Summary
LKB1 kinase is a tumor suppressor that is causally linked to Peutz-Jeghers (PJS) syndrome [1]. In complex with the pseudokinase STRAD and the scaffolding protein MO25, LKB1 phosphorylates and activates AMPK family kinases, which mediate many cellular processes [2, 3]. The prototypical family member AMPK regulates cell energy metabolism [4] and epithelial apico-basal polarity [5, 6]. This latter event is also dependent on E-cadherin-mediated adherens junctions (AJs) at lateral borders [7, 8]. Strikingly, overexpression of LKB1/STRAD can also trigger establishment of epithelial polarity in the absence of cell-cell or cell-matrix contacts [9]. However, the upstream factors that normally govern LKB1/STRAD function are unknown. Here, we show by immunostaining and fluorescence resonance energy transfer that active LKB1/STRAD kinase complex co-localizes with E-cadherin at AJs. LKB1/STRAD localization and AMPK phosphorylation require E-cadherin-dependent maturation of AJs. However, LKB1/STRAD complex kinase activity is E-cadherin-independent. These data suggest that in polarized epithelial cells, E-cadherin regulates AMPK phosphorylation by controlling the localization of the LKB1 complex. The LKB1 complex therefore appears to function downstream of E-cadherin in tumor suppression.
Results and Discussion
LKB1/STRAD kinase complex localizes at the level of adherens junctions
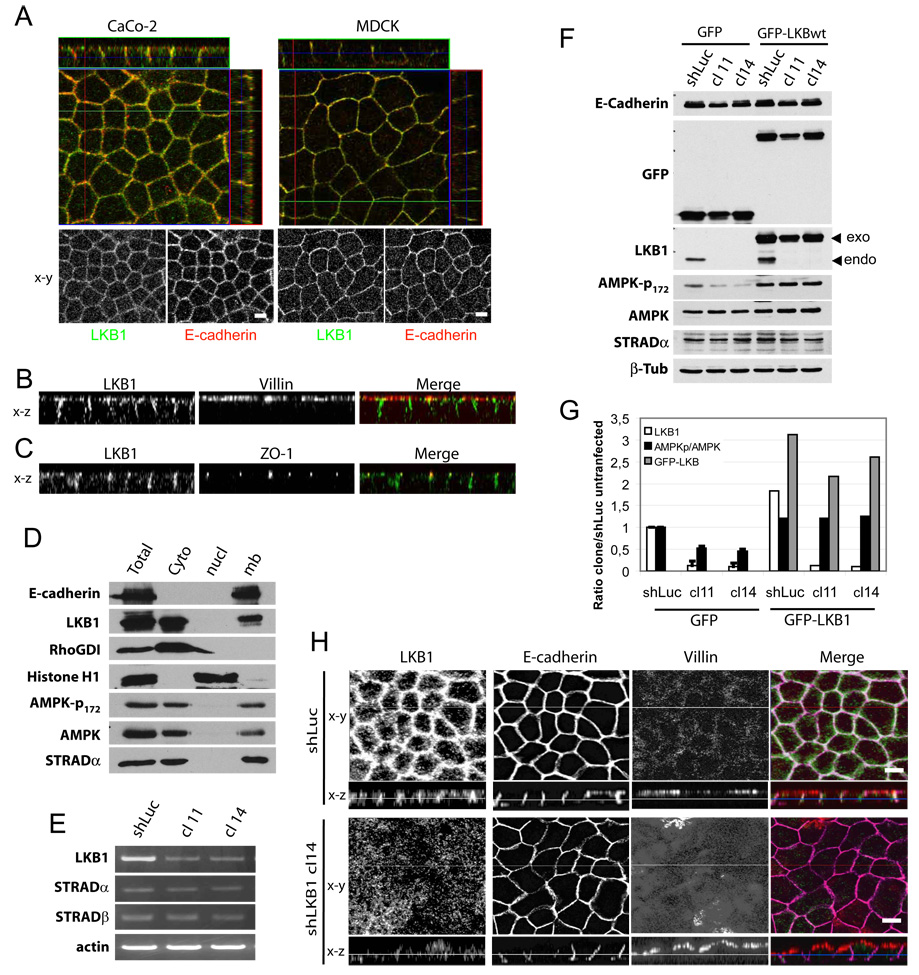
To investigate regulation of the LKB1/STRAD complex, we first examined its localization in polarized epithelial cells. Immunostained endogenous LKB1 localized predominantly at cell-cell junctions in polarized Caco-2 and MDCK II cells (fig 1A, G). Z axis projections showed that LKB1 staining was mainly present at adherens junctions where it co-localized with E-cadherin. LKB1 was minimal at both the apical domain, marked by villin, and the basal domain (fig 1B). Endogenous LKB1 was also poorly localized at tight junctions as marked by ZO-1 (fig 1C). To our knowledge, this is the first report of endogenous LKB1 localization in polarized epithelial cells.
