World consumption of soybean (Glycine max) in 2008 was over 221 million metric tons, with approximately 50% of this supply coming from U.S. production, where soybean plantings on an annual basis are over 77 million ha. Soybeans are desired on the marketplace as a valuable source of protein and oil. The former is primarily used as feed, with some food applications, while the latter is more broadly incorporated into food, feed, and some industrial applications (e.g. biodiesel). Protein and oil percentages in soybean, while influenced by both genotype and environmental cues, average approximately 40% and 20%, respectively. A strong indirect phenotypic correlation exists between these traits. In addition, variation in soybean germplasm for protein content is significantly greater than that observed for total oil content. Historically, soybean breeders have used total protein content as a selection criterion for germplasm development. However, recently, both oil content and quality have drawn much attention in soybean genetics and breeding programs, due to the increased demand for vegetable oils and increased consumer awareness of health issues around dietary fats. To this end, significant efforts have been made to increase oxidative stability of soybean oil as a means to avoid trans-fats generated through the hydrogenation process and to enhance ω-3 fatty acid content of the oil for use in both food and feed applications and increase the total oil content of seeds.
Commodity soybean prices have risen over 65% during the last decade, from $158.3 per metric ton in June 1999 to $445.2 per metric ton in June 2009. The world demand for soybean is driven by its highly valued protein and oil for use in food, feed, and industrial applications. During embryogenesis, carbon flux in soybean is primarily partitioned between protein and oil, such that at maturity approximately 40% and 20% of the dry matter is in one of these two respective carbon reserves. The remainder of the seed dry matter is largely carbohydrate, which possesses negligible economic value. While some starch accumulates early in embryogenesis, minimal amounts remain at maturity. The inverse relationship between total oil and protein content in soybean is well documented, where typically a 1% reduction in total oil content will lead to a 2% increase in total protein content. Thus, the regulation of carbon flux during embryogenesis will be shifted toward one or the other, which is impacted by both genetics and environment, although strong metabolic links between oil and storage protein synthesis are not apparent (Schwender et al., 2003). The phenotypic variation in protein and oil within the U.S. Department of Agriculture soybean germplasm collection has been reported to range from 34.1% to 56.8% and from 8.1% to 27.9% for protein and oil, respectively (Wilson, 2004). Interestingly, the “microenvironment” (i.e. the location of seeds on the plant) can also impact carbon flux during embryogenesis, with pods positioned at the top of the plant having seeds with a higher percentage of protein and lower oil content than in those positioned at the bottom of the plant (Bennett et al., 2003).
Soybean breeders have made significant progress in improving the overall yield of soybean, which translates into more protein and oil on a per ha basis. Despite this, minimal advancements have been made in the selection of high-yielding genotypes, with major shifts in carbon flux for improvements in total oil or protein content (Mahmoud et al., 2006). On the other hand, implementing the tools of molecular biology and biotechnology has opened the door to the development of improved end-use quality of the oil for food, feed, and industrial applications. These have been achieved by directed modification of fatty acid biosynthesis to alter relative amounts of fatty acids naturally found in soybean or to produce novel fatty acids (Jaworski and Cahoon, 2003; Damude and Kinney, 2008).
Modulating endogenous levels and/or production of novel fatty acids of oils has gained significant attention in recent years, due to the increasing awareness of consumers of the impact dietary lipids have on health. Commodity soybean oil is composed of five fatty acids: palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), and linolenic acid (18:3). The percentage of these five fatty acids in soybean oil averages 10%, 4%, 18%, 55%, and 13%, respectively. This fatty acid profile results in low oxidative stability that limits the uses of soybean oil in food products and industrial applications. Oxidative breakdown of soybean oil, for example, results in rancidity and off flavors in food products and the buildup of viscous materials in soybean-derived biodiesel that clogs oil filters (Canakci et al., 1999). Oxidative instability of soybean oil results from the relatively high percentage of the polyunsaturated fatty acids linoleic acid and linolenic acid. Historically, this problem has been addressed for food and feed applications through partial hydrogenation. This typically will reduce the polyunsaturated fatty acids below 18% of the total fatty acids, with linolenic acid generally below 2%, and a concomitant rise in oleic and stearic acids. The negative aspect with partial hydrogenation is that it generates a preponderance of trans-fatty acids, which have been linked with cardiovascular disease (Danaei et al., 2009). Moreover, a partially hydrogenated soybean oil possesses poor cold flow properties, reduced lubricity, and increased viscosity, thus limiting its value as a biodiesel (Moser et al., 2007). Genetic strategies that reduce polyunsaturated fatty acid synthesis can be used to improve oxidative stability of soybean oil, without the production of trans-fatty acids, while maintaining functionality for end use in food, feed, and industrial applications. To this end, the primary fatty acid profiles targeted for soybean are (1) low linolenic acid oil, (2) high oleic acid oil, and (3) elevated stearic acid combined with high oleic acid oil.
The production of novel fatty acids in seed oils for nutritional improvements for food and feed use or as a route for cost-effective/sustainable feedstock for industrial applications has been demonstrated (Kinney, 1998; Jaworski and Cahoon, 2003; Abbadi et al., 2004; Cahoon et al., 2007; Burgal et al., 2008). When considering feedstocks for the production of novel fatty acids for industrial applications, it is imperative to take into account the cropping system of the feedstock. Soybean as a feedstock for fatty acids with targeted industrial applications comes with distinct advantages. As a member of the Fabaceae, it possesses inherent reduced fertilization requirements due to its ability to fix nitrogen, and the infrastructure for production and transport is well established. However, if production of the novel fatty acid compromises the suitability of the protein component as a feed, the fatty acid would have to be of sufficient value to recoup the loss of the protein coproduct and the cost associated with identity preservation (Cahoon, 2003). Hence, the drawback of soybean as a feedstock for this purpose is the profit potential. Moreover, even if the profit margin of the novel fatty acid could overcome the potential reduced value of soybean meal, it would most likely be required in a relatively low volume, which in turn would mitigate the need for large production and transport capacity. For these reasons, if an industrial fatty acid is the target, and it is not compatible with feed usage, it may be prudent to use an alternative crop whose seeds are not used for animal feed as the host for this trait.
Nutritional enhancement of soybean oil has emphasized improving the ω-3 fatty acid levels for food and feed applications (Kinney, 2003; Cahoon et al., 2007). Significant progress has been made in assembling pathways for heterologous production of targeted ω-3 fatty acids, such as stearidonic acid (SDA; 18:4Δ6,9,12,15; Eckert et al., 2006), and the very-long-chain polyunsaturated fatty acids eicosapentaenoic acid (EPA; 20:5 Δ5,8,11,14,17) and docosahexaenoic acid (DHA; 22:6Δ4,7,10,13,16,19) in plant systems (Abbadi et al., 2004; Damude and Kinney, 2007; Napier, 2007). Soybean oils with enhanced ω-3 fatty acid levels have value in both food and feed applications. Currently, the primary sources for EPA and DHA are either algae or fish oil. Algae as a feedstock for EPA and DHA are currently grown heterotrophically, which adds significantly to cost. However, with renewed interest in algal systems as a feedstock for biofuels, a drive for research innovations will be stimulated that potentially will lead to the development of enabling technologies for cost-effective photoautotrophic and high-volume lipid extraction systems, which in turn could be translated toward reducing costs for the production of nutritional fatty acids. In addition, commercial sources of the ω-3 fatty acid SDA are currently restricted to oils from plant species such as echium (Echium plantagineum) that have limited potential for large-scale, cost-effective production (Griffiths et al., 1996; Berti et al., 2007).
