Abstract
The nature of H(H2O)n+ cations for n = 3 – 8 with weakly basic carborane counterions has been studied by IR spectroscopy in benzene and dichloroethane solution. Contrary to general expectation, neither Eigen-type H3O·3H2O+ nor Zundel-type H5O2+·4H2O ions are present. Rather, the core species is the H7O3+ ion.
Keywords: Proton hydrates, solvation, strong H-bonding, carborane acid, IR spectroscopy
Introduction
The current state of knowledge of the aquated proton H(aq)+ is in a curious position. In water, the “excess proton” is widely believed to exist in two limiting forms, the Eigen-type H3O+ ion1 and the Zundel-type H5O2+ ion,2 coexisting in a dynamic equilibrium. These ions are well characterized in crystalline salts,3,4 organic solvents5–7 and the gas phase8–10 but the extent to which they represent H(aq)+ in water has not been determined experimentally. The belief that H(aq)+ is an approx. 60:40 ratio of hydrated Eigen:Zundel ions is based on theory.11,12
The problem of developing an accurate molecular description of H+ in water lies in the difficulty of accurately separating its spectroscopic signature(s) from those of the background water, and in interpreting the resulting spectra. Infrared is the spectroscopy of choice because of its sensitivity and fast timescale but the IR spectrum of H(aq)+ is notoriously broad. A poorly understood continuous broad absorption (cba) associated with the vibrations of the excess proton extends over the much of the useful IR range. These difficulties have shifted attention to the gas phase. The underlying assumption is that an accurate molecular description of each ion-selected hydrate H(H2O)n+ via step-by-step increasing n will ultimately lead to an understanding of H(aq)+ in bulk water. Indeed, experiment and theory in vacuo come together very nicely to establish that the trihydrated Eigen ion (H9O4+ i.e. H3O+·3H2O) and the tetra-hydrated Zundel ion (H13O6+ i.e. H5O2+·4H2O) have extra stability in the gas phase.13,14 Other “magic number” clusters such as H(H2O)21+ have distinctive structural characteristics and heightened stability compared to their near n neighbors.15–18
While gas phase studies have obvious and direct importance in atmospheric chemistry there are reasons to doubt such a close relationship will hold in condensed phases – where so much acid chemistry is carried out. Gas phase ion chemistry (and theory) is carried out in the absence of counterions whereas all condensed phase chemistry must deal with the necessity of a conjugate base. Even strong acids that fully ionize in a solvent are subject to ion pairing. The uni-directional electric field created by the proximity of an anion will affect the structures of the H(H2O)n+ cations. Structural isomers of H(H2O)n+ for n = 5–8 values are calculated in vacuo to be very close in energy10,19–22 so their structures should be quite sensitive to environmental influences in condensed media.
In the solid state, X-ray crystal structures of hydrated acids were studied quite extensively in the late 1970s.23 In favorable circumstances they can be expected to reflect the structures of ions in other phases. For example, if an H(H2O)n+ ion is surrounded in a crystal by a spherically symmetric anion field, it might be expected to reflect the gas phase structure of the cation. In anion fields of lower symmetry, an H(H2O)n+ ion might be expected to reflect ion-paired structures of hydrated acids in solution.
Given these considerations, as well as the importance of the hydrated proton in acid catalysis, fuel cell electrolytes and acid/base chemistry in general, we are focusing on the nature of the hydrated proton in organic solvents, where studies are few. Just as in the gas phase, water molecules can be controlled and sequentially attached to the proton forming H(H2O)n+ clusters. The ease of formation, structure and composition of these cations depends strongly on the basicity of the solvent and the basicity of the anion, i.e. the strength of the conjugate acid. In weakly basic solvents like CH2Cl2, acids such as HCl are hydrated mainly without proton transfer to H2O molecules.24 Simple H-bonded solvates of the type HA·(H2O)n prevail rather than ionized H(H2O)n+ clusters. With solvents of higher basicity such as tributylphosphate (TBP), whose basicity is close to that of water, proton transfer to H2O becomes possible but is accompanied by waterless TBP·HA monosolvates.25 Stronger acids such as perchloric and triflic acid certainly protonate water in weakly basic solvents but the solubilities of the ionized species are low and often insufficient to study their full hydration by IR. Thus, only the mono- and dihydrates of triflic acid, H3O+OTf− and H5O2+OTf−, have been studied in dichloroethane.26 With increasing solvent basicity, the solubilities of strong acids increase. In TBP for example, HClO4 and HFeCl4 form a set of H(H2O)n+ clusters with step-by-step increasing n up to aggregates with high n that are like reverse nanomicelles.27,28 The protons are located in the water core of these micelles near the boundary with the TBP solvation shell in the form of tetrasolvated H5O2+ ions, [H5O2+·2H2O·2TBP]. The counterions are weakly ion paired to the water micelle in the case of ClO4− but remote and non-interacting in the case of the more weakly basic FeCl4− anion. These observations highlight the need for a strong acid whose H(H2O)n+ salts are soluble in low basicity solvents and whose anion will have minimal influence on the structures of the H(H2O)n+ cations.
Suitable acids have recently become available in carborane superacids, H(CHB11R5X6) (R = H, Cl, Br, I; X = Cl, Br, I; see Figure 1).29 They are presently the strongest known pure acids30,31 and their conjugate base anions impart good solubility to salts in low basicity solvents. Their large, non-polarizable anions are expected to have the weakest influence on the structure of the cations, possibly leading to H(H2O)n+ cations that reflect those in the gas phase. We have previously used carborane acids to establish the nature of the H3O+ and H5O2+ ions under controlled conditions of minimal water content.5,7 We now apply these methods to the more normal laboratory conditions of wet organic solvents where higher hydrates prevail. Benzene and dichloroethane are chosen because they are common solvents for synthetic chemistry.
Figure 1.
The conjugate base carborane anions of the type CHB11X5Hal6− used in this work (X/Hal = H/Br, Cl/Cl, I/I).
Experimental Section
Carborane acids H(CHB11H5Br6), H(CHB11Cl11) and H(CHB11I11) (abbrev. H{H5Br6}, H{Cl11} and H{I11}) and their arenium ion salts were prepared as previously described.30 Chlorinated cobalt(III) dicarbollide, Co(C2B9H8Cl3)2−, (abbrev. {CCD−}) (90% in H-form and 10% in Na-form) with an analysis of 9.35% Co, and 29.05% Cl was received from KatChem (Czech Republic) and converted into 100% H-form H{CCD} by shaking a dichloroethane (DCE) solution with 3M H2SO4 aqueous solution for 5 min and retaining the DCE layer. Benzene and DCE were purified and dried according to literature methods.32 H+(H2O)n{H5Br6} solutions in DCE were prepared by mixing calculated volumes of dry DCE, water-saturated DCE containing 0.1M H2O, and 0.025M C6Me6H+{H5Br6−} or C6H7+{H5Br6−} solutions in DCE to obtain solutions with constant acid concentration CHCarb, and defined water concentration C0H2O. For solutions with H2O/acid molar ratio equal to 2–3, the CHCarb was 0.015M. For H2O/acid > 3, CHCarb was 0.01M. Solutions with the largest fixed H2O/acid molar ratios were obtained by dissolving weighed portions of C6H7+{H5Br6−} in DCE containing 0.1M H2O. Benzene solutions of H+(H2O)n{H5Br6−} and H+(H2O)n{Cl11−} were prepared in a similar manner to the DCE solutions, using water-saturated benzene (0.2M) and weighed quantities of mesitylenium or hexamethylbenzenium salts dissolved in water-saturated benzene. The final benzene solutions contained 0.004M H+(H2O)n{H5Br6} or 0.005M H+(H2O)n{Cl11}. The spectrum of free hexamethylbenzene or mesitylene (formed in solution) was digitally subtracted. Water-saturated H+(H2O)nCarb− solutions with Carb− = {H5Br6−}, {Cl11−}, {I11−} and {CCD−} were obtained by extraction of the acids from aqueous solutions. The concentrations of all acids were measured by the intensity of the anion absorption bands in IR spectra.
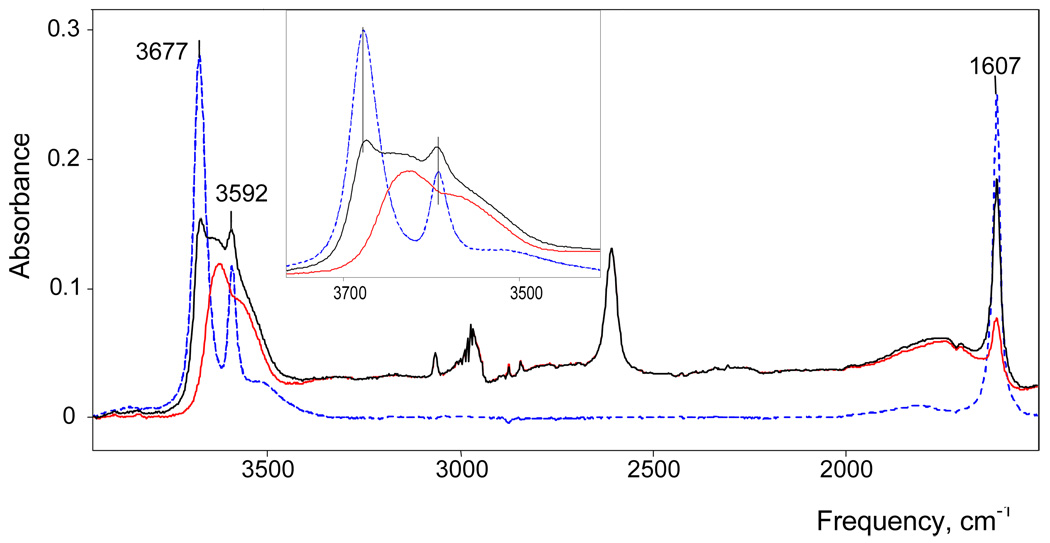
The IR spectra of these solutions consist of overlapping spectra of the following components: (i) solvent, (ii) water dissolved in the solvent, and (iii) hydrated acid, H(H2O)n+Carb−. To isolate the spectrum of a particular H+(H2O)nCarb− species, the spectrum of the solvent and dissolved water were successively subtracted from the initial spectrum. Figure 2 shows a typical example of this procedure. Subtraction of the dissolved water using spectra of 0.1M water-saturated DCE or 0.2M water-saturated benzene with a scaling factor fi allowed determination of the concentration of “free” water dissolved in the solutions under study: CH2Ofree = fi·Csat, where Csat = 0.1 or 0.2M for DCE and benzene, respectively. The concentration of the water involved in H(H2O)n+ cation is determined from the difference CH2OCat = C0H2O - CH2Ofree, where C0H2O is the total water concentration, known from the conditions of preparation. The ratio N = CH2OCat/CHCarb gives the average stoichiometry of H(H2O)n+ cations formed in solution. This method of spectral subtraction to determine the concentration of water (or methanol)33 bound with H+ and the average N stoichiometry of the formed H+·LN cations has been previously described in detail.27,28,34
Figure 2.
