Abstract
Cardiopulmonary exercise testing (CPET) in paediatric cardiology differs in many aspects from the tests performed in adult cardiology. Children's cardiovascular responses during exercise testing present different characteristics, particularly oxygen uptake, heart rate and blood pressure response, which are essential in interpreting haemodynamic data. Diseases that are associated with myocardial ischaemia are rare in children. The main indications for CPET in children are evaluation of exercise capacity and the identification of exercise-induced arrhythmias. In this article we will review the main indications for CPET in children with congenital heart disease, the contraindications for exercise testing and the indications for terminating an exercise test. Moreover, we will address the interpretation of gas exchange data from CPET in children with congenital heart disease. (Neth Heart J 2009;17:385-92.)
Keywords: exercise physiology, children, oxygen uptake, ergometry, exercise test
Children with congenital or acquired heart disease often have impairment of their functional capacity. This occurs in the preoperative, postoperative, as well as in the long-term setting and may be the result of the primary cardiac problem, treatment of that problem1 or hypoactivity leading to detraining.2 Lunt et al. found that adolescents with congenital heart disease (CHD) were less likely to reach minimum exercise requirements and perform vigorous exercises than were healthy adolescents.3
Cardiopulmonary exercise testing (CPET) provides objective information about the functional status of heart, lungs and peripheral muscle. This information can be of value in making clinical decisions resulting in a reduced use of hospital facilities, and improved quality of life and functional capacity.4
The aim of this article is to review the main indications for CPET in children with CHD, the contraindications for exercise testing and the indications for terminating an exercise test. Moreover, we will address the interpretation of gas-exchange data from CPET in children with CHD.
Contraindications for exercise testing
The risk of CPET in the paediatric population is believed to be low.5,6 In adults the risk of 1 myocardial infarction or death per 2500 exercise tests has been reported.7 Because ischaemic heart disease is rare in children with CHD, the risk in children is lower than in adults.6 Several absolute and relative contraindications can be distinguished for performing CPET (table 1).
Table 1.
Contraindications for exercise testing.
| Absolute contraindications |
| Active inflammatory heart disease |
| Active hepatitis |
| Acute myocardial infarction |
| Active pneumonia |
| Severe systemic hypertension for age |
| Acute orthopaedic injury to an exercise muscle group |
| Relative contraindications |
| Severe left ventricular outflow obstruction |
| Severe right ventricular outflow obstruction |
| Congestive heart disease |
| Pulmonary vascular obstructive disease |
| Severe aortic stenosis |
| Severe mitral stenosis |
| Ischaemic coronary artery disease |
| Cardiomyopathy |
| Certain inherited arrhythmia syndromes: LQTS, CPVT |
| Complex acquired ventricular arrhythmias |
LQTS=long-QT syndromes, CPVT=catecholaminergic polymorphic ventricular tachycardia. Modified from Stephens and Paridon.6
Before testing, all patients with a CHD must have undergone a complete medical evaluation by a paediatric cardiologist. Several conditions are described in the literature that warrant special consideration including severe aortic/pulmonary stenosis, unrepaired arterial abnormalities, bleeding diatheses, pulmonary hypertension and acute disease processes involving any vital organ.6 If a test can be highly life-threatening, CPET is strongly discouraged.
Indications for CPET in children with CHD
CPET in children with CHD has several indications (table 2). The first is to assess the physical capacity or aerobic capacity of a child with a CHD. This can be used to provide recommendations for physical activity in sports, occupation or rehabilitation. Recommendations for sports participation are available elsewhere.8 Moreover, a CPET can determine whether a patient's complaints of fatigue have a physical aetiology.
Table 2.
Indications for exercise testing in children.
| Assesses physical capacity for recreational, athletic and occupational recommendations |
| Evaluates specific pathophysiological characteristics |
| - Provides indications for surgery, therapy, or additional tests |
| - Evaluates functional postoperative success |
| - Diagnoses disease |
| Assesses adequacy of therapy |
| Assesses risk for future complications in existing disease |
| Instils confidence in child and parents |
| Motivates child for further exercise or weight loss |
Modified from Bar-Or.11
The second indication for exercise testing in CHD is to provide indications for surgery (e.g. pacemaker implantation, valve replacement), therapy (medication or rehabilitation) or additional more invasive/demanding tests (e.g. cardiac CT/MRI, nuclear imaging or heart catheterisation).
Moreover, the stress of the CPET can be used to evaluate the success of interventions such as pacemaker implantation, closure of shunt (normalising in exercise SaO2 %), or ablation of arrhythmogenic substrates. In addition, CPET can be used to diagnose inherited arrhythmia syndromes (e.g. LQTS and CPVT) or chronotropic incompetence.