GENETIC STRATEGIES FOR IMPROVING OXIDATIVE STABILITY OF SOYBEAN OIL
Low Linolenic Acid Soybean Oil
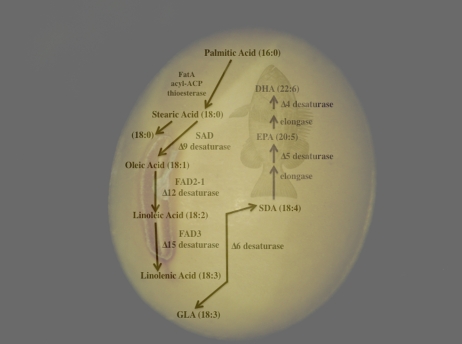
The amount of linolenic acid in commodity soybean oil is roughly 10% of the total fatty acids, which reduces oxidative stability of the oil, leading to rancidity and decreased shelf-life of products. A number of low linolenic acid soybean oil (low-lin) genotypes have been developed through mutational breeding (Hammond and Fehr, 1983a; Wilcox and Cavins, 1985; Fehr et al., 1992). Low-lin soybean oil typically has linolenic acid percentages below 4%, with ultra-low-lin under 2%. Mutations resulting in the low-lin oil phenotype in soybean have been shown to lie within the FAD3 gene (Chappell and Bilyeu, 2006; Reinprecht et al., 2009), which encodes for a Δ15 fatty acid desaturase that incorporates a third double bond into linoleic acid to produce linolenic acid (Fig. 1). In soybean, three FAD3 genes have been identified, designated GmFAD3A, GmFAD3B, and GmFAD3C. Although all three contribute to linolenic acid levels in seeds, GmFAD3A is highly expressed during embryogenesis and thus is the major determinant of the linolenic acid content of soybean oil (Bilyeu et al., 2003). Not surprisingly, both low-lin soybean ethyl methanesulfonate mutants A5 (Hammond and Fehr, 1983a) and RG10 (Stojsin et al., 1998) were shown to harbor lesions in GmFAD3A (Chappell and Bilyeu, 2006; Reinprecht et al., 2009). Using a targeted gene-silencing approach to deliberately down-regulate the GmFAD3 family in a seed-specific manner, Flores et al. (2008) were able to generate an ultra-low-lin phenotype in soybean by effectively suppressing the expression of all three GmFAD3 genes with a single RNA interference construct.
Figure 1.
Designing a better soybean: the steps for improved oil quality.
Low-lin soybean varieties have been on the market for a number of years, with plantings of low-lin genotypes increasing due to the 2006 Food and Drug Administration mandate requiring that percentage of trans-fats in food products be included on labels. While reducing linolenic acid levels of soybean oil improves oxidative stability compared with standard commodity soybean oil, the improvement is not sufficient to be classified as a high-stability oil. The oxidative stability index, broadly defined as the length of time before the onset of oxidation of an oil, is 8.9, 5.4, 22.7, and 16.7 h for ultra-low-lin, standard soybean oil, partially hydrogenated soybean oil, and high oleic acid (>80%) sunflower (Helianthus annuus) oil, respectively (Warner, 2009). Oils with a high percentage of oleic acid (>80%) require less processing, thereby offering a route to further decreased levels of trans-fats in food products. Moreover, commodity-type oil with a high percentage of oleic acid will provide a cost-effective source for applications requiring high-stability oils, such as nut roasting, flavor and color carriers, lubricants, and fried products requiring extended shelf-life (Lampert, 1999).
High Oleic Acid Soybean Oil
In developing soybean seeds, oleic acid is converted to linoleic acid in a single desaturation step carried out by Δ12 fatty acid desaturase encoded by the FAD2 gene (Heppard et al., 1996). Three FAD2 genes have been identified in soybean, designated FAD2-1A, FAD2-1B, and FAD2-2. FAD2-1A and FAD2-1B are expressed primarily during embryogenesis, while FAD2-2 is constitutively expressed (Heppard et al., 1996; Tang et al., 2005).
Soybean genotypes with mid-oleic acid phenotypes, 30% to 70%, have been developed through conventional breeding by exploiting the variation found in the soybean germplasm (Takagi and Rahman, 1996; Rahman et al., 2001; Alt et al., 2005b). However, commercial planting of mid-oleic acid germplasm has never materialized, primarily due to yield drag associated with the trait (Bachlava et al., 2008a; Scherder and Fehr, 2008). Oleic acid levels in this germplasm are also strongly influenced by growth temperature and require warmer climates to obtain the mid-oleic acid phenotype. The environmental instability of this trait is likely due to the impact that growth temperatures has on Δ12 desaturase activity (Heppard et al., 1996; Tang et al., 2005).
The yield drag associated with the mid-oleic acid soybean genotypes is not due to the fatty acid profile of the seed per se, given that targeted down-regulation of FAD2-1 in a seed-specific manner results in seeds with greater than 80% oleic acid that do not have compromised agronomic performance (Kinney and Knowlton, 1997; Graef et al., 2009). In the soybean M23 mutant, the mid-oleic acid phenotype is associated with a lesion in one of the FAD2-1 genes (Alt et al., 2005a). However, even under optimal environmental conditions, the M23 genotype and the other mid-oleic acid genotypes require pyramiding of multiple genetic alleles for full penetration of the mid-oleic acid phenotype (Bachlava et al., 2008b; Monteros et al., 2008). While the factors underlying the yield drag associated with soybean mid-oleic acid mutants is not understood, one plausible explanation is that the fatty acid profile of membranes in vegetative tissues is also altered. This may impact membrane fluidity and, in turn, affect the plant's ability to respond to environmental change, ultimately leading to reduced yield.
High oleic acid soybean oil has value, but the trait is not of sufficient value to compensate for a significant reduction in yield. Problems of yield drag and environmental instability of oleic acid levels in mid-oleic acid mutants have largely been solved through the use of biotechnology. This has been achieved by down-regulation of FAD2-1A and -1B via seed-specific expression of posttranscriptional gene-silencing elements (Mazur et al., 1999; Buhr et al., 2002; Graef et al., 2009). Seeds resulting from this approach have oleic acid content exceeding 80% of the total fatty acids, without alteration of the fatty acid composition of vegetative tissues. Moreover, the transgenic allele is inherited as a single dominant trait, thereby greatly facilitating breeding.
High Oleic Acid and Elevated Stearic Acid Soybean
Many baking applications require the functionality of saturated fats. To address this need, food processors have historically relied upon hydrogenated oils, given the requirements for both oxidative stability and relatively high melting temperatures. Vegetable oils with elevated saturated fatty acids combined with high oleic acid and low polyunsaturated fatty acids can meet this need and thereby serve as a replacement for hydrogenated oil in many confectionery applications. However, not all saturated fatty acids are alike with regard to their impacts on cardiovascular health. Saturated fats in general adversely impact cardiovascular fitness; however, stearic acid is considered cardiovascular neutral, as it tends to have minimal effect on blood cholesterol levels (Bonanome and Grundy, 1988; Kris-Etherton et al., 2005). Thus, a fatty acid profile that addresses the needs of the confectionery industry for high melting point and oxidative stability and does not negatively impact human health would have elevated stearic acid and high oleic acid coupled with low polyunsaturated fatty acids.
Two avenues have been successful for increasing stearic acid levels of seed oils: (1) heterologous expression of a stearoyl-ACP thioesterase (FatA; Hawkins and Kridl, 1998; Facciotti et al., 1999), and (2) mutations in (Bubeck et al., 1989; Rahman et al., 1995; Pantalone et al., 2002; Zhang et al., 2008) or targeted down-regulation of (Knutzon et al., 1992; Liu et al., 2002) Δ9-stearoyl-ACP desaturase (SAD). In the first approach, seeds of the tropical fruit mangosteen (Garcinia mangostana) accumulate stearic acid to amounts exceeding 46% of the total fatty acids. A key enzyme contributing to this high stearic acid oil phenotype is a variant FatA gene, which possesses high specificity for stearoyl-ACP. This activity releases stearic acid from de novo fatty acid synthesis in the plastid for eventual incorporation into triacylglycerols in the endoplasmic reticulum. Consistent with this, seed-specific expression of the mangosteen FatA transgene in canola (Brassica napus) resulted in a stearic acid content exceeding 20% of the total fatty acids (Hawkins and Kridl, 1998; Facciotti et al., 1999).
