that TSAd plays essential roles in α6 integrin-mediated T cell migration via association with non-integrin LBP.
Methods
Yeast two hybrid screen
The yeast two-hybrid assay was performed as described in earlier report (Choi et al., 1999). cDNA fragment encoding murine TSAd (mTSAd) was subcloned into pGBT9 vector and used for the bait in the yeast two-hybrid screens of murine T cell lymphoma cDNA library cloned into pACT (Clontech).
Antibodies
Rabbit anti-mTSAd antibody generated against a.a 208-366 is described in a previous report (Choi et al., 1999). Anti-human TSAd (hTSAd) Ab was generated by immunizing mice with GST fusion proteins encompassing the a.a 1-202 of hTSAd. Polyclonal anti-LBP Ab was generated by immunization of rabbits or mice with GST fusion proteins encompassing the aa 215-295 of LBP. The other antibodies used in this study were obtained from commercial sources: anti-phosphotyrosine (4G10, Upstate Biotechnology), anti-phospho-FAK (BioSource), anti-FAK (BD Biosciences), anti-integrin α6 (Santa Cruz), PE-conjugated anti-integrin α6 (BD Pharmingen), anti-phospho-ERK1/2, anti-ERK1/2 (Cell Signaling), anti-integrin α6 (GoH3, BD Pharmingen), and anti-integrin β1 (MAR4, BD Pharmingen) antibodies.
Cells and activation
For activation, tissue culture dishes (60 mm, Costar) were coated with appropriate combinations of 5 µg/ml laminin (Sigma), anti-integrin α6 (GoH3, BD Pharmigen), anti-ingetrin β1 (MAR4, BD Pharmigen), anti-CD3 Abs (BD Pharmingen) or poly-L-lysine (Sigma) at 4℃ for overnight. After washing, D1.1 cells were incubated for appropriate time on coated plates; 1-2 h for analysis of TSAd-LBP binding, 30 min for the analysis of TSAd or FAK phosphorylation.
Immunoprecipitation and western blot analysis
All procedures for immunoprecipitation and Western analysis were described previously (Hur et al., 2003).
Expression vectors and transfection
For binding assays, cDNA encoding full-length TSAd was subcloned into the expression vector, pEGFP-N1 (Clontech). For migration assays, cDNAs encoding wild-type TSAd or TSAd-SH2 domain were subcloned into the expression vector p3 × FLAG-CMV. The expression vectors were transiently transfected into cells by electroporation at 240V, 25 msec, 1 pulse using Electroporator (BTX T820). We routinely observed transfection efficiency of more than 50%.
The pSUPER plasmid was used for the synthesis of shRNAs (Brummelkamp et al., 2002). Two regions of 19-base pair corresponding to the nucleotides 194-212 (siA) and 871-889 (siB) in the coding sequence of human TSAd were selected for the construction of shRNA expressing plasmid.
siA: 5'-UCAUCCUGAAUCUGAUCUUdTdT-3', 5'-AAGAUCAGAUUCAGGAUGAdTdT-3', siB: 5'-GAUGCGGAAGCUCUUCAGUdTdT-3', 5'-ACUGAAGAGCUUCCGCAUCdTdT-3'.
Flow cytometry
For flow cytometry, 1 × 106 cells were washed twice with PBS and stained with the appropriate antibodies at 4℃ for 30 min. After washing with PBS, cells were fixed with 1% paraformaldehyde in PBS. Fluorescent intensity was measured with a flow cytometer (FACS caliber, BD) and analyzed using CellQuest software (BD). The following antibodies were used in flow cytometry; mouse anti-LBP, FITC-conjugated secondary and, PE-conjugated anti-integrin α6 antibodies.
