Nitroglycerin (glyceryl trinitrate, GTN) has been an important part of the management of patients with angina or heart failure for over 135 years. GTN works through a combined action on the venous circulation and coronary vasculature to reduce preload and improve myocardial blood flow.1 Its attributes include a potent vasodilatory action on diseased coronary vessels as well as anti-ischemic effects elicited in the microcirculation.1,2 Dilation of conduit vessels by GTN is mediated in large part through nitric oxide (NO) binding to heme within, and activation of, soluble guanylate cyclase (sGC) in vascular smooth muscle, thereby leading to induction of the second messenger, cyclic GMP. The microvascular action of GTN involves additional effects on red blood cells (RBCs) to improve rheology and oxygen delivery.2 GTN is an exceptionally potent vasodilator compared to other organic nitrates (isosorbide di- or mono-nitrates), but loses efficacy over time. Tachyphylaxis to GTN is initially specific to GTN (mechanism-based tolerance), but is ultimately associated with diminished responsiveness to other nitro(so)vasodilators (cross-tolerance) and even other classes of drugs (as a result of fluid retention and perhaps cellular injury).1,3,4 Tolerance and cross-tolerance have generally been thought of in terms of an NO deficiency, resulting in attenuated sGC activity.5,6 Sayed and co-workers had found recently that S-nitrosylation of sGC (the addition of an NO group to a cysteine thiol) by endothelium-derived NO inhibits sGC activity,7 and they now report that exposure to GTN can result in the S-nitrosylation and desensitization of sGC, thereby providing a mechanism for cross-tolerance.8 In other words, they suggest that aberrant or misdirected NO bioactivity, rather than NO deficiency per se, may contribute to cross-tolerance. These findings are consistent with an emerging paradigm in NO biology in which NO-based signaling is elicited in substantial part by S-nitrosothiols (SNOs) and accordingly, dysregulated protein S-nitrosylation contributes to cellular dysfunction and disease.9,10 These new results also help elucidate the long-recognized importance of S-nitrosothiols in GTN biotransformation and metabolism.
Classic studies by Murad, Ignarro and Furchgott originally identified the activity of GTN with that of the endothelium-derived vasodilator, NO.11 Both GTN and NO activated sGC in situ. It is now understood, however, that NO bioactivity cannot be readily differentiated from that of endogenous SNOs, which mediate vasorelaxation and whose role in regulation of vascular resistance has been established by stringent genetic criteria.12,13 SNO-based activity is transduced by sGC/cGMP and by S-nitrosylation of proteins. It is therefore of interest that a large part of the acetylcholine-mediated relaxation in the classic Furchgott bioassay (rabbit thoracic aorta) is in fact preserved after inhibition of sGC,14,15 and probably attributable to S-nitrosylation of the charybdotoxin-sensitive potassium channel and perhaps of calcium ATPase.12,14 The case for S-nitrosothiols is perhaps even stronger in the microcirculation. Harrison and colleagues noted long ago that coronary microvessels are far more responsive to low mass nitrosothiols such as S-nitrosocysteine than to NO itself.16 S-nitrosothiols are also impervious to the NO-scavenging chemistry of hemoglobin (Hb), which is of particular importance in small vessels where the effective concentration of Hb is highest. Interestingly, vasodilation by GTN is markedly less efficacious in small versus large coronary vessels and is greatly potentiated in microvessels by the addition of cysteine,16-18 which reacts with GTN to produce S-nitrosocysteine.19 Thus, the role of cGMP in the action of GTN in the microcirculation (especially during low flow states), and more generally in the control of microcirculatory blood flow, is poorly understood. In view of this, and of atypical features of the hamster cheek pouch preparation used by Sayed et al8 (which is not representative of vascular beds that contribute principally to the effects of nitro(so)vasodilators), their findings will need to be confirmed in more relevant vascular systems.