In soybean, elevated stearic acid phenotype is governed by recessive loci manifested via mutagenesis (Rahman et al., 1995, 2003; Spencer et al., 2003; Zhang et al., 2008), with one exception, genotype FAM94-41 (Pantalone et al., 2002). Homozygous lineages of soybean carrying the recessive elevated stearic acid alleles in soybean accumulate stearic acid in seed oil ranging from 19% to 30%, while the FAM94-41 genotype typically possesses 9% stearic acid. This is in contrast to standard soybean oil, with 2% to 4% stearic acid. In soybean, at least three SAD genes have been identified, designated SAD-A, -B, and -C. The first two are expressed constitutively (Byfield et al., 2006), while SAD-C is more highly expressed in seeds (Zhang et al., 2008). The elevated stearic acid mutants of soybean have recently been characterized at the molecular level and shown to carry changes in SAD-C. For example, in mutant genotype A6 (Hammond and Fehr, 1983b), the SAD-C locus is deleted (Zhang et al., 2008). In mutant genotype FAM94-41, the increased stearic acid content of seeds appears to be allelic with A6 (Pantalone et al., 2002), yet SAD-C expression is not altered and the locus appears to be intact (Zhang et al., 2008). However, a point mutation in the SAD-C open reading frame results in an Asp-to-Asn change at position 126 of the protein, which appears to enhance the in vitro enzymatic activity. Despite this, it is believed that the amino acid change leads to decreased stability of the SAD-C protein in FAM94-41 (Zhang et al., 2008).
The observation that the elevated stearic acid phenotype in the various soybean mutants is linked to SAD is not surprising, given that targeted down-regulation of the expression of SAD genes led to an elevated stearic acid phenotype in Brassica species (Knutzon et al., 1992). To meet the target fatty acid profile in soybean for downstream utility in baking and confectionery applications (i.e. oil with oxidative stability and sufficient percentage of saturated fatty acids for increased meting point), a stacking strategy combining down-regulation of FAD2-1 with either expression of an appropriate FatA and/or silencing of GmSAD-C may provide the desired soybean oil. The precedence for such a strategy was described by Liu et al. (2002); independent transgenic cotton (Gossypium hirsutum) events were generated that carried seed-specific silencing elements directed toward either the Δ12 desaturase (FAD2) or the Δ9 desaturase (SAD). The fatty acid profile of cotton seed from the FAD2 down-regulated events displayed oleic acid levels over 75%, while the seed from the down-regulated SAD events accumulated stearic acid levels up to 40%. Pyramiding of the silencing elements via sexual crossing generated a cotton oil with approximately 40% stearic acid and 37% oleic acid (Liu et al., 2002).
Producing a soybean with oil with sufficient levels of saturated fatty acids for direct use in baking and confectionery applications may be a challenge, given that perturbation of fatty acid flux toward saturates during embryogenesis will likely lead to changes in membrane fluidity, given their high melting points. This in turn may impact agronomics. For example, in soybean with elevated levels of saturated fatty acids, both palmitic acid and stearic acid tend to have germination and yield penalties associated with the phenotype (Fehr, 2007). However, the goal does not necessarily need to be a vegetable oil for direct use per se. The fatty acid profile only needs to serve as a feedstock for cost-effective blending with other saturates and/or interesterification of triacylglycerol to prepare a suitable margarine for baking and confectionery applications (List et al., 1996, 1997, 2001). Hence, a soybean with 20% to 25% stearic acid and 60% to 70% oleic acid may be a reasonable target for an oil that is suitable for blending and/or interesterification purposes (Kok et al., 1999; List et al., 2001) without compromising yield.
Metabolic Engineering for Enhanced Tocopherol Content and Composition
Although oxidative stability of soybean oil is largely determined by the relative amounts of polyunsaturated fatty acids, the content and composition of tocopherols in soybean oil also contribute to oil stability. Tocopherols are lipid-soluble antioxidants that are extracted with triacylglycerols and other oil components during the commercial processing of soybean seeds. These molecules contribute to both the nutritional value and the oxidative stability of soybean oil. Tocopherols are generated in plastids from the condensation of phytol diphosphate from the methylerythritol phosphate pathway and homogentisate from the shikimate pathway (Hunter and Cahoon, 2007). Through a cyclization step and up to two methylation steps, four forms of tocopherol can be produced that differ in the number and/or position of methyl groups on their aromatic head group. These include δ-tocopherol with a single methyl group, γ- and β-tocopherol with two methyl groups (at different positions), and α-tocopherol with three methyl groups. These molecules are collectively referred to as vitamin E. Of these, α-tocopherol is generally regarded as having the highest nutritional value or “vitamin E activity,” because it is the most readily absorbed and retained by human and other mammalian cells (Kamal-Eldin and Appelqvist, 1996). Relative to the other tocopherol forms, δ- and γ-tocopherol have been shown to confer the greatest degree of oxidative stability to vegetable oils for frying applications, although their vitamin E activities are only a fraction of that of α-tocopherol (Wagner and Elmadfa, 2000; Wagner et al., 2001; Warner et al., 2003).
Tocopherols compose approximately 300 μg g−1 of the soybean seed weight and consist of principally γ- and δ-forms, which account for 60% to 70% and 20% to 25% of the total tocopherols, respectively (Almonor et al., 1998; Van Eenennaam et al., 2003). α-Tocopherol is typically only a minor component, representing 10% or less of the tocopherols in soybeans seeds. Although tocopherols compose only approximately 1.5% of the oil extracted from soybean seeds, they are critical for its oxidative stability. For example, foods fried in vegetable oil lacking tocopherols develop lipid oxidation products more quickly that contribute to rancidity (Warner et al., 2003; Warner, 2005). Given that approximately 24% of the soybean oil consumed in the United States is used for baking and frying applications (American Soybean Association, 2009), the chemical antioxidant properties of tocopherols, particularly the γ- and δ-forms, represent an important quality trait. This trait is also of significance for the high-temperature performance of soybean oil in biodiesel and biobased lubricants, which are growing markets for soybean oil (Erhan and Asadauskas, 2000; Knothe et al., 2005).
To date, biotechnological enhancement of tocopherols in soybean seeds has focused on increasing their total content and the relative amounts of α-tocopherol. Efforts to increase the total tocopherol content of soybean seeds, however, have resulted in only marginal success to date. One focus of metabolic engineering strategies has been the up-regulation of homogentisate phytyltransferase (HPT) activity in seeds (Savidge et al., 2002; Karunanandaa et al., 2005). This enzyme catalyzes the first committed step in tocopherol synthesis, the condensation of phytyl-diphosphate and homogentisate, and thus represents a logical target for biotechnological study. However, expression of the Arabidopsis (Arabidopsis thaliana) VTE2 gene for HPT and the corresponding gene from Synechocystis under the control of strong seed-specific promoters in soybean resulted in modest increases of less than 1.5-fold in the total tocopherol content (Karunanandaa et al., 2005). The inability to obtain large enhancements in tocopherol content through HPT up-regulation is likely due to limiting substrate pools of phytyl-diphosphate and/or homogentisate. To address the possible limitation of homogentisate, a bacterial TYRA gene for the bifunctional chorismate mutase-prephenate dehydrogenase was expressed under the control of a seed-specific promoter in soybean (Karunanandaa et al., 2005). This enzyme bypasses Tyr aminotransferase to generate p-hydroxyphenylpyruvate, the immediate precursor of homogentisate. In these experiments, the TYRA gene was coexpressed with Arabidopsis genes for three other enzymes: (1) 4-hydroxyphenylpyruvate dioxygenase, which catalyzes the last step in homogentisate synthesis; (2) geranylgeranyl reductase, which catalyzes the conversion of geranylgeranyl diphosphate to phytol diphosphate; and (3) HPT. Expression of the four transgenes using strong seed-specific promoters yielded high levels of homogentisate and more than 10-fold increase in the total content of vitamin E-type molecules. The enhanced production of homogentisate was visually evidenced by the accumulation of its pigmented oxidation product melanin in the soybean seeds (Karunanandaa et al., 2005). Unexpectedly, the increase in vitamin E content was due almost entirely to the production of tocotrienols, an unsaturated form of vitamin E that is typically found in trace amounts in soybean seeds (Karunanandaa et al., 2005). This alternative form of vitamin E, which is commonly enriched in the endosperm of monocot seeds, is derived from the condensation of the geranylgeranyl diphosphate, rather than phytol diphosphate, with homogentisate (Hunter and Cahoon, 2007). Although this large enhancement in vitamin E-type molecules in soybean seeds represents a metabolic engineering success, it is currently unclear how tocotrienols are formed by strong up-regulation of homogentisate synthesis. The results also indicate that soybean seeds have the metabolic capacity for enhancement of vitamin E content in the form of tocotrienols but apparently lack sufficient pools of phytol diphosphate for the increased production of tocopherols.
