Abstract
Previous work has shown that continuous estradiol replacement in young ovariectomized rats enhances acquisition of a delayed matching-to-position (DMP) T-maze task over that of ovariectomized controls. The mechanism by which estradiol confers this benefit has not been fully elucidated. This study examined the role of selective estrogen receptor agonists of ERα, ERβ, and GPR30 in the enhancement of spatial learning on a DMP task by comparing continuous estradiol replacement with continuous administration of PPT (an agonist of ERα), DPN (an agonist of ERβ), or G-1 (an agonist of GPR30) relative to gonadally intact and ovariectomized vehicle-treated controls. It was found that ovariectomy impaired acquisition on this task, whereas all ER selective agonists restored the rate of acquisition to that of gonadally intact controls. These data suggest that estradiol can work through any of several estrogen receptors to enhance the rate of acquisition on this task.
Keywords: GPR30, estradiol, G-1, PPT, DPN, T-maze, working memory
Introduction
Animal studies have shown that estradiol can have beneficial effects on a variety of cognitive tasks and can even prevent age-related cognitive decline (Daniel, 2006; Dohanich, 2002; Gibbs and Gabor, 2003). Our own studies have shown repeatedly that estradiol has significant positive effects on acquisition of a delayed matching-to-position (DMP) T-maze task in young and middle-aged rats (Gibbs, 2000; Gibbs, 2007; Gibbs, Gabor, Cox, and Johnson, 2004; Gibbs, Mauk, Nelson, and Johnson, 2009). The DMP task is a spatial learning task in which rats are rewarded for returning to the same arm of the maze visited on the immediately preceding trial. The mechanisms that underlie the effect on the DMP task as well as other tasks remain unclear, although in some cases specific neural circuits have been implicated (Daniel, Hulst, and Lee, 2005; Fernandez, Lewis, Pechenino, Harburger, Orr, Gresack, Schafe, and Frick, 2008; Gibbs, 2007; Packard and Teather, 1997).
Estradiol exerts its effects by binding to specific receptors. Two nuclear receptors have been identified, ERα and ERβ (Kuiper, Enmark, Pelto-Huikko, Nilsson, and Gustafsson, 1996; Toran-Allerand, 2004). These receptors are part of a large superfamily of nuclear receptors that regulate gene transcription, but which also can associate with specific membrane compartments and activate specific second messenger signaling pathways such as MAPK, CamKII, and CREB (Manavathi and Kumar, 2006; McEwen, 2002). Both receptors are expressed in the brain in a variety of isoforms, studied mainly at the mRNA level (Lewandowski, Kalita, and Kaczmarek, 2002; Pfeffer, 1996; Pfeffer, Fecarotta, Arena, Forlani, and Vidali, 1996). In the adult brain, ERα is highly expressed in areas of the hypothalamus that are responsible for reproduction (Ogawa, Eng, Taylor, Lubahn, Korach, and Pfaff, 1998), as well as in areas involved in learning, memory, emotionality, and attention such as the hippocampus and amygdala (Fugger, Foster, Gustafsson, and Rissman, 2000; Osterlund, Keller, and Hurd, 2000). ERβ is more widely distributed throughout the adult brain and, in addition to the hypothalamus, hippocampus, and amygdala, is expressed in many regions of the neocortex (Li, Schwartz, and Rissman, 1997; Osterlund, Kuiper, Gustafsson, and Hurd, 1998b; Shughrue, Lane, and Merchenthaler, 1997).
It is assumed that binding to specific estrogen receptors results in the activation of neural circuits, which in turn enhance performance on specific cognitive tasks. Evidence that selective activation of ERα and/or ERβ can affect specific tasks has been reported(Rissman, 2008). For example, Walf et al. (Walf, Rhodes, and Frye, 2006) reported that PPT (an ERα-selective agonist) as well as DPN (an ERβ-selective agonist) were effective at enhancing object recognition memory in rats. Frye et al. (Frye, Duffy, and Walf, 2007), reported that PPT, but not DPN, enhances performance on a placement recognition task in rats. In contrast, Rhodes and Frye (Rhodes and Frye, 2006) reported that while DPN was effective at enhancing both inhibitory avoidance memory and place memory in rats, PPT was not. Several studies have shown that activity at ERβ can reduce measures of anxiety (Bodo and Rissman, 2006; Day, Sung, Logue, Bowlby, and Arias, 2005; Walf and Frye, 2006), and some of the cognitive tasks that have been used (e.g., inhibitory avoidance, contextual fear conditioning, water maze tasks) include a significant acute anxiety or stress-related component, which may account for some of the estrogen-mediated effects described.