Immunofluorescence staining and confocal laser scan microscopy
Slide glasses were coated with anti-CD3 Ab or laminin for 2 h at 37℃. D1.1 T cells were loaded onto slide glasses and incubated at 37℃ for 1 h. Subsequently, cells were fixed for 15 min at room temperature with 4% paraformaldehyde. After washing twice with PBS, cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature. Permeabilized cells were incubated with primary antibody for 40 min at 37℃, washed twice with PBS, and incubated with FITC-conjugated or TRITC-conjugated secondary antibody for 30 min at 30℃. After washing twice with PBS, prepared samples on slide glasses were mounted using mounting medium (Molecular Probes), and photographs were taken using a LSM510 Confocal Microscope (Carl Zeiss Co.).
Migration assay
Cell migration assays were performed using 6.5-mm-diameter Transwell™ inserts with polycarbonate filters (5-µm pore size) (Costar, Cambridge, MA). The filters were either left uncoated or coated overnight with anti-CD3 Ab. After washing, the filters were additionally coated with 5 µg/ml laminin at room temperature for 2 h. Transwell™ chambers were inserted into wells filled with RPMI 1640 containing 0.6% BSA, and 5 × 105 cells in 100 µl of the same medium were added to the upper chamber. After incubation at 37℃ for the appropriate duration of time, filters were removed and the numbers of the migrated cells were counted in four separate fields of each well. Data are presented as means ± standard deviation (SD) from experiments performed in triplicate.
Acknowledgements
We thank other members of the laboratory for materials and helpful discussions. We are grateful for Dr. Agami for pSUPER vector. This work was supported by the Korea research foundation grant (Basic Research promotion fund (KRF-2006-C00308), the Molecular and Cellular Biodiscovery Program (M1-06N0100-16210) and the KBRDG initiative Research Program grants (2006-08367) from the Korea Science and Engineering Foundation.
Abbreviations
- LAD
p56lck-interacting adaptor protein
- LBP
67 kDa laminin binding protein
- TSAd
T cell-specific adaptor protein
References
- 1.Ardini E, Tagliabue E, Magnifico A, Buto S, Castronovo V, Colnaghi MI, Menard S. Co-regulation and physical association of the 67-kDa monomeric laminin receptor and the alpha6beta4 integrin. J Biol Chem. 1997;272:2342–2345. doi: 10.1074/jbc.272.4.2342. [DOI] [PubMed] [Google Scholar]
- 2.Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51:280–301. doi: 10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 3.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 4.Buto S, Tagliabue E, Ardini E, Magnifico A, Ghirelli C, van den Brule F, Castronovo V, Colnaghi MI, Sobel ME, Menard S. Formation of the 67-kDa laminin receptor by acylation of the precursor. J Cell Biochem. 1998;69:244–251. doi: 10.1002/(sici)1097-4644(19980601)69:3<244::aid-jcb2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Canfield SM, Khakoo AY. The nonintegrin laminin binding protein (p67 LBP) is expressed on a subset of activated human T lymphocytes and, together with the integrin very late activation antigen-6, mediates avid cellular adherence to laminin. J Immunol. 1999;163:3430–3440. [PubMed] [Google Scholar]
- 6.Choi YB, Kim CK, Yun Y. Lad, an adapter protein interacting with the SH2 domain of p56lck, is required for T cell activation. J Immunol. 1999;163:5242–5249. [PubMed] [Google Scholar]
- 7.Clement B, Segui-Real B, Savagner P, Kleinman HK, Yamada Y. Hepatocyte attachment to laminin is mediated through multiple receptors. J Cell Biol. 1990;110:185–192. doi: 10.1083/jcb.110.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colantonio L, Iellem A, Clissi B, Pardi R, Rogge L, Sinigaglia F, D'Ambrosio D. Upregulation of integrin alpha6/beta1 and chemokine receptor CCR1 by interleukin-12 promotes the migration of human type 1 helper T cells. Blood. 1999;94:2981–2989. [PubMed] [Google Scholar]