The third indication for CPET is to evaluate the adequacy of medication, for instance β-blockers, angiotensin-converting enzyme inhibitors and digoxin in heart failure.
The fourth indication is to assess the risk for future disease complications, for instance complex Premature Ventriculae Contractions (PVCs) during exercise in hypertrophic cardiomyopathy; and the fifth indication for CPET is to instil confidence in children and parents. Parents of children with a CHD are often overprotective of their child, even though the underlying disorder is only small and might not be restrictive for performing physical activities including competitive sports (e.g. children with a small ventricular septum defect or atrial septum defects). It usually provides confidence in their child's ability to perform physical exercise when parents see their child running on a treadmill or cycling on an ergometer with high heart rates till exhaustion without any complications. In adult cardiac patients after myocardial infarction (MI), it has been shown that the confidence of the patient and their spouse can be significantly improved when a CPET is performed three weeks after the MI.9
Lastly, CPET (as well as field tests, such as the 6-minute walk test10) can be used to show improvement of physical rehabilitation in children with CHD. Furthermore, a fitness assessment including CPET can be helpful in motivating children with CHD to maintain a healthy body weight or to combat overweight/ obesity.
Interpretation of exercise test results
The use of CPET depends on obtaining a maximal exercise effort from the patient, although several important parameters can also be obtained during submaximal exercise (e.g. ventilatory threshold).
Maximal oxygen uptake
Maximal oxygen uptake (VO2max or VO2peak) is widely recognised as the best single indicator of cardiopulmonary function in children and adults.12 VO2peak is the highest possible oxygen consumption a patient can attain for a specific type of exercise.12 The VO2 plateau theory originates from A.V. Hill and coworkers.13 This theory proposes that oxygen uptake reaches a finite value during exhaustive aerobic exercise: a point at which, despite further increases in exercise intensity, there is no further increase in oxygen uptake. This levelling-off in oxygen uptake can be graphically defined by an asymptotic curve, thus giving exercise physiologists a clear indication of maximal cardiopulmonary exertion, a plateau in oxygen uptake.14 However, with up to 50% of children not reaching a levelling-off in oxygen uptake, the use of a plateau in oxygen uptake as a marker of maximal exertion is not supported in children and the term peak oxygen uptake (VO2peak) is thought to be more appropriate.15,16
Many factors influence VO2peak. The VO2peak can be described using the Fick equation17 in which the oxygen uptake of exercising muscle reflects the product of oxygen delivery by cardiac output and its cellular extraction as indicated by the difference in arterial and venous content:
VO2peak = SVmaxx HRmaxx (CaO2 − CvO2)
in which the cardiac output is a product of heart rate (HR) and stroke volume (SV), and the arterial-venous oxygen difference is the difference in oxygen content of arterial (CaO2) and mixed-venous blood (CvO2). Dynamic changes in one of these parameters related to exercise, therefore, might influence the maximal amount of oxygen uptake during exercise.
Measuring aerobic capacity
VO2peak is the reflection of the maximal oxygen flux through the lungs, transported by the circulation to the mitochondria of the exercising muscle. Based on the Fick principle (as described above), VO2peak is the product of cardiac output and the mixed arterio-venous oxygen difference.17 Thus VO2peak is dependent on cardiac function and the ability of the muscles to extract (utilise) oxygen from the Circulation. In healthy subjects, the cardiac output increases linearly with oxygen uptake, such that for every 1 L/minincrease in oxygen uptake, cardiac output increases by about 5 to 6 L/min.19 In order to increase the VO2, and therefore also VO2peak, either the cardiac output or the arterialvenous oxygen difference must rise.
The gold standard for determining absolute VO2peak in an individual is by metabolic measurement system analysis of O2 and CO2 concentrations in expired air at regular intervals and attainment of a plateau of VO2 during increasing workloads.20 However, in many subjects, including children, a plateau in VO2 is not observed.15 Several secondary parameters have therefore been established to determine VO2peak without a VO2 plateau. These parameters include:21
subjective criteria: Signs of intense effort (unsteady walking, running or biking; sweating; facial flushing; clear unwillingness to continue despite encouragement) and
objective criteria: HRpeak>180/minute and/or RERpeak>0.99.22
Reported values of VO2peak are approximately 38±7 ml/min/kg for 6- to 11-year-old healthy girls and 34±4 ml/kg/min for healthy girls >11 years. In healthy boys, a value of 42±6 ml/kg/min is found before the age of 13, and 50±8 ml/kg/min thereafter.23
Because VO2peak is strongly biased by body weight, it is usually expressed as VO2peak per millilitre per minute per kilogram body mass.20,24 This procedure, however, underestimates the fitness of overweight and obese subjects.25 Moreover, VO2peak/kg body mass also underestimates the VO2peak of taller subjects.26 Therefore, several other fractional-power relationships have been suggested such as per kg 0.66, kg 0.75 or kg 0.87.27,28 Adjusting bodyweight according to these formulas allows a more accurate estimation of an individual's VO2peak than when expressed as VO2peak/kg; however, the optimal scaling power for children is unclear.