Fernandez et al. (Fernandez et al., 2008) recently reported that estradiol enhances object recognition memory in mice via a mechanism that requires activation of pERK in the dorsal hippocampus, and that the effect is mediated by membrane-bound receptors. Evidence that ERα and ERβ can mediate rapid signaling at the cell membrane has been described (Kelly and Levin, 2001), and it has been suggested that the effects of estradiol involve both membrane and intracellular ER actions, which can potentiate each other (Vasudevan and Pfaff, 2007). Recently, a novel G-protein coupled receptor was described which is capable of mediating rapid signaling events in response to estradiol in a number of cell lines (Prossnitz, Arterburn, Smith, Oprea, Sklar, and Hathaway, 2008; Thomas, Pang, Filardo, and Dong, 2005). This receptor, referred to as GPR30, is a member of the seven transmembrane G-protein coupled receptor family, and is both genetically and structurally unrelated to ERα or ERβ. In addition to mediating estrogen signaling in various cell lines, GPR30 is expressed in brain, including areas of the brain that play an important role in learning and memory processes such as the hippocampus and frontal cortex (Brailoiu, Dun, Brailoiu, Mizuo, Sklar, Oprea, Prossnitz, and Dun, 2007). Our recent analysis also suggests that GPR30 is expressed by the majority of cholinergic neurons in the medial septum and nucleus basalis magnocellularis (Hammond et al., unpublished observations). These neurons are the source of cholinergic inputs to the hippocampus and frontal cortex, and have been shown to play an important role in learning and memory processes (Baxter and Chiba, 1999; Everitt and Robbins, 1997). These neurons also play an important role in estrogen-mediated effects on DMP acquisition (Gibbs, 2002; Gibbs, 2007). Note that a subset of these cholinergic neurons also express ERα (Mufson, Cai, Jaffar, Chen, Stebbins, Sendera, and Kordower, 1999; Shughrue, Scrimo, and Merchenthaler, 2000), but do not appear to express ERβ (Shughrue et al., 2000). A selective GPR30 agonist (G-1) has been developed which binds with high affinity to GPR30, activates GPR30-mediated signaling in specific cell lines, and has no appreciable binding to ERα or ERβ (Bologa, Revankar, Young, Edwards, Arterburn, Kiselyov, Parker, Tkachenko, Savchuck, Sklar, Oprea, and Prossnitz, 2006). Effects of this agonist on cognitive performance have not yet been reported. In the present study, our goal was to use the GPR30-selective agonist G-1, as well as agonists that are selective for ERα and ERβ, to identify which estrogen receptors are involved in mediating estrogen effects on DMP acquisition.
Materials and Methods
Animals
A total of sixty-eight 3–4 month old female Sprague-Dawley rats (300–350 g) were purchased from Hilltop Laboratories. Rats were ovariectomized (with the exception of the gonadally intact group) prior to delivery and individually housed on a 12-hour day/night cycle with food and water available ad libitum. All procedures were carried out in accordance with PHS policies on the use of animals in research, and with the approval of the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Treatments
Two weeks prior to behavioral training, animals were handled daily, food restricted, and maintained at 85% of normal body weight during acquisition and testing. Rats were administered estradiol (Sigma Chemicals; St Louis, MO), G-1 (Calbiochem; La Jolla, CA), DPN (Tocris Cookson; Ellisville, MO), PPT (Tocris Cookson; Ellisville, MO), or vehicle by Alzet model 2006 mini-osmotic pumps implanted s.c. in the dorsal neck region. Gonadally intact rats received sham surgeries. The pumps delivered a volume of 0.15 µl per hour over 42 days. G-1 has been shown to be an agonist specific to GPR30 – competitive binding studies showed that G-1 did not compete with estradiol binding in COS7 cells transfected with either ERα or ERβ, whereas as it did compete with binding in COS7 cells transfected with GPR30 (Bologa et al., 2006). The ERα agonist used was propylpyrazole triol (PPT), a triarylpyrazole found to be approximately 410-fold more selective for ERα than ERβ (Kraichely, Sun, Katzenellenbogen, and Katzenellenbogen, 2000; Stauffer, Coletta, Tedesco, Nishiguchi, Carlson, Sun, Katzenellenbogen, and Katzenellenbogen, 2000). The ERβ agonist used was diarylpropiolnitrile (DPN), a compound with 70-fold higher relative binding affinity and 170-fold higher relative potency for ERβ over ERα (Meyers, Sun, Carlson, Marriner, Katzenellenbogen, and Katzenellenbogen, 2001).
Estradiol, DPN, and PPT treatments were administered at a rate of 5 µg/day at concentrations of 1.39 mg/ml in vehicles consisting of 13.9% DMSO + 20% hydroxypropyl-β-cyclodextrin (HPβCD). G-1 treatments were also administered at a rate of 5 µg/day at a concentration of 1.39 mg/ml in a vehicle 33.7% DMSO+ 13.3% HPβCD. Previous literature was used to select doses of estradiol, PPT, and DPN (Gibbs et al., 2004; Walf, Rhodes, and Frye, 2004), but because no previous research has been published using G-1, the dose was chosen to be similar based on molecular weight. Vehicle controls contained either one of the vehicles listed above minus any drug treatment.