A more direct approach for the production of enhanced amounts of tocotrienols is the seed-specific expression of homogentisate geranylgeranyl transferase (HGGT; Cahoon et al., 2003). This enzyme catalyzes the committed step for the biosynthesis of tocotrienols in monocot seed endosperm, and transgenic expression of barley (Hordeum vulgare) HGGT in Arabidopsis leaves and maize (Zea mays) embryos conferred tocotrienol production at high levels (Cahoon et al., 2003). More recently, transgenic expression of HGGT in soybean resulted in tocotrienol accumulation and a 6- to 10-fold increase in total tocopherol and tocotrienol contents of soybean seeds and somatic embryos (Cahoon et al., 2006; Meyer, 2007). These levels achieved with only transgenic expression of HGGT were nearly comparable to those obtained through the four-transgene stack strategy described above.
Enhancement of the α-tocopherol content of soybean seeds has proven to be more amenable to metabolic engineering. This has involved shifting the high content of δ- and γ-tocopherol to the α-form by enhanced expression of genes for two enzymes that methylate the tocopherol head group: 2-methyl-6-phytylbenzoquinol methyltransferase (encoded by VTE3) and γ-tocopherol methyltransferase (encoded by VTE4; Van Eenennaam et al., 2003). The 2-methyl-6-phytylbenzoquinol methyltransferase converts the initial product of HPT to 2,3-dimethyl-5-phytylbenzoquinol. This effectively prevents the ultimate conversion of biosynthetic intermediates in the pathway to δ-tocopherol. The γ-tocopherol methyltransferase catalyzes the final methylation reaction for the formation of α-tocopherol. This enzyme is also able to generate β-tocopherol by methylation of δ-tocopherol. By seed-specific coexpression of the Arabidopsis VTE3 and VTE4 genes, it has been possible to engineer α-tocopherol levels to greater than 90% of the total tocopherol content of soybean seeds (Van Eenennaam et al., 2003). Although this does not increase the total amount of tocopherols in the seed, this shift in composition yields a more nutritious oil with an approximately 5-fold increase in vitamin E activity (Van Eenennaam et al., 2003). A similar approach of coexpression of VTE3 and VTE4 has also been used to generate significant increases in α-tocotrienol content in soybean seeds engineered to produce high levels of tocotrienols (Karunanandaa et al., 2005; Meyer, 2007). Overall, these results indicate that methyltransferase activity is the primary limitation in α-tocopherol/tocotrienol biosynthesis in soybean seeds.
GENETIC STRATEGIES FOR NUTRITIONALLY IMPROVED SOYBEAN OIL
Production of γ-Linolenic Acid and SDA in Soybean
The ability to combine or “stack” multiple genetic cassettes into a plant genome allows for the assembly of biochemical pathways. A vast diversity of fatty acids can be found within the Plantae, although the predominant fatty acids present in most plant species are palmitic, stearic, oleic, linoleic, and linolenic acids, at various ratios depending on species and genotypic variation. Many of the relatively rare fatty acids found in plants have commercial applications, but the relatively high cost of these oils are prohibitive for delivery to the marketplace, due to the low content of theses fatty acids in native sources and/or the limited ability to intensively cultivate the source species. In some plant species, a Δ6 desaturase has been shown to have activity on both linoleic and linolenic acids, leading to the production of γ-linolenic acid (GLA; 18:3Δ6,9,12) and SDA (18:4Δ6,9,12,15), respectively (Fig. 1; Sayanova et al., 1997, 2003). GLA, an ω-6 fatty acid, has been shown to have both pharmacological and nutraceutical properties (Horrobin, 1990, 1992). SDA, an ω-3 fatty acid, is an intermediate in the biosynthesis of the very-long-chain polyunsaturated ω-3 fatty acids EPA and DHA and therefore has nutritional value in food and feed applications.
Constitutive expression of a Δ6 desaturase transgene from the herb Borago officinalis in tobacco (Nicotiana tabacum) resulted in the accumulation in young leaves of 12.9% GLA and 8.5% SDA (Sayanova et al., 1997), with the maximum levels of over 27% GLA and just under 9% SDA in stem sections (Sayanova et al., 1999). Liu et al. (2001) stacked a Δ12 and a Δ6 desaturase gene from the fungus Mortierella alpina in canola. This led to the accumulation of GLA to over 40% and SDA of approximately 2% in seeds. The studies in tobacco with the borage Δ6 desaturase were translated to soybean, in which the data revealed accumulation of GLA and SDA in seeds at 27.7% and 3.4%, respectively (Sato et al., 2004). One of the transgenic soybean events carrying the borage Δ6 desaturase, designated 420-5, was subsequently evaluated under field conditions in Nebraska during the 2005 growing season. No major impact on yield, date to maturity, height, lodging, total oil, or total protein was observed (T.E. Clemente, unpublished data). Moreover, the fatty acid profile at harvest mirrored that observed under greenhouse conditions.
To increase metabolic flux toward SDA synthesis, Ursin (2003) described the stacking of three transgenic cassettes, harboring the M. alpina Δ6 and Δ12 and canola Δ15 desaturase genes, in canola. The transgenic stack resulted in GLA and SDA accumulation of 18% and 23%, respectively. Mimicking this strategy, Eckert et al. (2006) pyramided the Δ6 desaturase gene from borage with the Δ15 desaturase from Arabidopsis in soybean. Transgenic events carrying the Δ15 desaturase generated a fatty acid profile mirroring that of flax (Linum usitatissimum) oil, with percentage of linolenic acid ranging from approximately 35% up to 52% of the total fatty acids. The coordinated expression of the Δ6 and Δ15 genes in a seed-specific fashion produced a fatty acid profile with GLA, linolenic acid, and SDA of 5.8%, 33.5%, and 21.6%, respectively, under greenhouse conditions (Eckert et al., 2006).
Clearly, the main driver for the development of nutritionally enhanced soybean oil is food applications. However, significant market potential for nutritionally enhanced soybean oil may also be found in various feed applications, including poultry, companion pets, and aquaculture. In the latter case, it is estimated that approximately one-third of the world's wild fish harvest is used as feed supplements for various animals, including swine, poultry, and aquaculture (Delgado, 2003). About 25% of the world's fish oil and 33% of the fishmeal supplements used in aquaculture feeds is derived from Peruvian anchovies (Engraulis ringens; Delgado, 2003). Fish species used for both direct and indirect human consumption are facing declines in population because of overharvesting and unfavorable environmental conditions. This has led to a rapid expansion in aquaculture, with an estimated one-quarter of all fish directly consumed by humans arising from farm-raised production (Naylor et al., 2000). Aquaculture offers a great potential to offset the strain put on wild fish populations, but its impact will be marginal if feeds need to be supplemented with fishmeal and oil to ensure productivity and the nutritional quality of the harvested product (Naylor et al., 2000; Delgado, 2003).
To date, a limited number of studies have examined SDA-enriched oils in aquaculture feeds. These oils, however, have shown only limited efficacy in these studies. For example, displacement of fish oil with echium oil (9.0% SDA content) in feeding trials with Atlantic cod (Gadus morhua) led to the accumulation of the SDA in the flesh and a reduction in EPA as compared with fish oil-coated feed (Bell et al., 2006). In a separate feeding trial with Artic charr (Salvelinus alpinus), Tocher et al. (2006) blended fish oil and echium oil and monitored growth characteristic fatty acid profiles in the fish. The data revealed no significant variation in productivity or oil content; however, reduced levels of both EPA and DHA were observed in the fish fed feed coated with the echium oil blend (Tocher et al., 2006). While it can be argued that echium oil contains substantially lower levels of SDA, as compared with what can be achieved through biotechnology approaches in canola (Ursin, 2003) and soybean (Eckert et al., 2006), it is likely that SDA-enriched oil alone will not able to serve as a land-based sustainable source to displace fish oil for the aquaculture industry.
