Abstract
The formation of a nitrogen-fixing nodule requires the coordinated development of rhizobial colonization and nodule organogenesis. Based on its mutant phenotype, lumpy infections (lin), LIN functions at an early stage of the rhizobial symbiotic process, required for both infection thread growth in root hair cells and the further development of nodule primordia. We show that spontaneous nodulation activated by the calcium- and calmodulin-dependent protein kinase is independent of LIN; thus, LIN is not necessary for nodule organogenesis. From this, we infer that LIN predominantly functions during rhizobial colonization and that the abortion of this process in lin mutants leads to a suppression of nodule development. Here, we identify the LIN gene in Medicago truncatula and Lotus japonicus, showing that it codes for a predicted E3 ubiquitin ligase containing a highly conserved U-box and WD40 repeat domains. Ubiquitin-mediated protein degradation is a universal mechanism to regulate many biological processes by eliminating rate-limiting enzymes and key components such as transcription factors. We propose that LIN is a regulator of the component(s) of the nodulation factor signal transduction pathway and that its function is required for correct temporal and spatial activity of the target protein(s).
The soil bacteria rhizobia are able to establish nitrogen-fixing symbioses with leguminous plants by inducing the formation of a new organ, the nodule, on the roots of the host plant. Symbiotic infection is initiated and maintained by an exchange of signaling molecules between the host plant and the microsymbionts. In most legumes, flavonoid compounds produced by leguminous plants activate bacterial regulators to induce nodulation (nod) genes required for the synthesis of lipochitooligosaccharide Nod factors. Nod factors initiate multiple early responses on plant hosts, including a burst of intracellular calcium levels in root hairs, calcium oscillations (calcium spiking), and the induction of nodulation-specific genes whose products are referred to as “nodulins.” In addition, they induce a rearrangement of the root hair cytoskeleton, leading to root hair deformation and curling, which traps surface-attached rhizobia, establishing a site that acts as an infection focus. Bacteria penetrate into the curled root hairs toward the root cortex via host-derived tubular structures called infection threads. Simultaneously, Nod factors stimulate the reinitiation of mitosis in cortical cells, leading to the formation of nodule primordia, which give rise to the cells that receive the invading bacteria (Oldroyd and Downie, 2008). Exopolysaccharides, capsular polysaccharides (K antigens), and lipopolysaccharides serve as further bacterial signals required for successful infection of nodules in many legumes (Jones et al., 2007). The infecting rhizobia are released into intracellular membrane compartments of plant origin called symbiosomes, where they differentiate into bacteroids capable of reducing atmospheric nitrogen to ammonium, which is provided to the plants in exchange for carbon and amino acid compounds (Brewin, 2004).
Analysis of nodulation-defective Medicago truncatula, Lotus japonicus, and pea (Pisum sativum) mutants has led to insights into the mechanisms by which Nod factors are perceived and trigger subsequent signal transduction cascades (Geurts et al., 2005; Stacey et al., 2006; Oldroyd and Downie, 2008). In M. truncatula and L. japonicus, the Nod factor signal is probably sensed by LysM-type receptor kinases such as NFP/NFR5 (Amor et al., 2003; Radutoiu et al., 2003) and LYK3/NFR1 (Smit et al., 2007; Radutoiu et al., 2003). Another receptor-like kinase known as NORK/DMI2/SYMRK (Endre et al., 2002b; Stracke et al., 2002) is also involved in the transmission of the Nod factor signal. Downstream in the pathway, DMI1/POLLUX and CASTOR (Ane et al., 2004; Imaizumi-Anraku et al., 2005), putative ligand-gated ion channels (Charpentier et al., 2008), nucleoporins NUP133 and NUP85 (Saito et al., 2007), a Ca2+/calmodulin-dependent protein kinase (CCaMK; Levy et al., 2004; Mitra and Long, 2004; Tirichine et al., 2006a), and a protein of unknown function, Cyclops (Yano et al., 2008), are necessary for the activation of transcriptional regulators such as NSP1 (Smit et al., 2005; Heckmann et al., 2006), NSP2 (Kalo et al., 2005; Heckmann et al., 2006), and ERN1 (Middleton et al., 2007) responsible for the transcriptional changes that are required for the initiation of nodule morphogenesis. In addition to the signaling pathway outlined above, legumes have many genes required to enable rhizobia to infect the roots. Only a few of these genes have been identified; the RPG gene required for infection was identified, but the function of its product has yet to be defined (Arrighi et al., 2008). Some genes such as NIN and ERN encode predicted regulators that may affect both the Nod factor signaling and infection pathways (Schauser et al., 1999; Marsh et al., 2007; Middleton et al., 2007), and the hcl mutation (in the MtLYK3 gene) affecting a predicted Nod factor receptor also causes an infection defect (Smit et al., 2007). The NAP and PIR genes are required for normal infection, and their loss affects polar growth of some cell types and probably is required for the polar growth of the infection thread (Yokota et al., 2009). The NAP and PIR proteins have been identified as components of the SCAR/WAVE complex, which is involved in polymerization of actin-related proteins (Li et al., 2004).
The lumpy infections (lin) mutant (line C88) was identified as a M. truncatula mutant, in which infection was arrested and reduced to one-quarter of the frequency seen in wild-type plants (Penmetsa and Cook, 2000; Kuppusamy et al., 2004). In those cases when infections were initiated, bacteria were arrested after very limited progression within root hairs. Although nodule primordia were formed in the root cortex, differentiation was arrested at an early stage. Interestingly, infection and initiation of cortical cell division recurred continually in the presence of rhizobia, suggesting that LIN is required for appropriate regulation of nodule initiation and for control of nodule number (Kuppusamy et al., 2004). The early nodulins (RIP1, ENOD20, and ENOD40) were expressed in lin at comparable levels to the wild type, while late nodulins such as MtN6, ENOD2, and ENOD8 failed to be induced in lin. This suggests that LIN acts downstream of the early Nod factor signal transduction pathway but is required for infection initiation and persistence and nodule differentiation (Kuppusamy et al., 2004). Based on the phenotype of the mutant, LIN function is probably needed in the process of nodule development/maturation, with indirect effect on the block of infection thread growth or in the invasion process resulting in the interruption of nodule development. It is also possible that LIN acts at both levels as a synchronizing regulator between the parallel processes in the root epidermis and the cortex.
In this work, we show the identification of the LIN gene, revealing that LIN encodes a large protein containing multiple domains including a U-box (modified RING) domain, indicating a function as an E3 ubiquitin ligase. Spatiotemporal analysis of the promoter activity of LIN demonstrated that the expression of the gene correlated with the early nodule primordia formation and bacterial invasion during the symbiosis. We used a gain-of-function mutation in CCaMK that induces spontaneous nodulation in the absence of rhizobia to show that LIN is not required for nodule organogenesis. This indicates that LIN functions exclusively during rhizobial colonization and that the defect in nodule development in lin is a response to aborted infection.