Figure 1. LKB1 localizes at adherens junction level in polarized epithelial cells.
(A) Polarized Caco-2 stained for LKB1 (green) and E-cadherin (red). (B) xz projection of polarized Caco-2 cells stained for LKB1 (green) and villin (red) or (C) for LKB1 (green) and ZO-1 (red). (D) Polarized Caco-2 cells were fractionated as described in “experimental procedures” and analyzed for indicated proteins. Results are representative of two independent experiments. (E) MDCK clones stably knocked down for LKB1 (cl) by shRNA or control construct (shLuc) transiently transfected by either GFP alone or GFP-LKB1wt were analysed by Western blotting for indicated proteins. (F) AMPKp-172/total AMPK ratio (black bar) were determined and plotted with endogenous (endo, white bar) and exogenous (exo, grey bar) LKB1, all relative to control. Values are means ± S.D., (n=2). (G) Polarized MDCK shLuc and shLKB1 cl 14 were stained for LKB1 (green), E-cadherin (purple) and villin (red). Bars, 10µm.
Previous studies showed a small amount of endogenous LKB1 in membrane fractions [10]. In other work, overexpressed LKB1 distributed between the cytoplasm and nucleus [10–12]. Those studies proposed that LKB1 was a nuclear protein that re-localized to the cytoplasm in a STRAD-dependent manner [2, 11]. Since our immunostaining did not detect any nuclear LKB1, we analyzed its distribution by fractionating polarized Caco-2 cells. LKB1 was completely absent from the nuclear fraction (characterized by histone H1) but was abundant in the cytosolic and membrane fractions (characterized by RhoGDI1 and E-cadherin, respectively; Figure 1D), much like STRADα and the LKB1 substrate AMPK, as previously described for this latter [13]. We did observe that over-expressed LKB1 was partially nuclear (fig S2A). However, in the polarized or nonpolarized epithelial cell lines that we examined, endogenous LKB1 was not detected in the nucleus. These cells presumably have sufficient levels of STRAD to allow endogenous LKB1 to be constitutively cytoplasmic.
To confirm the immunolocalization, we prepared stable MDCK clones in which LKB1 was depleted using small hairpin RNAs (shRNA). Clones 11 and 14 showed large decreases in LKB1 at mRNA (not shown) and protein level (figures 1E) whereas E-cadherin and STRAD were unaffected. Recent papers described in drosophila a key role for AMPK, in epithelial polarity [5, 6]. We evaluated AMPK phosphorylation on threonine 172 (AMPK-T172), the site of LKB1 phosphorylation. Clone 14, in which LKB1 was depleted by ∼80% exhibited a ∼50% decrease in AMPK-T172 phosphorylation (fig 1F). Activation of AMPK by other kinases [14, 15] may account for this modest discrepancy. AMPK phosphorylation was rescued by transient transfection of GFP-tagged human LKB1 wild type (insensitive to canine shRNA) (figure 1E and quantified figure 1F.) In LKB1-depleted cells, staining for LKB1 was also greatly reduced, indicating that the signal was specific (fig 1G). Interestingly, E-cadherin and villin appeared less regular after LKB1 suppression, though their polarity appeared largely unaltered (fig 1G). Figure S1 shows comparable results in Caco-2 cells using the shRNA construct described in [9].