IR spectrum of H(H2O)n+{H5Br6−} in DCE solution with N = 3.7 after sequential subtraction of dry DCE and hexamethylbenzene (black), then free dissolved water, resulting in the final spectrum (red). For reference, the spectrum of 0.1 M water in DCE (after subtracting DCE) is shown in dashed blue.
The water-saturated DCE solutions of H(H2O)n+{I11−} and H(H2O)n+{CCD−} acids were prepared by extraction from water solution of their Cs{I11} or H/Na{CCD} salts containing 2–4 M H2SO4. The separated organic phase was washed 4–5 times with 1–2 M H2SO4 and then with distilled water. The molar concentrations of the acids were determined from the intensity of IR absorption of the {I11−} and {CCD−} anions compared to standard DCE solutions of (Oct)3NH+{I11−} or (Oct)3NH+{CCD−}. The total water concentration was determined using 1H NMR. The initial DCE solvent was made 0.2 M in chloroform as an internal standard. In the NMR spectra of water-saturated DCE solutions, the signal integrations of chloroform (at 7.26 ppm) and water (at 1.49 ppm) give the ratio 1.00:1.03, in good agreement with known water content of 0.1 M.35 In the spectra of H(H2O)n+{I11−} and H(H2O)n+{CCD−}, the ratio of these signals allowed determination of the total water concentration, C0H2O, and the molar ratio N = (C0H2O – 0.1)CHcarb.
With the exception of water-saturated solvents, all solutions were prepared in a Vacuum Atmospheres Corp. glove box under nitrogen (O2, H2O < 0.5 ppm). IR spectra in the 4000–450 cm−1 range were run on a Shimadzu-8300 FT-IR spectrometer housed inside a glovebox. A cell with Si windows having 0.036 mm separation at the beam transmission point was used. To avoid interference effects, the cell configuration was wedge-shaped. IR data were manipulated using GRAMMS software. NMR spectra were run on Varian INOVA 400.
X-ray crystals of [H7O3+][CHB11Cl11] were grown from o-dichlorobenzene under conditions of slow solvent evaporation at low pressure. Crystals of [H(CH3OH)3+][CHB11Cl11−] were grown from benzene solution with a 1:3 MeOH/H{Cl11} mole ratio.33 Crystals of [H9O4+][CHB11Cl11−] were grown from water-saturated H[CHB11Cl11] solution in a desiccator over CaCl2. CCDC 687306, 687307 and 687308 contain the supplementary crystallographic data for these salts. These data can be obtained free of charge in the Supporting Information or from The Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/data_request/cif.
Results and Discussion
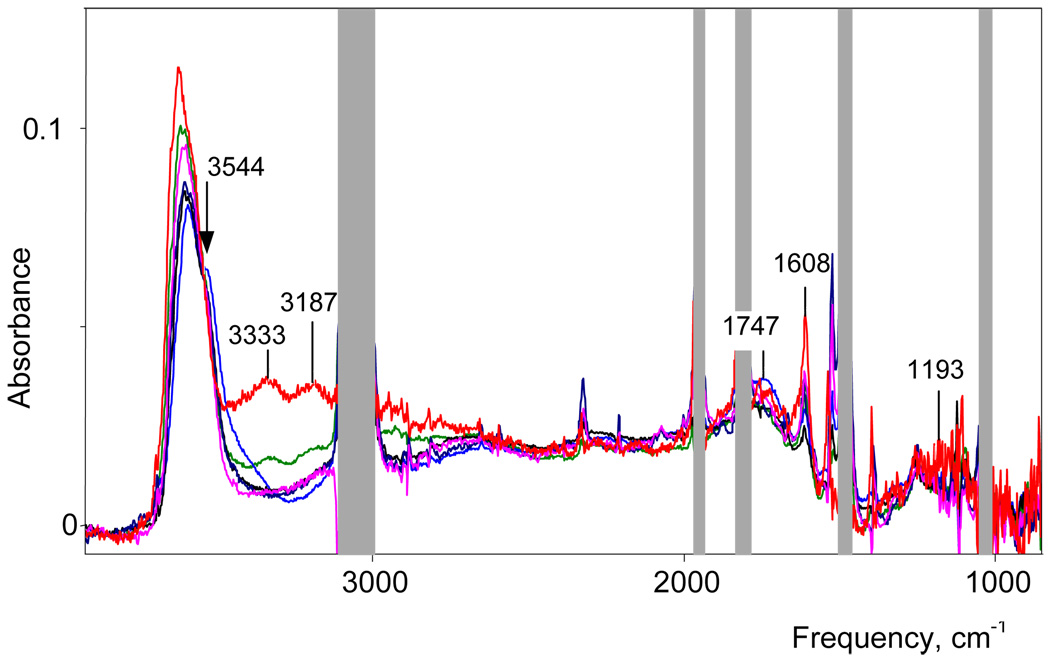
IR spectra of benzene and DCE solutions of carborane acids with different water/acid mole ratios N = H2/H(carborane) change regularly with increasing N, indicating sequential of hydration of H+. In addition, water molecules may be involved with solvation of the carborane anions. In order to examine this possibility we studied dichloroethane extracts of cesium carborane salts from water solutions. In addition to the bands from the carborane anions and dissolved water, IR spectra of these extracts developed two new narrow OH stretching bands νas at 3655 and νs at 3578 cm−1, arising from H2O molecules with free OH groups. Since these frequencies are independent of the nature of the carborane anion and are red shifted compared to those for dissolved monomeric water molecules in DCE (3675 and 3591 cm−1, respectively) they are assigned to H2O molecules bound to the Cs+ cation via the O-atom. Thus, there are no water molecules involved with hydrating the carborane anions. Taking into account that the νOH frequencies of the H(H2O)n+ cations discussed below are also independent of the nature of carborane anion, we can conclude that the OH groups of the cations are not H-bonded with counterion. This is consistent with our earlier studies on the H5O2+·4Solv cations7 where, although there is good evidence for ion pairing, H-bonding of the cation occurs only with solvent molecules. Thus, solvent separated ion pairs of the type [H+(H2O)n·mSolv]Carb− are formulated in the present work.