RESULTS
LIN Is Not Required for Nodule Organogenesis
Ethyl methanesulfonate (EMS) and fast-neutron-mutagenized M. truncatula populations were screened to identify new loci required for nodulation; two of the mutants identified had phenotypes similar to lin, namely impaired nodulation and infection with the infection threads arrested in the root hair cells. Although nodule primordia emerged 3 to 4 d after inoculating the roots with wild-type rhizobia, nodule development was always blocked before the stage of nodule differentiation. No nonsymbiotic phenotype was detected in these mutants. Genetic crosses revealed that the two mutants (EMS6:T7 and 14P) carried mutations allelic to lin-1 (Table I) but not allelic to the other nodulation mutations tested (data not shown). EMS6:T7 was derived from EMS mutagenesis and will be designated as lin-2, while 14P was derived from fast-neutron mutagenesis and will be designated as lin-3.
Table I.
Results of the lin allelism test in M. truncatula
All individuals were identified as symbiotic mutants in the progeny of the respective crosses. Values represent numbers of individuals tested; values in parentheses represent pod numbers from which seeds originated. na, Not applicable.
| Mutant | lin-1 C88 | lin-2 EMS6:T7 | lin-3 14P |
|---|---|---|---|
| lin-1 | na | 3 (1) | 17 (4) |
| lin-2 | 15 (4) | na | 11 (5) |
|
lin-3 |
15 (5) |
24 (7) |
na |
To determine if LIN is required for nodule organogenesis, we tested whether an autoactive form of CCaMK could induce spontaneous nodulation in lin mutants. In M. truncatula, transgenic expression of an autoactive CCaMK (DMI31–311), composed of the kinase domain alone, is capable of inducing spontaneous nodules and has been useful in determining the order of gene product function within the early Nod factor signaling pathway relative to CCaMK (Gleason et al., 2006). When we transformed lin-1, lin-2, and lin-3 mutants with this autoactive CCaMK construct, we could clearly detect nodules on the lin-1 (three of 21 plants), lin-2 (nine of 19 plants), and lin-3 (40 of 103 plants) mutants. The observation that spontaneous nodules were induced on 52 of 143 lin mutant plants transformed with autoactive CCaMK suggests that LIN is not essential for nodule organogenesis; therefore, the defects in nodule development observed in the lin mutants during rhizobial invasion are likely to be a result of the abortion of bacterial infection.
Positional Cloning of LIN in M. truncatula
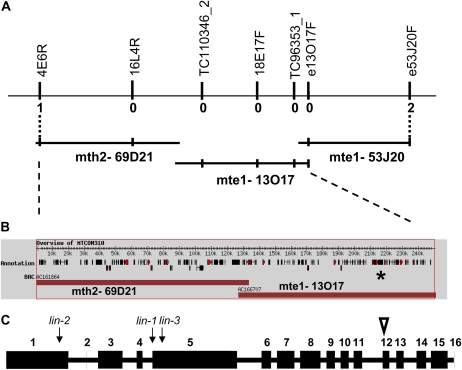
Preliminary mapping data previously positioned lin-1 (C88) between molecular markers DSI and SCP on the lower arm of linkage group 1 (LG1) within a relatively large distance of several centimorgan (Kuppusamy et al., 2004). Using newly generated markers and a new segregating population of 290 F2 individuals and 508 F3 individuals of selected F2 plants, we mapped LIN within the region on LG1 between markers 4E6R and e53J20F (Fig. 1A). This region is spanned by the sequenced bacterial artificial chromosome clones (BACs) mth2-69D21, mte1-13O17, and mte1-53J20 (Fig. 1, A and B). Within this region, there were numerous good candidates for LIN among the predicted genes, including genes encoding AP2 transcription factors, a bZIP transcription factor, a protein kinase, a putative RING zinc finger protein, and genes with unknown function, but represented with nodule ESTs in the Medicago database. No mutation was detected in lin-1 for any of these candidate genes.
Figure 1.
M. truncatula LIN identification by positional cloning. A, Genomic region around the lin mutation. The overlapping BAC clones mth2-69D21, mte1-13O17, and mte1-53J20 represent the region where lin was delimited by the identified recombination events. Vertical bars correspond to the polymorphic markers generated in the region and used for identifying recombinations (numbers under the bars) from lin to set the borders of the region. B, Predicted coding regions in MTCON310. The position of MTCON310-47 is indicated by the star. C, Gene model of MTCON310-47. For the exon-intron structure of the LIN coding region, numbered blocks are the coding exons and the white triangle shows the position of the extra 24 nucleotides in the actual cDNA compared with the predicted cDNA. Arrows indicate the positions of the mutations in the three M. truncatula lin alleles. [See online article for color version of this figure.]
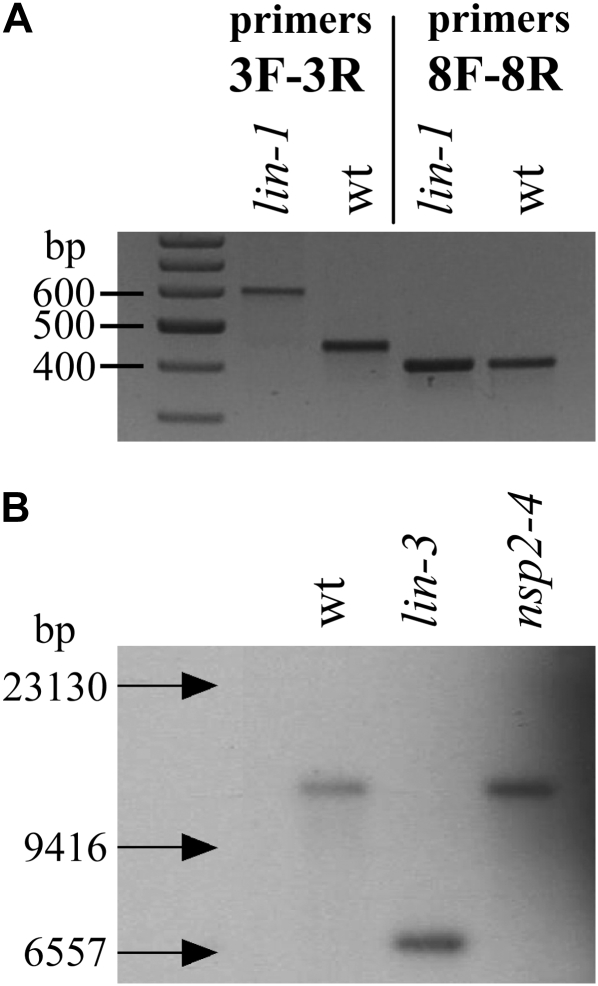
Further sequencing of this region in lin-1 and comparisons with the sequence from the wild type revealed a single nucleotide difference in lin-1 in the predicted coding region MTCON310-47 (indicated by the star in Fig. 1B). Since no EST had been reported for this gene, we validated that this region was actively transcribed in M. truncatula. PCR amplification products could easily be obtained from plant cDNA isolated from roots 4 d after Sinorhizobium meliloti inoculation. The cDNA of MTCON310-47 could be assembled from the sequences of overlapping fragments. The intron/exon boundaries were similar to what had previously been predicted for the genomic sequence, with the exception that exon 12 was found to be 24 bp longer (indicated by a white triangle in Fig. 1C), resulting in a predicted total protein of 1,488 amino acids. The mutation we identified in lin-1 was in the last nucleotide position of intron 4 (indicated by an arrow in Fig. 1C). Reverse transcription (RT)-PCR amplification of the affected region using primers Lin-3F (in exon 3) and Lin-3R (in exon 5) on lin-1 RNA samples of inoculated roots revealed that there was indeed an error in the RNA splicing, resulting in a longer transcript (Fig. 2A). Sequencing of this longer fragment showed that due to the mutation, intron 4 was not spliced out during mRNA processing (while correct splicing of intron 3 was detected in the same fragment), causing a premature stop codon to be introduced. Amplification of all other parts of the lin-1 cDNA resulted in the expected fragment sizes (e.g. the fragment amplified by Lin-8R and Lin-8R primers in Fig. 2A).