Behavior
The DMP task is a spatial learning and memory T-maze task. The T-maze consists of an approach alley (4 in. wide × 14 in. long) and two goal arms (4 in. wide × 12 in. long). The walls of the maze are 5 in. high, and the doors are constructed of clear plexiglass, thus allowing animals to view the surrounding room. Sliding doors are positioned 8 in. down the approach alley and at the entrance to each goal arm.
Behavior training was performed as previously described (Gibbs, 1999). Animals were first adapted to the maze by placing them in the maze with food (formula 5TUM 45 mg pellets from Test Diets, Inc.) for 5 days. Starting on day 6 through 9 animals were trained to run to the ends of the goal arms by using a series of forced choices and rewarding with four pellets. Right and left arms were alternated to avoid introducing a side bias. Next, animals began DMP testing, which was performed as 8 trial pairs per day. The first trial of each pair consisted of a forced choice in which one arm was blocked, forcing the animal to enter the unblocked arm to receive the food reward. The animal was then returned to the approach alley for the second trial in which both goal arms were open. A choice was defined as the animal placing both front legs and part of both rear legs into a goal arm. Returning to the same arm as the forced choice trial resulted in a food reward, while entering the incorrect arm resulted in no food reward and confinement for 10 seconds. Forced choices were randomized and balanced to avoid introducing a side bias. Animals were run in squads of 4 to 6. After each trial pair, the animal was returned to its home cage for 5–10 min while other animals were tested. Animals received 8 trial pairs per day until they reached the criterion of at least 15/16 correct choices over two days.
After reaching criterion, animals received a probe trial during which the T-maze was rotated 180° (relative to extramaze cues) between the forced and open trial. This was done to assess whether rats were using a place strategy (relying on extramaze cues) or a response strategy (independent of extramaze cues) to perform the task. Rats using a place strategy would be impaired by maze rotation, whereas rats using a response strategy would be unaffected. For analysis purposes, rats were given a score of 1 if they entered the same goal arm during the open choice and a score of 0 if they entered the opposite arm. After the probe trial, animals received 8 trial pairs per day for 4 days with increased intertrial delays (10, 30, 60, 90 seconds on each of the 4 consecutive days).
Following training, animals were given an overdose of ketamine (40 mg/kg) and xylazine (28 mg/kg) injected i.p. and euthanized by decapitation. Trunk blood was collected for the determination of serum estradiol and luteinizing hormone (LH) levels. For estradiol analysis, samples were extracted with ether and resuspended in buffer. Samples were then analyzed in duplicate by RIA using a kit from Diagnostic Systems Laboratories, Inc. (Webster, TX). All samples were analyzed in one assay. The estradiol assay was performed by the assay core of the Center for Reproductive Physiology at the University of Pittsburgh. The minimum detectable dose was 1.2 pg/ml, and the intra-assay CV was 5.8%. Samples also were analyzed for circulating levels of LH. Samples were assayed in singlet using a sensitive sandwich immunoradiometric assay by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. This assay had a reportable range of 0.07 – 37.4 ng/ml with a sensitivity of 0.07 ng/ml. The inter- and intra-assay CVs were 10% and 5%, respectively.
Data Analysis
Rate of acquisition was defined as the number of days to reach criterion (DTC). DTC was analyzed for each treatment group by one-way ANOVA. Learning curves were constructed by plotting the mean performance (percent correct) for each group across Blocks of training. Each Block represented average performance across three consecutive days of training. Upon reaching criterion, a value of 93.8% correct, reflecting the criterion of 15/16 correct choices over 2 days, was used in calculating group performance on subsequent days. The learning curves were compared using a two factor (Treatment×Block) ANOVA with repeated measures on Block. Performance on the probe trial was analyzed by contingency table and Chi-square. Performance during the increased intertrial delays was analyzed by ANOVA with repeated measures on Delay. Post-hoc comparisons were made using a Tukey test. Significance was set at p ≤ 0.05. All statistical analysis was performed using JMP software for Macintosh.
Results
Serum estradiol levels
The mean levels of estradiol in the estradiol treatment group were 116.0 ± 39.6 pg/ml, with a range of 65.0–166.1 pg/ml. Mean estradiol levels in the gonadally intact vehicle group were 15.0 ± 7.0 pg/ml, with a range of 4.7–23.1 pg/ml. Levels of estradiol in non-estradiol treatment groups were undetectable.
Serum LH levels
The mean serum LH levels were 0.96 ± 0.85, 0.25 ± 1.02, 8.45 ± 0.88, 10.16 ± 0.99, 9.08 ± 1.04, and 10.22 ± 0.95 ng/ml for the intact, estradiol, vehicle, G-1, DPN, and PPT treatment groups, respectively. ANOVA revealed a significant effect of Treatment (F(5,61)=14.9, p < 0.01), and post-hoc analysis revealed vehicle, G-1, DPN, and PPT treatment groups differed significantly from intact and estradiol groups.
