Abstract
This study investigated a possible role for ventral hippocampal corticotropin-releasing factor (CRF) in modulating both unconditioned and conditioned defensive behaviors by examining the effects of pre-training ventral hippocampal ovine-CRF (oCRF) or acidic-astressin ([Glu11,16]Ast) microinfusions in male Long Evans hooded rats exposed to various threat stimuli including the elevated plus-maze (EPM) (oCRF), cat odor (oCRF and [Glu11,16]Ast) and a live cat ([Glu11,16]Ast). Unconditioned defensive behaviors were assessed during threat exposure, while conditioned defensive behaviors were assessed in each predator context 24 h after the initial threat encounter. Pre-training infusions of the CRF1 and CRF2 receptor agonist oCRF significantly increased defensive behaviors during both the unconditioned and conditioned components of the cat odor test, as well as exposure to the EPM. In contrast to the behavioral effects of oCRF microinfusions, the CRF1 and CRF2 receptor antagonist [Glu11,16]Ast significantly decreased defensive behaviors during exposure to cat odor, while producing no discernible effects following a second injection in the cat exposure test. During conditioned test trials, pre-training infusions of [Glu11,16]Ast also significantly reduced defensive behaviors during re-exposure to both predator contexts. These results suggest a specific role for ventral hippocampal CRF receptors in modulating anxiety-like behaviors in several ethologically relevant animal models of defense.
Keywords: CRF, Hippocampus, Defense, Anxiety, Fear, Conditioning, Predator, EPM, Rats, Stress
Introduction
Small mammalian prey species such as rats and mice have developed specific species-typical antipredator defensive behaviors to facilitate predator detection (e.g., risk assessment) and increase avoidance (e.g., flight, freezing) of threat sources (Apfelbach et al., 2005; Blanchard and Blanchard, 2003, 1989; Blanchard and Blanchard, 1989). In laboratory settings, evaluation of antipredator defensive behaviors allows for an ethological analysis of fear and anxiety by exposing animals directly to a natural predator or potential threat sources, respectively. The defensive behaviors associated with these ethologically relevant threat stimuli can be divided into two broad categories based upon the intensity and/or ambiguity of the threat stimulus. In the presence of an immediate threat source, such as a live cat, flight (if possible) or freezing is the primary response pattern, while confrontation with ambiguous/potential threats, such as cat odor or predator-paired contexts, elicits risk assessment (Blanchard et al., 1989; Blanchard and Blanchard, 1989, 1988). Those defensive behaviors evoked during predator exposure have been used as an index of fear, while risk assessment behaviors, responsive to standard anxiolytics such as diazepam and 5-HT1A agonists, have been used as an index of anxiety (Blanchard et al., 2003, 2001; Griebel, et al., 1999a, 1999b).
Recent studies aimed at examining the neurocircuitry controlling the expression of unconditioned defensive behaviors have begun to focus on a possible modulatory role for the ventral hippocampus (VH) (Bertoglio et al., 2006; Pentkowski et al., 2006). Pentkowski and colleagues (2006) demonstrated that excitotoxic VH lesions failed to reliably alter behavioral responses to a live cat, but reduced defensiveness to its odor, suggesting a preferential role in mediating certain anxiety-like behaviors. In support of this assertion, VH lesions using either lidocaine (Bertoglio et al., 2006), tetrodotoxin (Degroot and Treit, 2004), ibotenic acid (Kjelstrup et al., 2002) or electrolytic techniques (Trivedi and Coover, 2004; Bannerman et al., 2002), all produced anxiolytic-like profiles in the elevated plus-maze (EPM). Concurrently, numerous VH lesion studies have demonstrated anxiolytic-like effects in the shock-probe burying, social interaction, light/dark and hyponeophagia tests of defense (Bannerman et al., 2003; Degroot & Treit, 2004; McHugh et al., 2004), with these results resembling the anxiolytic-like effects induced by benzodiazepines in many of the same behavioral tests (Menard & Treit, 1999; Gray & McNaughton, 2000). Collectively these results indicate that the neural circuitry mediating the expression of unconditioned defensive behaviors includes the VH.
In addition to modulating defensive responses to unconditioned threat stimuli, the VH also contributes to the expression of conditioned fear. Pre-training VH ibotenic acid lesions reduced the expression of contextual fear 24 h after exposure to cat odor, a live cat or footshock (Pentkowski et al., 2006). Similarly, reversible VH lesions using tetrodotoxin, the GABA receptor agonist muscimol (Bast et al., 2001) or the NMDA receptor antagonist MK-801 (Zhang et al., 2001), all reduced the expression of conditioned fear, while permanent pre-training excitotoxic VH lesions produced deficits in cue elicited conditioned freezing (Richmond et al., 1999). Collectively, these findings suggest that in addition to a role in modulating unconditioned defense behaviors, the VH may subserve the expression of conditioned fear.
The neuropeptide corticotropin-releasing factor (CRF) represents a possible mediator of the behavioral responses modulated by the VH during the aforementioned tests of defense. CRF is a 41 amino-acid peptide that functions to mediate the autonomic, behavioral, endocrine and immune responses to stress, via actions at two G protein-coupled receptor subtypes termed CRF1 and CRF2 (Hillhouse and Grammatopoulos, 2006; Carrasco and Van de Kar, 2003; Spiess et al.,1981). In rats, central CRF1 receptor mRNA expression is found throughout the isocortex, cerebellum, periaqueductal gray, amygdala and hippocampus, whereas CRF2 receptors are located predominantly in the lateral septum, ventromedial hypothalamus, bed nucleus of the stria terminalis, dorsal raphe nucleus and olfactory bulb, with moderate levels located throughout the hippocampus (Van Pett et al., 2000; Chalmers et al., 1995).
Overall, evidence has been relatively consistent for a role of CRF1 receptors in modulating anxiety-like defensive behaviors. Intracerebroventricular (i.c.v.) administration of the CRF agonists human/rat CRF (h/rCRF) or ovine-CRF (oCRF) increased fear or anxiety-like defensive behaviors during the acoustic startle (Risbrough et al., 2004), defensive withdrawal (Heinrichs and Joppa, 2001) and social interaction (Campbell et al., 2004) tests. Antalarmin, a non-peptidic CRF1 receptor antagonist, blocked the anxiogenic-like effects of CRF in the EPM, decreased defensive withdrawals in a brightly lit open field, and blocked the motor-activating effects of CRF without causing sedation (Zorrilla et al., 2002), indicating heightened defensiveness in response to central CRF1 receptor activation (Bale and Vale, 2004). However, while the central effects of CRF1 receptors appear consistent, the behavioral effects of site-specific microinjections of various CRF compounds have produced conflicting results (Todorovic et al., 2005; Bale and Vale, 2004). Injections of urocortin I, a CRF1 and CRF2 receptor agonist, into the basolateral amygdala (BLA) produced anxiogenic-like effects in the social interaction test (Spiga et al., 2006), while dorsal hippocampal and lateral septum infusions of h/rCRF were found to enhance and impair conditioned freezing (Radulovic et al., 1999), respectively. Collectively these results suggest that global activation or inactivation of CRF1 receptors via i.c.v. administration increases and decreases defensive behavior, respectively, while indicating regional specificity for the behavioral role of CRF1 receptors (Henry et al., 2006; Radulovic et al., 1999).
