Abstract
Objective
Epidemiological studies have shown that low folate levels are associated with a high body mass index (BMI). These findings have potentially important health implications and warrant further investigation to determine whether a causal relationship exists and the direction of this relationship. The methylenetetrahydrofolate reductase (MTHFR) C677T TT genotype is associated with reduced folate availability and may be a surrogate for measuring folate levels. We sought to determine whether MTHFR C677T genotype was associated with obesity.
Design
We carried out our study on four populations from three longitudinal studies based in the UK and Denmark in which DNA for genotyping was obtained along with measures of obesity.
Methods
Our subjects were taken from the British Women's Heart and Health Study (BWHHS), the Avon Longitudinal Study of Parents and Children (two populations: mothers and children) and the Copenhagen City Heart Study. We performed analyses separately by population, and then carried out a meta-analysis, combining similar populations.
Results
Initial findings in the BWHHS suggested that the TT genotype may be associated with an increased risk of obesity BMI≥30, however, no association was found with BMI or central adiposity in this cohort. This genotype was not associated with obesity in our other cohorts.
Conclusions
Our results suggest that the initial positive finding with obesity in the BWHHS was a chance finding. Our findings do not support a causal effect of low folate on obesity.
Introduction
Recent findings that low folate levels are associated with a high body mass index (BMI) (1–3) have potentially important health implications. The apparent association warrants further investigation to determine whether a causal relationship exists as the current obesity epidemic is a major public health issue, affecting the whole population irrespective of age, gender and ethnic group. Folate supplementation of commonly consumed foods (e.g. flour), currently being considered in the UK (Scientific Advisory Committee on Nutrition. Folate and Disease Prevention, TSO, London, 2006), could be a potential population level intervention to reverse the obesity epidemic if low folate levels are indeed causally related to obesity.
Lower serum folate levels have been found to be strongly associated with increased BMI in women of childbearing age in two waves of the National Health and Nutrition Examination Survey, each 10 kg/m2 increase in BMI was associated with a 15.6% decrease in serum folate (P<0.001), an association that persisted even after controlling for age, ethnicity, folate intake and red blood cell folate (1). A further large cross-sectional study of women in prenatal care replicated the finding of an association between low serum folate levels and obesity (2). Also, a small case–control study found much higher plasma homocysteine, a biomarker of low folate levels, among obese children and adolescents compared with non-obese controls (3). Further, indirect evidence for an association between low folate and obesity is that high pre-pregnancy BMI has been consistently found to be associated with neural tube defects (4, 5), which are caused by low perinatal folate levels (6).
It is possible that lower levels of serum folate are observed among heavier women, simply because their requirements are greater (1). Another plausible explanation for the association between low folate status and obesity could be confounding by dietary habits, since it is likely that those individuals who have a high-energy diet will eat less fruit, vegetables and cereals and thus have a lower folate intake. However, we have observed an association between the MTHFR C677T TT genotype, which is associated with reduced folate availability (7), and obesity in the British Women's Heart and Health Study (BWHHS) (8). Clearly, this genotype could not have been altered by adult BMI, and it is not subject to confounding by lifestyle factors (9, 10). Thus, our MTHFR genotype–obesity findings suggest that folate levels may be causally related to greater BMI and obesity.
A potential mechanism by which folate could influence body mass and obesity is via epigenetic control of gene expression. Methylation of cytosines in CpG dinucleotides is an important epigenetic modification, which affects gene expression and thus cellular function. To a certain extent, methylation patterns can be controlled by environmental factors such as intake of dietary folate, which is an important donor of methyl groups required for methylation. Folate depletion in humans has been observed to diminish genomic DNA methylation (11, 12). The hypothesis that epigenetic changes including methylation are linked to adult obesity is supported by the observation that in humans several genes have been shown to exhibit changes in expression that correlated closely with BMI and/or waist/hip ratio (13). Further, obesity is one of the symptoms of Prader–Willi syndrome that is caused by irregular DNA methylation patterns in a given region on chromosome 15q (14).