Since LKB1 membrane localization was reported to be dependant on its C-terminal CAAX sequence that serves as a site for prenylation [10, 16], we localized GFP-tagged WT LKB1 vs. a non-prenylated mutant (LKB1-CA) in MDCK cells. As described [2, 11] and mentioned above, GFP- LKB1-WT localized to the nucleus. Similar to endogenous LKB1, it was also at cell-cell contacts with E-cadherin, but little was localized to tight junctions (fig S2A). By contrast, GFP-LKB1-CA was diffusely distributed in the cytoplasm and the nucleus, with much less at the membrane (fig S2B, C). We conclude that in polarized epithelial cells, endogenous LKB1 localizes mainly at adherens junctions, with membrane association being prenylation-dependent.
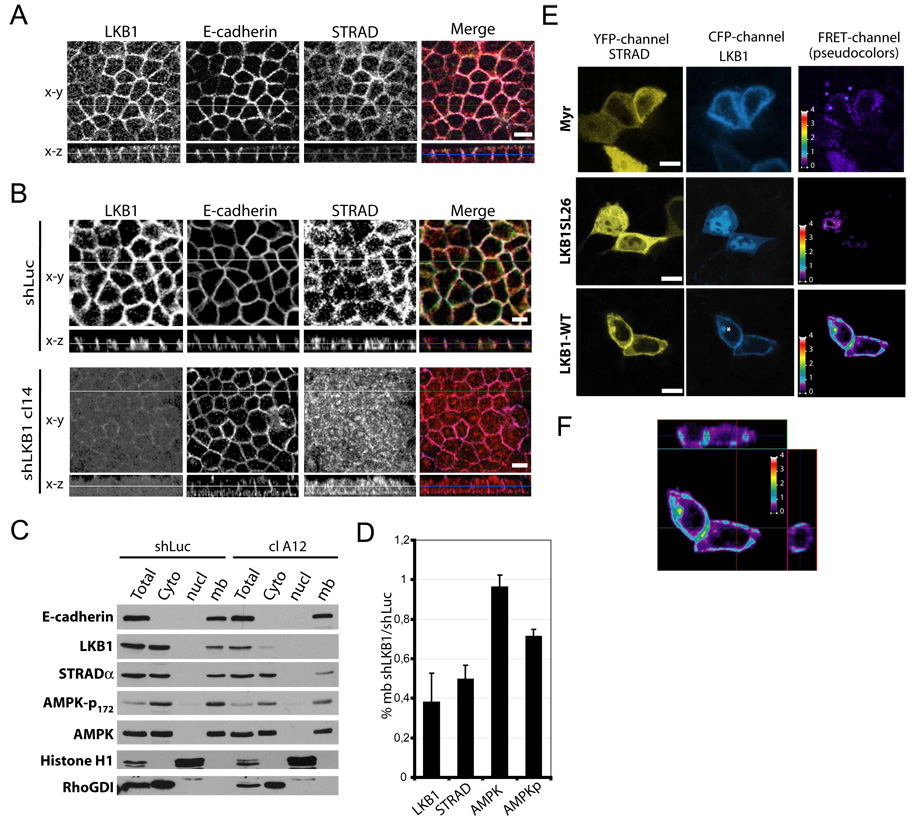
Since the LKB1 complex includes STRAD and Mo25 [2, 11], we wondered whether STRAD also localized to adherens junctions. We therefore raised a rabbit polyclonal antibody against the STRADα C-terminus. Western blotting showed that the antibody specifically recognized an endogenous band in MDCK and Caco2 cells at 45kD, which co-migrated with over-expressed Flag-STRADα (fig S3A). When MDCK cells transiently transfected with N-terminally Flag-tagged STRAD were fixed and stained, (fig S3B), we observed a bright and diffuse cytoplasmic signal for both Flag and STRAD in transfected cells, in agreement with previous reports [2, 11]. By contrast, almost all untransfected cells exhibited cortical membrane staining. Staining was completely abolished by an excess of soluble immunogenic protein, though Flag staining was unaffected. Staining for endogenous STRADα, LKB1 and E-cadherin in polarized Caco-2 (fig S1E) or MDCK cells (fig 2A, shLuc) showed that these proteins substantially co-localized. As with endogenous LKB1, almost no STRAD signal was detected at tight junctions (not shown) or at the apical domain (fig S1E and fig 2A, shLuc). Importantly, LKB1-depleted cells exhibited mainly cytoplasmic STRADα staining, suggesting that STRAD localization at adherens junctions depends on LKB1. Fractionation of control shLuc or LKB1-depleted Caco-2 cells, LKB also showed decreased STRADα at the membrane after LKB1 depletion (fig 2B,C). Total STRADα expression and AMPK distribution were unaffected. These results show that in polarized epithelial cells, STRADα localizes at adherens junctions in an LKB1-dependent manner.