Figure 2.
Visualization of two mutations in the M. truncatula LIN gene. A, RT-PCR products of lin-1 mutant and wild-type A17 plants on the RNA samples isolated from roots 4 d after S. meliloti inoculation. DNA fragments with primers Lin-3F and Lin-3R include intron 4, which is spliced out from the wild-type allele but not spliced from the mutant. Amplification with primers Lin-8F and Lin-8R resulted in identical fragments representing exons 11 to 14 on the same cDNA templates. B, Southern blot using EcoRV-digested genomic DNA of the wild type (wt) and lin-3 mutant plants (as well as the nsp2-4 mutant included as another control), and a probe of 500 bp from exon 5 amplified with primers Lin-3Fb and Lin-12Rc revealed an apparent deletion or rearrangement affecting the large part of the gene.
Sequencing of the genomic DNA amplified from the lin-2 mutant revealed a point mutation in the first exon that appears at nucleotide position 662 in the cDNA sequence, introducing a premature stop codon into MTCON310-47 very early in this gene (indicated by an arrow in Fig. 1C). In addition, Southern analysis of lin-3 revealed an apparent large deletion or rearrangement in this same gene (Fig. 2B). RT-PCR experiments on lin-3 demonstrated the presence of a short mRNA (1,568 nucleotides) transcribed from this allele (indicated by an arrow in Fig. 1C). Another mutant line, C105, originating from the same population (Penmetsa and Cook, 2000) and having a similar phenotype to C88 (lin-1), is allelic with C88 (R. Dickstein, personal communication); sequencing of MTCON310-47 in C105 revealed the same mutation, suggesting that C88 and C105 are siblings. The identification of mutation events in three different alleles of lin provides strong evidence that MTCON310-47 is indeed LIN.
Complementation of the Medicago lin Mutants on Transgenic Hairy Roots
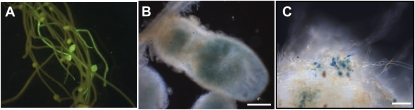
To confirm that MTCON310-47 corresponds to LIN, we tested complementation of the lin alleles with a construct carrying MTCON310-47 expressed off the 35S promoter and also harboring a constitutively expressed GFP reporter gene to facilitate the identification of the transgenic roots. The lin mutants were transformed using Agrobacterium rhizogenes-mediated hairy root transformation, and transgenic hairy roots were detected by the fluorescent GFP signal (Fig. 3A). For screening the symbiotic phenotype of the roots, wild-type symbiotic bacteria were applied on the well-grown root systems following 1 week of nitrogen starvation. Complementation of the symbiotic phenotype of all three lin mutant alleles was observed, indicated by the formation of mature nodules induced by S. meliloti strain 1021 carrying a hemA promoter:lacZ fusion (Fig. 3B). The lin mutant alleles do form bumps following S. meliloti inoculation; however, in these complementation experiments, we could clearly discriminate between the mature complemented nodules that were infected (Fig. 3B) and the nodule primordia bumps, where infections always arrested in the root hairs on the nontransgenic roots of lin mutants or on transgenic roots induced by A. rhizogenes carrying the empty vector (Fig. 3C). Using β-galactosidase staining of the symbiotic bacteria, we could clearly see the presence of rhizobia within the nodule and in infection threads ramifying throughout the nodules on complemented roots. The identification of mutations in MTCON310-47 in lin-1, lin-2, and lin-3, coupled with the complementation of these mutants by this gene, proves that MTCON310-47 encodes LIN.
Figure 3.
Positive complementation of the nodulation phenotype on the transgenic hairy roots of the lin-1 mutants. A, Detection of the transgenic roots and nodules by GFP fluorescence. Hairy roots were induced without antibiotic selection, allowing the emergence of both transgenic (bright green fluorescent roots) and nontransgenic (yellowish) roots. B, Mature nodule on the transgenic root of the lin-1 mutant occupied by S. meliloti strain 1021 carrying a hemA promoter:lacZ fusion, stained for β-galactosidase activity. Bacteria visualized in the nodule revealed that wild-type nodule development and an invasion process occurred in the complemented roots. C, Nodule primordia on control nontransgenic roots of the lin-1 mutant inoculated with the same bacteria showed the mutant phenotype with the nodule primordia and arrested infections in the root hairs. Bars = 200 μm (B) and 10 μm (C).
Identification and Transcomplementation of a L. japonicus lin Mutant
Mutants of L. japonicus with a phenotype similar to the M. truncatula lin mutant were described previously, and two of the mutant loci, sym7 and itd3, map to a 10-centimorgan region on the upper arm of chromosome 5 (Lombardo et al., 2006) in a region predicted to show synteny with the map location of LIN in M. truncatula (Choi et al., 2004). To identify a LIN-like gene from L. japonicus, The Institute for Genomic Research database of ESTs was checked for sequences similar to MtLIN. The most similar L. japonicus EST sequence was BP053520 (E-value of 8.7 e−47), and two reverse primers designed from this sequence were used in combination with various MtLIN forward primers to amplify cDNA from L. japonicus. This resulted in the amplification of about 3.5 kb of cDNA. Where the primer combinations produced multiple fragments, nested PCR was used to produce individual fragments for DNA sequencing. The 5′ end of the gene was obtained using the L. japonicus GenomeWalker library (Heckmann et al., 2006). The assembled L. japonicus cDNA sequence of 4,470 bp (GenBank accession no. EU926664) is 86% identical to the coding region of the M. truncatula LIN gene.
The MtLIN-like gene was amplified and sequenced from mutants SL1450-5 (carrying an allele of sym7) and SL1947-2 (carrying itd3), revealing one change, a G-to-A substitution at position 3,799, in SL1450-5 and no change in SL1947-2. This mutation in SL1450-5 resulted in the alteration of Asp-1267 (GAC) to Asn (AAC). Roots of SL1450-5 were transformed with the same MtLIN construct that was used for complementation of the M. truncatula lin mutants. MtLIN complemented both nodulation (Table II; Fig. 4A) and infection (Fig. 4B) of SL1450-5 in transformed hairy roots. Normal-looking pink nodules could be observed in the complemented plants, indicating that they were functional; nodulation, scored only using those transgenic roots showing GFP fluorescence, was significantly different from the controls lacking MtLIN (Table II). This shows that (1) the L. japonicus mutant phenotype can be rescued by the M. truncatula wild-type LIN gene, (2) MtLIN is the ortholog of LjSYM7, and (3) the identified mutation caused the nodulation-defective phenotype of SL1450-5. A few nodules were observed on hairy roots in the negative controls (Table II), but microscopy revealed only a few infected cells in these nodules, as had been seen previously with the SL1450-5 mutant (Lombardo et al., 2006). No nodules were observed on hairy roots of SL1947-2 (itd3) transformed with MtLIN, confirming previous observations (Lombardo et al., 2006) that SL1947-2 and SL1450-5 do not carry allelic mutations.