DMP acquisition
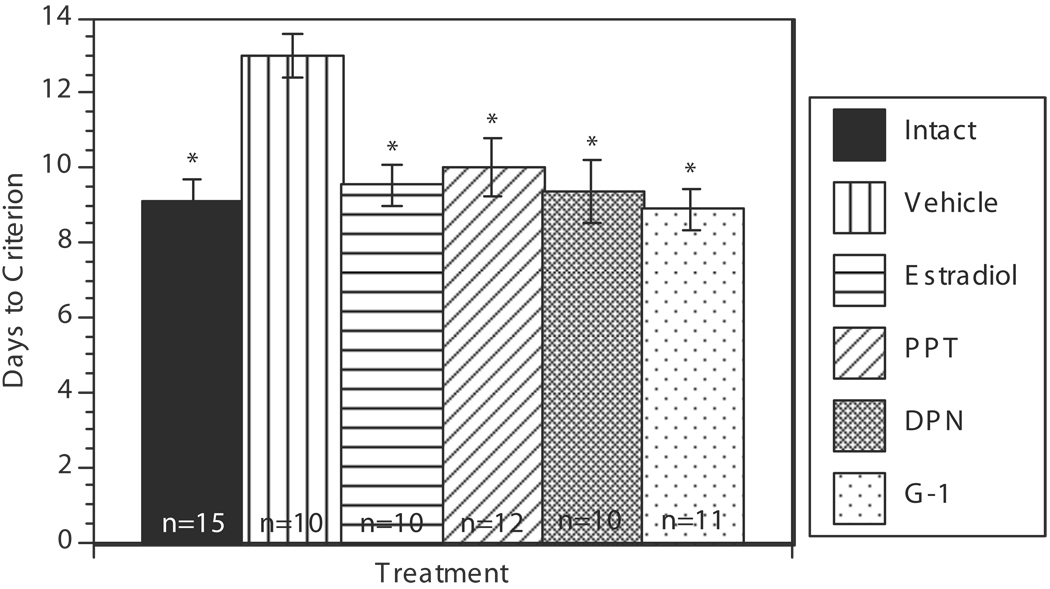
Results show that ovariectomized (OVX) animals took more days to reach criterion than gonadally intact animals, and that estradiol and all three selective estrogen receptor agonists were able to restore the rate of acquisition to that of gonadally intact controls (Fig. 1). On average, OVX controls took 13±0.59 days to learn the task. All other groups took an average of approximately 9 to 10 days to reach criterion on the task. ANOVA revealed a significant effect of Treatment (F(5,62)=6.60, p < 0.01). Post-hoc analysis revealed that estradiol treatment and all selective estrogen receptor agonist treatments differed significantly from OVX controls. Estradiol and selective estrogen receptor agonist treatments did not significantly differ from gonadally intact controls. There was no significant correlation between DTC and circulating estradiol levels in the E-treated or intact groups. Likewise there was no significant correlation between DTC and serum LH levels in any of the treatment groups.
FIG 1.
Bar graph summarizing Days to Criterion (DTC) for each treatment group. Bars indicate mean number of days ± s.e.m. *p < 0.05 relative to OVX vehicle.
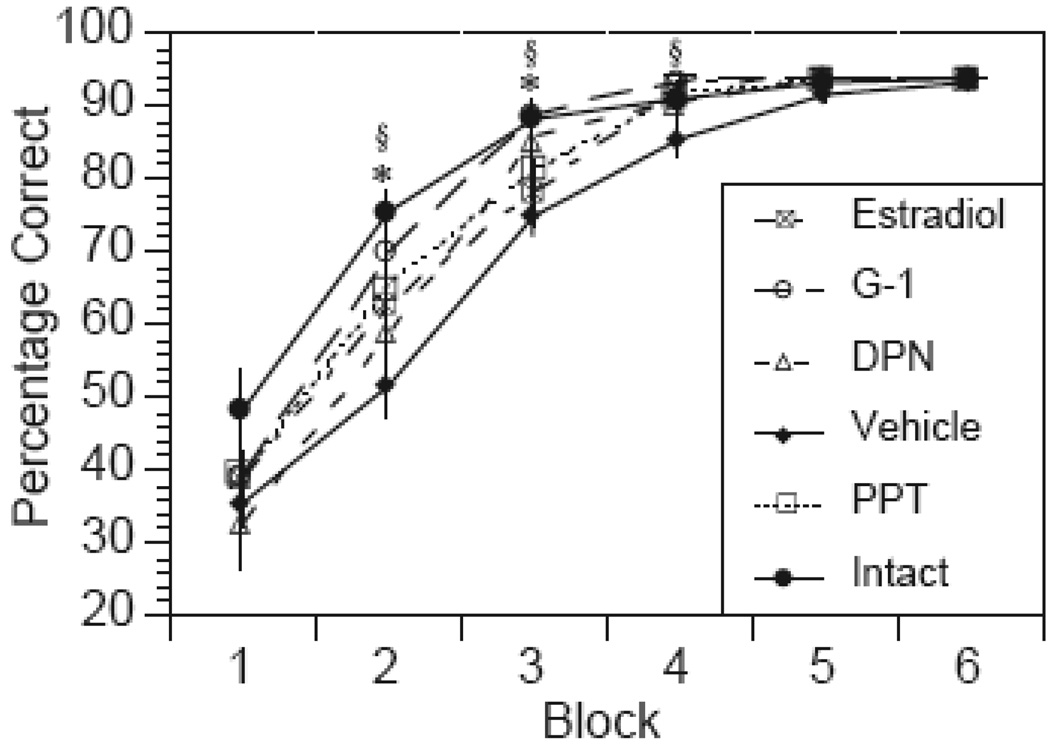
Examination of the learning curves revealed that all groups performed similarly below chance at the start of training (no effect of treatment on Block 1), and that rats receiving estradiol or agonist treatments acquired the task at a faster rate than the vehicle group (Fig. 2). ANOVA revealed a significant effect of Treatment (F(5,62)= 5.74, p < 0.01), a significant effect of Block (F(3,60)= 94.4, p < 0.01), and a significant Treatment×Block interaction (F15,166)=1.88, p<0.028). Post-hoc analysis of the main effects of treatments collapsed across Blocks 2–5 revealed significant differences between OVX controls and intact (p<0.01), estradiol (p<0.0041), G-1 (p<0.01), PPT (p<0.0025), and DPN (p<0.01) treatments. Significance was adjusted to p ≤ 0.01 to account for multiple comparisons. One way ANOVA for each block followed by a post-hoc Tukey test showed that the intact group was significantly different from OVX controls at Blocks 2–3, and that the G-1 treatment group was significantly different from OVX controls at Blocks 2–4.