DCE solutions
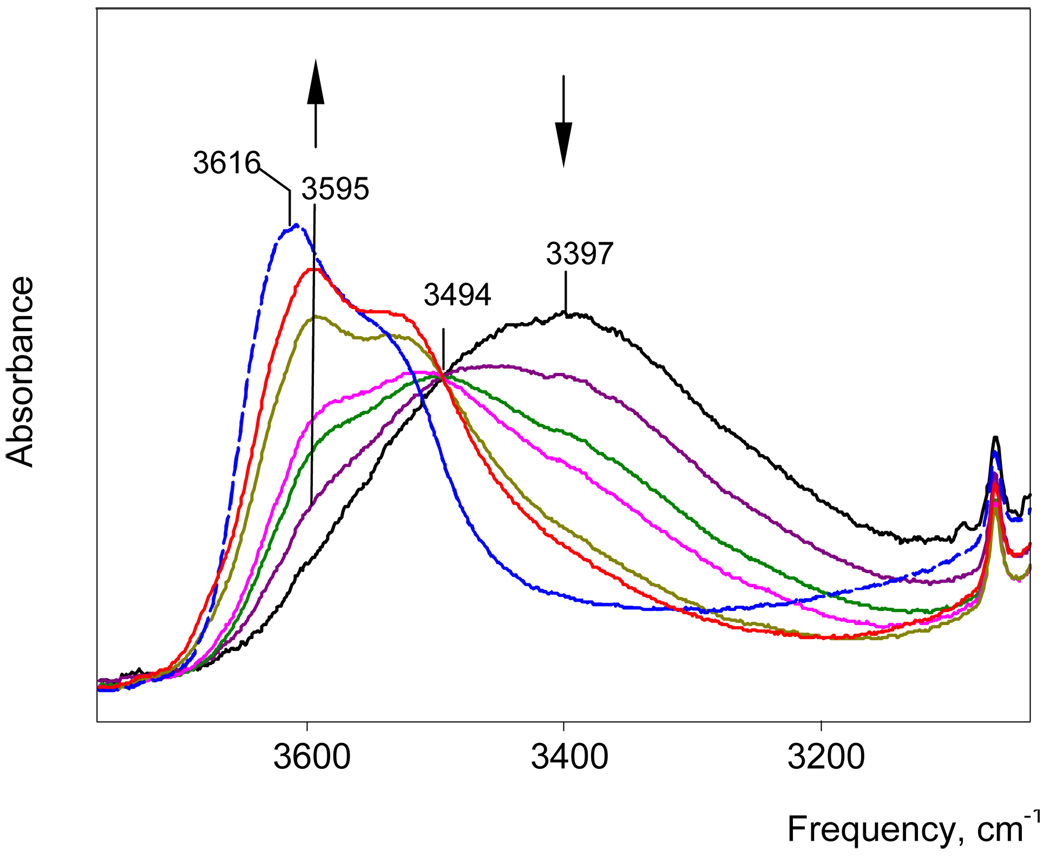
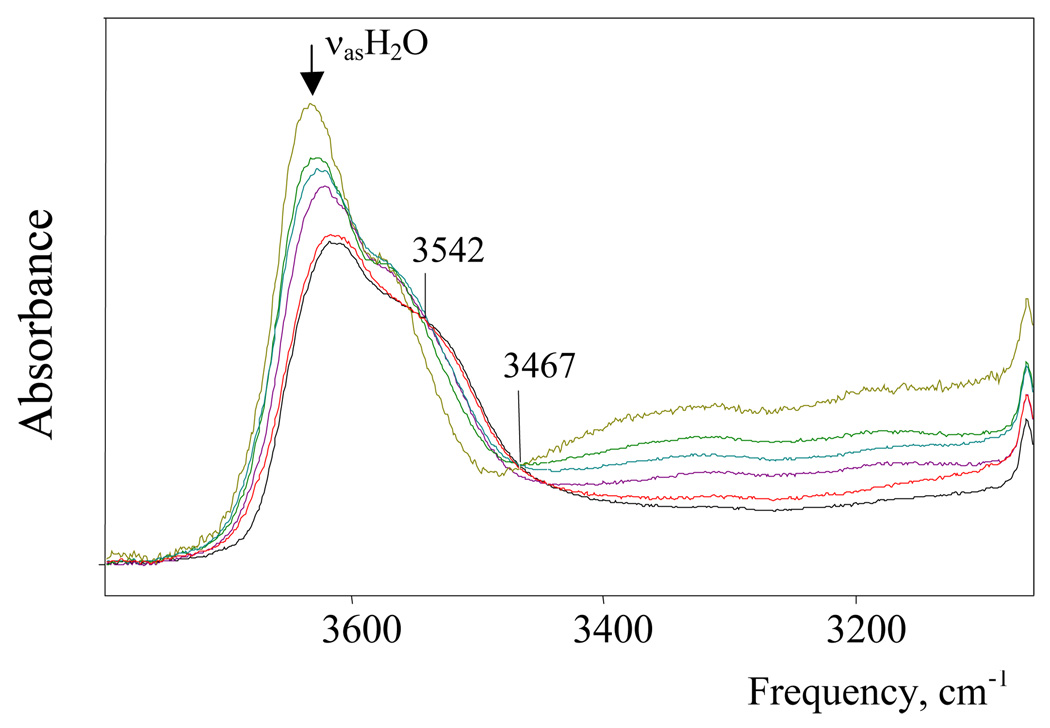
IR spectra of H(H2O)n+{H5Br6−} solutions with water/acid mole ratios N ≥ 2 have been studied. The spectrum of the solution with N = 2 belongs to the H5O2+·4DCE cation.7 With gradually increasing N to 3, the intensity of the H5O2+ spectrum decreases and disappears. At the same time the spectrum of the H7O3+ cation appears and reaches a maximum at N = 3. As shown in Figure 3, there is an isosbestic point at 3494 cm−1 up to N = 2.9, confirming that in this range only two cations, H5O2+ and H7O3+, are formed (i.e. N = n). As N = 3 is approached, there is a peculiar step change in the H7O3+ spectrum (Figure 3). This change is consistently reproducible and indicates the existence of two forms of the H7O3+ cation, the α isomer at N < 3 and the β isomer at N > 3. Their individual spectra are shown in Figure 4 and, as discussed in more detail below, are ascribed to different degrees of ion pairing. With further increase of N from 3 to 5.2 the spectrum of β-H7O3+ is transformed into that for the more highly hydrated H(H2O)n+ cations with isosbestic points at 3542 and 3467 cm−1 (Figure 5). This means either that H7O3+ is transformed into a single H(H2O)n+ cation with constant n, which seems improbable, or that a set of spectroscopically indistinguishable compounds with variable n ≥ 4 are formed, which will need a specific explanation.
Figure 3.
IR spectra of H(H2O)n+ cations with n = 2.0, 2.16, 2.30, 2.45, 2.75, 2.85 and 3.06 in DCE solutions. The initial spectrum (black) belongs to H5O2+. The red spectrum belongs to the α-H7O3+ isomer, the final (blue dashed) spectrum to the β-H7O3+ isomer.
Figure 4.
IR spectra of H7O3+ isomers α (red) and β (blue).
Figure 5.
IR spectra of H(H2O)n+ cations with n = 3.02–5.22.
Proton hydrates can be followed as a function of N via the change in frequency of νasH2O from the terminal H2O molecules, i.e. the “free” OH groups that are H-bonded to solvent. The νasH2O band is chosen over νsH2O for this analysis because it does not overlap with bands from other types of vibrations. As shown in Figure 6, for N = 2–3 the νasH2O frequency of α-H7O3+ is constant. At about N ∼ 3, the α → β isomerism of H7O3+ takes place and νasOH jumps steeply. In the range N > 3, nasH2O of β-H7O3+ and higher H(H2O)n+ hydrates are strongly overlapped and their joint maximum weakly increases in frequency as cations with n > 3 are formed. The procedures necessary to obtain samples of known water concentration (see Exp. Sect.) do not allow the total water concentration to exceed that of water-saturated DCE (0.1M) and maximum N value we can study is 5.2. On the other hand, a DCE extract from aqueous H{H5Br6} has a higher total concentration of water and the νasOH frequency is 3636±1 cm−1. Extrapolation using the Figure 6 dependence indicates that this frequency corresponds on average to the H(H2O)6+ cation.
Figure 6.
The dependence of νasH2O on the stoichiometry n of H(H2O)n+.
If successive H(H2O)n+ cations have strongly differing stability constants Kn, then the dependence of the free dissolved water concentration CH2Ofree on n should be stepped since in accordance with equilibria
the equilibrium constant Kn = Cn/Cn−1·CH2Ofree includes, in addition to Cn and Cn−1 concentrations of H(H2O)n+ and H(H2O)n−1+ cations respectively, the concentration of free dissolved water CH2Ofree. However, as shown in Figure 7, the dependence of CH2Ofree on n shows only one step at n = 3, when formation of H7O3+ cation is complete and a detectable concentration of free dissolved water appears. With further increasing n, the dependence increases steeply and linearly, indicating that the Kn constants for cations with n ≥ 4 are low and do not differ significantly. Extrapolation to water-saturated DCE (CH2Ofree = 0.1 M) results in n = 6, confirming that the highest formed proton hydrate is the H(H2O)6+ ion. The dependence of CH2Ofree on n allows the determination of Kn values for cations with n ≥ 4. For equal concentrations of H+(H2O)n and H+(H2O)n+1 cations Kn = 1/C'H2O, where C'H2O is the free water concentration for solutions with Cn = Cn−1. From CfreeH2O = f(n) one can determine that C'H2O = 0.0385, 0.0628 and 0.0876M respectively for solutions with N = 3.5, 4.5 and 5.5. Then K4 = 26.0, K5 = 15.9 and K6 = 11.4. The K3 value, for the formation of the H7O3+ cation, cannot be determined since the C'H2O concentration required for calculation is below the threshold of IR detectability.
Figure 7.
The dependence of the concentration of free dissolved water on stoichiometry of the H(H2O)n+ cations formed.
For all H(H2O)n+ cations with n ≥ 3 the frequency of the δH2O terminal water bending vibration is practically the same, 1608 cm−1. Its intensity (I1608) increases linearly with increasing H7O3+ cation concentration in solutions as N increases from 2 to 3 and extrapolates to zero at N = 2 (Figure 8). This confirms a peculiarity established earlier, namely, that in the IR spectrum of the H5O2+ cation the δH2O band is not observed.7 At N ∼ 3 the I1608 dependence on N changes slope and then continues to grow with increasing n in line with the increasing number of terminal water molecules in H(H2O)n+ cations. They can contain two types of terminal H2O: type (i) with both OH groups free or type (ii) with one free and the second H-bonded. Their δH2O frequencies coincide because the addition of a single H-bond in (ii) has minimal effect on the force constant of this vibration.
Figure 8.
Dependence of the δH2O band intensity on the stoichiometry of the H(H2O)n+ cations.
On the other hand, when both O-H groups are H-bonded to additional water molecules as in coordination type (iii), the δH2O frequency is detectably higher.
In summary, IR spectra of DCE solutions show that only the H5O2+ and H7O3+ cations have specific identities. H(H2O)n+ cations with n = 4–6 develop as a single family of compounds with spectroscopic properties sufficiently similar that it is impossible to detect their successive formation. They only differ slightly in the bands arising from their terminal water molecules.
Benzene solutions
The IR spectra of H(H2O)n+ cations in benzene solution are quite similar to those for DCE and are essentially independent of the nature of the counterion, {Cl11−} or {Me5Br6−}. Therefore, we will discuss the spectra of solutions of the H{Cl11} acid, whose hydrates are more soluble in benzene.