The mode of exercise testing and the age and gender of the subject determine oxygen uptake with exercise testing. In general, the highest oxygen uptake is achieved with the type of exercise that uses the greatest amount of muscle mass. In normal subjects, the highest VO2peak is obtained with treadmill testing due to the quantity of the muscle mass involved, followed by bicycle testing. VO2peak achieved by bicycle testing is reported to be 5 to 15% lower than with treadmill testing in normal subjects.29,30 Predicted VO2peak in ml/kg/min estimated from arm exercise testing is 60 to 70% of leg exercise in normal subjects. The intraarterial blood pressure during arm exercise is higher than in leg exercise at given oxygen uptake or cardiac output and the submaximal HR is also higher. The consequence is a heavier load on the heart.31 For completely untrained subjects, older subjects, or those whose cardiac status is unclear, this mode of exercise testing is not recommended. Normal females reach 65 to 75% of male VO2peak.24 The lower oxygen uptake capacity in women may be connected with their lower haemoglobin concentration and higher body fat content. Per kilogram lean body mass, VO2peak is not significantly different between men and women.
Heart rate
HR is mainly determined by both cardiac autonomic nervous activity and sinus nodal function. Dynamic change to some extent reflects the state of the cardiac autonomic nervous system.32
HRpeaknormally increases with exercise, varying according to age and sex. Highly conditioned athletes may have a delayed increase in HR, while deconditioned persons or patients with cardiac dysfunction may have a more rapid than normal HR response.1
The HR at rest and both submaximal and maximal exercise are higher in young subjects compared with adults. Although the HR at rest and the HR at a given workload progressively decreases as a child grows, HRpeakdoes not change. HR at exhaustion in a progressive test remains stable for both boys and girls during the growing years, and does not begin to decline until about the age of 16 years. Consequently, formulae for estimating HRpeak(e.g. 220 – age) are inappropriate for children and young adolescents. The maximal achievable HR during exhaustive exercise has been used extensively as a marker of exertion in normal children,33 and depends on testing modality and protocol. During treadmill running the HRpeakis typically 200 beats/min, whereas walking or cycling protocols usually elicit a HRpeakof approximately 195 beats/min. However, it should be recognised that wide interindividual variability exists in such values, and HRpeakof 185 to 225 beats/min are consistent with exhaustive exercise efforts in individual subjects, making adherence to rigorous criterion values unadvisable. The potential difficulties of using HRpeakas an indicator of maximum exertion are made more acute in children with CHD. The HR response in this population varies considerably, depending on the particular defect, largely as a result of chronotropic incompetence.33
Recently the use of HR recovery after maximal exercise has emerged. It has been described that attenuated HR recovery is a risk factor for cardiovascular disease in adults.34 One-minute HR recovery after exercise is attenuated with age in children. It was found that children with higher BMI, particularly those who are overweight, and those with lower endurance capacity, have slower HR recovery.34 Moreover, Singh et al. reported a significant improvement in HR recovery after maximal exercise in children with CHD following a 12-week cardiac rehabilitation programme.35
Cardiac output
Cardiac output is the product of HR and stroke volume (SV). Since maximal HR during childhood is independent of age and gender, maximal HR can be dismissed as a defining determinant of the increase of cardiac output with age in healthy children. The increase of cardiac output in healthy children is entirely due to maximal SV, which increases in parallel with growth of the left ventricle.11 The maximal SV during exercise shows a clear difference between children and adults; children show a smaller SV during maximal exercise.36 It is known that stroke volume rises progressively in the initial phase of upright exercise up to moderate submaximal intensities (± 40–50% of VO2peak) and then plateaus as exercise intensity increases.31 The further increase in cardiac output with increasing exercise intensity is regulated by the HR. In children with a CHD, the cardiac output might be reduced by a reduction in HRpeakand/or a reduction in SV (e.g. children with a Fontan type circulation or tetralogy of Fallot).