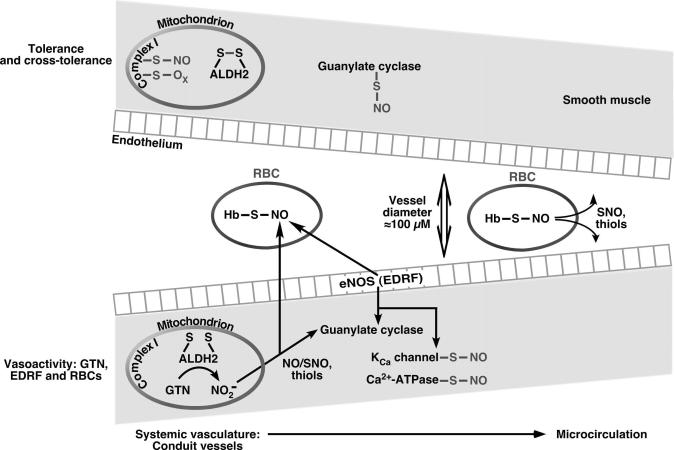
The observations of Sayed et al8 nonetheless shed new light on shared biochemical and physiological properties of GTN and S-nitrosocysteine with respect to cross-tolerance. These results are reminiscent of early work by Ignarro19 on the participation of S-nitrosothiols, particularly S-nitrosocysteine, in GTN biotransformation, and of work by Needleman,20 who suggested that oxidation of protein thiols may constitute a mechanism of GTN tolerance. Recent experiments by Kaul and colleagues2 further suggest that the principal function of these S-nitrosothiols may be in the microcirculation where they subserve RBC-mediated control of blood flow. Notably, GTN augments the S-nitrosylation of hemoglobin in tandem with the increases in oxygen delivery mediated by RBCs.2 S-nitrosohemoglobin is in equilibrium with low-mass SNOs, which convey NO bioactivity from RBCs,13,21,22 consistent with the accumulating evidence that SNOs play central roles in hypoxic vasodilation, a mechanism that is pivotal in relief from ischemia (Figure).
Figure.
The vasoactivity of nitroglycerin derives from its biotransformation (veins > arteries) by mitochondrial ALDH33,34 and through a microcirculatory effect that is mediated by RBCs2. S-nitrosothiols likely contribute substantively to the actions of GTN. The action spectrum of GTN thus overlaps that of NO bioactivity derived from the endothelium (shear- or G-protein coupled receptor-mediated) as well as RBCs (hypoxia regulated), which is conveyed at least in part through protein S-nitrosylation, as well as activation of guanylate cyclase. Prolonged use of GTN results in the aberrant S-nitrosylation and/or oxidation of MtALDH, mitochondrial complex 1, guanylate cyclase (as shown in present paper) and likely other proteins, which constitute the basis of tolerance and cross-tolerance (see text).
One of the great enigmas in the study of GTN has been the inability to detect NO as a byproduct.23 The likely solution is found in the recent discovery that GTN is bioactivated within mitochondria by the enzyme aldehyde dehydrogenase (MtALDH or ALDH 2).24,25 The main product is nitrite. However, whereas the cytosolic isoform of aldehyde dehydrogenase also generates nitrite, only the mitochondrial enzyme subserves vasodilation.4,24,25 Cytosolic nitrite is thus effectively inert. Rather, either nitrite within mitochondria or some other action of MtALDH26 generates vasodilatory NO bioactivity that is exported to dilate blood vessels, and that bioactivity is not conveyed by NO itself. It is therefore of interest that this activity is precisely replicated by S-nitrosoglutathione (GSNO) (which may undergo further biotransformation to S-nitrosocysteine).24 Furthermore, excessive amounts of GTN or SNO may oxidize the active site thiols of MtALDH, providing a mechanism for tolerance.4,27-29 Increased amounts of S-nitrosothiols will also S-nitrosylate and/or oxidize mitochondrial respiratory proteins (complex 1),30 leading to an oxidant leak4 that can accentuate cross-tolerance. Increased S-nitrosylation (nitrosative stress) thus begets oxidation (oxidative stress), a formula underlying tolerance and cross-tolerance.
