Abstract
Purpose. Therapeutic mild hypothermia (TMH) is indicated for comatose survivors of an out-ofhospital cardiac arrest (OHCA) to improve general outcome. Although widely used, there are not many reports on its use on a critical care unit (CCU) or on the comparison of cooling methods.
Methods. In a retrospective analysis covering January 2005 to December 2006, 75 consecutive comatose subjects post-OHCA due to ventricular fibrillation and nonventricular fibrillation rhythms (asystole/pulseless electrical activity) were studied in a single tertiary PCI centre. Subjects treated with conventional post-resuscitation care without TMH served as controls (n=26; Jan 2005–Sep 2005). Outcome from controls at hospital discharge was compared with subjects treated with TMH (n=49; Oct 2005–Dec 2006). During the study period, TMH was induced by either external (n=25; Oct 2005–Feb 2006) or endovascular (n=24; Mar 2006–Dec 2006) approach.
Results. Besides more females in the control group, there were no major differences in baseline characteristics present between all groups. TMH improved survival (OR 0.36 [0.13–0.95], p<0.05) and neurological outcome (OR 0.23 [0.07–0.70], p<0.01). After subanalysis, TMH-improved outcome did not differ between the two cooling methods used. However, the times to reach TMH and normothermia were shorter with the endovascular approach.
Conclusion. TMH induced on a CCU improves survival and neurological outcome after post-OHCA coma. TMH by endovascular approach was more feasible compared with external cooling, but the two cooling methods did not result in a different outcome. (Neth Heart J 2009;17:378-84.)
Keywords: resuscitation, heart arrest, hypothermia (induced), treatment outcome
Subjects presenting with an out-of-hospital cardiac arrest (OHCA) have a poor prognosis when adequate resuscitation and supporting measures are not timely initiated, especially when the surviving subject is comatose.1–4 The poor prognosis is related to the associated hypoxic cerebral damage during circulation arrest.5,6 Therapeutic mild hypothermia (TMH) is recommended by the current guidelines on the post-resuscitation care of comatose subjects post-OHCA from the International Liaison Committee on Resuscitation (ILCOR) and European Resuscitation Council (ERC)7,8 TMH post-OHCA is usually performed in an intensive care unit (ICU) and improves neurological outcome and decreases mortality.2,9,10
TMH can be induced in several ways. The most common methods include the endovascular route11 and external surface cooling by e.g. ice packs, cooling mattresses and other external surface cooling devices.9,10 Other methods include veno-venous extracorporeal blood shunt cooling12,13 and selective cooling of the cerebrum by a helmet device.2,14 Comparison of cooling methods is limited. Endovascular cooling has only been shown to be superior to external surface cooling in cerebrovascular surgery.15
To date, randomised controlled trials have only been performed to demonstrate the beneficial effect of TMH post-OHCA.2,9,10,14 In our hospital, TMH has been induced in eligible subjects since 2005. At first, we induced TMH by external surface cooling and later on by endovascular cooling. The switch in cooling method was done in part on the request by our nursing staff. As TMH was induced in a critical care unit (CCU) and not an ICU as done by others, a retrospective analysis was performed to assess our results. In retrospect, the goals were to assess (1) whether inducing TMH improves neurological outcome and/or survival post-OHCA compared with conventional post-resuscitation care in a CCU, and (2) which cooling method seems more effective in inducing TMH post-OHCA.
Methods
Definitions
Mild hypothermia is defined as a central body temperature between 32 and 34°C. Target temperature of TMH is 33°C. Deep hypothermia is defined as a temperature below 32°C. Normothermia is defined as a temperature above 36.5°C.2 The time to reach TMH or the time to ‘cool’ is defined as the time from the start of inducing TMH until the target temperature of TMH was reached. The time to reach normothermia or the time to ‘rewarm’ is defined as the time from discontinuing the maintenance of TMH until the arrival at normothermia. The Glasgow Coma Score (GCS) describes the state of a subject in terms of three aspects of responsiveness: eye opening, verbal response and best motor response, each stratified according to increasing impairment.16 The Glasgow-Pittsburgh Cerebral Performance Categories (CPC) describe the neurological and the thereby related functional outcome of comatose subjects, being (1) good cerebral performance allowing a normal life, (2) moderate cerebral disability allowing part-time work or independent activities of daily life, (3) severe cerebral disability rendering subjects dependent on others for daily support, (4) coma or vegetative state, and (5) death.16 A CPC score of 1–2 depicts a favourable outcome. Higher CPC scores are viewed as non-favourable outcome.