There are several acceptable noninvasive methods to measure cardiac output and SV in children: indirect Fick, acetylene rebreathing, electrical bioimpedance, and Doppler ultrasound.37
Oxygen (O2/HR) pulse
The O2 pulse is the amount of oxygen consumed per heartbeat(O2/HR). A reduced submaximal oxygen pulse may indicate reduced oxygen extraction at the cellular level (reduced arteriovenous difference) or simply a lower SV (O2/HR= SV × (CaO2 − CvO2). Oxygen pulse has been used as an indicator for cardiac output assessment when testing children with CHD.33 The O2 pulse has a decreased rise during exercise in conditions that reduce SV or that decrease arterial oxygen content, such as anaemia or hypoxaemia.1
Oxygen uptake efficiency slope
The oxygen uptake efficiency slope (OUES) represents the rate of increase of VO2 in response to a given VE during incremental exercise, indicating how effectively oxygen is extracted and taken into the body.38 OUES was originally developed by Baba et al. for children with CHD.39 OUES is determined from the linear relation of VO2 (y-axis) vs. the logarithm of minute ventilation (VE) (x-axis) during exercise, i.e. VO2 = a log10 VE+ b, where a is the OUES and b is the intercept.38 The logarithmic transformation of VE is aimed at linearising the otherwise curvilinear relation of VO2 vs. VE, so making the OUES theoretically independent of the patient-achieved effort level.
OUES is influenced by both the metabolic acidosis and the physiological pulmonary dead space.39 The OUES is a variable that indicates the status of both systemic and pulmonary perfusion, and which explains the high correlation with VO2peak. The advantage of the OUES is that it can be calculated from submaximal exercise test data and is therefore effort-independent.39 In healthy children the OUES increases from 1132±149 ml/min/logL in 7-year-old subjects to 2726±602 ml/min/logL in 18-year-old subjects, which shows that the results are influenced by development in children.40
Work efficiency
Work efficiency (ΔVO2/ΔWR) is a measure of the metabolic cost of performing external work. ΔVO2/ΔWR is calculated using the slope of the relationship between oxygen uptake and work rate during incremental exercise. The ΔVO2/ΔWR slope has remarkable linearity and is explained by the rigid physiological coupling of these parameters, especially below the ventilatory threshold (VT). In healthy subjects the relationship remains linear above the VT, while in children with CHD the relationship can be lowered above the VT, due to reduced oxygen delivery to working muscle.41 Paradoxically, a lower slope might be taken to indicate better work efficiency; however a shallow slope is considered abnormal.
In adults the ΔVO2/ΔWR is frequently reported,42 and a normal value of 10.3±1 ml O2/Watt is reported for healthy subjects.43 In children, however, less data are available. In children with a coarctation of the aorta, a significantly reduced value is reported compared with healthy subjects.44
Blood oxygen saturation
An important parameter to measure in children with CHD during exercise is arterial blood oxygen saturation (SaO2%). Using a transcutaneous saturation measurement, SaO2% can be estimated noninvasively. This value gives an estimate of the arterial and venous blood mixing in the heart. CHD with a right-to-left shunt in the heart (e.g. tetralogy of Fallot) are causing reduced SaO2% at rest but sometimes further decreasing during maximal exercise. Usually a drop in SaO2% of >4% during exercise is considered abnormal.45
Blood pressure response
Blood pressures at rest and during exercise are lower in children compared with adults.46 At maximal exercise, a child with a body surface area (BSA) of 1.25 m2 demonstrates a systolic blood pressure of about 140 mmHg, whereas 160 mmHg is expected in a subject with a BSA of 1.75 m2.
Maintaining proper perfusion pressure is a crucial and major role of the Circulation. Several mechanisms are needed during exercise, or in the setting of the failing heart, to maintain adequate pressures of perfusion.32 Systolic blood pressure should show a progressive increase during exercise and a progressive decline after exercise. A decrease in systolic pressure or a failure to increase systolic pressure is abnormal and may indicate clinically significant left ventricular dysfunction.1 Diastolic blood pressure should remain stable or decrease with exercise.1 The level of pressure maintained by arterial baroreflex mechanisms is set at a higher level during exercise. The rapid increase in HR following withdrawal of parasympathetic nervous activity is a major means of attaining adequate cardiac output to achieve the initial target for arterial blood pressure at the beginning of exercise, while metabolic reflexes from working muscle and vasoconstrictive mechanisms are additional stimuli involved in achieving higher arterial blood pressure during moderate to severe exercise.47 An impaired response in terms of the level of blood pressure, therefore, indicates an abnormal function of these regulators.