Figure 2. STRAD localizes with LKB1 at adherens junction level.
(A) Polarized MDCK shLuc or shLKB1 cl 14 cells were stained for LKB1 (green), E-cadherin (purple) and STRAD (red). (B) Polarized Caco-2 clone knocked down for LKB1 (cl A12) or not (shLuc) were fractionated as in figure 1. (C) Membranous shLKB1 cl A12/shLuc ratio for LKB1, STRADα, total or phosphorylated AMPK, normalized to E-cadherin. Values are means ± S.D., (n=3). (D) MDCK cells on fibronectin-coated coverslips were transiently transfected with YFP-STRADα (yellow) plus either CFP-Myr, wild type or SL26 CFP-LKB1 (blue). FRET was measured as described in “Experimental Procedures”. The asterisks mark an unidentified perinuclear compartment. (E) Projections of FRET images from CFP-LKB1-WT and YFP-STRADα. FRET intensity is displayed on a pseudocolor scale in arbitrary units. Bars, 10µm.
Active LKB1/STRAD kinase complex at adherens junctions
Whether LKB1 at adherens junctions is bound to STRAD is an important question since this interaction is required for LKB1 kinase activity [2, 11]. We therefore evaluated the proximity of LKB1 and STRADα using fluorescence resonance energy transfer (FRET), which provides higher spatial resolution than co-localization. LKB1 and STRAD were tagged with CFP and YFP respectively. As a negative control, we used the SL26 LKB1 mutation, isolated from a Peutz-Jeghers patient [17], which does not bind STRAD [11]. We also used CFP fused to the Fyn kinase myristoylation and palmitoylation membrane localization sequence (CFP-Myr) [18]. These constructs expressed equally well in MDCK cells (fig S4A). As expected, YFP-STRAD co-immunoprecipitated (IPed) with WT CFP-LKB1 but not with SL26 LKB1, CFP-Myr or CFP alone after immunoprecipitating LKB1 using limiting antibody to minimize IP of endogenous LKB1 (fig S4B). YFP-STRAD co-IP was observed by probing with an anti-GFP antibody or with anti-STRAD sera. Next, we analyzed these precipitates for LKB1 kinase activity using a recombinant AMPK fragment and probing for phospho-T172 [19]. LKB1 kinase activity was elevated only when WT LKB1 and STRAD were co-expressed, whereas all others conditions showed poor kinase activity (Fig S4B). As previously reported [10–12], high LKB1 kinase activity was associated with a shift in the LKB1 band to lower mobility, which was attributed to serine/threonine autophosphorylation, providing further evidence for activation. In the absence of transfected LKB1, endogenous LKB1 showed little kinase activity and no detectable associated YFP-STRAD at these exposures. Thus, endogenous LKB1 makes a negligible contribution compared to the transfected protein under these conditions.