Preliminary analysis has shown that the MTHFR C677T TT genotype was associated with an ∼20% increase in the prevalence of obesity in the BWHHS, although only very small differences in BMI were observed by genotype (8). Since initial positive genotype–phenotype associations frequently fail to replicate (15), we sought to examine the association of MTHFR C677T genotype with BMI and obesity in two further population-based cohorts and to present a full analysis in the BWHHS.
Methods
Study population
We examined the association between the MTHFR genotype in four distinct populations within three cohort studies, namely the BWHHS, the Avon Longitudinal Study of Parents and Children (two populations: mothers and children) and the Copenhagen City Heart Study (CCHS).
The British Women's Heart and Health Study (BWHHS)
Between 1999 and 2001 4286 women aged 60–79 years, who were randomly selected from 23 British towns were interviewed, examined, completed medical questionnaires and had detailed reviews of their medical records. Of 4286 women who participated in the BWHHS, 3938 (92%) had complete data on all anthropometric measurements and of these 3438 (87%) provided consent for genetic testing and had adequate MTHFR genotype assays. Twenty women were excluded because they had non-white ethnicity, leaving a final sample of 3416.
The Avon Longitudinal Study of Parents and Children (ALSPAC)
A population-based prospective study investigating factors that affect the health and development of children and their parents. Pregnant women living in Bristol, England, who had an expected date of delivery between April 1991 and December 1992, were eligible. A total of 14 541 women enrolled in the study. Extensive data have been collected on the children and their mothers from pregnancy onwards by questionnaire, abstraction from medical notes, record linkage and by attendance at research clinics. Of 8128 women with genotype data, information on pre-pregnancy BMI was available for 6952 (86%), 461 (7%) women were excluded due to non-white or unknown ethnicity, leaving a final sample of 6491 women. Of 8782 children with genotype data, information on BMI at around age 9 was available for 5619 (64%), a further 489 (9%) children were excluded due to non-white or unknown ethnicity, leaving a final sample of 5130 children.
Copenhagen City Heart Study (CCHS)
A prospective cardiovascular study of individuals selected from the Central-Population-Register Code designed to reflect the adult Danish general population, including both men and women. Those invited were stratified into 5-year age groups from 20 to 95 years, with main emphasis placed on the 35- to 70-year olds. In the CCHS, 9252 individuals were available for genotyping and 9238 received a final genotype, 9173 individuals of those genotyped had data on all measures of adiposity and so were included in the final analysis. The cohort is very ethnically homogenous so no participants were excluded on the basis of ethnicity. The Danish Ethics Committees for the cities of Copenhagen and Frederiksberg approved the study. Informed consent was obtained from participants.
Measurement of weight at height
Weight and height were measured using standard procedures and were used to calculate BMI; in all studies obesity was defined using the WHO standard threshold of 30 kg/m2 for adults. For the ALSPAC children, the following cut-offs were used; males >23.39 kg/m2 and females >23.46 kg/m2 (16). In the ALSPAC mothers, self-reported pre-pregnancy weight and waist and hip circumference were used, which was obtained at the 18-week antenatal clinic. In the ALSPAC children, weight and height at around age 9 years measured by trained nurses at the clinic were used in this analysis. In the BWHHS, all measurements were undertaken at the baseline clinical examination and were measured in the clinic by trained nurses. In the CCHS, the participants were interviewed at baseline at Rigshospitalet, Copenhagen University Hospital and weight and height were measured.
Genotyping
DNA was extracted by salting out procedure (17). Genotyping in the ALSPAC and the BWHHS was undertaken by KBioscience Ltd (www.kbioscience.co.uk), who use their own form of competitive allele-specific PCR system (KASPar) and Taqman (Applied Biosystem, Foster City, CA, USA) for SNP analysis. In the CCHS, the MTHFR C677T polymorphism was genotyped using restriction enzymes digesting the PCR product, which were then separated on a gel (18). Two independent investigators confirmed each genotype.