In contrast to the consistent anxiogenic-like effects produced by central CRF1 receptor activation, the precise role of CRF2 receptors remains less clear (Todorovic et al., 2005; Bale and Vale, 2004). Activation of CRF2 receptors via i.c.v. injections of urocortin II, a preferential CRF2 receptor agonist, produced delayed anxiolytic-like effects in the EPM (Valdez et al., 2002), open field and light/dark (Venihaki et al., 2004) tests. Conversely, central urocortin II administration enhanced anxiogenic-like responses in the EPM, marble burying and open field tests of defense (Pelleymounter et al., 2002, 2004). The behavioral effects of centrally administered CRF2 receptor antagonists also appear to be inconsistent, as central infusions of the preferential CRF2 receptor antagonist antisauvagine (aSVG-30) or inhibition of CRF2 receptor expression with antisense oligonucleotides both reduced conditioned freezing and produced anxiolytic-like effects in the EPM and defensive withdrawal tests (Ho et al., 2001). However, i.c.v. infusions of aSVG-30 have also been shown to produce an anxiogenic profile similar to CRF2 receptor knockouts (Todorovic et al., 2005; Kishimoto et al., 2000). These behavioral differences following various CRF manipulations may involve CRF acting on different neural circuits that mediate different defensive behaviors (Lowry and Moore, 2006), and indicate the importance of determining the effects of site-specific CRF manipulations on unconditioned as well as conditioned defensive behaviors related to fear and anxiety (Takahashi, 2001; Todorovic et al., 2005).
The present studies sought to examine the role of ventral hippocampal CRF receptors in modulating defensive behaviors, hypothesizing that microinfusions of the CRF agonist oCRF would increase defensiveness during exposure to the EPM and cat odor, while administration of the CRF antagonist acidic-astressin ([Glu11,16]Ast) would decrease similar responses to cat odor and a live cat. The EPM was substituted for cat exposure in the defensive test battery evaluating oCRF infusions, as cat exposure consistently produces an asymptotic freezing response, which would not have allowed for a measurable increase in defensive behavior, as expected with oCRF (Blanchard et al., 2005). In addition, considering the extensive body of evidence suggesting that the VH plays a crucial role in modulating both unconditioned and conditioned defensive behaviors, contextual fear conditioning for each of the predator tests was also examined by exposing the same subject to the relevant test chamber 24 h after the initial threat encounter. oCRF and [Glu11,16]Ast were used for these experiments based on similar selectivity for CRF receptors, improved solubility and higher bioavailability levels compared to other CRF compounds such as h/rCRF and astressin (Eckart et al., 2001). For example, h/rCRF and astressin possess over 100 fold higher affinity for the CRF binding protein compared to oCRF (Sutton et al., 1995) and [Glu11,16]Ast (Eckart et al., 2001), respectfully, allowing us to use lower doses/volumes to target CRF receptors. Furthermore, the active dose levels of h/rCRF and astressin at CRF receptors would likely vary since it would depend on the amount bound by the binding protein.
Materials and Methods
The housing conditions and care of the animals were in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). All testing procedures conducted on subjects in these experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Hawaii at Manoa. All efforts were made to minimize any animal pain and/or suffering.
Subjects
Subjects were male Long-Evans hooded rats born and reared in the Snyder Hall breeding colony at the University of Hawaii at Manoa. Animals weighed between 350 and 450 g at the time of surgery. Following weaning (21 days), all animals were singly housed under controlled temperature (21 ± 2 °C) and illumination (12 h light/dark cycle, lights on at 06:00 a.m.) with free access to food and water.
Surgery
Prior to surgery, animals were randomly assigned to either an experimental-CRF drug group (oCRF or [Glu11,16]Ast), or to their corresponding artificial cerebrospinal fluid (aCSF)-vehicle control group. Subjects were deeply anesthetized with an intraperitoneal injection of sodium pentobarbital (80 mg/kg; Sigma-Aldrich) and were mounted in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). The scalp was incised and retracted, and the head was positioned to place Bregma and Lambda in the same horizontal plane. Two sets of small burr holes (2.0 mm in diameter) were drilled in the skull in order to implant stainless steel guide cannulae (22G, Plastics One, Roanoke, VA) bilaterally into the VH; guide cannulae were implanted 1.0 mm dorsal to the desired injection site. Cannulae were implanted using a David Kopf micromanipulator aimed at intermediate regions of the VH using coordinates obtained from the Rat Brain Atlas (Paxinos and Watson, 1998); −5.2 mm posterior to bregma, +/− 5.1 mm from the midline and −6.0 mm ventral from the surface of the skull. Guide cannulae were affixed to the skull using dental cement and two 24 g jewelers’ screws, and following implantation dummy cannulae were inserted into each guide in order to prevent blockage and/or infection. Following surgery, animals were administered 3 ml of 0.9% saline in order to prevent dehydration and were returned to their home cage for a 1-wk recovery period prior to the start of behavioral testing.
Peptides
[Glu11,16]Ast (Mw 3637) and oCRF (Mw 4670) were obtained from Dr. Joachim Spiess at the Max-Planck Institute for Experimental Medicine (Gottingen, Germany) and from American Peptide Company (Sunnyvale, CA), respectively. Each peptide was diluted in an aCSF solution (CH3COOH/1xaCSF) at pH = 7.4 until the final low and high doses were obtained; 200 ng/0.5 μl and 400 ng/0.5 μl of [Glu11,16]Ast, and 100 ng/0.5 μl and 200 ng/0.5 μl of oCRF. Control animals received 0.5 μl infusions of the aCSF vehicle solution.
Peptide Infusion
Each subject was gently restrained by hand, the dummy cannulae were removed and 0.5 μl of the appropriate compound (vehicle, oCRF or [Glu11,16]Ast) was infused over a course of 60 s. The injectors were connected via polyethylene-20 tubing (Plastics One) to two 10 μl Hamilton microsyringes mounted in an infusion pump (Harvard Apparatus, Holliston, MA) in order to control the rate of infusion. The injectors extended 1.0 mm below the end of the guide cannulae to insure accurate peptide delivery. Before being slowly removed, injectors (Plastics One) remained in place for an additional 60 s to allow for complete drug diffusion.
Behavioral Testing
All testing was conducted between 09:00 and 12:00 h in one of the following two sequences. Subjects assigned to the oCRF experiments received vehicle or agonist infusions 15 min before testing in the EPM, and again 5 days later 15 min prior to cat odor exposure. Subjects in the [Glu11,16]Ast experiments were microinfused with the vehicle or antagonist 30 min prior to the cat odor test, and again 5 days later 30 min before cat exposure. Between every subject trial, each apparatus was thoroughly cleaned using a 10% ethanol solution. Unconditioned defensive behaviors were assessed during the EPM, cat odor and cat exposure tests, while conditioned defensive behaviors were assessed for both predator tests in each context 24 h after initial threat exposure. Each study (oCRF and [Glu11,16]Ast) was conducted using a separate group of animals, bred at different times and tested in different orders as well as partly different tests [cat odor-cat exposure [Glu11,16]Ast) or EPM-cat odor (oCRF)]. Therefore, variations among cat odor baseline control levels can be attributed to many varying/uncontrollable conditions.