Ventilatory indexes
Respiratory exchange ratio
The respiratory exchange ratio (RER) is a useful variable, both as a marker of effort and as an indicator of the contribution of anaerobic metabolism.33 RER is the ratio of CO2 exhaled to the O2 uptake per unit time. It is usually around 0.7–0.8 at rest. RER reflects both tissue level exchange of gasses (measured by the respiratory quotient or RQ) and transient changes in O2 and CO2.1 For normal children, RER values at VO2peak reported during cycle ergometry generally range from 1.0 to 1.10.48
Ventilatory threshold
The ventilatory threshold (VT) has traditionally been considered to be the point at which oxygen supply no longer meets the oxygen demands of the working muscle and the increasing contribution from anaerobic metabolism is reflected by a rapidly rising blood lactate level.33 The VT is the level of oxygen uptake above which anaerobic metabolism supplements aerobic energy consumption. This is characterised by an increase in CO2 production above that of aerobic metabolism. Graphically this appears as an abrupt increase in the slope of CO2 production during exercise.1 Usually, the VT occurs at an exercise intensity between 40 and 70% of VO2peak in healthy children.23 A variety of gas exchange criteria have been used, including an abrupt increase in ventilation (VE), in ventilatory equivalent for oxygen (VE/VO2), in the RER or the end-tidal pressure of oxygen (PETO2). These increases must be accompanied by a lack of increase in the ventilatory equivalent for carbon dioxide (VE/VCO2) or the endtidal pressure of carbon dioxide (PETCO2).33 Reybrouck et al. found that, irrespective of the type of heart defect, the VT was always below the normal value in children with CHD.49 The lowest values were recorded in patients after the Fontan operation (62±10% of the normal value).49
Ventilatory equivalents
The ventilatory equivalent for oxygen (VE/VO2) is the amount of ventilation needed for the uptake of a given amount of oxygen. It is an index of ventilatory efficiency and is increased in patients with congestive heart failure due to an increase in physiological dead space (large ventilation/perfusion mismatch).
The ventilatory equivalent for carbon dioxide (VE/VCO2) is the amount of ventilation needed for the elimination of a given amount of carbon dioxide produced by the metabolising tissues. It reflects dead space ventilation, CO2 production, and PCO2, and is higher than normal in heart failure.1
Algorithm for interpretation
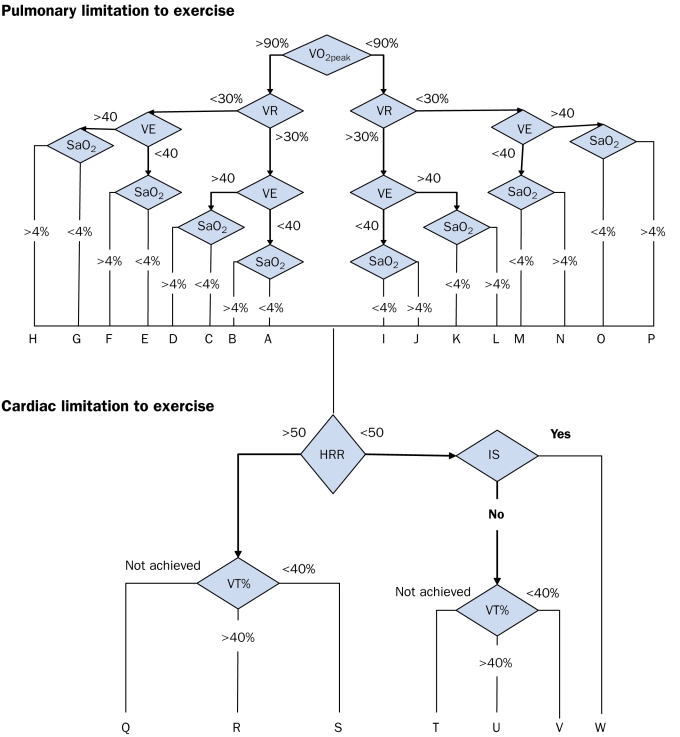
A tool facilitating the interpretation of the CPET data is the algorithm as described by Eschenbacher and Mannina.45 This algorithm uses cut-off points regarding standard outcomes from exercise testing to make a distinction between cardiac, pulmonary or ‘other’ limitations such as deconditioning or musculoskeletal limitations to explain maximal exercise capacity. The following parameters are used in the algorithm: VO2peak, VCO2peak, VEpeak, VEpeak/VO2peak, VEpeak/VCO2peak, HR response (HRR; = (HRpeak− HRrest)/VO2peak − VO2rest), and ventilatory threshold (VT) as percentage of predicted VO2peak. The interpretation of the algorithm can be found in figure 1 and in table 3.
Figure 1.