FRET was assessed in cells transfected with YFP-STRAD plus CFP-LKB1 or controls (fig 2D). Substantial FRET was observed when WT constructs were co-expressed, whereas minimal FRET was detected with CFP-LKB1-SL26 or CFP-Myr (fig 2D). FRET was highest at lateral membranes, indicating that LKB1 and STRAD are within ∼5 nm in this compartment. Little FRET was detected at apical or basal domains as shown in the Z axis projections (fig 2E). We also observed increased membrane localization of STRAD when co-expressed with WT LKB1 but not the SL26 LKB1 (Fig 2D). Some cells showed accumulation of both probes at a perinuclear localization where FRET was high (cf asterix). This location was not evident with endogenous proteins and was not further evaluated. As a further control, FRET was tested by acceptor photobleaching [20], (Fig S4D) which showed that after YFP-STRAD was bleached, CFP-LKB1 intensity inside the bleached area increased about 8% when normalized to the unbleached control area. These data are consistent with a physical interaction between LKB1 and STRAD at cell-cell junctions, suggesting that LKB1 kinase is likely to be active at this location.
LKB1/STRADα recruitment to adherens junctions level depends on E-cadherin
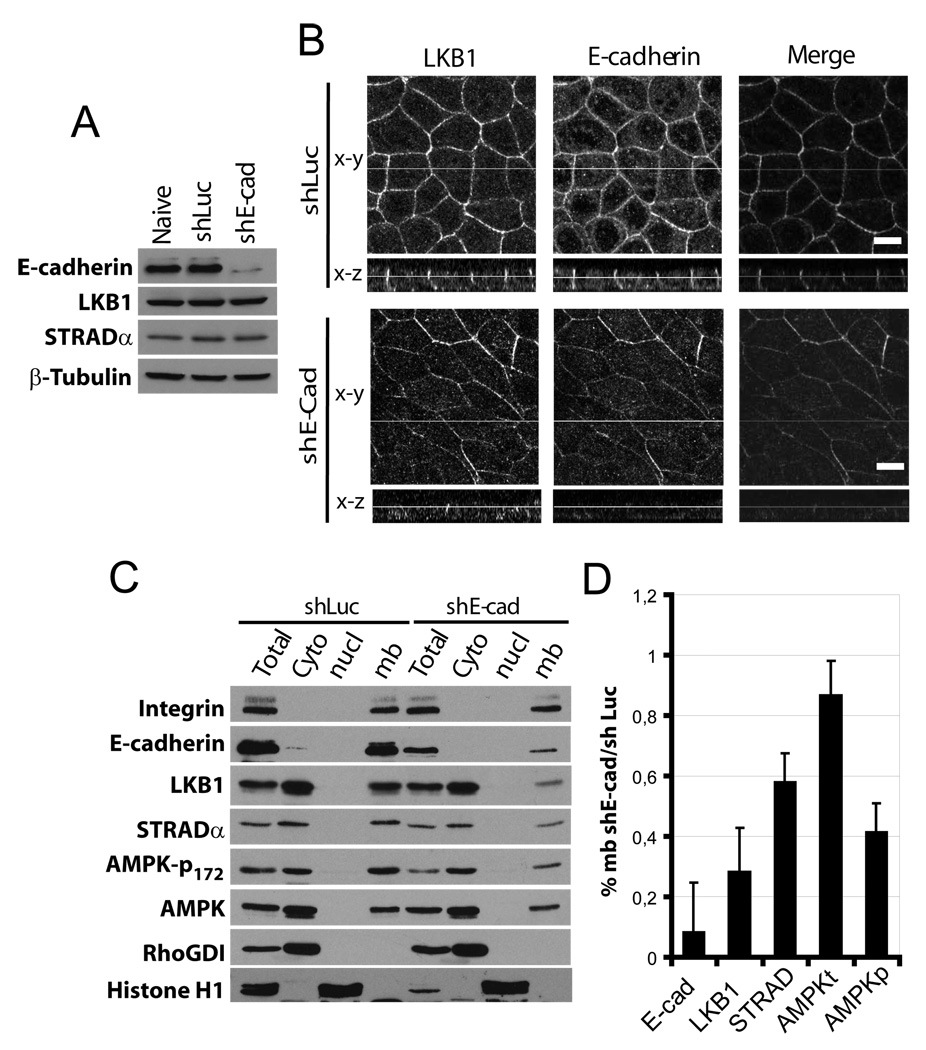
To test whether the lateral LKB1/STRADα localization is dependent on E-cadherin, we stably depleted E-cadherin in MDCK cells using a previously described shRNA [21]. Knockdown efficiency determined by Western blotting was ∼90% (fig 3A), whereas LKB1 and STRADα expression levels were not affected. In these cells, LKB1 immunostaining at cell-cell junctions decreased concomitant with loss of E-cadherin (fig 3B). Similar results were also observed using transient E-cadherin knockdown (not shown). Fractionation of these cells showed that loss of E-cadherin led to decreased LKB1 and STRADα at the membrane (figure 3C, D). As a further test, we examined human colon carcinoma SW480 cells that express very little E-cadherin, and two stably transfected SW480 clones that express higher levels of E-cadherin [22]. Membrane localization of transiently transfected GFP-LKB1 was minimal in SW480 cells but increased in E-cadherin overexpressing clones SW6A2 and SW8C1 (fig S5A,B,C). GFP alone showed only slight membrane localization. Taken together, these data show that LKB1/STRADα lateral membrane localization in epithelial cells requires E-cadherin. However, the fact that E-cadherin adherens junction formation occurs earlier than LKB1 membrane localization (fig S5D) suggests that E-cadherin ligation alone is not sufficient for LKB1 recruitment.