Subjects and study design
The induction of TMH was introduced to our critical care unit (CCU) in the course of 2004. After its pilot phase, subjects post-OHCA were treated according to conventional post-resuscitation care until the development of an approved and functional TMH protocol. Due to the extensive workload of external surface cooling on the nursing staff, endovascular cooling was introduced from March 2006. The protocol and (preventive) measures surrounding TMH itself did not change, only the means of cooling. Therefore, assignment to a certain cooling method occurred by date of the OHCA. When TMH was introduced, we choose to treat all comatose subjects after OHCA irrespective of primary rhythm as the hypothesised mechanism of damage to the central nervous system is not dependent on rhythm type, but due to circulatory insufficiency.2,17
A retrospective analysis was performed in which all subjects admitted to the CCU in a single tertiary PCI centre from January 2005 to December 2006 presenting with an OHCA were included. Inclusion criteria to start TMH were (1) an OHCA with a primary rhythm of VF or non-VF rhythms (asystole/ pulseless electrical activity), (2) a return of spontaneous and stable circulation (ROSC) within 60 minutes after the collapse, (3) a successful cardiopulmonary resuscitation (CPR) that was initiated within 15 minutes after the event, (4) a GCS <7 in an unconscious subject post-OHCA. Exclusion criteria were OHCA due to non-cardiac causes (e.g. haemorrhagic cerebrovascular disease), persisting haemodynamic instability under supporting measures (e.g. inotropes), deep hypothermia (temperature <32°C), terminal illness, pregnancy, and known thrombotic or bleeding tendency. All subjects received CPR, advanced cardiac life support (ACLS) and post-resuscitation care according to the contemporary guidelines from the ILCOR and ERC.7 Included subjects were divided into three groups: (1) historic controls meeting inclusion criteria that received conventional post-resuscitation care without TMH, (2) subjects meeting inclusion criteria who received TMH. In a subanalysis, the TMH group was divided in (i) those who received TMH induced by external surface cooling, and (ii) subjects who received TMH induced by endovascular cooling (see for details of TMH induction below).
Induction of therapeutic mild hypothermia
The rates for the induction of TMH and return to normothermia were taken from national guidelines for TMH (www.nvic.nl). For both endovascular and external surface cooling, the goal was to reach the target temperature of TMH by a rate of no more than 2°C/hour, after which it was maintained for 24 hours. The goal of rewarming to normothermia was by a rate of no more than 0.5°C/hour. TMH was always induced in the CCU without pre-cooling, e.g. the infusion of cold saline. The induction, maintenance and reversal of TMH via the endovascular route and external surface cooling have been described earlier by others.9–11
Additional measures to induction of TMH
All subjects were intubated in the field. Mechanical ventilation in the CCU was managed in accordance with predefined parameters. After starting TMH, a pulmonary artery catheter (Swan-Ganz) was inserted for the close monitoring of haemodynamic parameters and temperature. Subjects received a once-only bolus of dexamethasone (1 mg/kg), after which sedation and relaxation was achieved with midazolam (0.05 mg/ kg/hour) and pancuronium (2 mg/hour). Sedation and relaxation was continued until the completion of the TMH protocol at normothermia to prevent subjects from shivering. Furthermore, subjects received selective digestive tract decontamination (SDD)18 and cefotaxim (3 g/day) intravenously as a prophylaxis regimen. Electrolytes and glucose levels were monitored closely and when needed corrected. Measures were performed to monitor the possible physiological adaptations, changes in electrolyte levels, thrombotic and bleeding tendency, infection risk and haemodynamic changes that are associated with the induction of TMH.2 Other measures varied as these were dependent on the subject's status, comorbidity and the specific cause of the cardiac arrest (e.g. myocardial infarction).