Algorithm for interpretation of CPET data in adults. For an explanation of the interpretation see tables 3 and 4. Modified from Eschenbacher and Mannina.45 VE=Ventilator equivalent for carbon dioxide; SaO2=change in arterial saturation; IS=Ischemic Symptoms; HRR=Heart Rate Response; VT%=Ratio of VT to predicted VO2peak.
Table 3.
Interpretive results using the algorithm.
| Pulmonary limitation to exercise |
| A. No pulmonary limitation or decreased effort or cardiac limitation |
| B. Mild diffusion type limitation |
| C. Mild gas exchange abnormality |
| D. Mild gas exchange abnormality and diffusion-type limitation |
| E. Mild ventilatory mechanical limitation |
| F. Mild ventilatory mechanical limitation and diffusion-type limitation |
| G. Mild ventilatory mechanical limitation and gas exchange abnormality |
| H. Mild ventilatory mechanical limitation and gas exchange abnormality and diffusion-type limitation |
| I. Decreased effort or cardiac limitation |
| J. Moderate or severe diffusion-type limitation |
| K. Moderate or severe gas exchange abnormality |
| L. Moderate or severe gas exchange abnormality and diffusion-type limitation |
| M. Moderate or severe ventilatory mechanical limitation |
| N. Moderate or severe ventilatory mechanical limitation and diffusion-type limitation |
| O. Moderate or severe ventilatory mechanical limitation and gas exchange abnormality |
| P. Moderate or severe ventilatory mechanical limitation and gas exchange abnormality and diffusion type limitation. |
| Cardiac or circulatory limitation to exercise |
| Q. Moderate or severe cardiac ‘pump’ limitation (cardiomyopathy, deconditioning) |
| R. Cardiac pump limitation (cardiomyopathy, deconditioning) |
| S. Cardiac pump limitation and circulatory limitation (pulmonary vascular or peripheral vascular disease, or pump limitation |
| T. Moderate or severe pulmonary limitation (see J through P) or poor effort |
| U. No obvious cardiac or circulatory limitation |
| V. Circulatory limitation (pulmonary vascular or peripheral vascular disease or pump limitation |
| W. Ischaemic heart disease |
Modified from Eschenbacher and Maninna.45
We have modified the cut-off values for adults with values for healthy children.50 These results are displayed in table 4. Although, the algorithm needs further validation in children, it can be helpful for the interpretation of CPET data in children with CHD.
Table 4.
Cut-off points in Eschenbacher and Maninna's algorithm45 modified for healthy children.
| Parameter | Cut-off point |
|---|---|
| VO2max (%) | <90% of predicted VO2peak |
| VE/VCO2peak | >36 |
| HR response | >(−6.25 × age) + 150 |
| VT (%) | <44 % of predicted VO2peak |
Values for children from De Groot et al.50
Conclusion
In this article we have reviewed the main indications for cardiopulmonary exercise testing in children with congenital heart disease, the contraindications for exercise testing and the indications for terminating an exercise test. Moreover, we have addressed the interpretation of gas exchange data from cardiopulmonary exercise testing in children with congenital heart disease. The growing body of research information surrounding these issues has identified a number of biological responses to exercise that are unique to physically immature individuals. These have provided evidence that, physiologically, children are not simply small adults. It is important that these features be considered when performing clinical exercise testing in children as well as while designing exercise programmes for young subjects.
Acknowledgement
We would like to thank Professor Emeritus David A. de Wolf for his valuable comments on our manuscript.