Metabolic Engineering of EPA and DHA Production
The effectiveness of SDA as a nutritional fatty acid is largely dependent on the ability of humans and animals to convert SDA to EPA and DHA. Diets rich in these very-long-chain ω-3 fatty acids have been widely linked to cardiovascular fitness (Marik and Varon, 2009). DHA is also important for neuron synthesis and infant brain development (Uauy et al., 2001, 2003). The conversion of SDA to EPA requires elongation of the fatty acid chain to C20 and Δ5 desaturation, while DHA synthesis further elongated to C22 and the introduction of an additional Δ4 double bond. Partial conversion of dietary SDA to EPA, but not to DHA, has been shown in human and Atlantic salmon (James et al., 2003; Miller et al., 2007). However, higher levels of EPA accumulation were observed with diets containing oils enriched in EPA rather than SDA (James et al., 2003). Similarly, DHA levels in Atlantic salmon were enhanced with a fish oil-based diet that is rich in EPA and DHA (Miller et al., 2007). These results indicate that the inclusion of oils containing EPA and DHA in the diet yields greater nutritional efficacy than can be achieved with the use of SDA-rich oils. Such findings have provided impetus for the development of soybean seeds that accumulate oils with EPA and DHA. It is envisioned that these oils can be produced at a lower cost in soybean seeds compared with fish and algae, the current commercial sources of EPA- and DHA-containing oils. In addition, soybean offers a more sustainable production platform than fish. The likely use of soybean oil enriched in EPA and DHA is the manufacture of food and feed products, including salad oils, infant formulas, and feed rations for farmed fish.
Although a number of different strategies could be used to produce EPA and DHA in soybean seeds, a biosynthetic route that builds off of that described for SDA involves the introduction of a “condensing” enzyme or fatty acid “elongase” to initiate the elongation of SDA to eicosatetraenoic acid (20:4Δ8,11,14,17) and a Δ5 desaturase to generate EPA. The production of DHA through this pathway requires another condensing enzyme to form the C22 docosapentaenoic acid (22:5Δ7,10,13,16,19) and a Δ4 desaturase. Vascular plants do not produce EPA and DHA and therefore lack genes for the synthesis of these fatty acids from SDA. Instead, potential sources of EPA and DHA biosynthetic genes include fungi, marine microalgae, and protists that synthesize very-long-chain polyunsaturated fatty acids (Domergue et al., 2005; Napier, 2007; Damude and Kinney, 2008). Attempts to assemble pathways in soybean using these genes have yielded EPA levels approaching 20% of the total seed fatty acids (Kinney et al., 2004). This was achieved by seed-specific coexpression of transgenes for the Arabidopsis Δ15 desaturase (FAD3), Saprolegnia diclina C20-specific Δ17 desaturase, S. diclina Δ6 desaturase, M. alpina Δ5 desaturase, and M. alpina ELO-type condensing enzyme (Kinney et al., 2004). By addition of transgenes for the Schizochytrium aggregatum Δ4 desaturase and Pavlova species ELO-type condensing enzyme, levels of DHA up to 3% of the total fatty acids were obtained in soybean somatic embryos (Kinney et al., 2004). Similar strategies have recently been reported for the production of EPA to levels of up to 25% of the total oil in Brassica carinata seeds (Cheng et al., 2009). One limitation of this work is that, in addition to the production of EPA and DHA, fatty acid intermediates such as eicosatetraenoic acid and docosapentaenoic acid also accumulate to low but significant levels (Kinney et al., 2004; Cheng et al., 2009). The nutritional properties of these fatty acids and their mixtures with EPA and DHA are largely uncharacterized. Ultimately, the technical success of these metabolic engineering efforts will require the production of EPA and DHA at economically viable levels with minimal amounts of intermediates. Beyond this, potential regulatory approval of these traits is complicated by the requirement for at least four transgenes for EPA synthesis and six transgenes for DHA synthesis. If commercial production is targeted, the regulatory process for these traits will likely provide an important precedent for the delivery of other genetically complex nutritional traits to the marketplace.
Metabolic Engineering for Increased Total Oil Content
As described above, soybean is widely recognized as a dual-use crop because its seeds are enriched in protein and oil. The demand for soybean oil has increased dramatically, partially due to expanded use of biodiesel. In 2004, for example, 95% of the soybean oil in the United States was used by the food industry, but by 2008, 19% of the U.S. domestic soybean consumption was for nonfood uses, principally the manufacture of biodiesel (American Soybean Association, 2009). As U.S. soybean production has remained virtually flat during this period (American Soybean Association, 2009), the increased demand for soybean oil has in part contributed to large increases in the price of soybean oil.
The high demand for soybean oil has sparked an interest in increasing the relative content of oil per seed. Oil accounts for 18% to 20% of the weight of soybean seeds, which is low relative to most other oilseed crops. Seeds of peanut (Arachis hypogaea), another legume, for example, contain approximately 45% oil per seed weight. The prospects of retooling the metabolism of soybean seeds to yield such high content of oil are daunting. Instead, the current focus of both breeding and transgenic efforts has been on increasing the oil content of soybean seeds incrementally without affecting protein content. This strategy addresses the need for more oil yield from soybean without compromising the dual-use nature of this crop.
A number of transgenic approaches have been explored for increasing seed oil content. Although some of this research has been conducted with oilseed crops, most of these efforts have used Arabidopsis as a model for oilseeds. Seed oils are composed almost exclusively of triacylglycerols, but they do contain small amounts of other lipidic compounds such as phospholipids and tocopherols. Triacylglycerols consist of three fatty acid chains bound to a glycerol backbone. As such, metabolic engineering efforts for seed oil enhancement have focused on either increasing the synthesis of fatty acids or increasing their incorporation onto the glycerol backbone. In the former case, this has involved attempts to enhance the partitioning of carbon toward fatty acid synthesis and to increase the pool sizes of substrates for fatty acid synthesis. A variety of approaches have been used, including the overexpression of transcription factors, such as WRI1 (Cernac and Benning, 2004; Baud et al., 2007), and key metabolic enzymes, such as acetyl-CoA carboxylase (Roesler et al., 1997). Experiments to increase oil content by enhancing fatty acid sequestration onto the glycerol backbone have centered on acyltransferases, including diacylglycerol acyltransferase (DGAT). This enzyme catalyzes the last step of triacylglycerol biosynthesis, the incorporation of a fatty acyl-CoA onto diacylglycerol. Two structurally divergent classes of membrane-associated DGATs (DGAT1 and DGAT2) occur in plants and other organisms (Cases et al., 1998, 2001; Lardizabal et al., 2001).
The only transgenic success reported to date for enhancement of soybean oil content was achieved by the introduction of a seed-specific transgene for a DGAT2-type enzyme from the oil-accumulating fungus Umbelopsis ramanniana (Lardizabal et al., 2008). In these studies, the oil content was increased from approximately 20% of the seed weight to approximately 21.5%. This increase was stable over three growing seasons in two different geographical locations. Importantly, the increased oil content had little or no impact on the protein levels of seeds and the seed yield per acre. It was estimated that at March 2008 soybean oil prices ($0.61 U.S. [USD] per pound), this increase in oil content would translate into a $1.26 billion USD increase in the value of the U.S. soybean crop (Lardizabal et al., 2008). At current soybean oil prices (approximately $0.35 USD per pound), this would be a nearly $725 million USD increase in the value of the domestic soybean crop. It is anticipated that other metabolic engineering strategies will be translated from Arabidopsis to soybean to yield similar or perhaps more substantial increases in seed oil content.
PROSPECTS FOR A BETTER BEAN
Plantings of low-lin soybean began in 2005 on 60,700 ha and reached over 809,000 ha in 2007 (Debruyne, 2007). DuPont recently announced the market release of Plenish, a high oleic acid soybean (>80%) generated through down-regulation of FAD2-1A and -1B, for 2010, and Monsanto currently has SDA soybean oil in phase III development, with expected market release by 2015. The future of the other novel soybean traits is influenced by two criteria: (1) the ability to deliver the trait in high-yielding genotypes, and (2) the net projected value of the trait. The latter encompasses both the market potential of the trait and regulatory costs associated with national and international regulatory approval processes governing the commercialization of biotechnology-derived traits.
The future strides in making a better bean, though, will be greatly facilitated by the wealth of genomics tools available for soybean (Shoemaker et al., 2003; Jackson et al., 2006), including the recent release of the 8X draft sequence (www.phytozome.net/soybean). These resources combined with efforts targeting proteomics (Hajduch et al., 2005), metabolomics (Bino et al., 2004), and flux map analysis (Iyer et al., 2008), both within Glycine and comparative genomics efforts across genera, will allow researchers to gain insight into carbon flow during embryogenesis. This, in turn, will allow for the designing of experiments that target manipulation of the metabolic control points. Such studies will address questions underlying the relative partitioning of carbon between seed storage components and the intricacies of fatty acid metabolism in soybean seeds. This information will provide the blueprints for the designer soybean of the future.