Table II.
Hairy root complementation tests of L. japonicus mutants for nodulation with the M. truncatula LIN gene gave positive results for the sym7 mutation
Different letters signify significant differences (P < 0.001, χ2 test).
| L. japonicus Mutant | Transforming Binary Plasmid | Transformed Plants | No. of Plants Nodulated |
|---|---|---|---|
| SL1450-5 (sym7) | pK7WG2D | 77 | 5 a |
| SL1450-5 (sym7) | pK7WG2D-35S:LIN | 90 | 33 b |
| SL1947-2 (itd3) | pK7WG2D | 35 | 0 a |
| SL1947-2 (itd3) |
pK7WG2D-35S:LIN |
80 |
0 a |
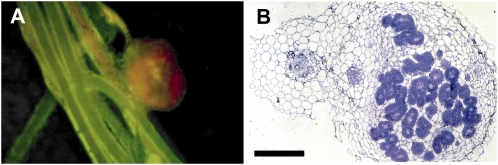
Figure 4.
Complementation of the L. japonicus sym7 mutant (SL1450-5) by MtLIN. A, Transgenic roots on the SL1450-5 mutant identified by GFP fluorescence. Pink nodules could be observed in the complemented plants, indicating that they were functional. B, Light microscopy of the SL1450-5 (sym7) nodules from MtLIN transgenic roots showing a regular infection pattern. Bar = 50 μm.
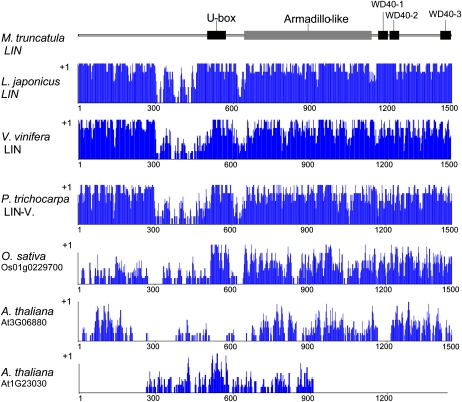
LIN Belongs to a Unique Family of Proteins Containing Domains with Homology to E3 Ubiquitin Ligases
Analysis of LIN revealed regions with high similarity to protein domains of known function. A U-box domain is present between residues 516 and 580 (Fig. 5). U-box domains are modified RING finger domains without the full complement of Zn2+-binding ligands and known for their E3 ubiquitin ligase activity. At the C-terminal region of the protein, the National Center for Biotechnology Information (NCBI) conserved domain search program identified a large region belonging to the WD40 superfamily (Fig. 5), while the predictions by InterProScan suggested three clear WD40 repeats with scores above the threshold from several programs (SMART, Pfam, SPRINT). According to the PANTHER classification system (www.pantherdb.org/panther/), this part shows homology to a more general family of F-box and WD40 domain proteins (PTHR22844) trained by 71 sequences from several organisms. Between these two main identified domains, a large Armadillo repeat-type region was predicted (Fig. 5) by only one protein analysis program (residues 662–1,001; InterPro-Gene3D: IPR011989 Armadillo-like helical domain). No further significant similarity to known domains was found. By comparing the predicted domain structure of LIN and the identified cDNA sequences of the three lin alleles in M. truncatula, it is clear that none of the possible truncated proteins would carry the U-box, Armadillo-like, and WD40 domains. The mutation identified in L. japonicus SL1450-5 mutant altered Asp-1267 (GAC) to Asn (AAC) in the second predicted WD40 repeat, indicating that the WD40 motif is essential for function.
Figure 5.
Schematic presentation of the domain structure of the M. truncatula LIN protein and similarity levels with closely related plant proteins. Predicted domains of the M. truncatula LIN protein are indicated in the schematic drawing at the top. Below the domain structure is a graphical representation of the alignment of the M. truncatula LIN protein with the best plant homologs plotted by the AlignX program of VectorNTI (Invitrogen) as follows: specific values (in a 0–1 range) are assigned to each residue at a given alignment position in each aligned sequence, depending on whether the residue is identical, similar, or weakly similar to the corresponding residue of the consensus sequence. [See online article for color version of this figure.]
When the Conserved Domain Architecture Retrieval Tool was used to find proteins with similar structural features, the closest group was proteins with the combination of RING finger and WD40 repeat domains (ring finger and WD40 repeat domain 3 Homo sapiens). Another set of proteins with a similar structural arrangement involves pre-mRNA processing factor 19 homologs (Bos taurus), which contain U-box and WD40 repeats, but always together with a PRP19/PSO4 domain. An additional large family includes proteins with the combination of F-box/WD40 repeat domains (like Pop1 from Schizosaccharomyces pombe). However, LIN is unusual when comparing with any of these families, as it contains a U-box instead of an F-box or RING domain and is exceptionally large with significant regions of unknown function. The structure of LIN (a large and novel N-terminal region, followed by a U-box, a probable Armadillo repeat, and WD40 repeats at the C-terminal domain) appears to be unique for plants, since genes with similar structure could not be found in other organisms. Genes homologous to LIN are clearly present in poplar (Populus trichocarpa) and grapevine (Vitis vinifera), identified as being duplicated in the Populus genome (Pt LGII, accession no. NC008468; Pt LGV, accession no. NC008471). The hypothetical protein product of the Vitis homolog is predicted under the GenBank accession number CAN74785. The amino acids of the predicted proteins are closely aligned, even the N-terminal region between residues 1 and 450 shows strong similarity with remarkably high conservation at positions 1 to 300 (Fig. 5; Supplemental Fig. S1). The next most similar protein to LIN was identified in rice (Oryza sativa; Os01g0229700; Fig. 5; Supplemental Fig. S2) and contains similar domain structure, but the level of similarity clearly drops at the N-terminal region, indicating that domain to be diverse. In the Arabidopsis (Arabidopsis thaliana) genome, no single homologous sequence could be identified, and no gene encoding a protein with similar domain structure was identified. Instead, two proteins show homologous regions with parts of the LIN protein: At3G06880 codes for a longer protein with WD40 repeats, while At1G23030 codes for a shorter U-box-containing protein (Fig. 5; Supplemental Figs. S3 and S4).
LIN Promoter Activity Is Associated with Infection and Primordium Formation
In order to reveal the promoter activity of the M. truncatula LIN gene throughout the nodulation process, a 1.2-kb segment upstream of the coding region of LIN was fused to the GUS reporter gene. This pLIN-GUS construct was subsequently introduced into M. truncatula A17 plants by A. rhizogenes-mediated transformation. Transformed hairy roots were monitored for GUS activity on uninfected roots and at different time points after inoculation with S. meliloti.
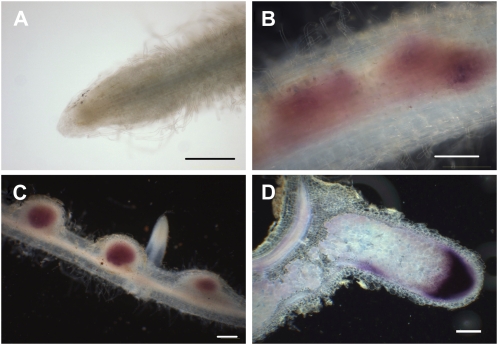
There was no detectable signal of GUS activity throughout most parts of the uninoculated roots, except a very faint signal just above the detection limit at the apical region (Fig. 6A). Three days after inoculation with rhizobia, GUS activity seemed to be associated with dividing cortical cells, leading to the formation of nodule primordia (Fig. 6B). Six days after inoculation, strong overall GUS staining was detected in the young, emerging nodules, where infection of plant cells by rhizobia takes place (Fig. 6C). In elongated mature nodules (21 d after inoculation), strong GUS activity was detected but was mainly restricted to a relatively broad area of nodule apices including the infection zone. Much lower expression was detected in the nitrogen-fixing zone (Fig. 6D).