The spectrum of the cation formed in solution with N = 2 belongs to H5O2+.7 With N increasing to 3 the intensity of the H5O2+ spectrum decreases as the spectrum of the H7O3+ cation increases with an isosbestic point at 3447 cm−1 (Figure 9). However, in the range of N = 2.6–2.9 the spectrum of H7O3+ cation changes rapidly such that at N = 3 the spectrum does not cross the isosbestic point. Just as in DCE solution, the H7O3+ cation must exist in two isomeric forms in benzene solution: α (N = 2–2.6) and β (N ≥ 2.9). Their spectra are given in Figure 10.
Figure 9.
IR spectra of H(H2O)n+ cations with n = 1.99, 2.35, 2.55, 2.91 in benzene solution in the frequency range of νOH of the terminal OH groups. The initial spectrum (black) belongs to H5O2+. The red spectrum belongs to the β-H7O3+ isomer.
Figure 10.
IR spectra of α (red) and β (blue) isomers of the H7O3+ ion, equalized to unit intensity of the {Cl11}− anion, and the difference spectrum (brown) with hatching showing positive and negative intensity.
With increasing N from 3 to 4, the spectra change with a new isosbestic point at 3544 cm−1 indicating transformation of the H7O3+ cation into H(H2O)n+ (Figure 11). The spectra with N > 4 do not cross exactly at 3544 cm−1 indicating that the H(H2O)4+ cation may differ slightly more from cations with n ≥ 5 in benzene compared to DCE.
Figure 11.
Spectra of H+(H2O)n{Cl11} in benzene solutions with N = 3.2; 3.42; 3.46; 3.91 and 4.52. The red spectrum is close to water saturated with undetermined N. The bands from {Cl11} anion were subtracted using the spectrum of (Oct)3NH{Cl11} solution in benzene.
Spectra and structures of the H(H2O)n+ cations
H7O3+
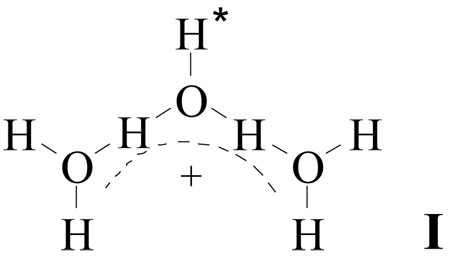
IR spectra of the α and β isomers of the H7O3+ cation are both in agreement with the symmetrical cation structure I.
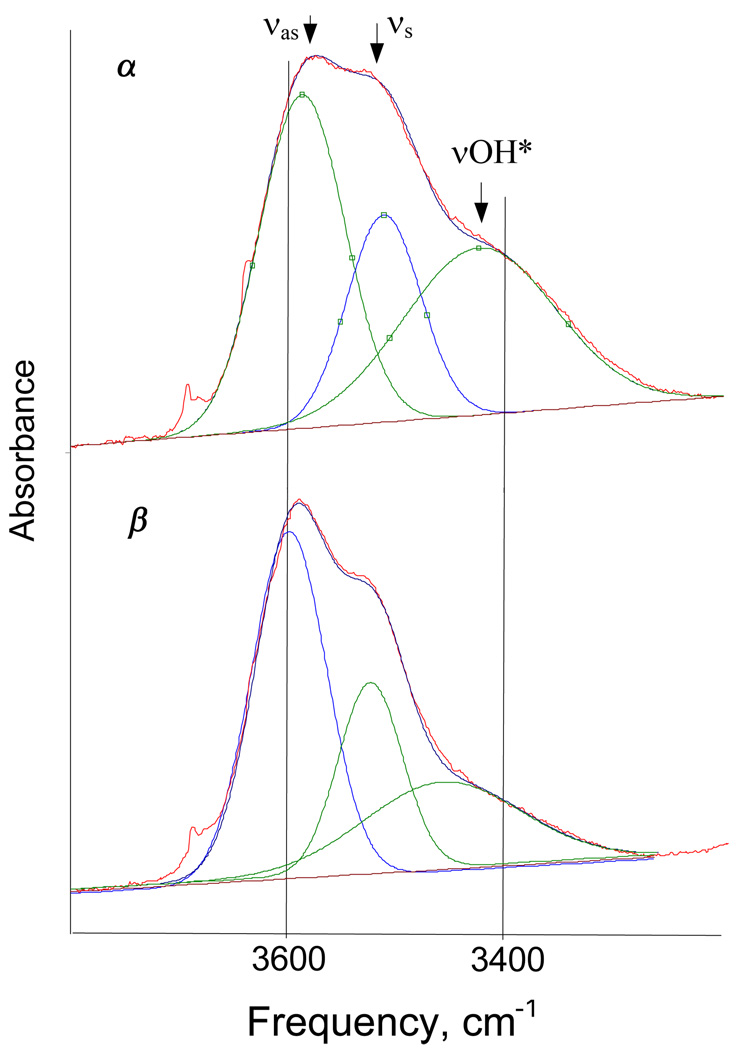
They show two types of OH stretching vibrations from terminal OH groups: firstly, νsH2O and νasH2O from the two equivalent H2O molecules and secondly, a lower frequency ν band from the group labeled OH* in I. The deconvolution is shown in Figure 12 and the data are listed in Table 1. The δH2O bend is at 1608 cm−1. Vibrations from the conjugated (O⋯H−O−H⋯O)+ group develop as a continuous broad absorption (cba) in the range 1400–3100 cm−1 (Figure 4 and Figure 10) with a particular shape (discussed below). The OH stretching frequencies of the terminal OH groups of the α isomer are somewhat red shifted compared with those for β isomer and band intensity is slightly higher (Figure 4, Figure 10 and Figure 12). This means that the α isomer experiences a somewhat stronger interaction with its environment. It is likely that α-H7O3+ forms mixed ion associates of the type (H7O3+)x(H5O2+)yCarb−x+y since the α isomer exists only in the presence of H5O2+ when N < 3. The destruction of these mixed associates as N approaches 3 can be understood in terms of the disappearance of the H5O2+ ion, resulting in the formation of the β isomer. Related associates were found in reverse nano-micelles formed in wet tributylphosphate solutions of HFeCl4 and HCl.28 They have a specific HFeCl4/HCl mole ratio and are destroyed when this molar ratio is not supplied.
Figure 12.
Deconvolution of the IR spectra of the α and β isomers of H7O3+ in benzene in the frequency range of the terminal OH groups.
Table 1.
Frequencies of terminal OH stretching vibrations in H7O3+.
| Solvent | Band | Frequency |
Δν(β - α) | |
|---|---|---|---|---|
| α isomer | β isomer | |||
| DCE | νasH2O | 3595 | 3612 | 17 |
| νsH2O | 3520 | 3535 | 14 | |
| νOH* | † | † | † | |
| Benzene | νasH2O | 3576 | 3597 | 21 |
| νsH2O | 3501 | 3523 | 21 | |
| νOH* | 3416 | 3458 | 42 | |
Cannot be determined with reliable accuracy
The distinctive continuous broad absorption (cba) in the spectrum of H7O3+ in the 1400–3100 cm−1 frequency range, which notably is absent in the spectrum of H5O2+ cation,7 derives from the (O⋯H−O−H⋯O)+ group vibrations. A similar feature is observed in the spectra of all other compounds containing the O−H⋯O group as long as the condition of O⋯O distance in the range 2.51–2.60 Å is fulfilled. As shown in Table 2, this group of compounds includes dimers of dialkylphosphoric and dialkylphosphinic acids, the salts of carboxylic acids with organic basics, and a variety of other acid salts. The IR spectra of compounds containing a conjugated (biprotonic) group of the (X⋯H−O−H⋯X)+ type with X = heteroatom, whose stretching and bending vibrations are strongly coupling, nevertheless develop the same cba.48,49 The shape of the cba is quite specific having so-called A (∼2600–2800), B (∼2200) and C (1700–1800 cm−1) structure.50
Table 2.
The frequencies of O-H⋯O groups for some compounds developed in IR spectra the OH stretching as a broad absorption with A, B and C contours.