Figure 3. E-cadherin is required to localize LKB1 at adherens junctions level.
(A) MDCK cells expressing control (shLuc) or E-cadherin (shE-cad) shRNA vectors were analysed by Western blot for the indicating proteins. (B) Cells were stained for LKB1 (green) and E-cadherin (red). Bars, 10µm. (C) MDCK cells described in A were fractionated as described in figure 1, (D) Ratio of membranous shE-cad/shLuc of LKB1, STRADα, total or phosphorylated AMPK, normalized to integrin. Values are means ± S.D., (n=2).
E-cadherin effects on LKB1/STRAD kinase activity and function
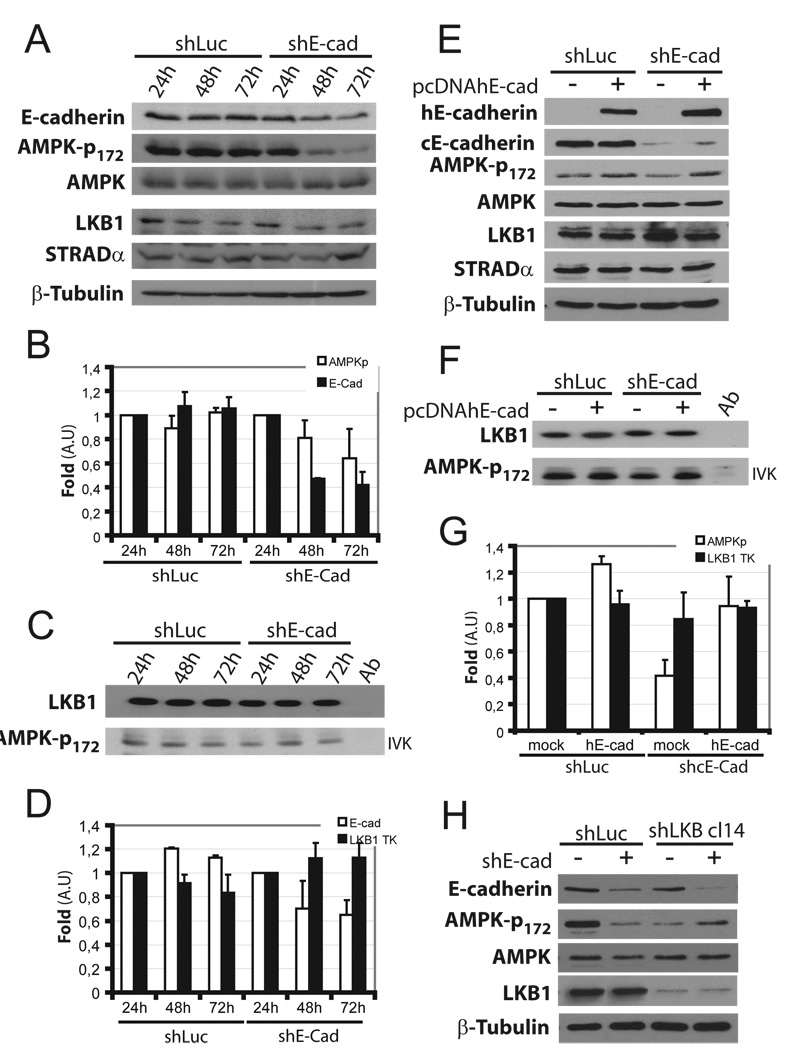
Since MDCK cells depleted for E-cadherin had decreased AMPK-T172 phosphorylation (Fig 3), we analyzed AMPK activity in these cells. Transient transfection with E-cadherin shRNA led to a time-dependent decrease of E-cadherin over 72h, whereas LKB1, STRAD and AMPK levels were unchanged (Fig 4A,B). AMPK T172 phosphorylation decreased as well. However, when LKB1 was IPed and kinase activity assessed in vitro, no change in kinase activity was observed (fig 4C and D). Re-expression of shRNA resistant human E-cadherin restored AMPK phosphorylation (fig 4E, G) without increasing LKB1 intrinsic kinase activity (fig 4F, G). This effect was LKB1-dependent since E-cadherin had no further effect on AMPK phosphorylation in stable MDCK LKB1 knock down cells. (fig 4H). Taken together, these results show that E-cadherin controls LKB1-dependent AMPK phosphorylation without a detectable effect on LKB1/STRAD complex intrinsic kinase activity.
Figure 4. E-cadherin knockdown decrease AMPK activation.
(A) MDCK cells were transiently transfected with control (shLuc) or shRNA targetting canine E-cadherin (shE-cad). Cells were extracted at indicated times and analyzed by Western blot for indicated proteins. (B) T172 AMPK phosphorylation and E-cadherin levels were quantified and normalized to total AMPK. Values are percent relative to levels in shLuc or shE-cad cells at 24h (means ± S.D. n=3). pAMPK: white bars; E-cadherin: black bars. (C) The same lysates were immunoprecipitated with anti-LKB1 and kinase activity analyzed in vitro (IVK). (D) Kinase activity was quantified and normalized to total immunoprecipitated LKB1, plotted as described in B (means ± S.D. n=4). Control (shLuc) and E-cadherin-depleted MDCK cells were transiently transfected or not with wild type