Statistical analysis
Assumptions of Hardy–Weinberg equilibrium were formally tested using a likelihood ratio test. Prevalence (for dichotomous variables) and means (for continuous variables) for obesity and other anthropometric measures were calculated by genotype. One-way ANOVA was used for testing differences between genotypes for continuous variables and χ2 tests were used for testing differences between genotypes for dichotomous variables. Pearson χ2 tests were used to test for Hardy–Weinberg equilibrium.
Unadjusted odds ratios were calculated for prevalence of obesity among TT versus CC genotypes, and these data were used to carry out a formal meta-analysis. Since the different studies were carried out on diverse age groups, we carried out three analyses for adults (CCHS and BWHHS), young women (ALSPAC mothers) and children (ALSPAC children). Because the results for the different studies and in different sexes in the ALSPAC were heterogeneous, we undertook random effects meta-analyses.
Results
We examined the association between obesity and other anthropometric measures and MTHFR C677T genotype in four population-based cohorts totalling 24 210 individuals from the United Kingdom and Denmark. The mean age of participants in the CCHS was 57.6 (s.d.=15.2) years (range 21–93) at the time that adiposity was measured. The mean age of women at early pregnancy and the time of recruitment into ALSPAC were 28.5 (s.d.=4.7) years (range 15–44), the age of children in the ALSPAC when BMI was measured was 9.9 (s.d.=0.3) years (range 8.8–11.8). The mean age of participants in the BWHHS was 68.8 (s.d.=5.5) years (range 59–80). The distribution of age did not differ by genotype in any cohort. T-allele frequencies ranged from 0.31 to 0.34 (Table 1), genotypes were in Hardy–Weinberg equilibrium in all populations.
Table 1.
Distribution of methylenetetrahydrofolate reductase (MTHFR) genotype among the cohorts in this study.
| CC | CT | TT | T-allele frequency | P for Hardy–Weinberg | |
|---|---|---|---|---|---|
| BWHHS | 1525 (44.7%) | 1496 (43.7%) | 395 (11.6%) | 0.33 | 0.28 |
| CCHS | 4400 (48.0%) | 3930 (42.8%) | 843 (9.2%) | 0.31 | 0.42 |
| ALSPAC mothers | 2870 (44.2%) | 2868 (44.1%) | 753 (11.6%) | 0.34 | 0.37 |
| ALSPAC boys | 1170 (45.2%) | 1138 (43.9%) | 282 (10.9%) | 0.33 | 0.83 |
| ALSPAC girls | 1100 (43.3%) | 1145 (45.1%) | 295 (11.6%) | 0.34 | 0.91 |
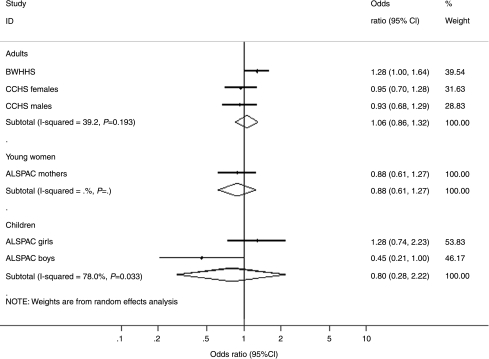
We found an association between genotype and prevalence of obesity (BMI >30 kg/m2) in the BWHHS, which was not replicated in the other cohorts (Table 2, and Fig. 1). In the BWHHS, as in the ALSPAC and CCHS, MTHFR C677T genotype was not associated with BMI, waist circumference or waist–hip ratio (all analysed as continuous variables) in adults. In the ALSPAC boys, there was weak evidence that the CT and TT genotypes were associated with a reduction in BMI relative to the CC genotype, and the risk of obesity appeared to reduce in a dose–response manner with the number of T-alleles. Our meta-analysis found an overall odds ratio for obesity in TT versus CC adults of 1.06 (95% CI 0.86–1.32). There was no evidence that MTHFR C677T was associated with social class, smoking, alcohol intake or physical activity factors that may confound an association between folate intake and obesity (data available from authors).