FIG 2.
Learning curves showing acquisition of the DMP task over time. Values represent the mean percent correct ± s.e.m. within a 3-day block of training for each treatment group. *p < 0.05 for intact group relative to OVX vehicle. §p < 0.05 for G-1 treatment relative to OVX vehicle.
Previous studies show that some rats will adopt a persistent turn early on during testing and that this can affect DTC (Gibbs, 2007; Gibbs and Johnson, 2007). To quantify this, we examined whether rats adopted a persistent turn, defined as entering the same arm at least 15/16 times during the choice trial over two days. Results show that a minority (26%) of rats adopted a persistent turn (consistently entering either arm of the maze). Treatments had no affect on the percentage of rats that adopted a persistent turn (X2 (5)=2.9, p=0.71). Among rats that did adopt a persistent turn, treatments did not affect the number of days that this pattern persisted (F(5,17)=1.3, p=0.32).
Post-criterion Testing
After reaching criterion, rotating the maze 180° between the forced and open choices significantly disrupted performance within most groups. Performance dropped from 93.8% (criterion level) to 60%, 44.4%, 72.7%, 72.7%, 83.3% and 53.3% for intact, estradiol, G-1, DPN, PPT, and vehicle-treated groups respectively as a result of rotating the maze 180° between the forced and open trials. Analysis showed that none of these values differed significantly from chance with the exception of the PPT-treated group, which scored significantly above chance (X2=9.7, p<0.02). Nevertheless, no significant overall effect of Treatment on performance during the probe trial was detected (X2=5.9, p > 0.4).
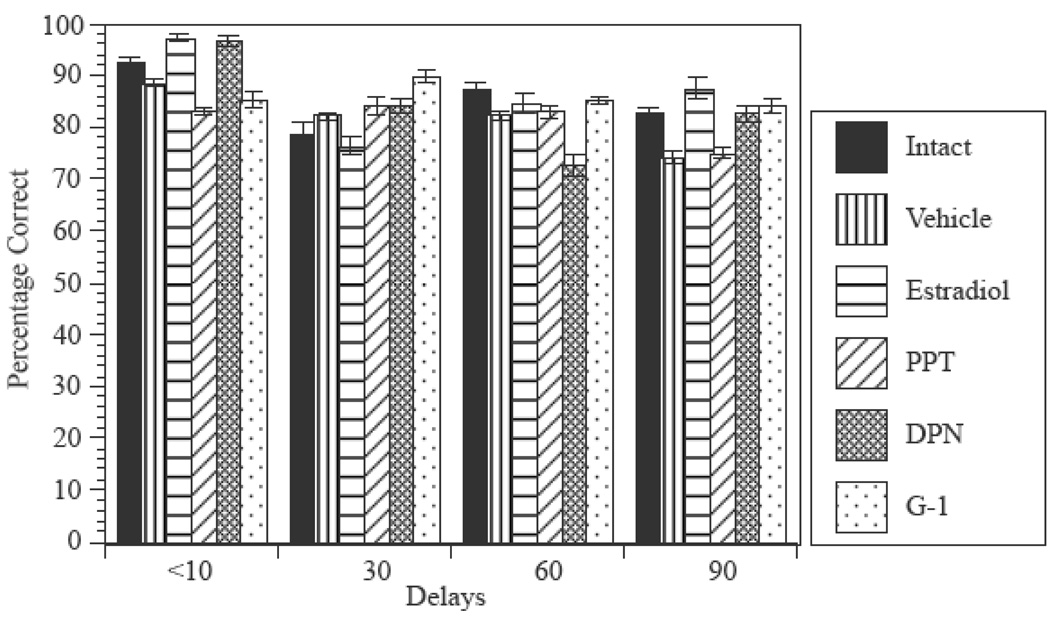
Once rats had reached criterion, increasing the intertrial delay impaired performance for all treatment groups (Fig. 3). ANOVA revealed a significant effect of Delay (F(3,60)=8.9, p < 0.01), no significant effect of Treatment (F(5,62)=0.7, p > 0.6), and no significant interaction between Treatment and Delay (F(15,166)=1.15, p > 0.3).