References
- 1.Connuck DM. The role of exercise stress testing in pediatric patients with heart disease. Pediatr Cardiol. 2005;20:45–52. [Google Scholar]
- 2.Bar-Or O. Pathophysiological factors which limit the exercise capacity of the sick child. Med Sci Sports Exerc. 1986;18:276–82. [DOI] [PubMed] [Google Scholar]
- 3.Lunt D, Briffa T, Briffa NK, Ramsay J. Physical activity levels of adolescents with congenital heart disease. Aust J Physiother. 2003; 49:43–50. [DOI] [PubMed] [Google Scholar]
- 4.Rich MW. Heart failure disease management: a critical review. J Card Fail. 1999;5:64–75. [DOI] [PubMed] [Google Scholar]
- 5.Alpert BS, Verrill DE, Flood NL, Boineau JP, Strong WB. Complications of ergometer exercise in children. Pediatr Cardiol. 1983; 4:91–6. [DOI] [PubMed] [Google Scholar]
- 6.Stephens P Jr, Paridon SM. Exercise testing in pediatrics. Pediatr Clin North Am. 2004;51:1569–87, viii. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers GP, Ayanian JZ, Balady G, Beasley JW, Brown KA, Gervino EV, et al. American College of Cardiology/American Heart Association Clinical Competence Statement on Stress Testing. A Report of the American College of Cardiology/American Heart Association/American College of Physicians-American Society of Internal Medicine Task Force on Clinical Competence. Circulation. 2000;102:1726–38. [DOI] [PubMed] [Google Scholar]
- 8.Pelliccia A, Fagard R, Bjornstad HH, Anastassakis A, Arbustini E, Assanelli D, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:1422–45. [DOI] [PubMed] [Google Scholar]
- 9.Taylor CB, Bandura A, Ewart CK, Miller NH, DeBusk RF. Exercise testing to enhance wives' confidence in their husbands' cardiac capability soon after clinically uncomplicated acute myocardial infarction. Am J Cardiol. 1985;55:635–8. [DOI] [PubMed] [Google Scholar]
- 10.Moalla W, Gauthier R, Maingourd Y, Ahmaidi S. Six-minute walking test to assess exercise tolerance and cardiorespiratory responses during training program in children with congenital heart disease. Int J Sports Med. 2005;26:756–62. [DOI] [PubMed] [Google Scholar]
- 11.Bar-Or O. Pediatric sports medicine for the practitioner. New York: Springer-Verlag; 1983. [Google Scholar]
- 12.Shephard RJ, Allen C, Benade AJ, Davies CT, Di Prampero PE, Hedman R, et al. The maximum oxygen intake. An international reference standard of cardiorespiratory fitness. Bull World Health Organ. 1968;38:757–64. [PMC free article] [PubMed] [Google Scholar]
- 13.Hill AV, Lupton H. Muscular exercise, lactic acid and the supply and utilization of oxygen. Q J Med. 1923;16:135–71. [Google Scholar]
- 14.Howley ET, Bassett DRJ, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27: 1292–301. [PubMed] [Google Scholar]
- 15.Rowland TW. Does peak VO2 reflect VO2max in children?: evidence from supramaximal testing. Med Sci Sports Exerc. 1993; 25:689–93. [PubMed] [Google Scholar]
- 16.Armstrong N, Welsman J, Winsley R. Is peak VO2 a maximal index of children's aerobic fitness? Int J Sports Med. 1996;17: 356–9. [DOI] [PubMed] [Google Scholar]
- 17.Fick A. Ueber die Messung des Blutquantums in den Herzventrikeln. Sitx Physik-Med Ges Wurzburg. 1870;2:16 [Google Scholar]
- 18.Shephard RJ, Allen C, Benade AJ, Davies CT, Di Prampero PE, Hedman R, et al. The maximum oxygen intake. An international reference standard of cardiorespiratory fitness. Bull World Health Organ. 1968;38:757–64. [PMC free article] [PubMed] [Google Scholar]
- 19.Haller RG, Lewis SF, Cook JD, Blomqvist CG. Hyperkinetic circulation during exercise in neuromuscular disease. Neurology. 1983;33:1283–7. [DOI] [PubMed] [Google Scholar]
- 20.Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardiorespiratory performance. J Appl Physiol. 1955;8:73–80. [DOI] [PubMed] [Google Scholar]
- 21.Franklin BA. Abnormal cardiorespiratory responses to acute aerobic exercise. In: Roitman JL, editor. ACSM's resource manual for guidelines for exercise testing and prescription. Third edition. Baltimore: Williams and Wilkins; 1998. p. 146–55. [Google Scholar]
- 22.Rowland TW. Aerobic exercise testing protocols. In: Rowland TW, editor. Pediatric Laboratory Exercise Testing: Clinical Guidelines. Champaign, Ill: Human Kinetics; 1993. p. 19–41. [Google Scholar]
- 23.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol. 1984;56:628–34. [DOI] [PubMed] [Google Scholar]
- 24.Astrand PO. Experimental studies of physical work capacity in relation to sex and age: Copenhagen, Munkgaard; 1952. [Google Scholar]