Elevated Plus-Maze
The EPM apparatus consisted of four Plexiglas arms arranged in a cross, elevated 75 cm above the floor. Each arm was 10 cm wide and 50 cm long, and each arm was joined at the center by a 10 cm square platform. The two opposite “open” arms contained no walls, while the other two “closed” arms had 40 cm high sides. Subjects were individually placed in the center arm of the apparatus facing one of the two closed arms. All sessions were 5 min in duration and were conducted under red light.
Cat Odor Exposure
The cat odor test apparatus consisted of a white Plexiglas runway (100 × 12 × 50 cm) with a clear front panel to permit observation and recording. A cloth-wrapped solid plastic block (9 × 9 × 2 cm) was rubbed for 5 min against the fur of laboratory-housed domestic male cat for three consecutive days and was then stored in a Ziploc plastic bag until serving as the cat odor stimulus. Both control and experimental animals were habituated to the apparatus for 10 min on three consecutive days without the presence of an odor block. On the fourth day (unconditioned behavior test), the cat odor block was placed at one end of the runway and a subject was placed at the opposite end, facing away from the odor block. 24 h later subjects were retested in the same apparatus without the cat odor stimulus (conditioned behavior test). A cloth-wrapped plastic block never exposed to cat odor served as the cue during conditioned test trials. All sessions were 10 min in duration and were conducted under red light in order to ensure that the threat stimulus remained as ambiguous as possible.
Cat Exposure
The test apparatus consisted of two adjacent subject chambers (50 × 20 × 30 cm) separated by an opaque white Plexiglas wall, with a wire-mesh screen separating these chambers from the adjoining cat compartment (55 × 40 × 35 cm). Two subjects were simultaneously placed, one in each subject chamber, facing away from the cat compartment (the same cat used in the cat odor test). Following a 5 min pre-cat exposure period, the cat was introduced into the cat compartment for 10 min (unconditioned behavior test). 24 h hours later, subjects were retested in the same apparatus without the cat stimulus (conditioned behavior test). Each test was conducted under white light to ensure the cat stimulus was as unambiguous as possible.
Behavioral Measures
Dependent measures analyzed in each test situation included freezing-complete cessation of movement other than respiration; grooming-movement of forepaws or tongue over the body; transits-crossing of lines marking the far, medium and near thirds of the apparatus (cat odor and cat exposure), or the number of open and closed arm entries (EPM), measured as any movement from one marked section of the apparatus to another; avoidance-duration of time spent in the far compartment relative to the threat stimulus (cat odor: block; cat exposure: wire-mesh), or the proportion of time (duration) spent in the closed versus open arms (EPM). Additional behavioral measures scored in both the cat odor and cat exposure tests included rearing-standing on rear paws with forepaws raised off the ground; and crouchsniff- olfactory investigation evidenced by vertical or lateral head movements, scoring initiated when nose visibly moved more than 1 cm. Head-dips-extension of the subjects head over the edge of an open arm; and risk assessment-combined measure of both stretch approach-forward ambulation with flat back and stretched neck, and stretch attend-standing on all four paws with flat back and stretched neck orientated toward the threat source-were additional measures scored in the EPM. Behavioral measures represent the durations of events in seconds (or numbers of transits) in either a 5 (EPM) or 10 min (cat odor and cat exposure) observation period.
Behavioral Analysis
All test trials were recorded using Pioneer DVD recorders, and were later analyzed using the behavioral analysis software Observer 5.0 (Noldus Information Technology, Wageningen, The Netherlands) by a highly trained observer blind to drug conditions. Use of this software allowed for a detailed, frame-by-frame analysis.
Statistical Analysis
One-way analysis of variance (ANOVA) was performed on each dependent measure in the EPM, cat odor and cat exposure paradigms; α was set at 0.05. Post-hoc Newman-Keuls tests were conducted in order to compare drug and vehicle control means; α was set at 0.05. In several instances where ANOVA approached but failed to reach statistical significance, planned comparisons using Dunnett’s test were conducted to test for significant differences between drug and control means.
Histology
Following the completion of behavioral testing, histological verification of cannulae placement was performed. All subjects were overdosed with sodium pentobarbital (100 mg/kg), and infused with 0.5 μl of 1% methylene blue using identical procedures as those during initial drug administration. Subjects were then perfused transcardially with 0.9% saline followed by 4% formalin. Following extraction from the skull, brains were placed into a 4% formalin solution for 48 h and then transferred to a 30% sucrose solution until blocking (at least 48 h) and sectioning on a cryostat (Leica). A series of coronal sections throughout the entire rostral–caudal extent of each cannulae placement were taken (40 μm thick, collected every 80 μm). After drying (24 h), the sections were analyzed under a microscope (Leica, USA) in order to verify cannulae tip placement.
Results
Histology
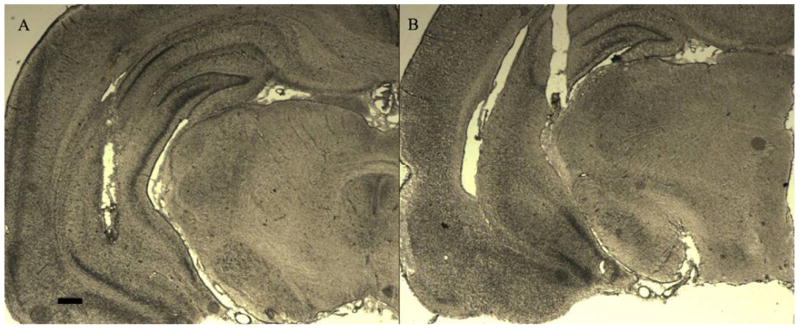
Figure 1 presents representative photomicrographs of injector tip placements within the VH for rats included in both the oCRF and [Glu11,16]Ast experiments. Only those subjects with correct cannulae placements (injector tip) located in the VH within the anterior–posterior range defined as −4.8 to −5.6 mm posterior to bregma, the medial–lateral range defined as ±4.8 to 6.0 mm lateral to the midsagittal suture, and the dorsal–ventral range defined as −5.0 to −7.0 mm ventral to the flat skull surface were included in the analyses (Paxinos and Watson, 1998). Three rats from the oCRF vehicle (n=9), two from the 100 ng (n=9) and three from the 200 ng (n=9) oCRF groups were excluded due to placements outside the circumscribed region. One additional subject from each oCRF drug group was excluded from the cat odor analysis due to a lost head cap (n=8 for each oCRF drug group; n=9 for vehicle controls). Three rats from the [Glu11,16]Ast vehicle (n=8), three from the 200 ng (n=9), and two from the 400 ng (n=8) drug groups were excluded due to misplaced cannulae tips outside the VH; one additional 200 ng subject (n=8) was excluded from the cat exposure analysis due to a lost head cap. Table 1 presents data from animals with cannulae tip placements located outside of the aforementioned range. These data illustrate ventral hippocampal specificity for the effects of oCRF and [Glu11,16]Ast injections, as opposed to that at neighboring regions; subjects did not differ from vehicle controls (F>0.05 in each case).