There are about 750 million individuals worldwide with the Asian variant of MtALDH. These individuals do not respond appropriately to GTN.31,32 Notably, isosorbide dinitrate is not metabolized by MtALDH,24 and may represent an appropriate first-line agent for these patients. The proper dosing of GTN during intravenous administration is not known, and it would seem appropriate to restudy this drug in light of the new information on mechanisms of biotransformation, tolerance and cross-tolerance. Monitoring of MtALDH activity may allow for therapeutic benefits of GTN without induction of tolerance.
Acknowledgments
Sources of Funding: NIH PO1-HL75443 and NIH R01 HL059130.
Footnotes
Disclosures: none
References
- 1.Gersh BJ, Braunwald E, Rutherford JD. Chronic coronary artery disese. In: Braunwald E, Zipes DP, Libby P, Bonow R, editors. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 5th edition Saunders; 1997. pp. 1302–1304. [Google Scholar]
- 2.Bin JP, Doctor A, Lindner J, Hendersen EM, Le DE, Leong-Poi H, Fisher NG, Christiansen J, Kaul S. Effects of nitroglycerin on erythrocyte rheology and oxygen unloading: novel role of S-nitrosohemoglobin in relieving myocardial ischemia. Circulation. 2006;113:2502–2508. doi: 10.1161/CIRCULATIONAHA.106.627091. [DOI] [PubMed] [Google Scholar]
- 3.Parker JD, Farrell B, Fenton T, Cohanim M, Parker JO. Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation. 1991;84:2336–2345. doi: 10.1161/01.cir.84.6.2336. [DOI] [PubMed] [Google Scholar]
- 4.Sydow K, Daiber A, Oelze M, Chen Z, August M, Wendt M, Ullrich V, Mulsch A, Schulz E, Keaney JF, Jr., Stamler JS, Munzel T. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest. 2004;113:482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forster S, Woditsch I, Schroder H, Schror K. Reduced nitric oxide release causes nitrate tolerance in the intact coronary circulation. J Cardiovasc Pharmacol. 1991;17:867–872. doi: 10.1097/00005344-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 7.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci U S A. 2007;104:12312–12317. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayed N, Kim DD, Fioramonti X, Iwahashi T, Duran WN, Beuve A. Nitroglycerin-Induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res. 2008;103:606–614. doi: 10.1161/CIRCRESAHA.108.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamler JS, Sun QA, Hess DT. A SNO storm in skeletal muscle. Cell. 2008;133:33–35. doi: 10.1016/j.cell.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen TT, Sorensen KE. Discovery of "endogenous nitroglycerin", NO, as cellular signal molecule. Ugeskr Laeger. 1998;160:7567. [PubMed] [Google Scholar]
- 12.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 14.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 15.Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, Radomski MW, Moncada S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci U S A. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sellke FW, Myers PR, Bates JN, Harrison DG. Influence of vessel size on the sensitivity of porcine coronary microvessels to nitroglycerin. Am J Physiol. 1990;258:H515–520. doi: 10.1152/ajpheart.1990.258.2.H515. [DOI] [PubMed] [Google Scholar]
- 17.Kurz MA, Lamping KG, Bates JN, Eastham CL, Marcus ML, Harrison DG. Mechanisms responsible for the heterogeneous coronary microvascular response to nitroglycerin. Circ Res. 1991;68:847–855. doi: 10.1161/01.res.68.3.847. [DOI] [PubMed] [Google Scholar]
- 18.Sellke FW, Tomanek RJ, Harrison DG. L-cysteine selectively potentiates nitroglycerin-induced dilation of small coronary microvessels. J Pharmacol Exp Ther. 1991;258:365–369. [PubMed] [Google Scholar]