Statistical analysis
Data were collected by retrospective analysis of patients' flowcharts. Variables were compared by the Χ2 test. Odds ratios (OR) were calculated with a logistic regression model in order to adjust the estimated effect of TMH on outcome for possible confounders. Primary outcome measures were survival and neurological outcome at hospital discharge. Neurological outcome was dichotomised in ‘favourable’ and ‘non-favourable’. Secondary outcome measures included hypothermic parameters of endovascular and external cooling induced TMH. Differences were assumed significant at a p value <0.05. Values are expressed as mean ± SD and where noted as 95% confidence interval (CI).
Results
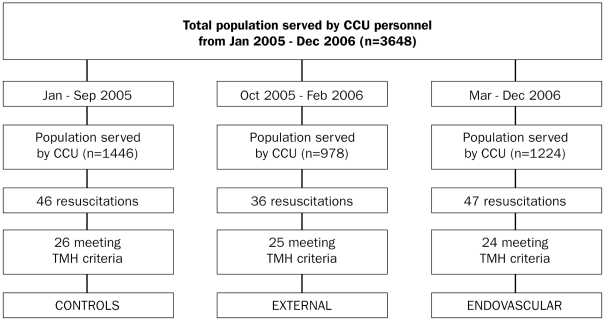
An overview of included subjects is shown in figure 1. Altogether, 75 subjects met the criteria to start TMH; 49 subjects received post-resuscitation care with TMH. In the period October 2005 to February 2006, TMH was induced by external cooling in 25 subjects. In the period March to December 2006, TMH was induced by endovascular cooling in 24 subjects.
Figure 1.
Overview of subjects. CCU=critical care unit, TMH=therapeutic mild hypothermia. See table 1 for further details.
Subject baseline parameters are shown in table 1. There were no differences between historic controls and subjects treated with TMH in age, body weight, referral from other centres or the presence of cardiovascular risk factors. There were more females in the group treated with TMH. In the TMH group, subjects with external surface cooling were more often referred from other centres than subjects with endovascular cooling. There was no major difference in cardiac or cerebrovascular history, except for the presence of more pacemakers in the historical control group. Almost all arrhythmias consisted of atrial fibrillation. One subject in the external surface cooling group had a previous cardiac arrest during transport by ambulance to a tertiary centre for primary PCI for an acute myocardial infarction, which did not result in coma.
Table 1.
Baseline parameters
| Controls (n=26) | Hypothermia (n=49) | P | External (n=25) | Endovascular (n=24) | P | |
|---|---|---|---|---|---|---|
| General characteristics | ||||||
| Age (years) | 60.7±13.5 | 65.7±20.9 | 0.27 | 66.9±15.4 | 64.4±11.6 | 0.53 |
| Gender (female) | 13 (50) | 12 (24.5) | 0.03† | 4 (16) | 8 (33.3) | 0.16 |
| Weight (kg) | 76.9±15.3 | 76.4±14.8 | 0.89 | 77.2±14.2 | 75.5±12.5 | 0.66 |
| Referral from other centre | 2 (7.7) | 4 (8.2) | 0.94 | 4 (16) | 0 (0) | 0.04† |
| Cardiovascular risk factors | ||||||
| Diabetes mellitus | 3 (11.5) | 4 (8.2) | 0.63 | 1 (4) | 3 (12.5) | 0.28 |
| Smoking | 3 (11.5) | 11 (22.4) | 0.25 | 5 (20) | 6 (25.0) | 0.68 |
| Hypertension | 5 (19.2) | 15 (30.6) | 0.29 | 8 (32) | 7 (29.2) | 0.83 |
| Hyperlipidaemia | 4 (15.4) | 7 (14.3) | 0.90 | 5 (20) | 2 (8.3) | 0.48 |
| Positive family history | 7 (26.9) | 9 (18.4) | 0.39 | 3 (12) | 6 (25.0) | 0.24 |