human E-cadherin (pcDNAhE-cad). After 24h cells, were extracted and treated as in A and C (E, F respectively) and quantified (G). T172 AMPK phosphorylation in cells (white bar), LKB1 IP kinase activity (TK) (black bar), control shLuc cells mock transfected was used as reference (means ± S.D. n=3). (H) Stable control (shLuc) and LKB1 depleted (shLKB cl14) MDCK cells were transiently transfected or not with shRNA targeting canine E-cadherin (shE-cad). After 72h, cells were lysed and analyzed by Western blotting with the specified antibodies (n=2).
In conclusion, we show that the active LKB1/STRADα kinase complex localizes to adherens junctions in an E-cadherin dependent manner in epithelial cells, which promotes phosphorylation of the LKB1 substrate AMPK. However, available evidence does not suggest a direct physical connection between E-cadherin and LKB1. LKB1 localization coincides with junctional maturation rather than initial formation. Maturation requires E-cadherin but clearly involves other components. Additionally, localization to cell-cell junctions requires LKB1 prenylation, which argues against a high affinity interaction. Thus, further work will be required to elucidate the mechanism by which LKB1 targets to cell-cell contacts and other potential locations.
Activated AMPK was recently described to promote epithelial polarity in drosophila [5, 6] and to negatively regulate the mTOR pathway [15], which is often upregulated in human tumors. Loss of E-cadherin in cancer is associated with poor prognosis through increased metastasis, although the mechanisms remain incompletely defined [23, 24]. Signalling through the Wnt/β-catenin pathway clearly contributes, as do other transcriptional pathways [23, 25]. Moreover, direct inhibition of cell migration by E-cadherin-dependent adhesion may also be involved. However, these effects may not fully explain the link to cancer. Given the known tumour suppressor activity of LKB1 and its role in promoting metastases [1, 26, 27], our results predict that loss of E-cadherin may promote tumorigenesis in part through inhibition of LKB1 function, which would lead to increased mTOR activity as well as contributing to the loss of polarity. Future work is warranted to test this hypothesis, better define the mechanisms regulating LKB1 localization by E-cadherin and explore its role in human cancers.