Figure 1.
Photomicrographs of representative ventral hippocampal cannulae placements included (A) and excluded (B) in the analysis. Scale bar, 420 μm.
Table 1.
Selected behaviors in the EPM, cat odor, cat odor cue + context conditioning, cat exposure and cat exposure context conditioning tests from subjects that received oCRF and [Glu11,16]Ast injections, yet whose cannulae were in regions outside of the VH. There were no reliable differences between CRF drug and vehicle control groups.
|
Elevated Plus Maze | ||
| Behavioral Measures | oCRF | |
| Percent Open Arm Time | 0.62 ± 0.13 | |
| Risk Assessment (s) | 26.00 ± 7.69 | |
| Head Dips (s) | 62.00 ± 28.78 | |
| Cat Odor Exposure | Cue + Context Conditioning | |
| Behavioral Measures | oCRF | oCRF |
| Freezing (s) | 64.80 ± 28.65 | 23.90 ± 5.67 |
| Crouch-Sniff (s) | 188.80 ± 35.30 | 176.60 ± 29.64 |
| Rearing (s) | 137.90 ± 25.84 | 210.20 ± 16.72 |
| Avoidance (s) | 401.60 ± 54.46 | 417.40 ± 55.30 |
| Cat Odor Exposure | Cue + Context Conditioning | |
| Behavioral Measures | [Glu11,16]Ast | [Glu11,16]Ast |
| Freezing (s) | 232.00 ± 47.75 | 136.20 ± 64.82 |
| Crouch-Sniff (s) | 226.40 ± 36.91 | 192.60 ± 35.89 |
| Rearing (s) | 43.20 ± 18.97 | 117.60 ± 48.65 |
| Avoidance (s) | 540.90 ± 16.89 | 381.50 ± 105.84 |
| Cat Exposure | Context Conditioning | |
| Behavioral Measures | [Glu11,16]Ast | [Glu11,16]Ast |
| Freezing (s) | 599.25 ± 0.75 | 419.00 ± 78.05 |
| Crouch-Sniff (s) | 0.00 ± 0.00 | 160.63 ± 67.35 |
| Rearing (s) | 0.00 ± 0.00 | 4.88 ± 3.57 |
| Avoidance (s) | 450.25 ± 150.08 | 580.75 ± 12.41 |
Elevated Plus-Maze (oCRF)
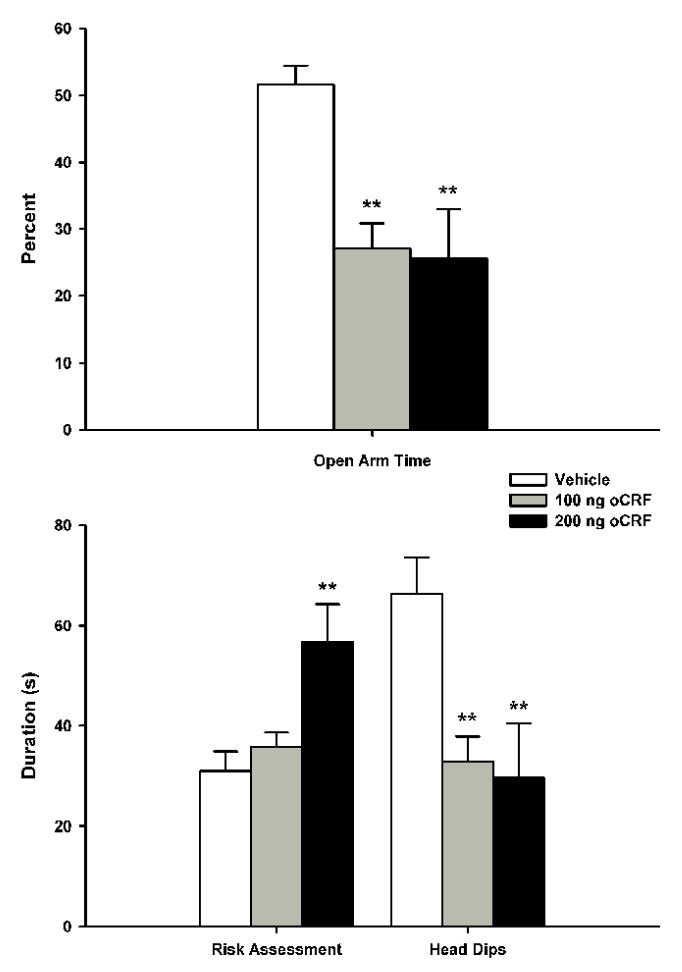
The data for both the behavioral and proximal measures in the EPM following pre-exposure VH oCRF microinfusions are summarized in figure 2. One-way ANOVA indicated that oCRF reliably decreased the percentage of time spent in the open arms [F(2, 24)=8.2380, p<0.005], with Newman-Keuls post-hoc tests revealing that both the 100 ng and 200 ng groups spent less time in the open arms compared to vehicle controls (p<0.005 in each case); there were no significant differences between the two oCRF drug groups. oCRF infusions significantly decreased head dips [F(2,24)=6.3904, p<0.01], with Newman-Keuls showing that both the 100 ng and 200 ng doses reduced levels compared to vehicle controls (p<0.01); there was no reliable difference between 100 ng and 200 ng oCRF groups. One-way ANOVA revealed a significant oCRF dose effect on risk assessment [F(2,24)=7.1937, p<0.005], with subsequent Newman-Keuls indicating that the 200 ng dose increased durations compared to both 100 ng (p<0.01) and vehicle control (p<0.005) groups; there were no reliable differences between the 100 ng and vehicle groups. VH oCRF infusions failed to significantly alter the number of transits (data not shown).
Figure 2.
Effects of ventral hippocampal oCRF infusions (mean + S.E.M.) on defensive behaviors during the EPM test trials. Animals (n=9/group) received their assigned dose of either vehicle or oCRF 15 min prior to testing. Differences for which p<0.01** compared to vehicle controls (Newman-Keuls).
Cat Odor Exposure (oCRF)
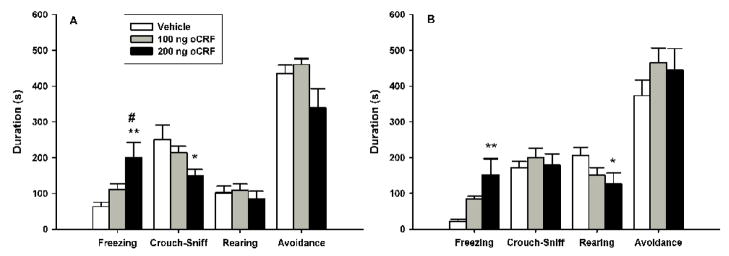
The data for both the behavioral and proximal measures in the cat odor exposure test following pre-exposure VH oCRF microinfusions are summarized in figure 3. One-way ANOVA revealed a significant main effect of drug on the duration of freezing [F(2, 22)=6.9520, p<0.005], with subsequent post-hoc Newman-Keuls tests showing that oCRF infusions produced a dose-dependent increase in freezing as 200 ng dosed rats exhibited higher levels than both the 100 ng (p<0.05) and vehicle control (p<0.005) groups; there was no reliable difference between the 100 ng and vehicle groups. Analysis of crouch-sniff duration using a one-way ANOVA indicated a near significant effect of drug [F(2, 22)=3.1712, p=.06]. Planned comparisons indicated that subjects receiving the 200 ng, but not 100 ng, dose of oCRF displayed significantly lower durations of crouch-sniff compared to vehicle controls (p<0.05). The durations of grooming (data not shown), rearing and avoidance were not reliably different between groups, and there was no significant difference in the number of transits (data not shown).