| History | ||||||
| None | 8 (30.8) | 25 (51.0) | 0.09 | 13 (52) | 12 (50.0) | 0.89 |
| Previous infarction | 5 (19.2) | 13 (26.5) | 0.77 | 8 (32) | 5 (20.8) | 0.38 |
| Cardiomyopathy | 3 (11.5) | 1 (2.0) | 0.08 | 0 (0) | 1 (4.2) | 0.30 |
| Angina pectoris | 3 (11.5) | 1 (4.1) | 0.22 | 0 (0) | 2 (8.3) | 0.14 |
| Previous PCI | 3 (11.5) | 7 (14.3) | 0.74 | 5 (20) | 2 (8.3) | 0.24 |
| Previous CABG | 4 (15.4) | 7 (14.3) | 0.90 | 4 (16) | 3 (12.5) | 0.73 |
| Cerebrovascular disease | 4 (15.4) | 3 (6.1) | 0.19 | 1 (4) | 2 (8.3) | 0.53 |
| Valvular disease | 2 (11.5) | 5 (10.2) | 0.72 | 3 (12) | 2 (8.3) | 0.32 |
| Pacemaker | 2 (7.7) | 0 (0) | 0.049† | 0 (0) | 0 (0) | 1.00 |
| Previous cardiac arrest | 0 (0) | 1 (2.0) | 0.46 | 1 (4) | 0 (0) | 0.32 |
| Arrhythmia | 2 (7.7) | 7 (14.3) | 0.40 | 5 (20) | 2 (8.3) | 0.24 |
| Electrical cardioversion | 0 (0) | 2 (4.1) | 0.30 | 2 (8) | 0 (0) | 0.16 |
Values are expressed as mean ± SD or in numbers with percentages of the whole (number of subjects in the cohort) in brackets. Variables significantly different are marked as †. ns=not significant, n=number, PCI=percutaneous coronary intervention, CABG=coronary artery bypass surgery.
About 70 to 80% of the subjects received CPR performed by bystanders. The use of automated external defibrillators (AED) was not reported. There was no difference in the time from the emergency call to the arrival of the emergency medical services (EMS). Delay ranged from 8 to 11 minutes. There was no clear relation between a relatively longer delay and outcome after TMH. Before the return of spontaneous circulation (ROSC), subjects received about three direct current shocks. Primary rhythm at arrival of the EMS was usually VF. About 20 to 40% of the subjects showed the presence of an acute myocardial infarction at the return of ROSC post-OHCA (table 2).
Table 2.
Resuscitation.
| Resuscitation | Controls (n=26) | Hypothermia (n=49) | P | External (n=25) | Endovascular (n=24) | P |
|---|---|---|---|---|---|---|
| General characteristics | ||||||
| Bystander-performed CPR | 18 (69.2) | 37 (75.5) | 0.56 | 17 (68) | 20 (83.3) | 0.21 |
| Time from emergency call to arrival | ||||||
| emergency medical services (min) | 8.3±4.8 | 10.4±8.9 | 0.26 | 8.0±4.8 | 11.2±8.1 | 0.10 |
| Number of DC Shocks before ROSC | 3.0±1.3 | 3.1±1.4 | 0.76 | 3.2±1.4 | 3.0±1.4 | 0.62 |
| Successful CPR | 26 (100) | 49 (100) | 1.00 | 25 (100) | 24 (100) | 1.00 |
| First rhythm | ||||||
| Pulseless VT | 3 (11.5) | 0.0 (0) | 0.02† | 0.0 (0) | 0.0 (0) | 1.00 |
| VF | 22 (84.6) | 46 (93.9) | 0.19 | 24 (96.0) | 22 (91.7) | 0.53 |
| Asystole | 1 (3.8) | 3 (6.1) | 0.68 | 1 (4.0) | 2 (8.3) | 0.68 |
| Infarction (new ST↑) | 5 (19.2) | 19 (38.8) | 0.08 | 9 (36.0) | 10 (41.7) | 0.68 |
CPR=cardio-pulmonary resuscitation, DC=direct current, ROSC=return of spontaneous circulation, VT=ventricular tachycardia, VF=ventricular fibrillation, min=minutes. See table 1 for further details.