FIG 3.
Effects of increasing the delay between the forced and open trial to 30, 60, and 90 seconds on performance. Bars represent the percentage of correct choices ± s.e.m. for each group.
Discussion
Effects of Estradiol and Estrogen Receptor Agonists on DMP Acquisition
Our results confirm that ovariectomy slowed the rate of DMP acquisition in young adult rats. PPT, DPN, G-1, and estradiol each were able to restore the rate of DMP acquisition to that of gonadally intact controls. This is consistent with previous studies showing that estradiol enhances DMP acquisition in young and middle-aged rats (Gibbs, 1999; Gibbs, 2007; Gibbs et al., 2004; Gibbs et al., 2009), and suggests that activity at any of the three established estrogen receptors might underlie this effect. Moreover, similar performance was observed in intact cycling rats and OVX rats with sustained estradiol replacement. Although we did not monitor cycles, we assume that the intact rats were undergoing estrous cycles. The fact that estradiol levels were low in some of these rats suggests that they were killed on diestrus, whereas others were killed at other phases of the cycle. This suggests that sustained elevated estradiol levels are not required to enhance acquisition of the DMP task. Controversy exists in regards to whether cyclical or sustained estradiol treatment is more effective in enhancing cognitive performance (Gresack and Frick, 2006; Iivonen, Heikkinen, Puolivali, Helisalmi, Hiltunen, Soininen, and Tanila, 2006; Rapp, Morrison, and Roberts, 2003). We also note that the effect of estradiol on the learning curve, though significant, was not as robust as in some previous reports, possibly due to the relatively high levels of estradiol achieved in the estradiol treatment group. Some studies have reported that high doses of estradiol can impair spatial working memory in rats (Wide, Hanratty, Ting, and Galea, 2004).
Recent studies suggest that elevated levels of LH that occur in response to ovariectomy may contribute to the cognitive impairment associated with ovariectomy, aging, and neurodegenerative diseases (Casadesus, Milliken, Webber, Bowen, Lei, Rao, Perry, Keri, and Smith, 2007; Webber, Stocco, Casadesus, Bowen, Atwood, Previll, Harris, Zhu, Perry, and Smith, 2006). It has been shown that intraperitoneal or intracerebroventricular administration of human chorionic gonadotropin produces deficits on T-maze tasks in rats (Lukacs, Hiatt, Lei, and Rao, 1995), and that increased LH levels in mice are associated with declines in cognitive performance on a Y-maze task (Casadesus et al., 2007). In the present study, we detected no correlation between LH levels and the number of days required to reach criterion on the task in any of the treatment groups. In addition, G-1, DPN, and PPT all significantly enhanced the rate of DMP acquisition despite the fact that LH levels were very high relative to intact and E-treated groups. This suggests that the effects of ovariectomy and estrogen treatments on the rate of DMP acquisition are not due to effects of LH.
Treatment Effects on Learning Strategy and Working Memory
Rats can use various strategies to solve T-maze tasks, such as place strategies, which use extramaze visual cues, and response strategies, which use body positioning and kinetic cues (Dudchenko, 2001; Korol, Malin, Borden, Busby, and Couper-Leo, 2004). Rats also have been shown to change strategy with repeated testing or in response to estradiol treatment (Korol and Kolo, 2002; Packard and McGaugh, 1996). Evidence suggests place learning is mediated by hippocampal circuits (Zurkovsky, Brown, and Korol, 2006) while response learning is mediated by extrapyramidal circuits, such as through the caudate (Packard and McGaugh, 1996; Squire, 1998). Moreover, place learning is acquired faster than response learning (Chang and Gold, 2003; Packard and McGaugh, 1996). Results of the probe trial suggest that upon reaching criterion most treatment groups used extramaze cues to a significant degree while performing the DMP task. PPT-treated rats were slightly less affected by rotating the maze than the other groups, which may indicate a slightly greater predisposition to use a response vs. a place-based strategy; however, this difference was not statistically significant. In addition, treatments had no effect on the predisposition to adopt a persistent turn. This suggests that the effects of the selective estrogen receptor agonists on DMP acquisition were not due to differential effects on strategy. The hippocampus also plays an important role in spatial working memory. Results of the delay trials indicate that the effects of the selective estrogen receptor agonists on DMP acquisition cannot be accounted for by effects on spatial working memory. These findings are consistent with a recent analysis of the effects of estradiol on DMP acquisition in young rats (Gibbs, 2007).