- 9.Guan JL. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 10.Hunter AJ, Ottoson N, Boerth N, Koretzky GA, Shimizu Y. Cutting edge: a novel function for the SLAP-130/FYB adapter protein in beta 1 integrin signaling and T lymphocyte migration. J Immunol. 2000;164:1143–1147. doi: 10.4049/jimmunol.164.3.1143. [DOI] [PubMed] [Google Scholar]
- 11.Hur EM, Son M, Lee OH, Choi YB, Park C, Lee H, Yun Y. LIME, a novel transmembrane adaptor protein, associates with p56lck and mediates T cell activation. J Exp Med. 2003;198:1463–1473. doi: 10.1084/jem.20030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menard S, Castronovo V, Tagliabue E, Sobel ME. New insights into the metastasis-associated 67 kD laminin receptor. J Cell Biochem. 1997;67:155–165. [PubMed] [Google Scholar]
- 13.Nelson J, McFerran NV, Pivato G, Chambers E, Doherty C, Steele D, Timson DJ. The 67 kDa laminin receptor: structure, function and role in disease. Biosci Rep. 2008;28:33–48. doi: 10.1042/BSR20070004. [DOI] [PubMed] [Google Scholar]
- 14.Ohashi Y, Iwata S, Kamiguchi K, Morimoto C. Tyrosine phosphorylation of Crk-associated substrate lymphocyte-type is a critical element in TCR- and beta 1 integrin-induced T lymphocyte migration. J Immunol. 1999;163:3727–3734. [PubMed] [Google Scholar]
- 15.Park D, Park I, Lee D, Choi YB, Lee H, Yun Y. The adaptor protein Lad associates with the G protein beta subunit and mediates chemokine-dependent T-cell migration. Blood. 2007;109:5122–5128. doi: 10.1182/blood-2005-10-061838. [DOI] [PubMed] [Google Scholar]
- 16.Rajagopal K, Sommers CL, Decker DC, Mitchell EO, Korthauer U, Sperling AI, Kozak CA, Love PE, Bluestone JA. RIBP, a novel Rlk/Txk- and itk-binding adaptor protein that regulates T cell activation. J Exp Med. 1999;190:1657–1668. doi: 10.1084/jem.190.11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao CN, Castronovo V, Schmitt MC, Wewer UM, Claysmith AP, Liotta LA, Sobel ME. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry. 1989;28:7476–7486. doi: 10.1021/bi00444a047. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu Y, Van Seventer GA, Horgan KJ, Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990;345:250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- 19.Spurkland A, Brinchmann JE, Markussen G, Pedeutour F, Munthe E, Lea T, Vartdal F, Aasheim HC. Molecular cloning of a T cell-specific adapter protein (TSAd) containing an Src homology (SH) 2 domain and putative SH3 and phosphotyrosine binding sites. J Biol Chem. 1998;273:4539–4546. doi: 10.1074/jbc.273.8.4539. [DOI] [PubMed] [Google Scholar]
- 20.Sun W, Kesavan K, Schaefer BC, Garrington TP, Ware M, Johnson NL, Gelfand EW, Johnson GL. MEKK2 associates with the adapter protein Lad/RIBP and regulates the MEK5-BMK1/ERK5 pathway. J Biol Chem. 2001;276:5093–5100. doi: 10.1074/jbc.M003719200. [DOI] [PubMed] [Google Scholar]
- 21.van Seventer GA, Salmen HJ, Law SF, O'Neill GM, Mullen MM, Franz AM, Kanner SB, Golemis EA, van Seventer JM. Focal adhesion kinase regulates beta1 integrin-dependent T cell migration through an HEF1 effector pathway. Eur J Immunol. 2001;31:1417–1427. doi: 10.1002/1521-4141(200105)31:5<1417::AID-IMMU1417>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 22.Wei J, Shaw LM, Mercurio AM. Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the alpha6 integrin subunit. J Biol Chem. 1998;273:5903–5907. doi: 10.1074/jbc.273.10.5903. [DOI] [PubMed] [Google Scholar]