- 25.Owens S, Gutin B. Exercise testing of the child with obesity. Pediatr Cardiol. 1999;20:79–83. [DOI] [PubMed] [Google Scholar]
- 26.Tanner JM. Fallacy of per weight and per surface area standards and their relation to spurius correlation. J Appl Physiol. 1949;2: 1–15. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Nielsen K. Scaling: Why is animal size so important? Cambridge: Cambridge University Press; 1984. [Google Scholar]
- 28.Weibel ER, Hoppeler H. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J Exp Biol. 2005;208: 1635–44. [DOI] [PubMed] [Google Scholar]
- 29.Hermansen L, Ekblom B, Saltin B. Cardiac output during submaximal and maximal treadmill and bicycle exercise. J Appl Physiol. 1970;29:82–6. [DOI] [PubMed] [Google Scholar]
- 30.Hermansen L, Saltin B. Oxygen uptake during maximal treadmill and bicycle exercise. J Appl Physiol. 1969;26:31–7. [DOI] [PubMed] [Google Scholar]
- 31.Astrand P, Rodahl K, Astrand PRK. Textbook of work physiology, physiological bases of exercise. New York: McGraw-Hill Book Company; 1986. [Google Scholar]
- 32.Ohuchi H. Cardiopulmonary response to exercise in patients with the Fontan Circulation. Cardiol Young. 2005;15 Suppl 3:39–44. [DOI] [PubMed] [Google Scholar]
- 33.McManus A, Leung M. Maximising the clinical use of exercise gaseous exchange testing in children with repaired cyanotic congenital heart defects: the development of an appropriate test strategy. Sports Med. 2000;29:229–44. [DOI] [PubMed] [Google Scholar]
- 34.Singh TP, Rhodes J, Gauvreau K. Determinants of heart rate recovery following exercise in children. Med Sci Sports Exerc. 2008;40:601–5. [DOI] [PubMed] [Google Scholar]
- 35.Singh TP, Curran TJ, Rhodes J. Cardiac rehabilitation improves heart rate recovery following peak exercise in children with repaired congenital heart disease. Pediatr Cardiol. 2007;28:276–9. [DOI] [PubMed] [Google Scholar]
- 36.Godfrey S. Exercise testing in children. London: W.B. Saunders Company Ltd.; 1974. [Google Scholar]
- 37.Driscoll DJ, Staats BA, Beck KC. Measurement of Cardiac Output in Children During Exercise: A Review. Pediatr Exerc Sci. 1989;1: 102–15. [Google Scholar]
- 38.Baba R, Nagashima M, Nagano Y, Ikoma M, Nishibata K. Role of the oxygen uptake efficiency slope in evaluating exercise tolerance. Arch Dis Child. 1999;81:73–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28:1567–72. [DOI] [PubMed] [Google Scholar]
- 40.Marinov B, Mandadzhieva S, Kostianev S. Oxygen-uptake efficiency slope in healthy 7- to 18-year-old children. Pediatr Exerc Sci. 2007;19:159–70. [DOI] [PubMed] [Google Scholar]
- 41.Groen WG, Hulzebos EH, Helders PJ, Takken, T. Oxygen Uptake To Work Rate Relationship During Exercise In Children With Lung, Heart or Muscle Disease. Med Sci Sports Exerc. 2009;41:S5, Abstract 1658 [Google Scholar]
- 42.Hansen JE, Sue DY, Oren A, Wasserman K. Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. Am J Cardiol. 1987;59:669–74. [DOI] [PubMed] [Google Scholar]
- 43.Wasserman K, Hansen JE, Sue DY, Casaburi R, Whipp BJ. Principles of Exercise Testing and Interpretation. Baltimore, MD: Lippincott, Williams and Wilkins; 1999. [Google Scholar]
- 44.Rhodes J, Geggel RL, Marx GR, Bevilacqua L, Dambach YB, Hijazi ZM. Excessive anaerobic metabolism during exercise after repair of aortic coarctation. J Pediatr. 1997;131:210–4. [DOI] [PubMed] [Google Scholar]
- 45.Eschenbacher WL, Mannina A. An algorithm for the interpretation of cardiopulmonary exercise tests. Chest. 1990;97:263–7. [DOI] [PubMed] [Google Scholar]
- 46.Riopel DA, Taylor AB, Hohn AR. Blood pressure, heart rate, pressure-rate product and electrocardiographic changes in healthy children during treadmill exercise. Am J Cardiol. 1979;44:697–704. [DOI] [PubMed] [Google Scholar]
- 47.Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990; 69:407–18. [DOI] [PubMed] [Google Scholar]
- 48.Turley KR, Wilmore JH. Cardiovascular responses to treadmill and cycle ergometer exercise in children and adults. J Appl Physiol. 1997;83:948–57. [DOI] [PubMed] [Google Scholar]
- 49.Reybrouck T, Rogers R, Weymans M, Dumoulin M, Vanhove M, Daenen W, et al. Serial cardiorespiratory exercise testing in patients with congenital heart disease. Eur J Pediatr. 1995;154:801–6. [DOI] [PubMed] [Google Scholar]
- 50.De Groot JF, Takken T, Schoenmakers MA, Vanhees L, Helders PJ. Limiting factors in peak oxygen uptake and the relationship with functional ambulation in ambulating children with Spina Bifida. Eur J Appl Physiol. 2008;104:657–65. [DOI] [PubMed] [Google Scholar]