Figure 3.
Effects of ventral hippocampal oCRF infusions (mean + S.E.M.) on defensive behaviors during the cat odor (A) and cat odor cue + context conditioning (B) test trials. Animals (n=8/group) received their assigned dose of either vehicle or oCRF 15 min prior to testing. Differences for which p<0.05* and p<0.01** compared to vehicle controls; p<0.05# compared to the 100 ng drug group (Newman-Keuls).
Cat Odor Exposure Cue + Context Conditioning (oCRF)
The data for both the behavioral and proximal measures in the cat odor exposure cue + context conditioning test following pre-training VH oCRF infusions are summarized in figure 3. One-way ANOVA revealed a significant drug effect on the duration of freezing [F(2,22)=6.3801, p<0.01], with Newman-Keuls post-hoc analysis indicating increased levels for the 200 ng group compared to vehicle controls (p<0.01); subsequent pair-wise comparisons failed to show further group differences. Analysis of rearing using a one-way ANOVA demonstrated a near significant main effect [F(2, 22)=2.8692, p=0.07]. Planned comparisons indicated a reliable reduction in the duration of rearing following infusions of 200 ng (p<0.05), but not 100 ng, of oCRF. VH oCRF infusions failed to reliably alter the durations of avoidance, crouch-sniff, grooming (data now shown) or the number of transits (data now shown).
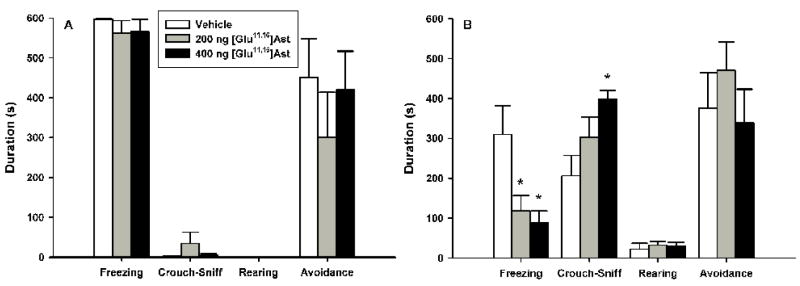
Cat Odor Exposure ([Glu11,16]Ast)
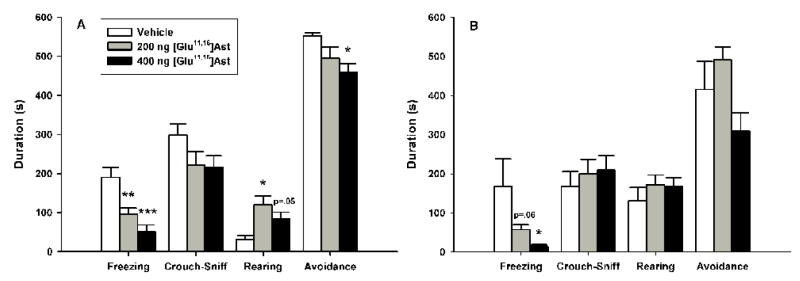
The data for both the behavioral and proximal measures in the cat odor exposure test following pre-exposure VH [Glu11,16]Ast microinfusions are summarized in figure 4. One-way ANOVA revealed a significant main effect of [Glu11,16]Ast on the duration of freezing [F(2, 22)=12.326, p<0.0005], with subsequent Newman-Keuls post-hoc tests showing that both the 200 ng (p<0.005) and 400 ng (p<0.0005) groups froze less than vehicle controls; there were no reliable differences between drug groups. One-way ANOVA revealed a significant dose effect on the duration of rearing [F(2,22)=5.9372, p<0.01], with Newman-Keuls showing that 200 ng infusions increased rearing durations compared to vehicle controls (p<0.05), while 400 ng approached, but failed to reach significance (p=.05); there were no reliable differences between [Glu11,16]Ast drug groups. There was a significant main effect of [Glu11,16]Ast on the number of transits [F(2, 22)=5.4878, p<0.05] (data now shown), with Newman-Keuls showing increased levels for both the 200 ng (p<0.05) and 400 ng (p<0.01) drug groups; there were no reliable differences between [Glu11,16]Ast dose groups. One-way ANOVA indicated that [Glu11,16]Ast infusions did not produce reliable group differences in the durations of crouch-sniff or grooming (data now shown). Analysis of [Glu11,16]Ast infusions using a one-way ANOVA revealed a significant main effect on the duration of avoidance [F(2, 22)=4.1469, p<0.05], with post-hoc Newman-Keuls revealing that 400 ng infusions significantly reduced avoidance compared to vehicle controls (p<0.05); subsequent pair-wise comparisons failed to show further group differences.
Figure 4.
Effects of ventral hippocampal [Glu11,16]Ast infusions (mean+S.E.M.) on defensive behaviors during the cat odor (A) and cat odor cue + context conditioning (B) test trials. Animals (n=8–9/group) received their assigned dose of either vehicle or [Glu11,16]Ast 30 min prior to testing. Differences for which p<0.05*, p<0.01** and p<0.001*** compared to vehicle controls (Newman-Keuls).
Cat Odor Exposure Cue + Context Conditioning ([Glu11,16]Ast)
The data for both the behavioral and proximal measures in the cat odor exposure cue + context conditioning test following pre-training VH [Glu11,16]Ast microinfusions are summarized in figure 4. One-way ANOVA demonstrated that [Glu11,16]Ast infusions significantly reduced the duration of conditioned freezing [F(2, 22)=3.8997, p<0.05], with post-hoc Newman-Keuls revealing a dose dependent effect as 400 ng dosed animals froze less than vehicle controls (p<0.05); 100 ng infusions approached but failed to reach significance (p=.06) and there were no differences between the two drug groups. [Glu11,16]Ast significantly increased the number of transits [F(2, 22)=4.0429, p<0.05] (data now shown), with Newman-Keuls showing that rats receiving the 400 ng dose exhibited more line crossings than both the vehicle control (p<0.05) and 200 ng groups, although the latter effect was not reliable (p=0.05); there were no differences between the 200 ng and vehicle control groups. One-way ANOVA indicated that there were no reliable differences in the durations of crouch-sniff, rearing or grooming (data now shown). One-way ANOVA indicated that differences in the duration of avoidance approached, but did not reach, statistical significance (p=.06).