This work was supported by the National Science Foundation (grant no. DBI 071919 to E.B.C.), the Nebraska Soybean Board (to T.E.C. and E.B.C.), and the United Soybean Board (to T.E.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Tom E. Clemente (tclemente1@unl.edu).
References
- Abbadi A, Domergue F, Bauer J, Napier JA, Welti R, Zahringer U, Cirpus P, Heinz E (2004) Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell 16 2734–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almonor GO, Fenner GP, Wilson RF (1998) Temperature effects on tocopherol composition in soybeans with genetically improved oil quality. J Am Oil Chem Soc 75 591–596 [Google Scholar]
- Alt JL, Fehr WR, Welke GA, Sandu D (2005. a) Phenotypic and molecular analysis of oleate content in the mutant soybean line M23. Crop Sci 45 1997–2000 [Google Scholar]
- Alt JL, Fehr WR, Welke GA, Shannon JG (2005. b) Transgressive segregation for oleate content in three soybean populations. Crop Sci 45 2005–2007 [Google Scholar]
- American Soybean Association (2009) SoyStats, A Reference Guide to Important Soybean Facts and Figures. http://www.soystats.com/2009/ (October 6, 2009)
- Bachlava E, Burton JW, Brownie C, Wang S, Auclair J, Cardinal AJ (2008. a) Heritability of oleic acid content in soybean seed oil and its genetic correlation with fatty acid and agronomics traits. Crop Sci 48 1764–1772 [Google Scholar]
- Bachlava E, Dewey RE, Auclair J, Wang S, Burton JW, Cardinal AJ (2008. b) Mapping genes encoding microsomal w-6 desaturase enzymes and their cosegregation with QTL affecting oleate content in soybean. Crop Sci 48 640–650 [Google Scholar]
- Baud S, Mendoza MS, To A, Harscoet E, Lepiniec L, Dubreucq B (2007) WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50 825–838 [DOI] [PubMed] [Google Scholar]
- Bell JG, Strachan F, Good JE, Tocher DR (2006) Effect of dietary echium oil on growth, fatty acid composition and metabolism, gill prostaglandin production and macrophage activity in Atlantic cod (Gadus morhua L.). Aquacult Res 37 606–617 [Google Scholar]
- Bennett JO, Krishnan HK, Wiebold WJ, Krishnan HB (2003) Positional effect on protein and oil content and composition of soybeans. J Agric Food Chem 51 6882–6886 [DOI] [PubMed] [Google Scholar]
- Berti M, Johnson BL, Dash S, Fischer S, Wilckens R, Hevia F (2007) Echium: a source of stearidonic acid adapted to the northern great plains in the US. In J Janick, A Whipkey, eds, Issues in New Crops and New Uses. ASHS Press, Alexandria, VA, pp 120–125
- Bilyeu KD, Palavalli L, Sleper DA, Beuselinck PR (2003) Three microsomal omega-3 fatty-acid desaturase genes contribute to soybean linolenic acid levels. Crop Sci 43 1833–1838 [Google Scholar]
- Bino RJ, Hall RD, Fiehn O, Kopka J, Saito K, Draper J, Nikolau BJ, Mendes P, Roessner-Tunali U, Beale MH, et al (2004) Potential of metabolomics as a functional genomics tool. Trends Plant Sci 9 418–425 [DOI] [PubMed] [Google Scholar]
- Bonanome A, Grundy SM (1988) Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N Engl J Med 318 1244–1248 [DOI] [PubMed] [Google Scholar]
- Bubeck DM, Fehr WR, Hammond EG (1989) Inheritance of palmitic and stearic acid mutants of soybean. Crop Sci 29 652–656 [Google Scholar]
- Buhr T, Sato S, Ebrahim F, Xing A, Zhou Y, Mathiesen M, Schweiger B, Kinney AJ, Staswick P, Clemente T (2002) Ribozyme termination of RNA transcripts down-regulate seed fatty acid genes in transgenic soybean. Plant J 30 155–163 [DOI] [PubMed] [Google Scholar]
- Burgal J, Shockey J, Lu C, Dyer JM, Larson T, Graham IA, Browse J (2008) Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J 6 819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byfield GE, Xue H, Upchurch RG (2006) Two genes from soybean encoding soluble Δ9 stearoyl-ACP desaturases. Crop Sci 46 840–846 [Google Scholar]
- Cahoon EB (2003) Genetic enhancement of soybean oil for industrial uses: prospects and challenges. AgBioForum 6 11–13 [Google Scholar]
- Cahoon EB, Coughlan SJ, Cahoon RE, Butler KH (2006) Compositions and methods for altering tocotrienol content. United States Patent No. 7154029
- Cahoon EB, Hall SE, Ripp KG, Ganzke TS, Hitz WD, Coughlan SJ (2003) Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat Biotechnol 21 1082–1087 [DOI] [PubMed] [Google Scholar]
- Cahoon EB, Shockley JM, Dietrich CR, Gidda SK, Mullen RT, Dyer JM (2007) Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: solving bottlenecks in fatty acid flux. Curr Opin Biotechnol 10 236–244 [DOI] [PubMed] [Google Scholar]
- Canakci M, Monyem A, Van Gerpen J (1999) Accelerated oxidation processes in biodiesel. Trans ASAE 42 1565–1572 [Google Scholar]
- Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, et al (1998) Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA 95 13018–13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV Jr (2001) Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem 276 38870–38876 [DOI] [PubMed] [Google Scholar]
- Cernac A, Benning C (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40 575–585 [DOI] [PubMed] [Google Scholar]
- Chappell AS, Bilyeu KD (2006) A GmFAD3A mutation in the low linolenic acid mutant C1640. Plant Breed 125 535–536 [Google Scholar]
- Cheng B, Wu G, Vrinten P, Falk K, Bauer J, Qiu X (2009) Towards the production of high levels of eicosapentaenoic acid in transgenic plants: the effects of different host species, genes and promoters. Transgenic Res (in press) [DOI] [PubMed]
- Damude HG, Kinney AJ (2007) Engineering oilseed plants for sustainable, land-based source of long chain polyunsaturated fatty acids. Lipids 42 179–185 [DOI] [PubMed] [Google Scholar]
- Damude HG, Kinney AJ (2008) Engineering oilseeds to produce nutritional fatty acids. Physiol Plant 132 1–10 [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJL, Ezzati M (2009) The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 6 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne I (2007) Novel soybean oil products for healthier nutrition: recent developments, market introduction and targeted commercialization. Lipid Technol 19 128–131 [Google Scholar]
- Delgado CL (2003) Implications for fisheries technology needs and prospects. In CL Delgado, N Wada, MW Rosegrant, S Meijer, M Ahmed, eds, Fish to 2020: Supply and Demand in Changing Global Markets. Technical Report 62. Worldfish Center, Washington, DC, pp 81–104
- Domergue F, Abbadi A, Heinz E (2005) Relief for fish stocks: oceanic fatty acids in transgenic oilseeds. Trends Plant Sci 10 112–116 [DOI] [PubMed] [Google Scholar]
- Eckert H, LaVallee BJ, Schweiger BJ, Kinney AJ, Cahoon EB, Clemente T (2006) Co-expression of the borage Δ6 desaturase and the Arabidopsis Δ15 desaturase results in high accumulation of stearidonic acid in the seeds of transgenic soybean. Planta 224 1050–1057 [DOI] [PubMed] [Google Scholar]
- Erhan SZ, Asadauskas S (2000) Lubricant basestocks from vegetable oils. Ind Crops Prod 11 277–282 [Google Scholar]
- Facciotti MT, Bertain PB, Yuan L (1999) Improved stearate phenotype in transgenic canola expressing a modified acyl-acyl carrier protein thioesterase. Nat Biotechnol 17 593–597 [DOI] [PubMed] [Google Scholar]
- Fehr WR (2007) Breeding for modified fatty acid composition in soybean. Crop Sci (Suppl 3) 47: S72–S87 [Google Scholar]