Table 2.
Distribution of indicators of adiposity by methylenetetrahydrofolate reductase (MTHFR) genotype.
| Number (%) for dichotomous variables or mean (s.d.) for continuous variables by genotype | ||||
|---|---|---|---|---|
| Outcome variables | CC | CT | TT | P |
| Obese (>30 kg/m2) | N (%) | N (%) | N (%) | |
| BWHHS | 360/1525 (23.6) | 410/1496 (27.5) | 112/395 (28.4) | 0.02 |
| CCHS females | 315/2454 (12.8) | 308/2145 (14.4) | 57/465 (12.3) | 0.24 |
| CCHS males | 273/1946 (14.0) | 266/1785 (14.9) | 50/378 (13.2) | 0.61 |
| CCHS ALL | 588/4400 (13.4) | 574/3930 (14.6) | 107/843 (12.7) | 0.16 |
| ALSPAC mothers | 163/2870 (5.7) | 155/2868 (5.4) | 38/753 (5.0) | 0.77 |
| ALSPAC girlsa | 53/1100 (4.8) | 51/1145 (4.4) | 18/295 (6.1) | 0.50 |
| ALSPAC boysa | 62/1170 (5.3) | 42/1138 (3.7) | 7/282 (2.5) | 0.05 |
| ALSPAC children ALL | 115/2270 (5.1) | 93/2283 (4.1) | 25/577 (4.3) | 0.27 |
| Body mass index (kg/m2) | Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | |
| BWHHS | 27.4 (4.7) N=1525 | 27.7 (5.2) N=1496 | 27.8 (5.2) N=395 | 0.14 |
| CCHS females | 25.0 (4.5) N=2454 | 25.2 (4.6) N=2145 | 24.9 (4.5) N=465 | 0.13 |
| CCHS males | 26.0 (3.9) N=1946 | 26.0 (4.0) N=1785 | 25.9 (3.9) N=378 | 0.90 |
| CCHS ALL | 25.4 (4.3) N=4400 | 25.6 (4.4) N=3930 | 25 (4.3) N=843 | 0.18 |
| ALSPAC mothers | 23.0 (3.9) N=2870 | 23.0 (3.8) N=2868 | 22.9 (3.7) N=753 | 0.64 |
| ALSPAC girls | 17.8 (2.9) N=1100 | 17.8 (2.9) N=1145 | 17.9 (3.1) N=295 | 0.86 |
| ALSPAC boys | 17.6 (2.9) N=1170 | 17.3 (2.5) N=1138 | 17.3 (2.7) N=282 | 0.06 |
| ALSPAC children ALL | 17.7 (2.9) N=2270 | 17.6 (2.7) N=2283 | 17.6 (2.9) N=577 | 0.50 |
| Waist–hip ratio | ||||
| BWHHS | 0.82 (0.07) N=1525 | 0.82 (0.07) N=1496 | 0.82 (0.07) N=395 | 0.27 |
| CCHS females | 0.83 (0.08) N=2454 | 0.83 (0.08) N=2145 | 0.83 (0.08) N=465 | 0.96 |
| CCHS males | 0.94 (0.08) N=1946 | 0.95 (0.08) N=1785 | 0.95 (0.07) N=378 | 0.57 |
| CCHS ALL | 0.88 (0.10) N=4400 | 0.88 (0.10) N=3930 | 0.88 (0.10) N=843 | 0.59 |
| ALSPAC mothers | 0.74 (0.07) N=1634 | 0.74 (0.06) N=1615 | 0.74 (0.07) N=407 | 0.45 |
| Waist circumference (cm) | ||||
| BWHHS | 85.7 (11.6) N=1525 | 86.5 (12.5) N=1496 | 86.5 (12.7) N=395 | 0.15 |
| CCHS females | 99.5 (9.5) N=2454 | 99.9 (9.8) N=2145 | 99.1 (9.3) N=465 | 0.18 |
| CCHS males | 100.2 (7.7) N=1946 | 100.0 (7.4) N=1785 | 99.9 (7.5) N=378 | 0.50 |
| CCHS ALL | 99.8 (8.7) N=4400 | 99.9 (8.8) N=3930 | 99.5 (8.5) N=843 | 0.36 |
| ALSPAC mothers | 68.6 (7.2) N=1891 | 68.3 (6.8) N=1830 | 68.3 (7.0) N=491 | 0.40 |
>23.39 kg/m2 males and >23.46 kg/m2 females. P values are for evidence of heterogeneity across categories (i.e. two degrees of freedom).