- 19.Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 20.Needleman P, Johnson EM., Jr. Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther. 1973;184:709–715. [PubMed] [Google Scholar]
- 21.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells. Evidence for an S-Nitrosothiol-based signal. Circ Res. 2008;103:545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer LA, Doctor A, Chhabra P, Sheram ML, Laubach VE, Karlinsey MZ, Forbes MS, Macdonald T, Gaston B. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest. 2007;117:2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunez C, Victor VM, Tur R, Alvarez-Barrientos A, Moncada S, Esplugues JV, D'Ocon P. Discrepancies between nitroglycerin and NO-releasing drugs on mitochondrial oxygen consumption, vasoactivity, and the release of NO. Circ Res. 2005;97:1063–1069. doi: 10.1161/01.RES.0000190588.84680.34. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Foster MW, Zhang J, Mao L, Rockman HA, Kawamoto T, Kitagawa K, Nakayama KI, Hess DT, Stamler JS. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2005;102:12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer B, Beretta M. The enigma of nitroglycerin bioactivation and nitrate tolerance: news, views and troubles. Br J Pharmacol. 2008;155:170–184. doi: 10.1038/bjp.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon KH, Kim BJ, Song BJ. Inhibition of mitochondrial aldehyde dehydrogenase by nitric oxide-mediated S-nitrosylation. FEBS Lett. 2005;579:6115–6120. doi: 10.1016/j.febslet.2005.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy TC, Arntzen R, Picklo MJ., Sr. Nitrate-based vasodilators inhibit multiple vascular aldehyde dehydrogenases. Cardiovasc Toxicol. 2005;5:321–332. doi: 10.1385/ct:5:3:321. [DOI] [PubMed] [Google Scholar]
- 29.Towell J, Garthwaite T, Wang R. Erythrocyte aldehyde dehydrogenase and disulfiram-like side effects of hypoglycemics and antianginals. Alcohol Clin Exp Res. 1985;9:438–442. doi: 10.1111/j.1530-0277.1985.tb05579.x. [DOI] [PubMed] [Google Scholar]
- 30.Esplugues JV, Rocha M, Nunez C, Bosca I, Ibiza S, Herance JR, Ortega A, Serrador JM, D'Ocon P, Victor VM. Complex I dysfunction and tolerance to nitroglycerin: an approach based on mitochondrial-targeted antioxidants. Circ Res. 2006;99:1067–1075. doi: 10.1161/01.RES.0000250430.62775.99. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Zhang D, Jin W, Shao C, Yan P, Xu C, Sheng H, Liu Y, Yu J, Xie Y, Zhao Y, Lu D, Nebert DW, Harrison DC, Huang W, Jin L. Mitochondrial aldehyde dehydrogenase-2 (ALDH2) Glu504Lys polymorphism contributes to the variation in efficacy of sublingual nitroglycerin. J Clin Invest. 2006;116:506–511. doi: 10.1172/JCI26564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackenzie IS, Maki-Petaja KM, McEniery CM, Bao YP, Wallace SM, Cheriyan J, Monteith S, Brown MJ, Wilkinson IB. Aldehyde dehydrogenase 2 plays a role in the bioactivation of nitroglycerin in humans. Arterioscler Thromb Vasc Biol. 2005;25:1891–1895. doi: 10.1161/01.ATV.0000179599.71086.89. [DOI] [PubMed] [Google Scholar]
- 33.Huellner MW, Schrepfer S, Weyand M, Weiner H, Wimplinger I, Eschenhagen T, Rau T. Inhibition of aldehyde dehydrogenase type 2 attenuates vasodilatory action of nitroglycerin in human veins. Faseb J. 2008;22:2561–2568. doi: 10.1096/fj.07-098830. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Chen Z, Cobb FR, Stamler JS. Role of mitochondrial aldehyde dehydrogenase in nitroglycerin-induced vasodilation of coronary and systemic vessels: an intact canine model. Circulation. 2004;110:750–755. doi: 10.1161/01.CIR.0000138105.17864.6B. [DOI] [PubMed] [Google Scholar]