| Type of O-H⋯O group | Compound | Method | Stretching vibration | Bending vibration | RO⋯O, Å | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | δ(OHO) | γ(OHO) | |||||
| (C)O-H⋯O(X) | H-complexes of carboxylic acids | IR | 2800 | 2500 | 1900 | 1200–1600 | 900–1100 | 36–38 | |
| (O3P)O-H⋯O(PO3) | K2HPO4·3H2O | IR | 2700 | 2330 2265 |
1670 1700 |
1220 1212 |
800 | 2.578 | 39 |
| (O3P)O-H⋯O(PO3) | Ca(H2PO4)2·H2O | IR | 2920 | 2375 | 1700 | * | * | 2.596 | 40 |
| (S)O-H⋯O(S) | CsHSO4 | IR, Raman | 2850 | 2500 | 1680 | 1252 | 884 | 2.572 | 41 |
| (Se)O-H⋯O(Se) | CsHSeO4 | IR, Raman | 2840 | 2415 | 1600 | 1258 | 805 | 2.603 | 42 |
| (Se)O-H⋯O(Se) | C6H5SeOOH | IR | 2740 | 2270 | 1650 | * | * | 2.52 | 43 |
| (P)O-H⋯O(P) | dialkylphosphoric and dialkylphosphinic acids | IR44,45 | 2600–2800 | 2200–2400 | 1610–1700 | * | * | 2.5346 2.5147 |
|
There are different points of views on the interpretation of the A, B, C structure of the cba band51 but Fermi resonance between the νOH stretch and the overtones of low frequency vibrations of the O−⋯ group, namely, with the first overtones of the in-plane δ(OHO) (A and B bands) and out-of-plane γ(OHO) (C band) vibrations is gaining wide acceptance.36–38,52,53 The Fermi resonance nature of the A, B and C bands and the correct assignments of the δ(OHO) and γ(OHO) bands have been rigorously confirmed based on polarization data in the IR and Raman bands in the spectra of acid sulfates, phosphates and selenates of potassium and cesium at 300 and 20 K.39,41,42
Since the cba represents a very broad OH stretching band, and its shape is distorted by Fermi resonance, νOH is determined as the centre of gravity of cba absorption.37,38 The cba does not significantly depend on the energy/enthalpy of the H-bonding, the temperature (80–550 K) or the state of matter.45 However, the center of gravity (i.e. νOH) depends on O⋯O distance. With O⋯O decreasing to 2.41–2.42 Å, as in H5O2+ cation3,4 or to 2.39 Å as in proton disolvates L-H+-L with L = strong base,54 νOH decreases in frequency. As O⋯O decreases, bands A and B decrease in intensity and disappear when H7O3+ becomes H5O2+. Band C is retained and new bands in the low frequency 800–1200 cm−1 region appear in the spectrum of H5O2+.7
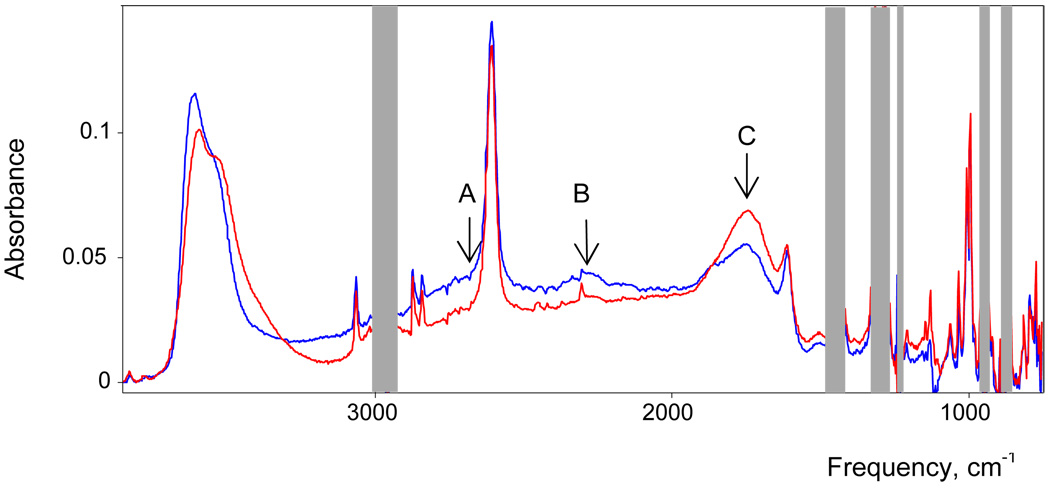
In crystalline H7O3+{Cl11} the H7O3+ cation is asymmetric with one O⋯O distance longer, 2.518(2) Å, and the second shorter, 2.439(2) Å, reflecting the asymmetrical environment of {Cl11−} anions. Such asymmetry is common for H7O3+ cations in the crystalline state e.g. [H7O3·(15-crown-5)][AuCl4]55 has 2.536(7) and 2.423(7) Å O⋯O distances). The proximity of shorter distance to that in the H5O2+ cation (2.40–2.42 Å)3,4,7 suggests formulation of these salts as H5O2+ monohydrates, H5O2+·H2O. However, the IR spectrum of the distorted H7O3+ cation in crystalline H7O3+{Cl11} shows a strong cba with A,B,C structure, not seen for the H5O2+ cation, arising from the O⋯H−O−H⋯O chromophore (Figure 13). Thus, it has a separate identity. Since its IR spectrum differs considerably from that in solution (Figure 13) we should compare it to that in a more symmetrical crystalline environment.
Figure 13.
Comparison of IR spectrum of α-H7O3+ cation in benzene solution (red) with that of solid H(CH3OH)3+{Cl11−} (blue) and crystalline H7O3+{Cl11} (black dashed). The red and black spectra are normalized to unit absorption of the {Cl11-} anion.
The structure reported to have the most symmetrical H7O3+ cation is found in [H7O3+][H9O4+]Br−2·H2O where the O⋯O distances are 2.47(1) and 2.50(1) Å (ave. 2.481 Å).56 The symmetry arises from a nearly symmetrical anion/hydrate field as shown in (iv).
The proton labeled H* is more tightly H-bonded to bromide than those from the terminal water molecules, reflecting its closer proximity to the positive charge. The interaction with two water solvate molecules is weak. Unfortunately, because of the presence of H9O4+ (and H2O) in these crystals, they are not suitable for IR investigation of the H7O3+ ion. Lacking good hydrates for structure/spectra correlation, we turn to the methanol analogue of the H3O3+ ion, namely the H(CH3OH)3+ cation, which has a similar cba band and has been characterized by X-ray crystallography.
The structure of the H(CH3OH)3+ cation in crystalline H(CH3OH)3+{Cl11−} with O⋯O distances 2.446(2) and 2.491(2) Å is shown in Figure 14.
Figure 14.
X-ray crystal structure of one cation of [H(MeOH)3][CHB11Cl11] in the unit cell. Thermal ellipsoids are shown at the 50% probability level.
The overall average O⋯O distance of 2.455 Å is close to the average of 2.481 Å in the nearly symmetrical H7O3+ cation in [H7O3+][H9O4+]Br−2 ·H2O.56 The spectrum of the H(CH3OH)3+ cation in benzene solution and in the crystal phase as the {Cl11}− salt is essentially the same so the structure of the cation must be very similar in both phases. Since the O⋯O distances in the H(CH3OH)3+ cation are shorter than those in compounds containing isolated O−H⋯O groups (Table 2), the νOH frequency (cba center of gravity, 1920±40 cm−1) is lower than, for example, in phosphinic acid dimers (2000–2070 cm−1).45 Comparing the spectrum of the H(CH3OH)3+ cation with the α-H7O3+ cation in benzene solution (Figure 13) close similarity can be seen.
The bands at ∼1300 and 1732 cm−1 in α-H7O3+ cation correspond to those at ∼960 and ∼1655 cm−1 in H(CH3OH)3+ and the center of gravity of the cba of α-H7O3+ practically coincides with that of H(CH3OH)3+, but in case of β-H7O3+ it is higher (Table 3). Therefore, the O⋯O distance of α-H7O3+ must be close to that of 2.455 Å in H+(CH3OH)3 cation while that in β-H7O3+ must be longer, approaching the value of 2.481 Å observed in the H7O3+ ion of [H7O3+][H9O4+]Br2−·H2O. This demonstration of diminished H-bond strength in the (O⋯H−O−H⋯O)+ group of β-H7O3+ compared to that in H(CH3OH)3+ can be understood in terms of the weaker intrinsic basicity of water versus methanol. Nevertheless, the availability of five terminal OH groups in H7O3+ instead of two in H(CH3OH)3+ for H-bonding with the environment must also play an important role. The H7O3+ cation can more effectively transfer positive charge from (O⋯H−O−H⋯O)+ group to the environment via a greater number of H-bonds.
Table 3.
νOH of H(H2O)n+ and H(CH3OH)3+ cations determined from the cba center of gravity.
| Sample | Solvent | νOH |
|---|---|---|
| α-H7O3+{Cl11−} | benzene | 1911 ± 40 |
| α-H7O3+{H5Br6−} | DChE | 2110 ± 40 |
| β-H7O3+{H5Br6−} | DChE | 2285 ± 40 |
| H3O+(H2O)3{Cl11−} | crystal | 2390 ± 40 |
| H7O3+·H2O{Cl11−} | benzene | 2270 ± 40 |
| H(CH3OH)3+{Cl11−} | benzene | 1920 ± 40 |
A weak blue shift of the cba center of gravity and the νOH frequencies of the terminal OH groups of the β isomer of H7O3+ compared to those for the α isomer (Table 1, Table 3) indicates a slight weakening of both the internal O⋯H−O−H⋯O core H-bonds and the external H-bonds with the environment in the β isomer. This may reflect different H7O3+/anion interactions. If aggregates of α-H7O3+{Cl11−} ion pairs are associated with H5O2+{Cl11−} ion pairs, α-H7O3+ may experience a more spherically symmetric anion field than the unidirectional field of the simple ion paired structure of β-H7O3+{Cl11−}. As shown schematically by II, the polarization of the β cation by the anion weakens its interaction with environment.
H(H2O)n+ cations with n ≥ 4
The formation of the H(H2O)4+ cation as an individual entity is detected with higher certainty in the lower permittivity solvent, benzene rather than DCE. As shown in Figure 15, its IR spectrum is very similar to that of β-H7O3+. The two differences are (a) the νOH* band of the “free” OH* group at 3458 cm−1 is red shifted to the region of ∼3200 cm−1 and (b) there is an increase in the intensity of the νasH2O, νsH2O and δH2O (1610 cm−1) bands from an added peripheral H2O molecule. These changes are readily understood in terms of structure III.
Figure 15.
Spectrum of H(H2O)4+ (red) compared to β-H7O3+ (blue) after subtraction of solvent (benzene) and counterion {Cl11−}.
Compared to H7O3+, the OH* group is now H-bonded to H2O rather than solvent so the νOH* frequency is decreases. The fourth water molecule is not equivalent to the other two peripheral H2O groups and νasH2O and νsH2O develop as one broad asymmetric band. The absorptions from the core (O⋯H−O−H⋯O)+ group are retained nearly unchanged indicating that the H7O3+ ion remains the fundamental building block. The H7O3+−H2O interaction is relatively weak so the H(H2O)4+ cation is more correctly represented as the monohydrated H7O3+ cation, i.e. H7O3+·H2O.