Figure 1.
Forest plot showing risk of obesity amongst MTHFR C677T TT homozygotes versus CC homozygotes.
Discussion
We found no evidence that the TT genotype of the MTHFR gene was associated with BMI or obesity in two out of three of our adult cohorts, which suggests that the initial positive finding with obesity in the BWHHS was a chance finding arising due to the large number of association tests that were performed between this genotype and phenotypes in the BWHHS. This emphasizes the need to replicate positive associations in other studies in order to guard against the problem of multiple testing. We found that among boys in the ALSPAC cohort, who were around age 9 at the time of measurement, the T-allele was associated with a reduction in the prevalence of obesity and a reduction in BMI. This most likely could also be a chance finding, but requires further investigation to clarify whether this is the case or whether there is in fact evidence of a protective effect of the T-allele in pre-adolescent boys.
We have used a genetic variant that influences circulating folate levels to test whether low folate availability is a risk factor for obesity. This analysis is not subject to reverse causation since an individual's genotype is determined at conception and cannot be determined by an individual's weight later in life. In addition, this genetic variant that affects the metabolism of folate, for instance, does not appear to be associated with other dietary and lifestyle factors, which are typically associated with dietary folate intake (8–10).
Our findings are consistent with a small study on obesity carried out in Spain (19) and also an Italian study, which found no association between this genotype and metabolic syndrome (20). These findings argue against a role for methylation in determining adult and childhood obesity, particularly as the MTHFR C677T TT homozygotes have been shown to exhibit a diminished level of DNA methylation compared with CC homozygotes (21). However, we are unable to shed any light on whether those who have a greater BMI also have a greater requirement for folate, this question requires further research. A recent family study has suggested that polymorphisms in this gene may be related to lean body mass rather than fat mass (22), whilst our study of BMI (which is a composite of the two) does not support these findings; in this analysis, we did not look at lean body mass separately as lean mass was not measured in all our populations, this hypothesis therefore requires further investigation.
Acknowledgements
We are extremely grateful to all the families who took part in the ALSPAC study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council, the Wellcome Trust and the University of Bristol provide core support for ALSPAC. The British Women's Heart & Health Study is co-directed by Prof. Shah Ebrahim, Dr Debbie Lawlor, Prof. Peter Whincup and Dr Goya Wannamethee. We thank Carol Bedford, Alison Emerton, Nicola Frecknall, Karen Jones, Rita Patel, Mark Taylor and Katherine Wornell for collecting and entering data, all of the general practitioners and their staff who have supported data collection, and the women who have participated in the study. We also thank all participants of the Copenhagen City Heart Study for their continued support. This study was supported by The Danish Medical Research Council, The Danish Heart Foundation and Chief Physician Johan Boserup and Lise Boserup's Fund.