In table 3, the hypothermic parameters of the two TMH methods are shown. The general body temperature at arrival was about 35.7°C, probably as a result of patients lying flat on the floor (usually outside) during the event and CPR. Endovascular cooling was faster in reaching TMH than external cooling. The time to reach normothermia was also shorter in endovascular controlled compared with passive rewarming in the External group. Due to software problems in the Coolgard™ unit, which were resolved after a software update, two subjects in the Endovascular group reached target temperature after four hours. When excluded, the time to cool for the Endovascular group was 1.6±1.2 hours (p<0.0001). The difference between the cooling and rewarming rates by the two TMH methods was not intended. The difference was not due to interruptions in the process by diagnostic procedures. Nor was there a structural protocol violation causing the differences. Some people were simply harder to cool or rewarm due to unknown causes that seem unrelated to body weight as one might note.
Table 3.
Hypothermic parameters.
| Hypothermic parameters | Controls (n=26) | Hypothermia (n=49) | P | External (n=25) | Endovascular (n=24) | P |
|---|---|---|---|---|---|---|
| Start temperature (°C) | 35.4±1.6 | 35.8±1.2 | 0.23 | 35.9±1.1 | 35.7±1.2 | 0.55 |
| Time to cool (hours) | N/A | N/A | - | 4.5±2.0 | 2.5±1.8 | 0.0006† |
| Time to rewarm (hours) | N/A | N/A | - | 8.5±2.5 | 5.4±1.5 | <0.0001† |
N/A=not applicable. See table 1 for further details.
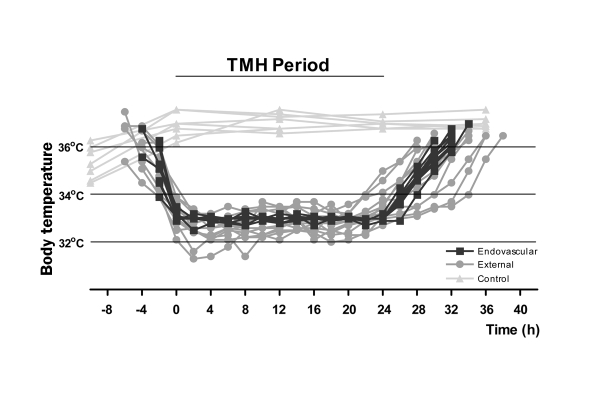
Individual temperature curves of the subjects are shown in figure 2 to illustrate the course of TMH. As shown earlier in table 3 the time to cool and rewarm was shorter in endovascular-controlled TMH. Overshoot to deep hypothermia occurred in two subjects with external surface cooling. Overshoot did not occur in endovascular-induced TMH. The short periods of severe hypothermia were not accompanied by adverse events that are related to severe hypothermia.
Figure 2.
Temperature characteristics of induced hypothermia. Temperature was monitored and shown here in 2 hourly intervals during therapeutic mild hypothermia (TMH) as described in the Methods section. ‘t=0’ was defined as arrival at the target temperature of TMH of 33°C that was maintained for 24 hours. See table 3 for further details.
TMH improved survival and neurological outcome. About 42% of the controls survived the OHCA in contrast to 67% of the subjects in the TMH group. Neurological outcome post-OHCA was favourable in 19% of controls in contrast to 51% of the subjects in the TMH group. In subanalysis, survival and neurological outcome was not different between subjects with either external or endovascular cooling (table 4).
Table 4.
Outcome at hospital discharge.
| Outcome | Controls (n=26) | Hypothermia (n=49) | P | External (n=25) | Endovascular (n=24) | P |
|---|---|---|---|---|---|---|
| Survival of hospital discharge | ||||||
| Alive | 11 (42.3) | 33 (67.3) | 0.04† | 16 (64.0) | 17 (70.8) | 0.61 |
| OR (95% CI) | 1 | 0.36 [0.13–0.95] | 1 | 0.73 [0.22–2.43] | ||
| Neurological outcome at hospital discharge | ||||||
| Favourable | 5 (19.2) | 25 (51.0) | 0.008† | 10 (40.0) | 15 (62.5) | 0.12 |
| OR (95% CI) | 1 | 0.23 [0.07–0.70] | 1 | 0.40 [0.13–1.26] |
p value calculated for differences in odds ratio [OR]. Neurological outcome was favourable when functional status was sufficient to allow independent activities and a discharge home (CPC category 1–2). Percentages in brackets. See table 1 for further details.