As noted earlier, the close similarity of the spectroscopic properties of H(H2O)n+ cations with n ≥ 4 means that these cations all belong to one family of compounds. Their spectra show isosbestic points and the only significant differences arise from absorptions from the increasing number of peripheral water molecules (Figure 16). All the data can be rationalized by the sequential attachment of three water molecules to the H7O3+ cation in structures III, IV and V for n = 4, 5 and 6 respectively (L = solvent). In each cation, the (O⋯H−O−H⋯O)+ structural unit of the H7O3+ ion is retained as the basic building block.
Figure 16.
IR spectra (red through black) of DCE solutions of H(H2O)n+{H5Br6−} with n = 3.02; 3.1; 3.4; 3.7; 4.2; 5.2.
Consistent with these structures, only the spectra for N = 5 and 6 show an increase in intensity of the broad band νOH at 3327 cm−1 (Figure 16) arising from the H-bonded OH groups of semi-hydrated water molecules (designated earlier as type (ii) and indicated in blue in IV and V). This is accompanied by a Fermi resonance band at 3180 cm−1 from the overtone of 2δH2O. Finally, the constancy of the δH2O frequency at 1608–1610 cm−1 for all cations with n = 3–6 that is in agreement with structures IV and V since they contain closely related peripheral water molecules only of types (i) and (ii).
The H(H2O)6+ cation with {H5Br6−} counterion is formed in DCE solution under conditions of water saturation. However, by using alternative carborane acids and extracting them from aqueous solution at varying temperatures, H(H2O)n+ cations with n > 6 may by obtained. Figure 17 presents the spectra of cations from aqueous H{CCD} extracts with N varying from 5.5–6.5 as well as the spectrum of an H{I11} extract in which N reaches 8.2. In all spectra the absorptions from the core (O⋯H−O−H⋯O)+ group remain unchanged as indicated by the constancy of the cba from 3000-1500 cm−1.
Figure 17.
IR spectra of H(H2O)n+ cations in water-saturated DCE: black, n = 5.5; green, n = 6.0; red, n = 6.5; blue, N = 8.2. The anion is {CCD}− in the black, green and red spectra, {I11}− in the blue. In the inset, the difference of the spectra with n = 8.2 and 5.5 is given to show with higher certainty the appearance of the δH2O band at 1634 cm−1 of the water molecules of type (iii) in cation VI.
Let us now consider the interaction between the terminal water molecules and H7O3+ group in more detail. Figure 18 shows the difference spectrum of cations with n = 4 and n = 4.74 in order to reveal the bands associated with the fifth added water molecule. The νasH2O band appears at 3650 cm−1, the lower frequency νsH2O band is masked by other νOH vibrations, and the νOH band from the OH group of the H7O3+ cation to which the fifth H2O molecule is H-bonded (type (ii), marked blue in IV) appears at 3350 cm−1. A small shift in the cba to higher frequency (in the range of the A band) results in a broad band at 2980 cm−1. With the exception of a weak δH2O band from fifth H2O molecule, there are no changes below 2500 cm−1. Similar difference spectra of cations are obtained with n = 6 (counterion {CCD−}, DCE) and n = 5.2 (counterion {H5Br6−}, DCE), except that the relative intensity of the band at 2980 cm−1 is weaker. So all changes in the spectra are in accordance with structures III, IV and V. In the difference spectra of cations with n = 6.5 and 5.5 (DCE, CCD− as counterion) the intensities of the bands at 3655, 3400 and 1608 cm−1 increase in accordance with additional water molecules of type (i) or (ii). The cba is not changed. However, for the first time, a δH2O band appears at ∼1630 cm−1 (Figure 17 inset). This is assigned to water molecules of type (iii) having both OH groups H-bonded to solvating H2O molecules. A similar band with higher intensity appears in the difference spectrum of cations with n = 8–9 and n = 5.5. Thus, attachment of the seventh and eight water molecules to H(H2O)6+ cation leads to cation VI.
Figure 18.
Deconvolution of the difference spectrum between cations with n = 4.52 and n = 4 for benzene solution (counterion {Cl11−}).
The structure of the H(H2O)7+ cation has been determined by X-ray in the crystalline hydrate H2SiF6·9.5H2O.57 It comprises the H7O3+ building block surrounded with five H2O molecules and one F atom from a SiF62− anion as shown in (v).
This is very similar to structure VI proposed for H(H2O)8+ in solution. The O⋯O distances in the O⋯H−O−H⋯O group of (v) are lengthened to 2.505(2) Å compared with the average of 2.481(x) Å in [H7O3+][H9O4+]Br−2 ·H2O (iv) where the H7O3+ ion is bonded with only two water molecules. It is evident that increasing the number of H-bonded water molecules solvating the H7O3+ cation delocalizes the positive charge and weakens H-bonding in the core O⋯H−O−H⋯O unit. The 2.505 Å O⋯O distance is close to that in dimers of dialkylphosphinic acids (2.51–2.52 Å) which is why their cba are quite similar. Structure v reflects the weak H-bonding between the H7O3+ cation and three solvating water molecules (RO⋯O = 2.77–2.81 Å). They are comparable to those in neutral liquid water (RO⋯O = 2.78 Å).58 The OH* group forms a somewhat stronger H-bond with RO⋯O = 2.62 Å, which nevertheless is insufficient to contribute to the cba below 2600 cm−1.
Comparison with gas and solid phases
The structures of H(H2O)n+ cations in acidified organic solutions are similar to those in the gas phase only when n = 1, 27,9 and 3. For n ≥ 4 they can be quite different. In the gas phase, the Gibbs energy dependence of the addition of the n-th molecule of H2O to the H(H2O)n−1+ hydrate indicates extra stability of the H(H2O)4+ and H(H2O)6+ cations.13 Indeed, the stability of H(H2O)4+ is higher than that of H7O3+. In organic solvents, the situation is different. The stability of H3O+, H5O2+ and H7O3+ is high but starting with the H(H2O)4+ cation, stability sharply decreases without any particular stability of cations with further increasing n.
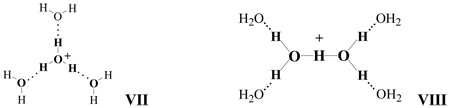
In accordance with quantum mechanical calculations10,19,59 and confirmed by IR spectroscopy,10,19 the most stable structures for the H(H2O)4+ and H(H2O)6+ cations in vacuo are symmetrical structures VII and VIII respectively. However, some calculations (G3B3 and CBS-QB322 or DFT21) predict a slightly higher stability for structure V compared to VIII for H(H2O)6+ cation. There energy difference is so small that both V and VIII may coexist. In any case, cations H3O+·3H2O (VII) and H5O2+·4H2O (VIII) have also been characterized in the solid phase by X-ray diffraction.60,61
The gas phase spectrum of VII develops two bands νsH2O = 3644 and νasH2O = 3730 cm−1 from the three equivalent H2O molecules surrounding the H3O+ ion and a broad band of νas and νs at 2665 cm−1 for the central H3O+.10 In the crystalline state of [H3O+·3H2O]{Cl11}− the OH stretch frequencies from H3O+ occur as a coalesced broad band at lower frequency, 2576 cm−1 (Figure 19), because the three H-bonded water molecules are made more basic by the anion environment. A combination band appears at 2291 cm−1. The frequencies of the three terminal water molecules of H3O+·3H2O appear at νas 3637, νs 3578 and δH2O 1605 cm−1 bands. In the low frequency region, a weak band appears ∼1200 cm−1, possibly ν2H3O, overlapped with strong bands from counterion. The spectrum is also very similar to that of the C3v symmetric H3O+·3TBP cation, an analogue of VII.27 The slightly higher basicity of TBP compared to H2O results in a small red shift νOH to 2530 cm−1 and the combination band to 2206 cm−1.
Figure 19.
IR spectra of H(H2O)4+{Cl11−} (N = 3.91) in benzene (red), crystalline [H3O+·3H2O] {Cl11−} (blue) and [H3O+·3TBP] FeCl4− in CCl4 (black dashed). Red and blue are normalized to unit intensity of anion.
Comparing the spectrum of the symmetrical H(H2O)4+ cation in [H3O+·3H2O]{Cl11}− with that in solution reveals differences ascribable to different structures: the symmetrical Eigen-type H3O+·3H2O cation in the crystal and the less symmetrical H7O3+·H2O cation in benzene or DCE solution (Figure 19). They show some similarities in the cba region, originating from conjugated O−H⋯O group vibrations, but nevertheless there are important distinctions in the distribution of the cba intensity that defines the νOH frequencies. For the H7O3+·H2O cation in benzene solution, νOH is about 120 cm−1 lower than in the H3O+·3H2O cation (Table 3). This reflects shorter O⋯O distances in the O⋯H−O−H⋯O core of the H7O3+·H2O cation compared to O−H⋯O in H3O+·3H2O. In the X-ray structure of [H7O3+][H9O4+]Br−2·H2O56 where both cations appear in the same crystal this difference is 2.48(1) versus 2.56(1) Å (averaged over equivalent bonds). The average O⋯O distance in the H3O+·3H2O cation is 2.51(2) Å60 with {H5Br6}− as counterion and 2.530(2) Å with {Cl11}−.