- Fehr WR, Welke GA, Hammond EG, Duvick DN, Cianzio SR (1992) Inheritance of reduced linolenic acid content in soybean genotypes A16 and A17. Crop Sci 32 903–906 [Google Scholar]
- Flores T, Karpova O, Su X, Zheng P, Bilyeu K, Sleper DA, Nguyen HT, Zhang ZJ (2008) Silencing of the GmFAD3 gene by siRNA leads to low a-linolenic acids (18:3) of fad3-mutant phenotype in soybean Glycine max (Merr.). Transgenic Res 17 839–850 [DOI] [PubMed] [Google Scholar]
- Graef G, LaVallee BJ, Tenopir P, Tat ME, Schweiger BJ, Kinney AJ, Van Gerpen J, Clemente TE (2009) A high oleic acid and low palmitic acid soybean: agronomic performance and evaluation as a feedstock for biodiesel. Plant Biotechnol J 7 411–421 [DOI] [PubMed] [Google Scholar]
- Griffiths G, Brechany EY, Jackson FM, Christie WW, Stymne S (1996) Distribution and biosynthesis of stearidonic acid in leaves of Borago officinalis. Phytochemistry 43 381–386 [Google Scholar]
- Hajduch M, Ganapathy A, Stein JW, Thelen JJ (2005) A systematic proteomic study of seed filling in soybean: establishment of high-resolution two-dimensional reference maps, expression profiles, and an interactive proteome database. Plant Physiol 137 1397–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond EG, Fehr WR (1983. a) Registration of A5 germplasm line of soybean. Crop Sci 23 192 [Google Scholar]
- Hammond EG, Fehr WR (1983. b) Registration of A6 germplasm line of soybean. Crop Sci 23 192–193 [Google Scholar]
- Hawkins DJ, Kridl JC (1998) Characterization of acyl-ACP thioesterases of mangosteen (Garcinia mangostana) seed and high levels of stearate production in transgenic canola. Plant J 13 743–752 [DOI] [PubMed] [Google Scholar]
- Heppard EP, Kinney AJ, Stecca KL, Miao GH (1996) Developmental and growth temperature regulation of two different microsomal ω-6 desaturase genes in soybeans. Plant Physiol 110 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrobin DF (1990) Gamma linolenic acid: an intermediate in essential fatty acid metabolism with potential as an ethical pharmaceutical and as a food. Rev Contemp Pharmacother 1 1–45 [Google Scholar]
- Horrobin DF (1992) Nutritional and medical importance of gamma-linolenic acid. Prog Lipid Res 31 163–194 [DOI] [PubMed] [Google Scholar]
- Hunter SC, Cahoon EB (2007) Enhancing vitamin E in oilseeds: unraveling tocopherol and tocotrienol biosynthesis. Lipids 42 97–108 [DOI] [PubMed] [Google Scholar]
- Iyer VV, Sriram G, Fulton DB, Zhou R, Westgate ME, Shanks JV (2008) Metabolic flux maps comparing the effect of temperature on protein and oil biosynthesis in developing soybean cotyledons. Plant Cell Environ 31 506–517 [DOI] [PubMed] [Google Scholar]
- Jackson SA, Rokhsar D, Stacey G, Shoemaker R, Schmutz J, Grimwood J (2006) Toward a reference sequence of the soybean genome: a multiagency effort. Plant Genome 46 S-55–S-61 [Google Scholar]
- James MJ, Ursin VM, Cleland LG (2003) Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am J Clin Nutr 77 1140–1145 [DOI] [PubMed] [Google Scholar]
- Jaworski J, Cahoon EB (2003) Industrial oils from transgenic plants. Curr Opin Plant Biol 6 178–184 [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A, Appelqvist LA (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31 671–701 [DOI] [PubMed] [Google Scholar]
- Karunanandaa B, Qi Q, Hao M, Baszis SR, Jensen PK, Wong YH, Jiang J, Venkatramesh M, Gruys KJ, Moshiri F, et al (2005) Metabolically engineered oilseed crops with enhanced seed tocopherol. Metab Eng 7 384–400 [DOI] [PubMed] [Google Scholar]
- Kinney AJ (1998) Plants as industrial chemical factories: new oils from genetically engineered soybeans. Fett/Lipid 100 173–179 [Google Scholar]
- Kinney AJ (2003) Engineering soybeans for food and health. AgBioForum 6 1–5 [Google Scholar]
- Kinney AJ, Cahoon EB, Damude HG, Hitz WD, Kolar CW, Liu ZB (2004) Production of very long chain polyunsaturated fatty acids in oilseed plants. World Patent Application No. WO 2004/071467
- Kinney AJ, Knowlton S (1997) Designer oils: the high oleic soybean. In S Harander, S Roller, eds, Genetic Engineering for Food Industry: A Strategy for Food Quality Improvement. Blackie Academic, London, pp 193–213
- Knothe G, Van Gerpen JH, Krahl J (2005) The Biodiesel Handbook. AOCS Press, Champaign, IL
- Knutzon DS, Thompson GA, Radke SE, Johnson WB, Knauf VC, Kridl JC (1992) Modification of Brassica seed oil by antisense expression of a stearoyl-acyl carrier protein desaturase gene. Proc Natl Acad Sci USA 89 2624–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok LL, Fehr WR, Hammond EG, White PJ (1999) Trans-free margarine from highly saturated soybean oil. J Am Oil Chem Soc 76 1175–1181 [Google Scholar]
- Kris-Etherton PM, Griel AE, Psota TL, Gebauer SK, Zhang J, Etherton TD (2005) Dietary stearic acid and risk of cardiovascular disease: intake, sources, digestion, and adsorption. Lipids 40 1193–1200 [DOI] [PubMed] [Google Scholar]
- Lampert D (1999) High stability oils: What are they? How are they made? Why do we need them? In N Widlak, ed, Physical Properties of Fats, Oils and Emulsifiers. AOCS Press, Champaign, IL, pp 238–246
- Lardizabal K, Effertz R, Levering C, Mai J, Pedroso MC, Jury T, Aasen E, Gruys K, Bennett K (2008) Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol 148 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardizabal KD, Mai JT, Wagner NW, Wyrick A, Voelker T, Hawkins DJ (2001) DGAT2 is a new diacylglycerol acyltransferase gene family: purification, cloning, and expression in insect cells of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity. J Biol Chem 276 38862–38869 [DOI] [PubMed] [Google Scholar]
- List GR, Mounts TL, Orthoefer F, Neff WE (1996) Potential margarine oils from genetically modified soybeans. J Am Oil Chem Soc 73 729–732 [Google Scholar]
- List GR, Mounts TL, Orthoefer F, Neff WE (1997) Effect of interesterification on the structure and physical properties of high-stearic acid soybean oils. J Am Oil Chem Soc 74 327–329 [Google Scholar]
- List GR, Pelloso T, Orthoefer F, Warner K, Neff WE (2001) Soft margarines from high stearic acid soybean oils. J Am Oil Chem Soc 78 103–104 [Google Scholar]
- Liu JW, DeMichele S, Bergana M, Bobik E Jr, Hastilow C, Chuang LT, Mukerji P, Huang YS (2001) Characterization of oil exhibiting high γ-linolenic acid from a genetically transformed canola strain. J Am Oil Chem Soc 78 489–493 [Google Scholar]
- Liu Q, Singh S, Green A (2002) High-oleic and high-stearic cottonseed oils: nutritionally improved cooking oils developed using gene silencing. J Am Coll Nutr 21 205S–211S [DOI] [PubMed] [Google Scholar]
- Mahmoud AA, Natarajan SS, Bennett JO, Mawhinney TP, Wiebold WJ, Krishnan HB (2006) Effect of six decades of selective breeding on soybean protein composition and quality: a biochemical and molecular analysis. J Agric Food Chem 54 3916–3922 [DOI] [PubMed] [Google Scholar]
- Marik PE, Varon J (2009) Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol 32 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B, Krebbers E, Tingey S (1999) Gene discovery and product development for grain quality traits. Science 285 372–375 [DOI] [PubMed] [Google Scholar]
- Meyer K (2007) Compositions and methods for altering alpha- and beta-tocotrienol content. World Patent Application No. WO 2007/059077