Discussion
Here we confirm that TMH improves survival and neurological outcome of comatose subjects post-OHCA when induced in a CCU. Endovascular cooling differs from external cooling in terms of a faster cooling and rewarming rate next to the appearance of being a more controlled process. Even though more subjects in the group with external surface cooling were referred from other centres that might cause a delay in treatment, there was no difference in outcome when cooling methods were compared. TMH is generally advocated as essential in post-resuscitation care but precaution is needed. A recent report showed that unintentional overcooling occurs in a number of cases in which subjects were treated with TMH by external cooling. Overcooling is an underestimated complication of TMH. Improved mechanisms for temperature control in external cooling are required to prevent potential complications of more profound hypothermia, and to improve its effectiveness.19
Animal studies show that a critical time window exists in which hypothermia has its beneficial effects. The suggested time window is short, lasts a few hours and is directly adjacent to the OHCA.1,20 Therefore, the early and rapid application of TMH with only a difference of, for instance, two hours can have significant effects on the prognosis post-OHCA. Although showing an effect of TMH overall, we could not confirm these results from animal studies in our retrospective study. One possibility is that the proposed time window is larger in humans, rendering more time to stabilise the subject and to prepare TMH. Another possibility is that subjects were already slightly hypothermic at presentation to our CCU thus facilitating TMH. There are sufficient resuscitation strategies and protocols for post-resuscitation support. However, there is a lot to gain from optimising the track between ROSC and arrival at an ICU or CCU. Some studies have already explored pre-hospital induction of TMH and show promising results.21–23
At the end of 2005, new resuscitation strategies were advised by the ILCOR and the ERC to achieve more success at Resuscitation.7,8 In the course of 2006, resuscitation strategy changed in the Netherlands. The protocol to induce TMH in our CCU was not changed during the period of the analysis. There is a probability that survival and neurological data might be biased with the changed pre-hospital management. However, improvements in resuscitation success do not automatically lead to improvements in neurological outcome when ROSC is achieved sooner. The Public Access Defibrillation trial investigators showed that the use of AEDs significantly improved the success rate of resuscitation, but the functional status of surviving subjects at hospital discharge was not improved.24 Therefore, additional measures such as TMH are necessary to improve outcome. It is not sufficient to just reach ROSC.
In conclusion, TMH improves survival and neurological outcome when compared with conventional post-resuscitation care. Even though the analysis is in retrospect and not powered to investigate outcome, it opens a new debate. The ideal cooling protocol does not yet exist.2,5 More research is needed on the comparison of methods to induce TMH.
Acknowledgements
The authors wish to thank the Ambulance Service RAV IJssel-Vecht and Ms. E. Kolkman for their assistance.
Financial disclosure
The work performed in this study was not sponsored.