Similarly, a comparison of the H(H2O)6+ cation in solution versus the gas phase or a symmetrical crystal environment reveals considerable structural differences. For the gas phase, the measured and calculated IR spectra provide evidence that the H(H2O)6+ cation has a H5O2+ core.10,19 However, other calculations based on different levels of theory predict that the lowest energy state in vacuo of the tetrahydrated H5O2+ ion, namely H5O2+·4H2O cation VIII, is insignificantly lower (or even higher) than that for the H7O3+·3H2O ion (V).21,22 For the crystal state, only one example of a type VIII cation is known. It is found in the strictly symmetric Cl− anion environment of a cage compound [(C9H18)3(NH2)2Cl]+Cl− crystallized from HCl solution.61 In the solutions of the present study, the IR data on H(H2O)6+ retain the characteristics of the H7O3+ core, indicating formulation as the H7O3+·3H2O ion, structure V. Since the vibrations of the O⋯H−O−H⋯O core of H7O3+·nH2O cations in solution with n ≥ 6 are very similar to those from the O−H⋯O group of phosphinic acid dimers, the O⋯O distances should be similar. They are ∼2.49–2.53 Å for phosphinic acid dimers.46,47,62 The average O⋯O distance in the core of the H7O3+·4H2O cation in the SiF62− salt is 2.505 Å.57 Therefore, in H(H2O)n+ cations with n ≥ 6 in solution the O⋯O distances should be ca. 2.51 Å. By comparison, the central O⋯O distance in H5O2+·4H2O should be ca. 2.39(2) Å as in crystal state.61 This distance is typical for L−H+−L proton disolvates with strong L bases, such as diethylether.54,63 These cations, including H5O2+, develop intense bands in the 800–1000 cm−1 region from O−H+−O group vibrations7,54,63. In accordance with DFT/B3LYP and MP2 calculations,64 complexation of the H5O2+ cation with four H2O molecules only marginally influences the frequency and strong intensity of these bands. However, they are absent in the spectra of H7O3+·3H2O in solution.
Thus, the Eigen and Zundel-type symmetrical cations H3O+·3H2O VII and H5O2+·4H2O VIII exist in gas phase where counterions are absent. Rare cases also exist in the solid phase where the cation happens to be nearly symmetrically surrounded by anions or other ligands. In solutions with aprotic solvents such as benzene and chlorinated hydrocarbons, the one-dimensional electrostatic influence of the anion in solvent-separated ion pairs results in higher stability of the H7O3+·H2O cation with structure III and H7O3+·3H2O with structure V.
The present results better allow us to appreciate the similarities and differences of the excess proton in water versus methanol.33 In benzene solution with carborane counterions, the H(H2O)n+ and H(CH3OH)n+ cations have similar spectra and structures for n = 2 and 3. At n = 4, however, different core structures become the building blocks for higher solvation. This occurs because the O-H* group in H7O3+ is replaced by an O-CH3 group in H(CH3OH)3+ and is therefore unavailable for H-bonding. Water clusters can grow in dendritic fashion whereas methanol clusters are forced to grow as linear chains. As a result, methanol clusters grow linearly as unique cations up to n = 4, each with individual spectral properties. Solvation continues up to n = 8 chains retaining the properties of the H(CH3OH)4+ core.33 On the other hand, water clusters cease to develop unique cations after n = 3, retaining the H7O3+ core.
Conclusions
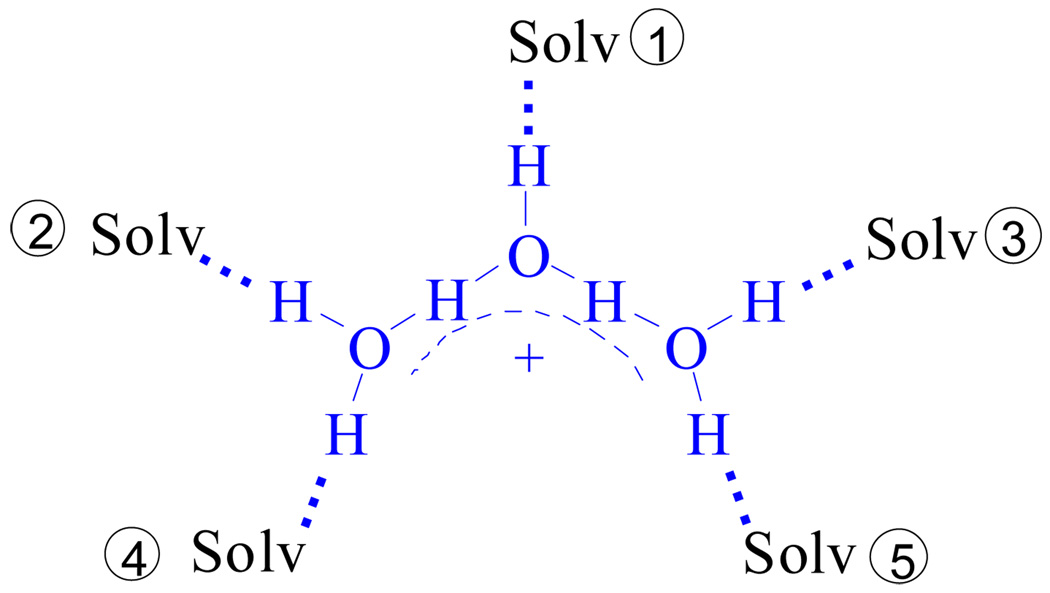
Study of the stepwise bonding of water molecules to H+ in weakly basic solvents with weakly basic anions shows that the first three hydrates, namely H3O+, H5O2+ and H7O3+, behave as individual ions with unique and distinctive structures and IR spectra. Additional water molecules are H-bonded to the H7O3+ cation via stepwise replacement of organic solvent molecules from its first coordination sphere via Eq. 1 and Figure 20.
| (1) |
Figure 20.
The H7O3+·(Solv)5 cation showing the structurally distinct sites of the first solvation shell. Site 1 is hydrated first, then sites 2 and 3, and finally sites 4 and 5.
Hydration of the H7O3+·(Solv)5 ion occurs first at the strongest coordination position, site 1. The second and third H2O molecules replace solvent molecules at positions 2 and 3 forming the H+(H2O)6 cation which is stable with all studied counterions in both benzene and DCE. Finally, with some of the studied counterions in DCE solvent, two further H2O molecules can be introduced in positions 4 and 5 to form the less stable H+(H2O)8 cluster, completing the first coordination sphere of H7O3+ cation. There are detectable changes in the spectrum of the H7O3+ ion as it is solvated by 1–3 water molecules but the core structure is retained for all m. Comparisons of IR data and X-ray data lead to the conclusion that in H(H2O)n+ cations with n > 4 the two O⋯O distances in conjugated O⋯H−O−H⋯O group, where the excess proton resides, are ca. 2.51 Å.
Notably, neither Eigen-type structures with the H3O+ core nor Zundel-type structures with the H5O2+ core are present. This finding is contrary to popular expectation and contrary to calculation and experiment in the gas phase. It reflects the unfortunate fact that perceptions regarding the nature of the aquated proton in solution have become too biased by calculations and experiments in the gas phase, where counterions are absent. The present work suggests that the obligatory presence of a counterion in solution, no matter how weakly coordinating, exerts an anisotropic electrostatic influence on H(H2O)n+ cations that has been largely ignored. The presence of the conjugate base of the acidic H(H2O)n+ cations is the principal reason that different structures exist in the condensed phase vis à vis the gas phase. The question about where the positive charge is localized has been typically been phrased in terms of Eigen and/or Zundel type ions, i.e. on one water molecule, or shared between two. For the presently studied H(H2O)n+ clusters the excess proton is localized between three oxygen atoms and the positive charge influences up to six water molecules. Additional water molecules above n = 6 are essentially indistinguishable from bulk water.
The next challenge will be to determine experimentally the nature of H(H2O)n+ in liquid water. Most theory focuses on Eigen versus Zundel ions,11.12 or a continuum of structures in between,65 although one study favors H7O3+ as the major ion present.66 The present work provides an excellent experimental fingerprint for this ion.
Acknowledgements
We thank Drs. Kee-Chan Kim for experimental assistance and the National Science Foundation (CHE-039878) and the National Institutes of Health (GM 23851) for support.
Footnotes
Supporting Information. Additional IR spectra and crystallographic data. These can be obtained free of charge at http://pubs.acs.org. CCDC-687306 for [H9O4][CHB11Cl11], CCDC-687307 for [H(CH3OH)3][CHB11Cl11] and CCDC-687308 for [H7O3][CHB11Cl11] contain the cif files which can be obtained free of charge from the Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/data_request/cif.