Cat Exposure ([Glu11,16]Ast)
The data for both the behavioral and proximal measures in the cat exposure test following pre-exposure VH [Glu11,16]Ast microinfusions are summarized in figure 5. [Glu11,16]Ast failed to produce significant differences between vehicle and drug dose groups on any of the behavioral measures during the cat exposure test.
Figure 5.
Effects of ventral hippocampal [Glu11,16]Ast infusions (mean + S.E.M.) on defensive behaviors during the cat exposure (A) and cat exposure context conditioning (B) test trials. Animals (n=8/group) received their assigned dose of either vehicle or [Glu11,16]Ast 30 min prior to testing. Differences for which p<0.05* compared to vehicle controls (Newman-Keuls).
Cat Exposure Context Conditioning ([Glu11,16]Ast)
The data for both the behavioral and proximal measures in the cat exposure context conditioning test following pre-training VH [Glu11,16]Ast microinfusions are summarized in figure 5. One-way ANOVA revealed that[Glu11,16]Ast significantly reduced conditioned freezing [F(2, 21)=5.6600, p<0.05], with Newman-Keuls post-hoc analysis indicating that both drug groups exhibited lower levels relative to vehicle controls (p<0.05 in each case); there were no significant differences between the two drug groups. There was a significant main effect of [Glu11,16]Ast on the duration of crouch-sniff [F(2, 21)=4.8712, p<0.05], with 400 ng of [Glu11,16]Ast increasing durations compared to vehicle controls (p<0.05); subsequent pair-wise comparisons failed to show further group differences. There were no reliable effects of [Glu11,16]Ast infusions on the durations of grooming (data now shown), rearing, avoidance or the number of transits (data now shown).
Discussion
The present results indicate that ventral hippocampal CRF receptors modulate both unconditioned and conditioned behaviors in several ethologically relevant tests of defense. Activation of ventral hippocampal CRF receptors following site-specific microinfusions of oCRF increased defensive behaviors during exposure to potential (EPM, cat odor and cat odor context) threats, while inhibition of CRF receptors following [Glu11,16]Ast microinfusions decreased defensive behaviors to potential (cat odor and both predator contexts), but not present (cat exposure), threat stimuli. While other studies have evaluated the effects of intra-hippocampal CRF infusions (Blank et al., 2002; Radulovic et al., 1999; Lee and Davis, 1997) this is the first in-vivo study to specifically evaluate the function of endogenous ventral hippocampal CRF receptors in mediating the expression of unconditioned defensive behaviors, as well as the role of these receptors during conditioning to the aforementioned predatory threats.
Ventral hippocampal oCRF infusions produced robust increases in anxiety-like defensive behaviors in the EPM, reducing the proportion of time spent in the open-arms and the number of head dips, while dose dependently increasing the duration of risk assessment, without altering locomotion. Following a second injection, oCRF also potentiated defensive behaviors during exposure to cat odor, dose dependently increasing and decreasing the durations of freezing and sniffing, respectively, suggesting that oCRF shifted the pattern of defensiveness such that the behavioral responses elicited by cat odor resembled those appropriate if heightened levels of environmental threat were present (i.e. a live predator). In contrast to the anxiogenic-like profile produced by oCRF, microinfusions of [Glu11,16]Ast preferentially attenuated defensive behaviors during exposure to cat odor, reducing the duration of freezing to, and avoidance of, the cat odor stimulus, while increasing the number of approaches to the odor block (transits); a shift in defensive responsivity similar to reducing the level of environmental threat (i.e. a return to non-defensive behaviors). During live cat exposure, [Glu11,16]Ast failed to alter any behavioral response, indicating that antagonism of VH CRF receptors reduces defensiveness during exposure to potential threats, but not to a present predator. The lack of an effect following VH [Glu11,16]Ast infusions during cat exposure is consistent with lesion data (Pentkowski et al., 2006) suggesting that the modulation of defensive behaviors during exposure to a potent immediate threat is not VH dependent, and that other neural systems can support defensiveness to a clearly present predator, in the absence of VH activity. Alternatively, during the cat-exposure test [Glu11,16]Ast injected animals may have been less defensive to olfactory cues, while the additional auditory and visual cues of the live cat stimulus were sufficiently potent as to elicit a near maximal freezing response.
Pre-training antagonism of ventral hippocampal CRF receptors also affected the expression of conditioned defensive behaviors. Similar to the effects of VH lesions (Pentkowski et al., 2006; Trivedi and Coover, 2004), pre-training microinfusions of [Glu11,16]Ast reduced conditioned defensive behaviors during re-exposure to both predator-paired contexts, with oCRF administration producing the opposite effect during the cat odor cue + context conditioning test. Pre-training [Glu11,16]Ast infusions dose-dependently decreased the duration of freezing and increased the number of approaches to the non-odor block cue (transits), while reducing and increasing the durations of freezing and sniffing, respectively, during re-exposure to the cat context. In cat odor cue + context conditioning test trials, pre-training oCRF infusions potentiated defensive behaviors in a dose dependent manner, increasing and decreasing the durations of freezing and rearing, respectively. This enhancement or attenuation in the expression of defensive behavior following pre-training oCRF or [Glu11,16]Ast microinfusions, respectively, suggests that the formation of conditioned fear involves VH CRF receptor activation. These changes in the expression of conditioned defensive behaviors may have resulted from changes in levels of defensiveness during the unconditioned test trials, or changes in situational learning from altered VH functioning (Blank et al., 2002).
In each unconditioned and conditioned test situation oCRF and [Glu11,16]Ast failed to alter non-defensive behaviors (grooming), suggesting a preferential role for ventral hippocampal CRF receptors in mediating the modulatory role of the VH during exposure to potential threats, including cat odor, contexts associated with predators (Pentkowski et al., 2006) and the EPM (Bertoglio et al., 2006). Although the increase in transits (locomotion) during both the unconditioned and conditioned cat odor tests following ventral hippocampal [Glu11,16]Ast microinjections could indicate general locomotor effects of the drug, results from the cat exposure test argue against this possibility. During unconditioned test trials, both [Glu11,16]Ast and vehicle infused animals froze for almost the entire duration of the test (figure 4), and increases in general locomotion would be anticipated following a reduction in levels of freezing. Furthermore, numerous studies have demonstrated anxiolytic-like effects without accompanying changes in locomotion following inactivation of the VH (Pentkowski et al., 2006; Degroot and Treit, 2004; Bannerman et al., 2003; Bast and Feldon, 2003; Kjelstrup et al., 2002).