Figure 6.
Promoter activity of the cloned upstream region of LIN in roots and nodules. Samples were collected from transformed hairy roots carrying the LIN promoter-GUS fusion, from uninoculated roots (A) and 3 (B), 6 (C), and 21 (D) d after inoculation with wild-type S. meliloti 1021. Histochemical staining of samples was carried out using Magenta-Gluc as substrate. Bars = 200 μm.
These data indicated that the cloned promoter segment enabled a gene expression associated with the bacterial infection as well as with the cortical cell division, leading to the formation of symbiotic nodules. Although this showed an activity that strongly correlated to the position where the expression of the LIN gene would be needed to recondition the mutant phenotype (i.e. early nodule primordia), to further support its activity, a complementation experiment was carried out using the wild-type LIN cDNA driven by the same promoter segment. Fully developed nodules appeared on the transgenic roots of lin-1 mutant plants, and bacterial invasion was detected in the apical zone of the nodules (Supplemental Fig. S5). This confirmed that promoter activity of this segment ensures a LIN expression necessary for nodule development and restoration of the infection process during early nodule invasion, but not sufficient for the complete occupation of the nodule by bacteria. This suggests that the function of LIN might also be needed at later steps of nodule invasion and occupation, and further regulatory elements in a larger promoter segment in the 5′ upstream region of LIN would be responsible for its expression and function at these later stages.
DISCUSSION
The formation of a nodule requires the coordinated development of rhizobial colonization and nodule organogenesis. These two processes are coordinated both spatially and temporally to ensure rhizobial infection of the developing nodule. However, rhizobial infection and nodule organogenesis can be separated genetically (Gleason et al., 2006; Murray et al., 2006; Tirichine et al., 2006a, 2006b), indicating that these two processes constitute different developmental pathways. Based on its mutant phenotype, LIN functions at an early stage of the rhizobial symbiotic process, required for both infection thread growth in root hair cells and the further development of nodule primordia. The lin mutation in L. japonicus SL1450-5 did not affect Nod factor-induced calcium spiking (Lombardo et al., 2006). No apparent nonsymbiotic phenotypes were observed in any of the three M. truncatula lin mutants, and colonization of the lin-1 mutant by mycorrhizal fungi is similar to that of wild-type M. truncatula (M. Harrison, personal communication). Gain-of-function mutations in CCaMK (autoactive CCaMK) allowed nodule development in the absence of rhizobial infection, and the use of this mutant revealed that LIN is not necessary for nodule organogenesis. From this, we infer that LIN predominantly functions during rhizobial colonization and that the abortion of this process in lin leads to a suppression of nodule development.
Detailed genetic studies in M. truncatula, L. japonicus, and pea have revealed an ordered array of loci functioning at different stages of nodulation (Tsyganov et al., 2002; Jones et al., 2007; Oldroyd and Downie, 2008). From studies in pea, it has been proposed that three potential checkpoints for rhizobial infection exist, while only a single checkpoint exists for cortical cell division, and this occurs as a result of a malfunctioning infection process. In the mutants where infection thread growth in the root hair is abolished (pea mutants sym2, sym36, sym37, and sym38), nodule tissue development is aborted after nodule primordia development and before nodule meristem formation. The lin mutants show an equivalent phenotype, and our work with autoactive CCaMK validates this hypothesis, indicating that the level of nodule organogenesis is dictated by the extent of infection thread invasion. Further supporting this assumption, promoter-reporter gene fusion experiments revealed that expression of LIN is associated with the dividing cortical cells, leading to the formation of nodule primordia, and in nodule tissues where infection of plant cells by rhizobia takes place.
The LIN protein contains many domains in which the presence of a U-box places it among E3 ubiquitin ligases, while other regions might be responsible for different interactions with target/substrate proteins or regulation of LIN by posttranslational modifications. E3 ubiquitin ligases play roles in the ubiquitination of the target protein achieved by enzymatic reactions that act in concert. The substrate proteins are labeled with ubiquitin, which serves as a degradation tag, in three consecutive steps catalyzed by the enzymes E1, E2, and E3, leading to proteolysis of target proteins by the 26S proteasome complex (Glickman and Ciechanover, 2002). This system plays a key role in the control of cellular functions as diverse as cell cycle progression, endocytosis, protein sorting, embryogenesis, hormone responses, defense against pathogens, and senescence through degradation of a wide range of proteins in the nucleus and cytoplasm (Pickart, 2001; Frugis and Chua, 2002; Vierstra, 2003; Smalle and Vierstra, 2004). In higher plants, a large gene family composed of diverse isoforms encodes the E3 ubiquitin ligases. In the Arabidopsis genome, there are 1,200 genes encoding the E3 components, while 41 genes encode E2 components and only two genes encode the E1 components (Vierstra, 2003; Kraft et al., 2005). Therefore, it is thought that the E3 ubiquitin ligases must play a central role in selecting the appropriate candidate proteins during the ubiquitination process (Zeng et al., 2006). On the basis of subunit composition, E3 ligases can be divided into four groups: HECT, SCF, APC, and RING/U-box. However, regardless of their subunit types, E3 ubiquitin ligases are responsible for identifying proteins that should be ubiquitinated, thereby determining target specificity (Callis and Vierstra, 2000; Vierstra, 2003; Smalle and Vierstra, 2004; Kraft et al., 2005). For this function, another domain is responsible that usually guides protein-protein interactions (e.g.WD40 repeats, Armadillo repeats, Leu-rich repeats). Besides its U-box domain, responsible for linking and presenting the ubiquitin tag onto the target protein, LIN has several additional domains, including WD40 repeats, that are likely to define the specificity of target substrates.
The complete structure of LIN appears to be unique to plants, since genes coding for proteins with similar structures could not be found in other organisms. Even in the plant kingdom, it is sometimes absent (e.g. in the Arabidopsis genome, no single homologous sequence could be identified). Instead, two proteins show homologous regions with parts of the LIN protein: At3G06880 codes for a longer protein with WD40 repeats, while At1G23030 codes for a shorter U-box-containing protein. On the other hand, the genome of the phylogenetically more distant rice does carry a gene coding for a protein with a domain structure (Os01g0229700) similar to LIN, although the level of similarity is poor at the N terminus. L. japonicus, P. trichocarpa, and V. vinifera show genes with a high degree of similarity to LIN. We show that the homologous protein in L. japonicus has an equivalent function to LIN, although the presence of homologs in nonlegumes suggests functions unrelated to nodulation in these species.