References
- 1.Eigen M. Angew. Chem. Int. Ed. Engl. 1964;3:1–19. [Google Scholar]
- 2.Zundel G, Metzer HZ. Phys. Chem. 1968;58:225–241. [Google Scholar]
- 3.Ortwein R, Schmidt AZ. Anorg. Allg. Chem. 1976;425:10–16. [Google Scholar]
- 4.Jones DJ, Roziere J. J. Mol. Struct. 1989;195:283–291. [Google Scholar]
- 5.Stoyanov ES, Kim K-C, Reed CA. J. Am. Chem. Soc. 2006;128:1948–1958. doi: 10.1021/ja0551335. [DOI] [PubMed] [Google Scholar]
- 6.Stoyanov ES, Smirnov IV, Fedotov MA. J. Phys. Chem. A. 2006;110:9505–9512. doi: 10.1021/jp0609838. [DOI] [PubMed] [Google Scholar]
- 7.Stoyanov ES, Reed CA. J. Phys. Chem. A. 2006;110:12992–13002. doi: 10.1021/jp062879w. [DOI] [PubMed] [Google Scholar]
- 8.Okumura M, Yen LI, Myers JD, Lee YT. J. Phys. Chem. 1990;94:3416–3424. [Google Scholar]
- 9.Roscioli JR, McCunn LR, Johnson MA. Science. 2007;316:249–254. doi: 10.1126/science.1138962. and references therein. [DOI] [PubMed] [Google Scholar]
- 10.Headrick JM, Diken EG, Walters RS, Hammer NI, Christie RA, Cui J, Myshakin EM, Duncan MA, Johnson MA, Jordan KD. Science. 2005;308:1765–1769. doi: 10.1126/science.1113094. and references therein. [DOI] [PubMed] [Google Scholar]
- 11.Tuckerman M, Laasonen K, Sprik M, Parinello MJ. J. Chem. Phys. 1995;103:150–161. [Google Scholar]
- 12.Voth GA. Acc. Chem. Res. 2006;39:143–150. doi: 10.1021/ar0402098. and references therein. [DOI] [PubMed] [Google Scholar]
- 13.Grimsrud EP, Kebarle P. J. Am. Chem. Soc. 1973;95:7939–7943. [Google Scholar]
- 14.Mizuse K, Fujii A, Mikami N. J. Chem. Phys. 2007;126:231101–231104. doi: 10.1063/1.2750669. and references therein. [DOI] [PubMed] [Google Scholar]
- 15.Searcy JQ, Fenn JB. J. Chem. Phys. 1974;61:5282–5288. [Google Scholar]
- 16.Zwier TS. Science. 2004;304:1119–1120. doi: 10.1126/science.1098129. [DOI] [PubMed] [Google Scholar]
- 17.Shin J-W, Hammer NI, Diken EG, Johnson MA, Walters RS, Jaeger TD, Duncan MA, Christie RA, Jordan KD. Science. 2004;304:1137–1140. doi: 10.1126/science.1096466. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki M, Fujii A, Ebata T, Mikami N. Science. 2004;304:1134–1137. doi: 10.1126/science.1096037. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J-C, Wang Y-S, Chang H-C, Lin SH, Lee YT, Niedner-Schatteburg G, Chang H-C. J. Am. Chem. Soc. 2000;122:1398–1410. [Google Scholar]
- 20.Christie RA, Jordan KD. J. Phys. Chem. A. 2001;105:7551–7558. [Google Scholar]
- 21.Wei D, Salahub DR. J. Chem. Phys. 1994;101:7633–7642. [Google Scholar]
- 22.Likholyot A, Lemke KH, Hovey JK, Seward TM. Geochim. Cosmochim. Acta. 2007;71:2436–2447. [Google Scholar]
- 23.Lungdren J-O, Ollovsson I. In: The Hydrogen Bond: II. Structure and Spectroscopy. Schuster P, Zundel G, Sandorfy C, editors. North-Holland: Amsterdam; 1976. Chap. 10. [Google Scholar]
- 24.Pankov AA, Borovkov VYu, Kazanski VB. J. Appl. Spectroscopy (Russian) 1982;37:824–825. [Google Scholar]
- 25.Stoyanov ES, Mikhailov VA, Chekmarev AM. Zr. Neorg. Khimii (Russian) 1990;35:1442–1450. [Google Scholar]
- 26.Stoyanov ES, Kim K-C, Reed CA. J. Phys. Chem. A. 2004;108:9310–9315. [Google Scholar]
- 27.Stoyanov ES. J. Chem. Soc. Faraday Trans. 1997;93:4165–4175. [Google Scholar]
- 28.Stoyanov ES. J. Chem. Soc. Faraday Trans. 1998;94:2803–2812. [Google Scholar]
- 29.Reed C. A. Chem. Commun. 2005:1669–1677. doi: 10.1039/b415425h. [DOI] [PubMed] [Google Scholar]
- 30.Juhasz M, Hoffmann S, Stoyanov E, Kim K-C, Reed CA. Angew. Chem. Int. Ed. 2004;43:5352–5355. doi: 10.1002/anie.200460005. [DOI] [PubMed] [Google Scholar]
- 31.Stoyanov ES, Kim K-C, Reed C. A. J. Am. Chem. Soc. 2006;128:8500–8508. doi: 10.1021/ja060714v. [DOI] [PubMed] [Google Scholar]
- 32.Armarego WLF, Perrin DD. Purification of Laboratory Chemicals. 4th Edition. Oxford: Educational and Professional Publishing Ltd; 1996. [Google Scholar]
- 33.Stoyanov ES, Stoyanova IV, Reed C. A. Chem. Eur. J. 2008;14:3596–3604. doi: 10.1002/chem.200701746. [DOI] [PubMed] [Google Scholar]
- 34.Stoyanov ES. Phys. Chem. Chem. Phys. 1999;1:2961–2966. [Google Scholar]
- 35.a Staverman AJ. Recl. Trav. Chim. Pays-Bas. 1941;60:836–841. [Google Scholar]; b Barr RS, Newsham DMT. Fluid Phase Equilibr. 1987;35:189–205. [Google Scholar]
- 36.Odinokov SE, Iogansen AV. Spectrochim. Acta. 1972;28A:2343–2350. [Google Scholar]
- 37.Glazunov VP, Mashkovskii AA, Odinokov SE. Zr. Prikl. Spektroskopii. 1975;22:696–702. [Google Scholar]
- 38.Mashkovskii AA, Glazunov VP, Odinokov SE. Zr. Prikl. Spektroskopii. 1974;20:852–856. [Google Scholar]
- 39.Baran J, Lis T, Ratajczak H. J. Mol. Struct. 1989;195:159–174. [Google Scholar]
- 40.Bertoluzza A, Battaglia MA, Bonora S, Monti H. J. Mol. Struct. 1985;127:35–45. [Google Scholar]
- 41.Baran J. J. Mol. Struct. 1987;162:211–228. [Google Scholar]
- 42.Baran J. J. Mol. Struct. 1987;162:229–245. [Google Scholar]
- 43.Detoni S, Hadzi D. J. Chem. Phys. 1956;53:760–764. [Google Scholar]
- 44.Stoyanov ES, Popov VM, Mikhailov VA. Zr. Prikl. Spektroskopii. 1984;40:77–84. [Google Scholar]
- 45.Asfin RA, Denisov GS, Tokhadze KG. J. Mol. Struct. 2002;608:161–168. [Google Scholar]
- 46.Solka JL, Reis AH, Mason GW, Lewey SM, Peppard DF. J. Inorg. Nucl. Chem. 1978;40:663–668. [Google Scholar]
- 47.Gebert E, Reis AH, Peterson SW, Katzin LI, Mason GW, Peppard DF. J. Inorg. Nucl. Chem. 1981;43:1451–1464. [Google Scholar]
- 48.Brzezinski B, Maciejewska H, Zundel G, Kramer R. J. Phys. Chem. 1990;94:528–531. [Google Scholar]
- 49.Brzezinski B, Maciejewska H, Zundel G. J. Phys. Chem. 1990;94:6983–6986. [Google Scholar]
- 50.Hadzi D, Kobilarov N. J. Chem.Soc. A. 1966:439–445. [Google Scholar]
- 51.Hofacker GL, Marechal Y, Ratner MA. In: The Hydrogen Bond. Shuster P, Zundel G, Sandorfy C, editors. Vol. 1. North-Holland: Amsterdam; 1976. pp. 683–766. [Google Scholar]
- 52.Claydon MF, Sheppard N. J. Chem. Soc. D. 1969:1431–1433. [Google Scholar]
- 53.Bratos S, Lascombe J, Novak A. In: Molecular Interactions. Ratajczak H, Orvill-Thomas WJ, editors. Wiley Chichester; 1980. pp. 301–346. [Google Scholar]
- 54.Stasko D, Hoffmann SP, Kim K-Ch, Fackler NLP, Larsen AS, Drovetskaya T, Tham FS, Reed CA, Rickard CEF, Boyd PDW, Stoyanov ES. J. Am. Chem. Soc. 2002;124:13869–13876. doi: 10.1021/ja012671i. [DOI] [PubMed] [Google Scholar]
- 55.Calleja M, Johnson K, Belcher WJ, Steed JW. Inorg. Chem. 2001;40:4978–4985. doi: 10.1021/ic010468i. [DOI] [PubMed] [Google Scholar]
- 56.Lundgren J-O, Olovsson I. J. Chem. Phys. 1968;49:1068–1074. [Google Scholar]
- 57.Mootz D, Oellers E-JZ. Anorg. Allg. Chem. 1988;559:27–39. [Google Scholar]
- 58.Head-Gordon T, Hura G. Chem. Rev. 2002;102:2651–2670. doi: 10.1021/cr0006831. [DOI] [PubMed] [Google Scholar]
- 59.Christie RA, Jordan KD. J. Phys. Chem. B. 2002;106:8376–8381. [Google Scholar]
- 60.Xie Z, Bau R, Reed CA. Inorg. Chem. 1995;34:5403–5404. [Google Scholar]
- 61.Bell RA, Christoph GG, Fronczek FR, Marsh RE. Science. 1975;190:151–152. [Google Scholar]
- 62.Reis AH, Peterson SW, Druyan ME, Gebert E, Mason GW, Peppard DF. Inorg. Chem. 1976;15:2748–2752. [Google Scholar]
- 63.Stoyanov ES. Phys. Chem. Chem. Phys. 2000;2:1137–1145. [Google Scholar]
- 64.Sobolewski AL, Domcke W. J. Phys. Chem. A. 2002;106:4158–4167. [Google Scholar]
- 65.Bush V, Dubrovsky A, Mohamed F, Parrinello M, Sadlej J, Hammerich AD, Devlin JP. J. Phys. Chem. A. 2008;112:2144–2161. doi: 10.1021/jp076391m. [DOI] [PubMed] [Google Scholar]
- 66.Heuft JM, Meijer E. J. Phys. Chem. Chem. Phys. 2006;8:3116–3123. doi: 10.1039/b603059a. [DOI] [PubMed] [Google Scholar]