The contrasting effects following [Glu11,16]Ast infusions during cat odor and cat exposure may indicate that VH CRF receptors mediate defensive behaviors sensitive to tests of anxiety, without affecting behaviors responsive to tests of fear. In support of this notion, Blanchard & Blanchard (1990) suggested that situations in which rats are exposed to specific immediate threat stimuli (cat exposure) elicit fear-like responses, whereas tests exposing rats to situations of potential or anticipated threat (cat odor, cat-paired contexts) elicit anxiety-like behaviors, an assertion supported by pharmacological data (Blanchard et al., 2003). For instance, classic anxiolytics such as the benzodiazepines diazepam and chlordiazepoxide (Blanchard et al., 1990c), alcohol (Blanchard et al., 1990a), the 5-HT1A agonists 8-OH-DPAT and Gepirone (Blanchard et al., 1992) and the tricyclic antidepressant imipramine (Blanchard et al., 1993) decrease freezing and avoidance while increasing risk assessment during exposure to contexts associated with a cat, while the benzodiazepine midazolam decreases freezing and avoidance during cat odor exposure (McGregor et al., 2004). In contrast, during exposure to an unavoidable predator diazepam failed to reliably alter levels of risk assessment or freezing, but again increased measures of risk assessment immediately following predator removal (Blanchard et al., 1990c).
The biological mechanisms of endogenous hippocampal CRF may explain the behavioral effects produced by CRF manipulations in the current studies. Recent research has implicated CRF in hippocampal cellular excitation (Blank et al., 2002; Kortekaas et al., 1999), as in-vitro application of h/rCRF to hippocampal slice preparations primed long-term potentiation (LTP) of population spikes (Blank et al., 2002), which was prevented by pre-application of [Glu11,16]Ast. Furthermore, Blank and colleagues (2002) showed that this priming was required for contextual fear conditioning, which may explain the current effects of CRF during the conditioning test trials. In-vivo CRF infusions in anesthetized rats have also been shown to increase hippocampal theta activity (Kortekaas et al., 1999), a mechanism believed to be involved in emotional regulation (McNaughton et al., 2007; Gray and McNaughton, 2000), particularly in modulating anxiety-like defensive behaviors (Deroot and Treit, 2002). In fact, all known classes of drugs that are clinically effective in treating generalized anxiety disorder (e.g. barbiturates, benzodiazepines, 5-HT1A receptor agonists, SSRIs) decrease the frequency of reticular-elicited theta, while drugs that are antipsychotic or sedative but not anxiolytic (haloperidol, chlorpromazine) do not have this effect (McNaughton et al, 2007). Collectively, these electrophysiological results suggest that the current effects obtained following CRF infusions may have resulted from VH neural excitation (oCRF) or inhibition ([Glu11,16]Ast), with the latter finding corroborated from numerous studies showing reduced defensiveness following inactivation of the VH (Bertoglio et al., 2006; Pentkowski et al., 2006; Degroot and Treit, 2004; McHugh et al., 2004; Bannerman et al., 2002).
Neuroanatomically the VH could modulate unconditioned and conditioned defensive behaviors via direct or indirect efferent projections to various hypothalamic and/or amygdaloidal nuclei. Intermediate regions of ventral CA1 and the subiculum project to the BLA, lateral, medial (MeA) and posterior basomedial amygdalar nuclei (Canteras & Swanson, 1992; Petrovich et al., 1996, 2001; Pikkarainen et al., 1999), structures all implicated in mediating various aspects of unconditioned and conditioned defense (Phelps and LeDoux, 2005; Rosen, 2004). Of these, the MeA is of particular importance in relation to cat odor, as it receives heavy projections from the vomeronasal organ, a structure known to play a critical role in the detection of predator odors, and lesions restricted to the MeA dramatically reduce defense behaviors during cat odor exposure (Blanchard et al., 2005; Li et al., 2004). Intermediate regions of ventral CA1 and the subiculum also send efferent projections indirectly to the hypothalamus via projections through the dorsal region of the ventrolateral zone of the rostral part of the lateral septal nucleus (Risold and Swanson, 1997) to the anterior hypothalamic nucleus and dorsal premammillary nucleus [PMd] (Canteras et al., 2001; Comoli et al., 2000; Risold and Swanson, 1997). These hypothalamic structures, along with the dorsomedial portion of the ventromedial hypothalamus, form the medial hypothalamic zone, a core circuit hypothesized to play a central role in the integration and initiation of defensive behaviors to innate threat stimuli (Canteras, 2002). Indeed recent research has supported a role for this circuit in modulating unconditioned defensive behaviors to natural predatory threats with PMd lesions abolishing freezing to both a live cat and its odor (Markham et al., 2004; Blanchard et al., 2003; Canteras et al., 1997).
Using the fear potentiated startle paradigm, Lee and Davis (1997) failed to demonstrate increased conditioned fear following oCRF microinfusions into the VH, a finding seeming at variance with the present results. However, they used lower doses (40 ng and 80 ng) and volumes (0.3 μl) than the present study, and a somewhat different infusion location: The current study targeted intermediate regions of ventral CA1 (main source of amygdaloidal and hypothalamic projections), while the Lee and Davis (1997) study targeted anterior ventral regions of the VH. Additionally, the current study infused oCRF rather than h/rCRH, suggesting that the heightened cat odor induced conditioned defensiveness may have been due to increased CRF1 receptor activation as oCRF is more selective for CRF1 than CRF2 receptors and possesses lower affinity for the CRF binding protein in comparison to h/rCRF (Radulovic et al., 2001). Finally, inconsistencies obtained between these studies could be due to differences in testing conditions, specifically the use of footshock pain as the unconditioned threat stimulus in the Lee and Davis (1997) study.