Supplementary Material
Acknowledgments
We warmly thank Drs. Barry Gumbiner, Gary D. Lopaschuk, Hans Clevers and Ian G. Macara for providing constructs. Antibodies RR1 and E7 were from the Developmental Studies Hybridoma Bank. We also thank A Pacheco and K Moissoglu for helpful discussions. This work was supported by USPHS grant RO1 GM47214 to M.A.S. and by Ligue Nationale Contre le Cancer (Label Ligue 2007), Inserm and Institut Paoli-Calmettes to J-P.B. M.S. is supported by “La Ligue Nationale Contre le Cancer”.
References
- 1.Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007;26:7825–7832. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- 2.Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, Prescott AR, Clevers HC, Alessi DR. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. Embo J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, Chung J. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 6.Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol. 2007;177:387–392. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol. 2000;1:91–100. doi: 10.1038/35040042. [DOI] [PubMed] [Google Scholar]
- 9.Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 10.Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, Morrice N, Deak M, Alessi DR. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth. J Biol Chem. 2001;276:19469–19482. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- 11.Baas AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA, Alessi DR, Clevers HC. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. Embo J. 2003;22:3062–3072. doi: 10.1093/emboj/cdg292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nezu J, Oku A, Shimane M. Loss of cytoplasmic retention ability of mutant LKB1 found in Peutz-Jeghers syndrome patients. Biochem Biophys Res Commun. 1999;261:750–755. doi: 10.1006/bbrc.1999.1047. [DOI] [PubMed] [Google Scholar]
- 13.Mitchelhill KI, Michell BJ, House CM, Stapleton D, Dyck J, Gamble J, Ullrich C, Witters LA, Kemp BE. Posttranslational modifications of the 5'-AMP-activated protein kinase beta1 subunit. J Biol Chem. 1997;272:24475–24479. doi: 10.1074/jbc.272.39.24475. [DOI] [PubMed] [Google Scholar]
- 14.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 15.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 16.Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 17.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelin K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 18.Moissoglu K, Slepchenko BM, Meller N, Horwitz AF, Schwartz MA. In vivo dynamics of Rac-membrane interactions. Mol Biol Cell. 2006;17:2770–2779. doi: 10.1091/mbc.E06-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altarejos JY, Taniguchi M, Clanachan AS, Lopaschuk GD. Myocardial ischemia differentially regulates LKB1 and an alternate 5'-AMP-activated protein kinase kinase. J Biol Chem. 2005;280:183–190. doi: 10.1074/jbc.M411810200. [DOI] [PubMed] [Google Scholar]
- 20.Siegel RM, Chan FK, Zacharias DA, Swofford R, Holmes KL, Tsien RY, Lenardo MJ. Measurement of molecular interactions in living cells by fluorescence resonance energy transfer between variants of the green fluorescent protein. Sci STKE. 2000;2000:PL1. doi: 10.1126/stke.2000.38.pl1. [DOI] [PubMed] [Google Scholar]
- 21.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrais M, Chen X, Perez-Moreno M, Gumbiner BM. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell. 2007;18:2013–2025. doi: 10.1091/mbc.E06-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 24.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 25.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 26.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang MC, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR, Padera RF, Bronson RT, Lindeman NI, Christiani DC, Lin X, Shapiro GI, Janne PA, Johnson BE, Meyerson M, Kwiatkowski DJ, Castrillon DH, Bardeesy N, Sharpless NE, Wong KK. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 27.Katajisto P, Vallenius T, Vaahtomeri K, Ekman N, Udd L, Tiainen M, Makela TP. The LKB1 tumor suppressor kinase in human disease. Biochim Biophys Acta. 2007;1775:63–75. doi: 10.1016/j.bbcan.2006.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.