References
- Mojtabai R. Body mass index and serum folate in childbearing age women. European Journal of Epidemiology. 2004;19:1029–1036. doi: 10.1007/s10654-004-2253-z. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Watkins ML, Chiu V, Erickson D, Petitti DB. Do racial and ethnic differences in serum folate values exist after food fortification with folic acid? American Journal of Obstetrics and Gynecology. 2006;194:520–526. doi: 10.1016/j.ajog.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Narin F, Atabek ME, Karakukcu M, Narin N, Kurtoglu S, Gumus H, Coksevim B, Erez R. The association of plasma homocysteine levels with serum leptin and apolipoprotein B levels in childhood obesity. Annals of Saudi Medicine. 2005;25:209–214. doi: 10.5144/0256-4947.2005.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Velie EM, Schaffer D. Risk of neural tube defect-affected pregnancies among obese women. Journal of the American Medical Association. 1996;275:1093–1096. doi: 10.1001/jama.1996.03530380035028. [DOI] [PubMed] [Google Scholar]
- Werler MM, Louik C, Shapiro S, Mitchell AA. Prepregnant weight in relation to risk of neural tube defects. Journal of the American Medical Association. 1996;275:1089–1092. doi: 10.1001/jama.1996.03530380031027. [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council vitamin study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, Rozen R. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nature Genetics. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Lawlor DA, Davey Smith G, Araya R, Timpson N, Day INM, Ebrahim S. An association between the thermolabile variant of MTHFR and depression; new evidence from the British Women's Heart and Health Study plus a meta-analysis of existing data. Molecular Psychiatry. 2006;11:352–360. doi: 10.1038/sj.mp.4001790. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? International Journal of Epidemiology. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. International Journal of Epidemiology. 2004;33:30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- Rampersaud GC, Kauwell GP, Hutson AD, Cerda JJ, Bailey LB. Genomic DNA methylation decreases in response to moderate folate depletion in elderly women. American Journal of Clinical Nutrition. 2000;72:998–1003. doi: 10.1093/ajcn/72.4.998. [DOI] [PubMed] [Google Scholar]
- Jacob RA, Pianalto FS, Henning SM, Zhang JZ, Swendseid ME. In vivo methylation capacity is not impaired in healthy men during short-term dietary folate and methyl group restriction. Journal of Nutrition. 1995;125:1495–1502. doi: 10.1093/jn/125.6.1495. [DOI] [PubMed] [Google Scholar]
- Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. PNAS. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. Prader–Willi syndrome: current understanding of cause and diagnosis. American Journal of Medical Genetics. 1990;35:319–332. doi: 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikalinos TA, Ntzani EE, Contopoulos-Ioannidis DG, Ioannidis JP. Establishment of genetic associations for complex diseases is independent of early study findings. European Journal of Human Genetics. 2004;12:762–769. doi: 10.1038/sj.ejhg.5201227. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen J, Juul K, Grande P, Jensen GB, Schroeder TV, Tybjærg-Hansen A, Nordestgaard BG. Methylenetetrahydrofolate reductase polymorphism (C677T), hyperhomocysteinemia, and risk of ischemic cardiovascular disease and venous thromboembolism: prospective and case–control studies from the Copenhagen City Heart Study. Blood. 2004;104:3046–3051. doi: 10.1182/blood-2004-03-0897. [DOI] [PubMed] [Google Scholar]
- Guillén M, Corella D, Portolés O, González JI, Mulet F, Sáiz C. Prevalence of the methylenetetrahydrofolate reductase 677C>T mutation in the Mediterranean Spanish population. Association with cardiovascular risk factors. European Journal of Epidemiology. 2001;17:255–261. doi: 10.1023/a:1017978503416. [DOI] [PubMed] [Google Scholar]
- Russo GT, Di Benedetto A, Alessi E, Ientile R, Antico A, Nicocia G, La Scala R, Di Cesare E, Raimondo G, Cucinotta D. Mild hyperhomocysteinemia and the common C677T polymorphism of methylene tetrahydrofolate reductase gene are not associated with the metabolic syndrome in Type 2 diabetes. Journal of Endocrinological Investigation. 2006;29:201–207. doi: 10.1007/BF03345540. [DOI] [PubMed] [Google Scholar]
- Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, Olivieri O, Jacques PF, Rosenberg IH, Corrocher R, Selhub J. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. PNAS. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhao LJ, Liu YJ, Xiong DH, Recker RR, Deng HW. The MTHFR gene polymorphism is associated with lean body mass but not fat body mass. Human Genetics. 2008;123:189–196. doi: 10.1007/s00439-007-0463-7. [DOI] [PubMed] [Google Scholar]