- Miller MR, Nichols PD, Carter CG (2007) Replacement of dietary fish oil for Atlantic salmon parr (Salmo salar L.) with a stearidonic acid containing oil has no effect on omega-3 long-chain polyunsaturated fatty acid concentrations. Comp Biochem Physiol B Biochem Mol Biol 146 197–206 [DOI] [PubMed] [Google Scholar]
- Monteros MJ, Burton JW, Boerma HR (2008) Molecular mapping and confirmation of QTLs associated with oleic acid content in N00-3350 soybean. Crop Sci 48 2223–2234 [Google Scholar]
- Moser BR, Haas MJ, Jackson MA, Erhan SV, List GR (2007) Evaluation of partially hydrogenated methyl esters of soybean oil as biodiesel. Eur J Lipid Sci Technol 109 17–24 [Google Scholar]
- Napier JA (2007) The production of unusual fatty acids in transgenic plants. Annu Rev Plant Biol 58 295–319 [DOI] [PubMed] [Google Scholar]
- Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MC, Clay J, Folke C, Lubchenco J, Mooney H, Troell M (2000) Effect of aquaculture on world fish supplies. Nature 405 1017–1024 [DOI] [PubMed] [Google Scholar]
- Pantalone VR, Wilson RF, Novitzky WP, Burton JW (2002) Genetic regulation of elevated stearic acid concentration in soybean oil. J Am Oil Chem Soc 2002 549–553 [Google Scholar]
- Rahman SM, Anai T, Kinoshita T, Takagi Y (2003) A novel soybean germplasm with elevated saturated fatty acids. Crop Sci 43 527–531 [Google Scholar]
- Rahman SM, Kinoshita T, Anai T, Takagi Y (2001) Combining ability in loci for high oleic and low linolenic acids in soybean. Crop Sci 41 26–29 [Google Scholar]
- Rahman SM, Takagi Y, Miyamoto K, Kawakita T (1995) High stearic acid soybean mutant induced by x-ray irradiation. Biosci Biotechnol Biochem 59 922–923 [Google Scholar]
- Reinprecht Y, Luk-Labey SY, Larsen J, Poysa VW, Yu K, Rajcan I, Ablett GR, Pauls KP (2009) Molecular basis of the low linolenic acid trait on soybean EMS mutant line RG10. Plant Breed 128 253–258 [Google Scholar]
- Roesler K, Shintani D, Savage L, Boddupalli S, Ohlrogge J (1997) Targeting of the Arabidopsis homomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds. Plant Physiol 113 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Xing A, Ye X, Schweiger B, Kinney A, Graef G, Clemente T (2004) Production of γ-linolenic acid and stearidonic acid in seeds of marker-free transgenic soybean. Crop Sci 44 646–652 [Google Scholar]
- Savidge B, Weiss JD, Wong YH, Lassner MW, Mitsky TA, Shewmaker CK, Post-Beittenmiller D, Valentin HE (2002) Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol 129 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayanova O, Davies GM, Smith MA, Griffiths G, Stobart AK, Shewry PR, Napier JA (1999) Accumulation of Δ6-unsaturated fatty acids in transgenic tobacco plants expressing a Δ6-desaturase from Borago officinalis. J Exp Bot 50 1647–1652 [Google Scholar]
- Sayanova O, Smith MA, Lapinskas P, Stobart AK, Dobson G, Christie WW, Shewry PR, Napier JA (1997) Expression of a borage desaturase cDNA containing an N-terminal cytochrome b5 domain results in the accumulation of high levels of Δ6-desaturated fatty acids in transgenic tobacco. Proc Natl Acad Sci USA 94 4211–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayanova OV, Beaudoin F, Michaelson LV, Shewry PR, Napier JA (2003) Identification of Primula fatty acid Δ6-desaturases with n-3 substrate preferences. FEBS Lett 542 100–104 [DOI] [PubMed] [Google Scholar]
- Scherder CW, Fehr WR (2008) Agronomic and seed characteristics of soybean lines with increased oleate content. Crop Sci 48 1755–1758 [Google Scholar]
- Schwender J, Ohlrogge JB, Shachar-Hill Y (2003) A flux model of glycolysis and the oxidative pentosephosphate pathway in developing Brassica napus embryos. J Biol Chem 278 29442–29453 [DOI] [PubMed] [Google Scholar]
- Shoemaker RC, Schlueter JA, Cregan P, Vodkin L (2003) The status of soybean genomics and its role in the development of soybean biotechnologies. AgBioForum 6 4–7 [Google Scholar]
- Spencer MM, Pantalone VR, Meyer EJ, Landau-Ellis D, Hyten DL Jr (2003) Mapping the fas locus controlling stearic acid content in soybean. Theor Appl Genet 106 615–619 [DOI] [PubMed] [Google Scholar]
- Stojsin D, Luzzi BM, Ablett GR, Tanner JW (1998) Inheritance of low linolenic acid levels in the soybean line RG10. Crop Sci 38 1441–1444 [Google Scholar]
- Takagi Y, Rahman SM (1996) Inheritance of high oleic acid content in the seed oil of soybean mutant M23. Theor Appl Genet 92 179–182 [DOI] [PubMed] [Google Scholar]
- Tang GQ, Novitzky WP, Griffin HC, Huber SC, Dewey RE (2005) Oleate desaturase enzymes of soybean: evidence of regulation through differential stability and phosphorylation. Plant J 44 433–446 [DOI] [PubMed] [Google Scholar]
- Tocher DR, Dick JR, MacGlaughlin P, Bell JG (2006) Effect of diets enriched in Δ6 desaturated fatty acids (18:3n-6 and 18:4n-3), on growth, fatty acid composition and highly unsaturated fatty acid synthesis in two populations of Arctic charr (Salvelinus alpinus L.). Comp Biochem Physiol B 144 245–253 [DOI] [PubMed] [Google Scholar]
- Uauy R, Hoffman DR, Mena P, Llanos A, Birch EE (2003) Term infant studies of DHA and ARA supplementation on neurodevelopment: results of randomized controlled trials. J Pediatr 143 S17–S25 [DOI] [PubMed] [Google Scholar]
- Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE (2001) Essential fatty acids in visual and brain development. Lipids 36 885–895 [DOI] [PubMed] [Google Scholar]
- Ursin VM (2003) Modification of plant lipids for human health: development of functional land-based omega-3 fatty acids. J Nutr 133 4271–4274 [DOI] [PubMed] [Google Scholar]
- Van Eenennaam AL, Lincoln K, Durrett TP, Valentin HE, Shewmaker CK, Thorne GM, Jiang J, Baszis SR, Levering CK, Aasen ED, et al (2003) Engineering vitamin E content: from Arabidopsis mutant to soy oil. Plant Cell 15 3007–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KH, Elmadfa I (2000) Effects of tocopherols and their mixtures on the oxidative stability of olive oil and linseed oil under heating. Eur J Lipid Sci Technol 102 624–629 [Google Scholar]
- Wagner KH, Wotruba F, Elmadfa I (2001) Antioxidative potential of tocotrienols and tocopherols in coconut fat at different oxidation temperatures. Eur J Lipid Sci Technol 103 746–751 [Google Scholar]
- Warner K (2005) Effects on the flavor and oxidative stability of stripped soybean and sunflower oils with added pure tocopherols. J Agric Food Chem 53 9906–9910 [DOI] [PubMed] [Google Scholar]
- Warner K (2009) Oxidative and flavor stability of tortilla chips fried in expeller pressed low linolenic acid soybean oil. J Food Lipids 16 133–147 [Google Scholar]
- Warner K, Neff WE, Eller FJ (2003) Enhancing quality and oxidative stability of aged fried food with g-tocopherol. J Agric Food Chem 51 623–627 [DOI] [PubMed] [Google Scholar]
- Wilcox JR, Cavins JF (1985) Inheritance of low linolenic acid content of the seed oil of a mutant Glycine max. Theor Appl Genet 71 74–78 [DOI] [PubMed] [Google Scholar]
- Wilson RF (2004) Seed composition. In HR Boerma, JE Specht, eds, Soybeans: Improvement, Production, and Uses, Vol 3. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, WI, pp 621–677
- Zhang P, Burton JW, Upchurch RG, Whittle E, Shanklin J, Dewey RE (2008) Mutations in a Δ9-stearoyl-ACP-desaturase gene are associated with enhanced stearic acid levels in soybean seeds. Crop Sci 48 2305–2313 [Google Scholar]