Comparison with Previous Studies
The present study showed that chronic estradiol treatment throughout training and testing enhanced acquisition of the DMP task. Others have used a post-training regimen and have shown that estradiol can be administered in the period immediately after training to enhance performance. For example, ovariectomized rats that were trained in a hidden platform water maze and given estradiol injections less than two hours post training performed better than vehicle controls when returned to the maze 24 hours later for a retention test. This enhancement in retention did not occur when estradiol was given later than two hours post training (Packard, 1998). In another case, ovariectomized rats were trained in an inhibitory avoidance task and tested following a 24 hour delay, and it was shown that estradiol administration immediately, but not 1, 2, or 3 hours post training, increased crossover latencies compared to vehicle (Rhodes and Frye, 2004). Taken together, these suggest that estradiol may affect both acquisition and memory consolidation.
In addition to spatial learning and memory, others have examined the effects of DPN and PPT on stress and anxiety. Daily treatment with DPN has been shown to decrease anxiety-related behaviors on tasks such as the elevated plus maze or open field, suggesting that ERβ mediates select anxiolytic effects of estradiol (Lund, Rovis, Chung, and Handa, 2005; Walf and Frye, 2005). In contrast, daily injections of PPT have been reported to increase anxiogenic behaviors such as the number of fecal boli and time spent grooming (Lund et al., 2005). Thus, it is important when analyzing effects of PPT and DPN on learning tasks to consider the degree to which stress and anxiety play a role in each task. Though we did not measure corticosterone levels, to our knowledge stress and anxiety are not major components of the DMP task, analogous to other food-motivated land-based navigational tasks. While rats do experience the stress of food deprivation, the task does not include an acute stressor such as foot shock or swimming, which are major components of standard inhibitory avoidance and water maze tasks.
The parallel effect of the three agonists in facilitating acquisition raises the possibility that estradiol and the three agonists may affect non-mnemonic processes such as sensory-motor functions, motivational factors, or attentional mechanisms. Previous work from our lab has shown that ovariectomy and estradiol treatments that affect DMP acquisition have no effect on a configural association operant conditioning task (Gibbs and Gabor, 2003). This task requires rats to distinguish between visual and auditory stimuli and is motivated by the same food reward associated with the DMP task. The fact that ovariectomy and estradiol treatments have no effect on this task reduces the likelihood that effects on motivation and sensory perception underlie the effects on DMP acquisition.
What circuits underlie the effects of the selective estrogen receptor agonists on DMP acquisition is unknown. As mentioned earlier, ERα immunoreactivity and mRNA have been found in the hippocampus, and ERβ immunoreactivity and mRNA have been found in the hippocampus and neocortex (Mitra, Hoskin, Yudkovitz, Pear, Wilkinson, Hayashi, Pfaff, Ogawa, Rohrer, Schaeffer, McEwen, and Alves, 2003; Osterlund, Kuiper, Gustafsson, and Hurd, 1998a). This raises the possibility that DPN and PPT may act directly at one of these two regions to affect DMP acquisition.
To our knowledge, this is the first study to show an effect of G-1 administration on a cognitive task. Our results show that G-1 had as much or more of a beneficial effect on DMP acquisition as any of the other agonists, suggesting that activation of GPR30 is a viable strategy for enhancing performance within specific cognitive domains. G-1 is still in the early stages of development; however, like other selective estrogen receptor agonists, it may offer numerous advantages over standard estrogen therapy, most notably with respect to cancer risks and stroke. Also, unlike ERα and ERβ, GPR30 is a membrane-bound receptor which can mediate rapid estrogen signaling via activation of adenylate cyclase and the liberation of membrane-bound EGF (Filardo, Quinn, Frackelton, and Bland, 2002). Where in the brain G-1 acts to affect DMP acquisition is not known. Further studies that evaluate the functional consequences of GPR30 expression and activation within specific regions of the brain are needed to fully elucidate the potential of GPR30 agonists to enhance neuronal function and cognitive performance.