The emerging picture for ubiquitin regulation in plant-microbe interactions is supported so far mostly by examples in plant defense, suggesting multiple levels of regulation: from the resistance proteins to downstream signaling components and regulators (Zeng et al., 2006). Several U-box-type E3 ligases are identified as components that regulate plant defenses. It is likely that an equivalent level of regulation exists in nodulation signaling as well. Based on the symbiotic phenotype of the lin mutants, it can be hypothesized that inappropriate regulation of plant defenses during rhizobial invasion in the root hairs might be causing the block in infection. Therefore, a possible function of LIN may be the fine-tuning of the pathogen response upon rhizobial infection.
There are already indications for an involvement of E3 ligases in the legume/rhizobia symbiosis, with two E3 ligases showing a role in nodule development. Recently, nsRING, a novel RING finger protein required for rhizobial invasion and nodule formation, was identified in L. japonicus (Shimomura et al., 2006). The possible involvement of nsRING in phytohormone-related signaling was suggested. In M. truncatula, it has been shown that the SINA family of E3 ligases is important for infection thread growth and symbiosome differentiation (Den Herder et al., 2008). Overexpressing SINAT5DN from Arabidopsis caused a significant reduction in nodule number, indicating a negative regulatory role for this protein. Based on the mutant phenotype, we can assume that LIN negatively regulates certain transcription factors and/or signal transduction proteins required for bacterial invasion, which need fine-tuned regulation for their proper action. Good candidates were identified in a suppression subtractive hybridization approach that revealed candidate transcription factors: a bHLH, a WRKY, and a C2H2 zinc-finger protein, which were up-regulated following S. meliloti inoculation in lin (Godiard et al., 2007). Understanding of LIN targets should provide insights into the maintenance of infection thread growth and may also provide insights into the mechanisms by which infection thread growth and nodule organogenesis are coordinated.
MATERIALS AND METHODS
Plant Growth and Bacterial Strains
Medicago truncatula ‘Jemalong’ genotype A17 was used as the wild-type control for phenotypic and genotypic analyses. The plants were grown as described previously by Cook et al. (1995). Plants were infected with Sinorhizobium meliloti 1021 carrying pXLGD4 plasmid, expressing the lacZ reporter gene under a hemA promoter. In the cloning procedures, Escherichia coli DH5α (Gibco BRL) and One Shot Omnimax 2T1 (Invitrogen) strains were used. S. meliloti and E. coli strains were grown at 30°C and 37°C, respectively, in Luria-Bertani medium supplemented with antibiotics when required. In hairy root transformation experiments, Agrobacterium rhizogenes ARqua1 strains electroporated with the appropriate binary vector constructs were used.
Gene Isolation
Mapping was done on an F2 segregating population originating from a cross between C88 and A17 as described by Kuppusamy et al. (2004), but on a different set of 290 individuals. For testing more individuals with close recombination events to the lin-1 mutation, 508 F3 individuals of selected F2 plants were also genotyped. Genetic markers used in this study (LP, length polymorphism; SNP, single nucleotide polymorphism) with their respective primer pairs are as follows. 4E6R (LP): 4E6R_F, 5′-TGAGCGTCCAACTAATTGAC-3′; 4E6R_R, 5′-TTCACAATACAAAACCCTCAC-3′. 16L4R (SNP): 16L4R_F, 5′-GGCATTTCAAGGTTTTTTTTG-3′; 16L4R_R, 5′-TAACTCATTTGATGCATAAAGAAG-3′. TC110346_2 (SNP): TC110346-2_F, 5′-TGATTCAAACAAAATGGACAGG-3′; TC110346-2_R, 5′-CATGAAAATGAAAACCTTAATCTG-3′. 18E17F (SNP): 18E17F_F, 5′-TAGCTCTCTAACACCTCCG-3′; 18E17F_R, 5′-CTCCGTCTACACTTCGTTC-3′. TC96353-1 (LP): TC96353-1_F, 5′-GAATAGTTGAGATAAACACGGAG-3′; TC96353-1_R, 5′-TTGTGGACAACAGAAAGAAGC-3′. e13O17F (SNP): e13O17F_F, 5′-CTTAGGGCGATTCGATCTGG-3′; e13O17F_R, 5′-CAAGCTGAACGAACACATGG-3′. e53J20F (SNP): e53J20F_F, 5′-GAGGGAAATGAAACGATGAAG-3′; e53J20F_R, 5′-AGAACTCACACGCAAGCAGAG-3′. Zero recombination was found for all tested markers with lin between flanking markers 4E6R and e53J20F; therefore, the region carrying the LIN gene could not be further delimited. The identified BAC clones were sent to and sequenced by the group at the University of Oklahoma (led by B. Roe) responsible for sequencing M. truncatula chromosome 1. Gene predictions of the region are from the IMGAG annotation of the two sequenced BAC clones (www.tigr.org/tigr-scripts/medicago/CONTIGS/GBROWSE/ gbrowse2?name=MTCON310).
The following primer pairs were used to demonstrate the imperfect splicing of the mRNA in the lin-1 mutant: Lin-3F, 5′-GGATGAAGATGTTGAACCAA-3′, and Lin-3R, 5′-CCTGTGATTGGACAAACAA-3′; Lin-8F, 5′-GGACGCAAGGAAGAGAAT-3′, and Lin-8R, 5′-CCAGTGAAGAATGAAGTTGATG-3′. Primers Lin-3Fb (5′-TTGTTTGTCCAATCACAGG-3′) and Lin-12Rc (5′-ACAGTCCTGCAAGTTTTCTGATGT-3′) were used to amplify fragments for the Southern hybridization. All amplification reactions were carried out as described earlier (Endre et al., 2002a) using adequate annealing temperature for the respective primer pairs.
Sequence Analysis
LIN sequences and homologs were aligned using VectorNTI. For different domain, motif, and structure predictions, the following programs/Web sites were used: NCBI conserved domain search program on A Conserved Domain Database and Search Service, version 2.16; Conserved Domain Architecture Retrieval Tool (searches the NCBI Entrez Protein Database for similarity based on domain architecture, defined as the sequential order of conserved domains in proteins; Geer et al., 2002); http://www.ncbi.nlm.nih.gov/Structure/lexington/lexington.cgi; and the PSORT II program (http://psort.ims.u-tokyo.ac.jp/form2.html).
A. rhizogenes-Mediated Complementation of Different lin Mutants
For complementation experiments, LIN cDNA was recombined from the pCR8GW-TOPO entry clone into the pK7WG2D vector (Karimi et al., 2002) under the control of the cauliflower mosaic virus 35S promoter. This construct also harbors a constitutively expressed GFP gene to facilitate the identification of the transgenic roots. For the pLIN-LIN cDNA construct, the 1.2-kb LIN promoter was amplified from genomic DNA using the following oligonucleotides, 5′-GGACTAGTCATCTATCAAGAAAAATCACAA-3′ and 5′-CCCAAGCTTATAATCTATGTGCCTTAGTCCG-3′, carrying the SpeI and HindIII restriction sites, respectively. LIN cDNA was also recombined from the pCR8GW-TOPO entry clone into the pK7WGF2 vector (Karimi et al., 2002) in which the 35S promoter was exchanged with the 1.2-kb LIN promoter segment using HindIII and SpeI. The resulting destination clones were electroporated into A. rhizogenes ARqua1 and used for hairy root transformation of M. truncatula plants as described earlier (Boisson-Dernier et al., 2001), only without using antibiotic selection for the transgenic roots. Plants harboring 4- to 5-cm-long hairy roots (approximately 2 weeks after A. rhizogenes treatment) were transferred into pots and allowed to grow and strengthen for another 3 to 4 weeks in Turface. One week before the date of inoculation by rhizobia, nitrogen was omitted from the liquid medium. Plants were inoculated afterward with S. meliloti 1021 strain carrying a hemA promoter:lacZ fusion (Leong et al., 1985) allowing the detection of bacteria within plant tissues by histochemical staining for lacZ activity. Roots were screened and scored for the presence of nodules or nodule primordia 21 d after inoculation. Transgenic hairy roots expressing GFP constitutively from the inserted T-DNA were selected and monitored for nodulation and for nodule occupation by S. meliloti.