References
- 1.Nozari A, Safar P, Stezoski SW, Wu X, Kostelnik S, Radovsky A, et al. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary Resuscitation. Circulation. 2006;113:2690–6. [DOI] [PubMed] [Google Scholar]
- 2.Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med. 2005;33:414–8. [DOI] [PubMed] [Google Scholar]
- 3.Arrich J. Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35:1041–7. [DOI] [PubMed] [Google Scholar]
- 4.Estner HL, Gunzel C, Ndrepepa G, William F, Blaumeiser D, Rupprecht B, et al. Outcome after out-of-hospital cardiac arrest in a physician-staffed emergency medical system according to the Utstein style. Am Heart J. 2007;153:792–9. [DOI] [PubMed] [Google Scholar]
- 5.van Zanten AR, Polderman KH. Early induction of hypothermia: will sooner be better? Crit Care Med. 2005;33:1449–52. [DOI] [PubMed] [Google Scholar]
- 6.Suarez JI. Outcome in neurocritical care: advances in monitoring and treatment and effect of a specialized neurocritical care team. Crit Care Med. 2006;34:S232–8. [DOI] [PubMed] [Google Scholar]
- 7.International Liaison Committee on Resuscitation. Advanced life support. Resuscitation. 2005;67:213–47. [Google Scholar]
- 8.Nolan JP, Deakin CD, Soar J, Bottiger BW, Smith G. European Resuscitation Council guidelines for resuscitation 2005. Section 4. Adult advanced life support. Resuscitation. 2005;67 Suppl 1:S39–86. [DOI] [PubMed] [Google Scholar]
- 9.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-ofhospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. [DOI] [PubMed] [Google Scholar]
- 10.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. [DOI] [PubMed] [Google Scholar]
- 11.Al Senani FM, Graffagnino C, Grotta JC, Saiki R, Wood D, Chung W, et al. A prospective, multicenter pilot study to evaluate the feasibility and safety of using the CoolGard System and Icy catheter following cardiac arrest. Resuscitation. 2004;62:143–50. [DOI] [PubMed] [Google Scholar]
- 12.Ichinose K, Okamoto T, Tanimoto H, Taguchi H, Tashiro M, Sugita M, et al. A moderate dose of propofol and rapidly induced mild hypothermia with extracorporeal lung and heart assist (ECLHA) improve the neurological outcome after prolonged cardiac arrest in dogs. Resuscitation. 2006;70:275–84. [DOI] [PubMed] [Google Scholar]
- 13.Holzer M, Behringer W, Janata A, Bayegan K, Schima H, Deckert Z, et al. Extracorporeal venovenous cooling for induction of mild hypothermia in human-sized swine. Crit Care Med. 2005;33: 1346–50. [DOI] [PubMed] [Google Scholar]
- 14.Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation. 2001;51:275–81. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg GK, Ogilvy CS, Shuer LM, Connolly ES, Solomon RA, Lam A, et al. Comparison of endovascular and surface cooling during unruptured cerebral aneurysm repair. Neurosurgery. 2004; 55:307–15. [DOI] [PubMed] [Google Scholar]
- 16.Prohl J, Rother J, Kluge S, de Heer G, Liepert J, Bodenburg S, et al. Prediction of short-term and long-term outcomes after cardiac arrest: a prospective multivariate approach combining biochemical, clinical, electrophysiological, and neuropsychological investigations. Crit Care Med. 2007;35:1230–7. [DOI] [PubMed] [Google Scholar]
- 17.Fries M, Weil MH, Chang YT, Castillo C, Tang W. Microcirculation during cardiac arrest and Resuscitation. Crit Care Med. 2006;34:S454–7. [DOI] [PubMed] [Google Scholar]
- 18.de Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362:1011–6. [DOI] [PubMed] [Google Scholar]
- 19.Merchant RM, Abella BS, Peberdy MA, Soar J, Ong ME, Schmidt GA, et al. Therapeutic hypothermia after cardiac arrest: Unintentional overcooling is common using ice packs and conventional cooling blankets. Crit Care Med. 2006;34:S490–4. [DOI] [PubMed] [Google Scholar]
- 20.Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–58. [DOI] [PubMed] [Google Scholar]
- 21.Kamarainen A, Virkkunen I, Tenhunen J, Yli-Hankala A, Silfvast T. Prehospital induction of therapeutic hypothermia during CPR: a pilot study. Resuscitation. 2008;76:360–3. [DOI] [PubMed] [Google Scholar]
- 22.Schefold JC, Storm C, Hasper D. Prehospital therapeutic hypothermia in cardiac arrest: will there ever be evidence? Crit Care. 2008;12:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim F, Olsufka M, Longstreth WT Jr, Maynard C, Carlbom D, Deem S, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation. 2007;115:3064–70. [DOI] [PubMed] [Google Scholar]
- 24.Hallstrom AP, Ornato JP, Weisfeldt M, Travers A, Christenson J, McBurnie MA, et al. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351:637–46. [DOI] [PubMed] [Google Scholar]