Acknowledgments
This work was supported by NIH grant R01 AG021471. Work by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCRIR) Grant U54-HD28934.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9(2):178–183. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27(2):217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2(4):207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193(2):311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269(1–2):107–111. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23(7):3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18(10):787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience. 2005;132(1):57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Day M, Sung A, Logue S, Bowlby M, Arias R. Beta estrogen receptor knockout (BERKO) mice present attenuated hippocampal CA1 long-term potentiation and related memory deficits in contextual fear conditioning. Behav Brain Res. 2005;164(1):128–131. doi: 10.1016/j.bbr.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Dohanich GP. Gonadal steroids, learning, and memory. Vol. 2. San Diego: Academic Press; 2002. [Google Scholar]
- Dudchenko PA. How do animals actually solve the T maze? Behav Neurosci. 2001;115(4):850–860. [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu. Rev. Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28(35):8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16(1):70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88(2):208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883(2):258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36(3):222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol. of Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42(3):245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52(3):352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74(5):637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Cholinergic lesions produce task-selective effects on delayed matching to position and configural association learning related to response pattern and strategy. Neurobiol Learn Mem. 2007;88(1):19–32. doi: 10.1016/j.nlm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Mauk R, Nelson D, Johnson DA. Donepezil Treatment Restores the Ability of Estradiol to Enhance Cognitive Performance in Aged Rats: Evidence for the Cholinergic Basis of the Critical Period Hypothesis. Hormones & Behavior. 2009 doi: 10.1016/j.yhbeh.2009.03.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res. 2006;1115(1):135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Iivonen S, Heikkinen T, Puolivali J, Helisalmi S, Hiltunen M, Soininen H, Tanila H. Effects of estradiol on spatial learning, hippocampal cytochrome P450 19, and estrogen alpha and beta mRNA levels in ovariectomized female mice. Neuroscience. 2006;137(4):1143–1152. doi: 10.1016/j.neuroscience.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12(4):152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116(3):411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45(5):330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kraichely DM, Sun J, Katzenellenbogen JA, Katzenellenbogen BS. Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-alpha and estrogen receptor-beta: correlations with biological character and distinct differences among SRC coactivator family members. Endocrinology. 2000;141(10):3534–3545. doi: 10.1210/endo.141.10.7698. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Nat. Acad. Sci., USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski S, Kalita K, Kaczmarek L. Estrogen receptor beta. Potential functional significance of a variety of mRNA isoforms. FEBS Lett. 2002;524(1–3):1–5. doi: 10.1016/s0014-5793(02)03015-6. [DOI] [PubMed] [Google Scholar]
- Li X, Schwartz PE, Rissman EF. Distribution of estrogen receptor-beta-like immunoreactivity in rat forebrain. Neuroendocrinology. 1997;66(2):63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- Lukacs H, Hiatt ES, Lei ZM, Rao CV. Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm Behav. 1995;29(1):42–58. doi: 10.1006/hbeh.1995.1004. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146(2):797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J Cell Physiol. 2006;207(3):594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44(24):4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144(5):2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Cai WJ, Jaffar S, Chen EY, Stebbins G, Sendera T, Kordower JH. Estrogen receptor immunoreactivity within subregions of the rat forebrain: neuronal distribution and association with perikarya containing choline acetyltransferase. Brain Research. 1999;849(1–2):253–274. doi: 10.1016/s0006-8993(99)01960-5. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139(12):5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998a;54(1):175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res. Mol. Brain Res. 1998b;54(1):175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95(2):333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34(2):126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. NeuroReport. 1997;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Pfeffer U. Estrogen receptor mRNA variants. Do they have a physiological role? Ann N Y Acad Sci. 1996;784:304–313. doi: 10.1111/j.1749-6632.1996.tb16245.x. [DOI] [PubMed] [Google Scholar]
- Pfeffer U, Fecarotta E, Arena G, Forlani A, Vidali G. Alternative splicing of the estrogen receptor primary transcript normally occurs in estrogen receptor positive tissues and cell lines. J Steroid Biochem Mol Biol. 1996;56(1–6 Spec No):99–105. doi: 10.1016/0960-0760(95)00227-8. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23(13):5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has mnemonic-enhancing effects in the inhibitory avoidance task. Pharmacol Biochem Behav. 2004;78(3):551–558. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85(2):183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J Neuroendocrinol. 2008;20(6):873–879. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERa and ERβ) in the cholinergic neurons of the rat basal forebrain. Neurosci. 2000;96(1):41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems. C R Acad Sci III. 1998;321(2–3):153–156. doi: 10.1016/s0764-4469(97)89814-9. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43(26):4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145(3):1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28(1):1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30(9):1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31(6):1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78(3):523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86(1):35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber KM, Stocco DM, Casadesus G, Bowen RL, Atwood CS, Previll LA, Harris PL, Zhu X, Perry G, Smith MA. Steroidogenic acute regulatory protein (StAR): evidence of gonadotropin-induced steroidogenesis in Alzheimer disease. Mol Neurodegener. 2006;1:14. doi: 10.1186/1750-1326-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res. 2004;155(1):45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol Learn Mem. 2006;86(3):336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]