Histochemical Localization of the LIN Promoter Activity
To generate the pLIN-GUS fusion construct, a 1.2-kb segment upstream the coding region of LIN was amplified and cloned into the binary vector pMDC164 (Curtis and Grossniklaus, 2003). The resulting plasmid constructs were introduced into M. truncatula by A. rhizogenes-mediated hairy root transformation as described above. In order to detect the activity of the cloned promoter in the transgenic hairy roots, root and nodulated root samples were collected 0, 3, 6, and 21 d after inoculation with S. meliloti 1021. Histochemical detection of GUS activity was carried out using a standard protocol and 5-bromo-6-chloro-3-indolyl-β-d-GlcUA cyclohexylammonium salt (Magenta-Gluc CHA salt; Duchefa Biochemie) as substrate (Jefferson and Wilson, 1991). Uninoculated roots and 6-d-old nodules were studied as intact organs. Developed nodules were embedded in 4% agarose, and 100-μm longitudinal sections were made with a microcut H1200 (Bio-Rad). Microscopic analysis was carried out with a Leica DM LB2 light microscope (Leica Microsystems) coupled with a MicroPublisher 3.3 RTV (Q imaging) digital camera.
Sequence data from this article are deposited in the GenBank/EMBL data libraries under the following accession numbers: MtLIN, EU926660; MtLIN_CDS, EU926661; Mtlin-1, EU926662; Mtlin-2, EU926663; Ljlin_CDS, EU926664; and Ljlin-1, EU926665.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figures S1 to S4. Multiple sequence alignments of the LIN proteins and their respective homologs.
Supplemental Figure S5. Positive complementation of the nodulation phenotype on the transgenic hairy roots on the lin-1 mutants using the pLIN-LIN construct.
Supplementary Material
Acknowledgments
We greatly appreciate the technical assistance of Sandor Jenei, Andrea Toth, Zsuzsa Liptay, and Erika Veres. We thank Kavitha Kuppusamy and Kathryn A. VandenBosch for the F2 seeds of the M. truncatula lin-1 segregating population.
This work was supported by the Hungarian Scientific Research Fund (grant nos. OTKA T046819, D048451, and K76843); by the National Research and Development Program (grant no. NKFP 4/031/2004), the Economic Competitiveness Operative Programs (grant no. GVOP–3.1.1–2004–05–0101/3.0), and the Biotechnology and Biological Research Council in the United Kingdom via a grant in aid and grant no. BB/D521749/1; by the European Union (grant nos. RTN–CT–2003–505227 and MRTN–CT–2006–035546); and by Janos Bolyai postdoctoral fellowships to E.K. and G.E.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Gabriella Endre (endre@brc.hu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Amor BB, Shaw SL, Oldroyd GE, Maillet F, Penmetsa RV, Cook D, Long SR, Dénarié J, Gough C (2003) The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J 34 495–506 [DOI] [PubMed] [Google Scholar]
- Ane JM, Kiss GB, Riely BK, Penmetsa RV, Oldroyd GED, Ayax C, Levy J, Debelle F, Baek JM, Kalo P, et al (2004) Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303 1364–1367 [DOI] [PubMed] [Google Scholar]
- Arrighi JF, Godfroy O, de Billy F, Saurat O, Jauneau A, Gough C (2008) The RPG gene of Medicago truncatula controls Rhizobium-directed polar growth during infection. Proc Natl Acad Sci USA 105 9817–9822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Chabaud M, Garcia F, Becard G, Rosenberg C, Barker DG (2001) Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol Plant Microbe Interact 14 695–700 [DOI] [PubMed] [Google Scholar]
- Brewin NJ (2004) Plant cell wall remodelling in the rhizobium-legume symbiosis. Crit Rev Plant Sci 23 293–316 [Google Scholar]
- Callis J, Vierstra RD (2000) Protein degradation in signaling. Curr Opin Plant Biol 3 381–386 [DOI] [PubMed] [Google Scholar]
- Charpentier M, Bredemeier R, Wanner G, Takeda N, Schleiff E, Parniske M (2008) Lotus japonicus CASTOR and POLLUX are ion channels essential for perinuclear calcium spiking in legume root endosymbiosis. Plant Cell 20 3467–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Mun JH, Kim DJ, Zhu H, Baek JM, Mudge J, Roe B, Ellis N, Doyle J, Kiss GB, et al (2004) Estimating genome conservation between crop and model legume species. Proc Natl Acad Sci USA 101 15289–15294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Dreyer D, Bonnet D, Howell M, Nony E, VandenBosch K (1995) Transient induction of a peroxidase gene in Medicago truncatula precedes infection by Rhizobium meliloti. Plant Cell 7 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U (2003) A Gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Herder G, De Keyser A, De Rycke R, Rombauts S, Van de Velde W, Clemente MR, Verplancke C, Mergaert P, Kondorosi E, Holsters M, et al (2008) Seven in absentia proteins affect plant growth and nodulation in Medicago truncatula. Plant Physiol 148 369–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endre G, Kalo P, Kevei Z, Kiss P, Mihacea S, Szakal B, Kereszt A, Kiss GB (2002. a) Genetic mapping of the non-nodulation phenotype of the mutant MN-1008 in tetraploid alfalfa (Medicago sativa). Mol Genet Genomics 266 1012–1019 [DOI] [PubMed] [Google Scholar]
- Endre G, Kereszt A, Kevei Z, Mihacea S, Kalo P, Kiss GB (2002. b) A receptor kinase gene regulating symbiotic nodule development. Nature 417 962–966 [DOI] [PubMed] [Google Scholar]
- Frugis G, Chua NH (2002) Ubiquitin-mediated proteolysis in plant hormone signal transduction. Trends Cell Biol 12 308–311 [DOI] [PubMed] [Google Scholar]
- Geer LY, Domrachev M, Lipman DJ, Bryant SH (2002) CDART: protein homology by domain architecture. Genome Res 12 1619–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts R, Fedorova E, Bisseling T (2005) Nod factor signaling genes and their function in the early stages of Rhizobium infection. Curr Opin Plant Biol 8 346–352 [DOI] [PubMed] [Google Scholar]
- Gleason C, Chaudhuri S, Yang T, Munoz A, Poovaiah BW, Oldroyd GE (2006) Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 441 1149–1152 [DOI] [PubMed] [Google Scholar]
- Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82 373–428 [DOI] [PubMed] [Google Scholar]
- Godiard L, Niebel A, Micheli F, Gouzy J, Ott T, Gamas P (2007) Identification of new potential regulators of the Medicago truncatula-Sinorhizobium meliloti symbiosis using a large-scale suppression subtractive hybridization approach. Mol Plant Microbe Interact 20 321–332 [DOI] [PubMed] [Google Scholar]
- Heckmann AB, Lombardo F, Miwa H, Perry JA, Bunnewell S, Parniske M, Wang TL, Downie JA (2006) Lotus japonicus nodulation requires two GRAS domain regulators, one of which is functionally conserved in a non-legume. Plant Physiol 142 1739–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi-Anraku H, Takeda N, Charpentier M, Perry J, Miwa H, Umehara Y, Kouchi H, Murakami Y, Mulder L, Vickers K, et al (2005) Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature 433 527–531 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Wilson KJ (1991) The GUS gene fusion system. Plant Mol Biol B14 1–33 [Google Scholar]
- Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC (2007) How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat Rev Microbiol 5 619–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalo P, Gleason C, Edwards A, Marsh J, Mitra RM, Hirsch S, Jakab J, Sims S, Long SR, Rogers J, et al (2005) Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308 1786–1789 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7 193–195 [DOI] [PubMed] [Google Scholar]
- Kraft E, Stone SL, Ma L, Su N, Gao Y, Lau OS, Deng XW, Callis J (2005) Genome analysis and functional characterization of the E2 and RING-type E3 ligase ubiquitination enzymes of Arabidopsis. Plant Physiol 139 1597–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppusamy KT, Endre G, Prabhu R, Penmetsa RV, Veereshlingam H, Cook DR, Dickstein R, Vandenbosch KA (2004) LIN, a Medicago truncatula gene required for nodule differentiation and persistence of rhizobial infections. Plant Physiol 136 3682–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SA, Williams PH, Ditta GS (1985) Analysis of the 5′ regulatory region of the gene for delta-aminolevulinic acid synthetase of Rhizobium meliloti. Nucleic Acids Res 13 5965–5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Bres C, Geurts R, Chalhoub B, Kulikova O, Duc G, Journet EP, Ane JM, Lauber E, Bisseling T, et al (2004) A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303 1361–1364 [DOI] [PubMed] [Google Scholar]
- Li Y, Sorefan K, Hemmann G, Bevan MW (2004) Arabidopsis NAP and PIR regulate actin-based cell morphogenesis and multiple developmental processes. Plant Physiol 136 3616–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo F, Heckmann AB, Miwa H, Perry JA, Yano K, Hayashi M, Parniske M, Wang TL, Downie JA (2006) Identification of symbiotically defective mutants of Lotus japonicus affected in infection thread growth. Mol Plant Microbe Interact 19 1444–1450 [DOI] [PubMed] [Google Scholar]
- Marsh JF, Rakocevic A, Mitra RM, Brocard L, Sun J, Eschstruth A, Long SR, Schultze M, Ratet P, Oldroyd GE (2007) Medicago truncatula NIN is essential for rhizobial-independent nodule organogenesis induced by autoactive calcium/calmodulin-dependent protein kinase. Plant Physiol 144 324–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton PH, Jakab J, Penmetsa RV, Starker CG, Doll J, Kalo P, Prabhu R, Marsh JF, Mitra RM, Kereszt A, et al (2007) An ERF transcription factor in Medicago truncatula that is essential for Nod factor signal transduction. Plant Cell 19 1221–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra RM, Long SR (2004) Plant and bacterial symbiotic mutants define three transcriptionally distinct stages in the development of the Medicago truncatula/Sinorhizobium meliloti symbiosis. Plant Physiol 134 595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J, Karas B, Ross L, Brachmann A, Wagg C, Geil R, Perry J, Nowakowski K, MacGillivary M, Held M, et al (2006) Genetic suppressors of the Lotus japonicus har1-1 hypernodulation phenotype. Mol Plant Microbe Interact 19 1082–1091 [DOI] [PubMed] [Google Scholar]
- Oldroyd GE, Downie JA (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 59 519–546 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR (2000) Production and characterization of diverse developmental mutants of Medicago truncatula. Plant Physiol 123 1387–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart CM (2001) Mechanisms underlying ubiquitination. Annu Rev Biochem 70 503–533 [DOI] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, Felle HH, Umehara Y, Gronlund M, Sato S, Nakamura Y, Tabata S, Sandal N, et al (2003) Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425 585–592 [DOI] [PubMed] [Google Scholar]
- Saito K, Yoshikawa M, Yano K, Miwa H, Uchida H, Asamizu E, Sato S, Tabata S, Imaizumi-Anraku H, Umehara Y, et al (2007) NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell 19 610–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauser L, Roussis A, Stiller J, Stougaard J (1999) A plant regulator controlling development of symbiotic root nodules. Nature 402 191–195 [DOI] [PubMed] [Google Scholar]
- Shimomura K, Nomura M, Tajima S, Kouchi H (2006) LjnsRING, a novel RING finger protein, is required for symbiotic interactions between Mesorhizobium loti and Lotus japonicus. Plant Cell Physiol 47 1572–1581 [DOI] [PubMed] [Google Scholar]
- Smalle J, Vierstra RD (2004) The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol 55 555–590 [DOI] [PubMed] [Google Scholar]
- Smit P, Limpens E, Geurts R, Fedorova E, Dolgikh E, Gough C, Bisseling T (2007) Medicago LYK3, an entry receptor in rhizobial nodulation factor signaling. Plant Physiol 145 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit P, Raedts J, Portyanko V, Debelle F, Gough C, Bisseling T, Geurts R (2005) NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science 308 1789–1791 [DOI] [PubMed] [Google Scholar]
- Stacey G, Libault M, Brechenmacher L, Wan J, May GD (2006) Genetics and functional genomics of legume nodulation. Curr Opin Plant Biol 9 110–121 [DOI] [PubMed] [Google Scholar]
- Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Szczyglowski K, et al (2002) A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417 959–962 [DOI] [PubMed] [Google Scholar]
- Tirichine L, Imaizumi-Anraku H, Yoshida S, Murakami Y, Madsen LH, Miwa H, Nakagawa T, Sandal N, Albrektsen AS, Kawaguchi M, et al (2006. a) Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 441 1153–1156 [DOI] [PubMed] [Google Scholar]
- Tirichine L, James EK, Sandal N, Stougaard J (2006. b) Spontaneous root-nodule formation in the model legume Lotus japonicus: a novel class of mutants nodulates in the absence of rhizobia. Mol Plant Microbe Interact 19 373–382 [DOI] [PubMed] [Google Scholar]
- Tsyganov VE, Voroshilova VA, Priefer UB, Borisov AY, Tikhonovich IA (2002) Genetic dissection of the initiation of the infection process and nodule tissue development in the Rhizobium-pea (Pisum sativum L.) symbiosis. Ann Bot (Lond) 89 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD (2003) The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins. Trends Plant Sci 8 135–142 [DOI] [PubMed] [Google Scholar]
- Yano K, Yoshida S, Muller J, Singh S, Banba M, Vickers K, Markmann K, White C, Schuller B, Sato S, et al (2008) CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc Natl Acad Sci USA 105 20540–20545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota K, Fukai E, Madsen LH, Jurkiewicz A, Rueda P, Radutoiu S, Held M, Hossain MS, Szczyglowski K, Morieri G, et al (2009) Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell 21 267–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LR, Vega-Sanchez ME, Zhu T, Wang GL (2006) Ubiquitination-mediated protein degradation and modification: an emerging theme in plant-microbe interactions. Cell Res 16 413–426 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.