Conclusion
The effects of ventral hippocampal oCRF and [Glu11,16]Ast microinjections on defensive behaviors in Long-Evans hooded rats were evaluated in the present studies. Our findings demonstrate that oCRF potentiated, while [Glu11,16]Ast attenuated, defensive behaviors in a situation-specific, dose-related manner, suggesting that activation of endogenous ventral hippocampal CRF receptors modulate the expression of unconditioned defensive behaviors during testing in paradigms more closely related to anxiety (anticipated or potential threat) rather than fear (immediate and clear threat). Furthermore, these results indicate that endogenous ventral hippocampal CRF receptors mediate conditioning processes related to predatory threats.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- aSVG-30
antisauvagine
- BLA
basolateral amygdala
- CRF
corticotropin-releasing factor
- EPM
elevated plus-maze
- [Glu11,16]Ast
acidic-astressin
- h/rCRF
human/rat CRF
- i.c.v
intracerebroventricular
- LTP
long-term potentiation
- oCRF
ovine-CRF
- PMd
dorsal premammillary nucleus
- VH
ventral hippocampus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apfelbach R, Blanchard DC, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: A review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF Receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RMJ, Yee BK, Feldon J, Rawlins JNP. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bast T, Feldon J. Hippocampal modulation of sensorimotor processes. Prog Neurobiol. 2003;70:319–345. doi: 10.1016/s0301-0082(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABAA agonist muscimol. Exp Brain Res. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Joca SRL, Guimaraes FS. Further evidence that anxiety and memory are regionally dissociated within the hippocampus. Behav Brain Res. 2006;175:183–188. doi: 10.1016/j.bbr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Bringing natural behaviors into the laboratory: a tribute to Paul MacLean. Physiol Behav. 2003;79:515–524. doi: 10.1016/s0031-9384(03)00157-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Ethoexperimental approaches to the biology of emotion. Annu Rev Psychol. 1988;39:43–68. doi: 10.1146/annurev.ps.39.020188.000355. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. In: An ethoexperimental analysis of defense, fear and anxiety. McNaughton N, Andrews G, editors. Anxiety, Dunedin: Otago University Press; 1990. pp. 124–133. [Google Scholar]
- Blanchard RJ, Blanchard DC, Hori K. An ethoexperimental approach to the study of defense. In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, editors. Ethoexperimental Approaches to the Study of Behavior. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1989. pp. 114–136. [Google Scholar]
- Blanchard DC, Blanchard RJ, Tom P, Rodgers RJ. Diazepam changes risk assessment in an anxiety/defense test battery. Psychopharmacology (Berl) 1990c;101:511–518. doi: 10.1007/BF02244230. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Weiss SM. Ethanol effects in an anxiety/defense test battery. Alcohol. 1990a;7:375–381. doi: 10.1016/0741-8329(90)90019-9. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Weiss SM, Meyer S. The effects of ethanol and Diazepam on reactions to predatory odors. Pharmacol Biochem Behav. 1990b;35:775–780. doi: 10.1016/0091-3057(90)90357-n. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: Effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. The mouse defense test battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Shepherd JK, Rodgers RJ, Blanchard RJ. Evidence for differential effects of 8-OH-DPAT on male and female rats in the anxiety/defense test battery. Psychopharmacology (Berl) 1992;106:531–539. doi: 10.1007/BF02244826. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Shepherd JK, Rodgers RJ, Magee L, Blanchard DC. Attenuation of Antipredator defensive behavior in rats following chronic treatment with imipramine. Psychopharmacology (Berl) 1993;110:245–253. doi: 10.1007/BF02246981. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: Implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BM, Morrison JL, Walker EL, Merchant KM. Differential regulation of behavioral, genomic, and neuroendocrine responses by CRF infusions in rats. Pharmacol Biochem Behav. 2004;77:447–455. doi: 10.1016/j.pbb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: Hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Chiavegatto S, Ribeiro Do Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Ribeiro-Barbosa ER, Comoli E. Tracing from the dorsal premammillary nucleus prosencephalic systems involved in the organization of innate fear responses. Neurosci Biobehav Rev. 2001;25:661–668. doi: 10.1016/s0149-7634(01)00048-3. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study of the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E, Ribeiro-Barbosa ER, Canteras NS. Afferent connections of the dorsal premammillary nucleus. J Comp Neurol. 2000;423:83–98. doi: 10.1002/1096-9861(20000717)423:1<83::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Degroot A, Treit D. Anxiety is functionally segregated within the septo-hippocampal system. Brain Res. 2004;1001:60–71. doi: 10.1016/j.brainres.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Eckart K, Jahn O, Radulovic J, Tezval H, van Werven L, Spiess J. A single amino acid serves as an affinity switch between the receptor and the binding protein of corticotropin-releasing factor: implications for the design of agonists and antagonists. PNAS. 2001;98:11142–7. doi: 10.1073/pnas.211424998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety; 2; Oxford: Oxford University Press; 2000. [Google Scholar]
- Griebel G, Perrault G, Sanger DJ. Study of the modulatory activity of BZ (omega) receptor ligands on defensive behaviors in mice: evaluation of the importance of intrinsic efficacy and receptor subtype selectivity. Prog Neuropsychopharmacol Biol Psychiatry. 1999a;23:81–98. doi: 10.1016/s0278-5846(98)00093-1. [DOI] [PubMed] [Google Scholar]
- Griebel G, Rodgers RJ, Perrault G, Sanger DJ. Behavioural profiles in the mouse defense test battery suggest anxiolytic potential of 5-HT1A receptor antagonists. Psychopharmacology (Berl) 1999b;144:121–130. doi: 10.1007/s002130050984. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: A role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse E, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Ho SP, Takahashi LK, Livanov V, Spencer K, Lesher T, Maciag C, Smith MA, Rohrbach KW, Hartig PR, Arneric SP. Attenuation of fear conditioning by antisense inhibition of brain corticotropin releasing factor-2 receptor. Mol Brain Res. 2001;89:29–40. doi: 10.1016/s0169-328x(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. PNAS. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas R, Costall B, Smythe JW. Changes in hippocampal theta following intrahippocampal corticotropin-releasing hormone (CRH) infusions in the rat. Brain Res Bull. 1999;48:603–607. doi: 10.1016/s0361-9230(99)00039-8. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Moore FL. Regulation of behavioral responses by corticotropin-releasing factor. Gen Comp Endocrinol. 2006;146:19–27. doi: 10.1016/j.ygcen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Markham CM, Blanchard DC, Canteras NS, Cuyno CD, Blanchard RJ. Modulation of predatory odor processing following lesions to the dorsal premammillary nucleus. Neurosci Lett. 2004;372:22–26. doi: 10.1016/j.neulet.2004.09.006. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JNP, Bannerman DM. Amygdala and ventral hippocampal lesions contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Kocsis B, Hajos M. Elicited hippocampal theta rhythm: a screen for anxiolytic and procognitive drugs through changes in hippocampal function? Behav Pharmacol. 2007;18:329–346. doi: 10.1097/FBP.0b013e3282ee82e3. [DOI] [PubMed] [Google Scholar]
- Menard J, Treit D. Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci Biobehav Rev. 1999;23:591–613. doi: 10.1016/s0149-7634(98)00056-6. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1998. [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor (2) receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302:145–152. doi: 10.1124/jpet.302.1.145. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW. Organization of the projections of the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1996;374:387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotional processing: from animal models to human behaviors. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Radulovic J, Ruhmann R, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: Differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JNP, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Robers AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: A neurobehavioral system analysis of the amygdale. Behav Cog Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. PNAS. 1981;78:6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F, Lightman SL, Shekhar A, Lowry CA. Injections of urocortin 1 into the basolateral amygdala induce anxiety-like behavior and c-Fos expression in brainstem serotonergic neurons. Neuroscience. 2006;138:1265–1276. doi: 10.1016/j.neuroscience.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Sutton SW, Behan DP, Lahrichi SL, Kaiser R, Corrigan A, Lowry P, Potter E, Perrin MH, Rivier J, Vale WW. Ligand requirements of the human corticotropin-releasing factor-binding protein. Endocrinology. 1995;136:1097–102. doi: 10.1210/endo.136.3.7867564. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF1 and CRF2 receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Todorovic C, Jahn O, Tezval H, Hippel C, Spiess J. The role of CRF receptors in anxiety and depression: Implications of the novel CRF1 agonist Cortagine. Neurosci Biobehav Rev. 2005;29:1323–1333. doi: 10.1016/j.neubiorev.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem. 2004;81:172–184. doi: 10.1016/j.nlm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Inoue K, Koob GF, Rivier J, Vale WW, Zorrilla EP. Human urocortin II: mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002;943:142–150. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale WW, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Venihaki M, Sakihara S, Subramanian S, Dikkes P, Weninger SC, Liapakis G, Graf T, Majzoub JA. Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: modulation by stress and attenuation of some anxiety-like behaviours. J Neuroendocrinol. 2004;16:411–422. doi: 10.1111/j.1365-2826.2004.01170.x. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or in noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126:159–174. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]